Generation Dependent Effects and Entrance to Mitochondria of Hybrid Dendrimers on Normal and Cancer Neuronal Cells In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Dendrimers
2.2. Cell Culture
2.3. Cytotoxicity Evaluation
2.4. Measurement of Reactive Oxygen Species (ROS)
2.5. Assessment of Mitochondrial Membrane Potential (Ψm)
2.6. Visualisation of Cell Morphology by Confocal Microscopy Imaging
2.7. Determination of Apoptosis and Necrosis by OA/EB Double Staining—Confocal Microscopy Imaging
2.8. Determination of Apoptotic and Dead Cells by Annexin V Staining-Flow Cytometry Analysis
2.9. TEM
2.10. Statistical Analysis
3. Results
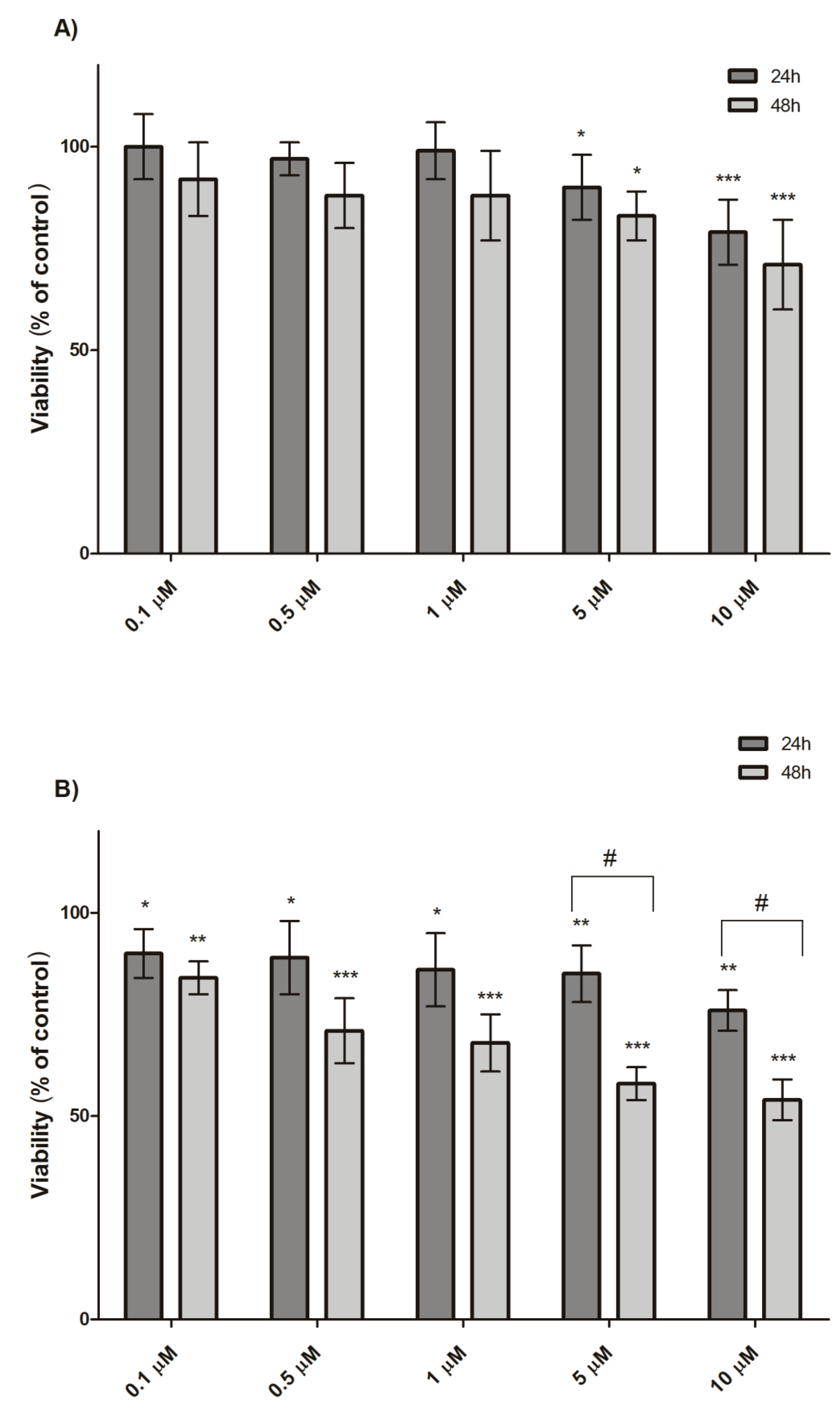
3.1. Cytotoxicity Evaluation
3.2. Measurement of Reactive Oxygen Species (ROS)
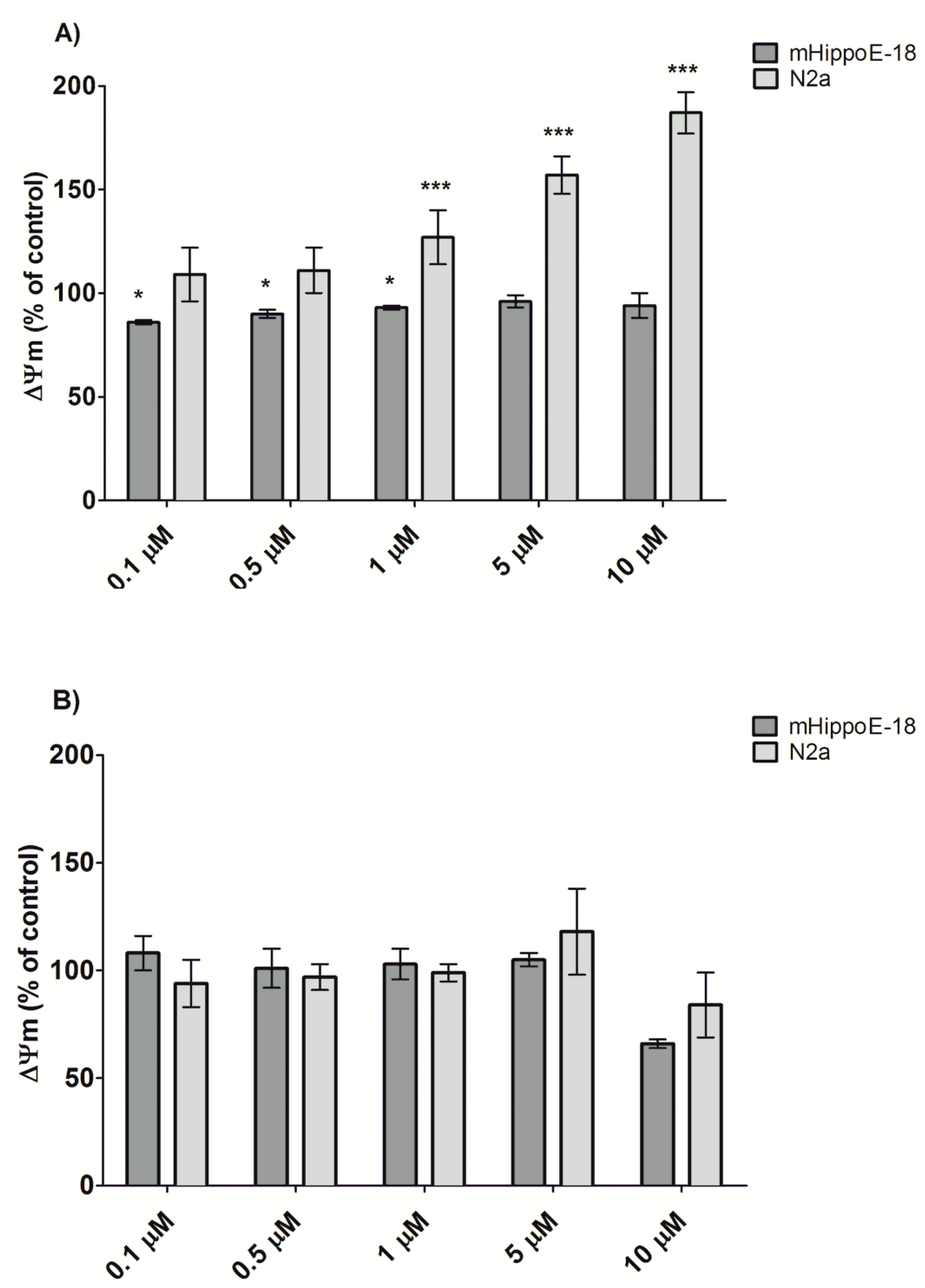
3.3. Alteration in Mitochondrial Membrane Potential (Ψm)
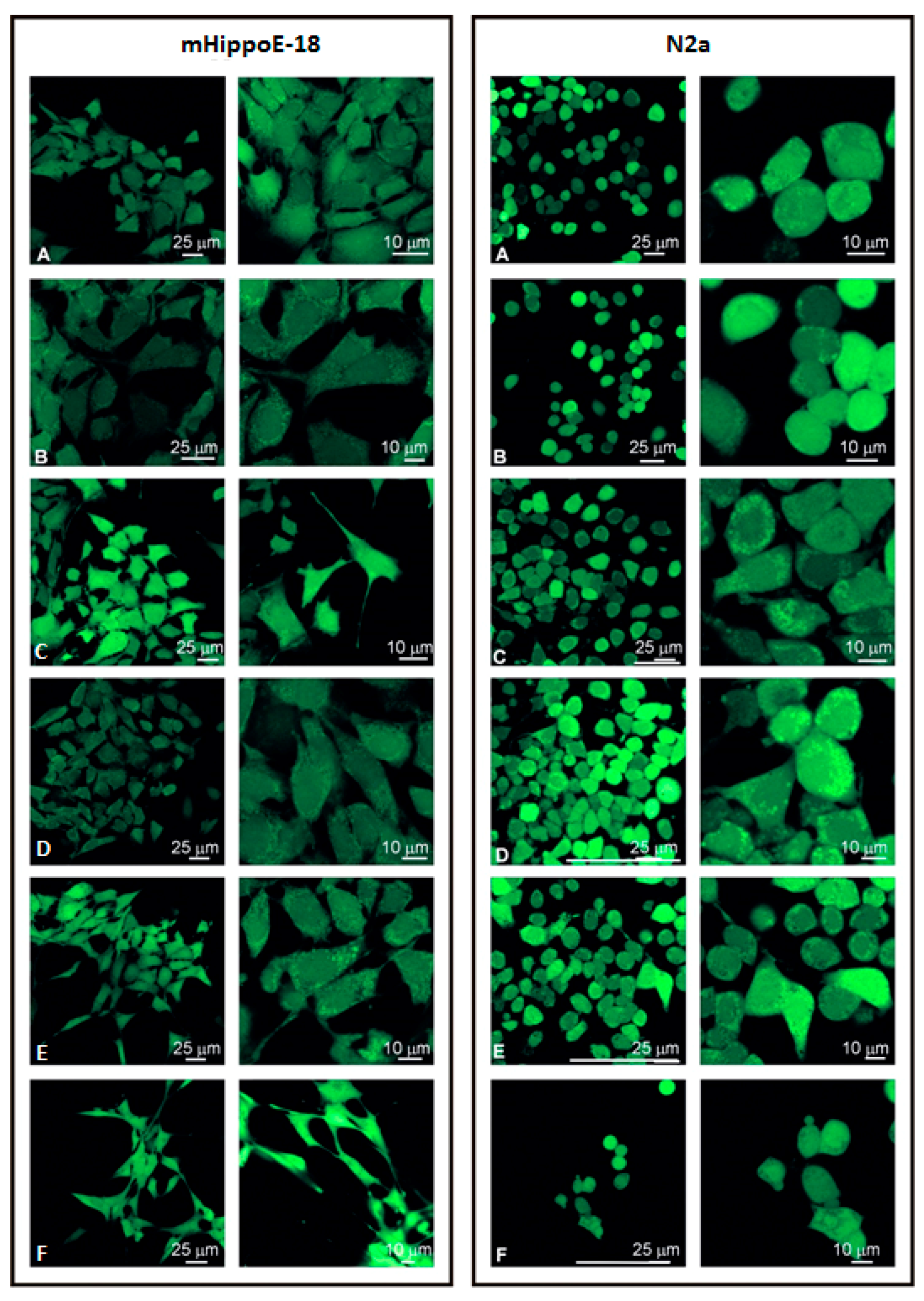
3.4. Visualisation of Cell Morphology by Confocal Microscopy Imaging
3.5. Determination of Apoptosis and Necrosis by Orange Acridine/Ethidium Bromide (OA/EB) Double Staining—Confocal Microscopy Imaging
3.6. Determination of Apoptotic and Dead Cells by annexin-V and propidium iodide Staining—Flow Cytometry Analysis
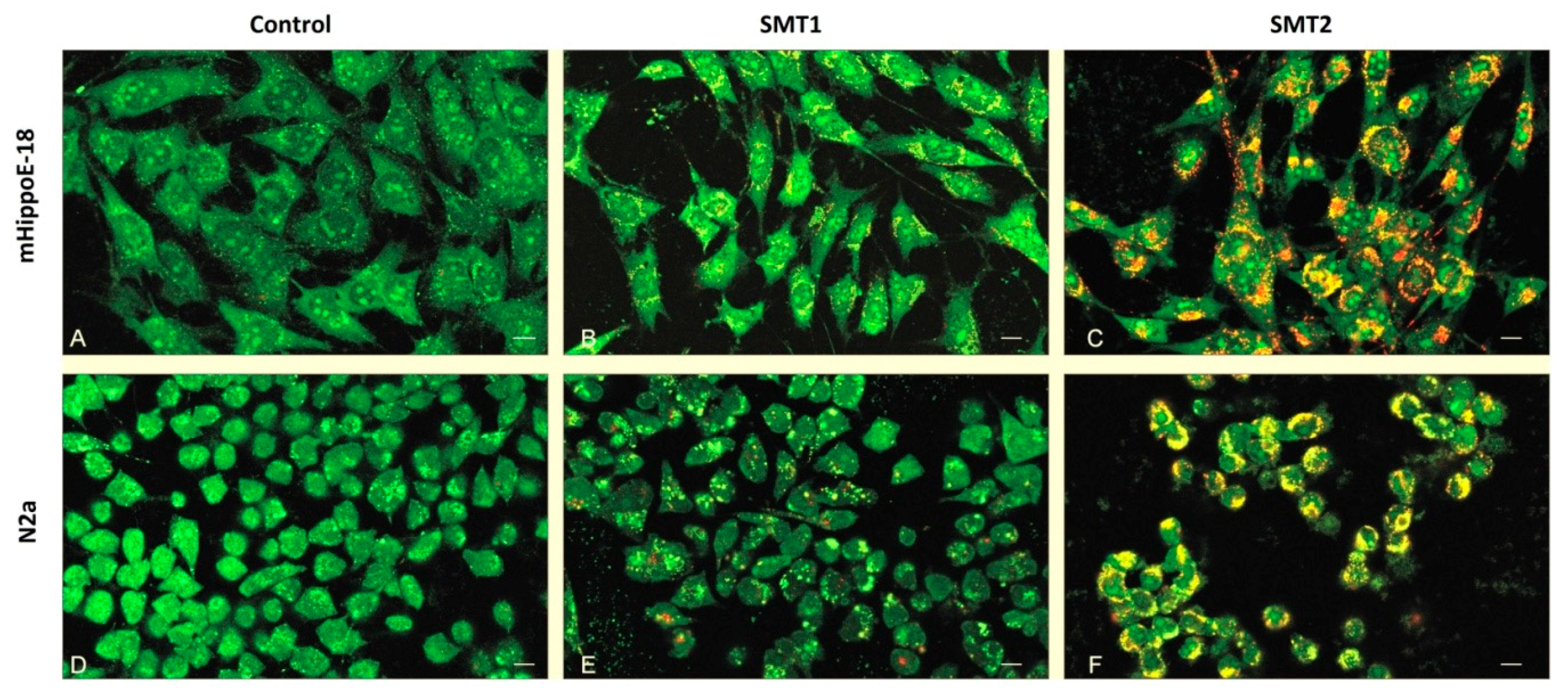
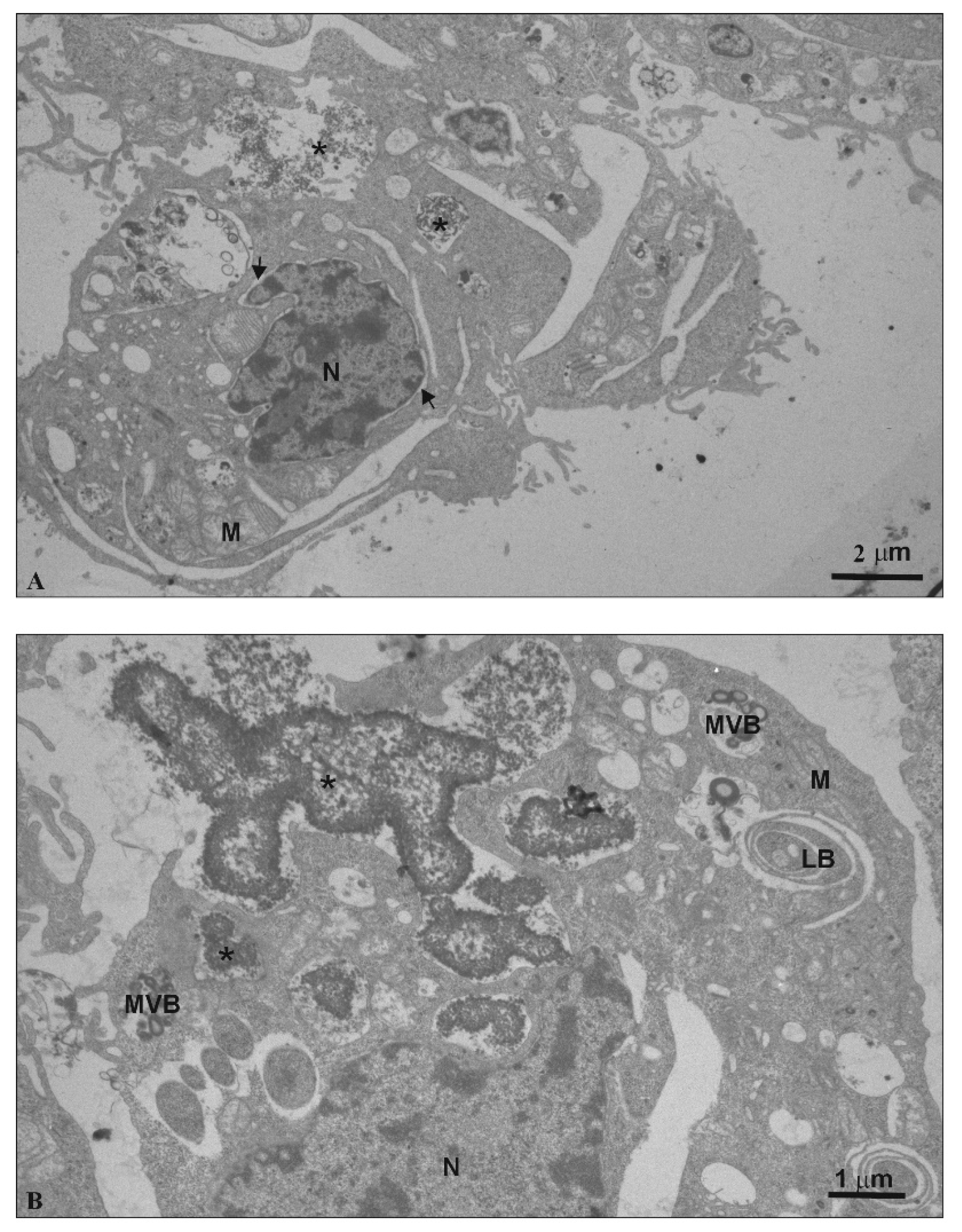
3.7. TEM—Ultra-Thin Sections
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Karande, P.; Trasatti, J.P.; Chandra, D. Novel Approaches for the Delivery of Biologics to the Central Nervous System. In Novel Approaches and Strategies For Biologics, Vaccines and Cancer Therapies; Manmohan, S., Maya, S., Eds.; Academic Press: London, UK, 2015; Volume I, pp. 59–88. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Sun, X.; Punj, V.; Sriramoju, B.; Mohan, R.R.; Zhou, S.F.; Chauhan, A.; Kanwar, R.K. Nanoparticles in the treatment and diagnosis of neurological disorders: Untamed dragon with fire power to heal. Nanomedicine 2012, 8, 399–414. [Google Scholar] [CrossRef]
- Mignani, S.; Bryszewska, M.; Zablocka, M.; Klajnert-Maculewicz, B.; Cladera, J.; Shcharbin, D.; Majoral, J.P. Can dendrimer-based nanoparticles fight neurodegenerative diseases? Current situation versus other established approaches. Prog. Polym. Sci. 2017, 64, 23–51. [Google Scholar] [CrossRef]
- Gendelman, H.E.; Anantharam, V.; Bronich, T.; Ghaisas, S.; Jin, H.; Kanthasamy, A.G.; Liu, X.; McMillan, J.; Mosley, R.L.; Narasimhan, B.; et al. Nanoneuromedicines for degenerative, inflammatory, and infectious nervous system diseases. Nanomedicine 2015, 11, 751–767. [Google Scholar] [CrossRef]
- Wohlfart, S.; Gelperina, S.; Kreuter, J. Transport of drugs across the blood-brain barrier by nanoparticles. J. Control. Release 2012, 161, 264–273. [Google Scholar] [CrossRef]
- Gao, H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta. Pharm. Sin. B 2016, 6, 268–286. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Dzmitruk, V.; Apartsin, E.; Ihnatsyeu-Kachan, A.; Abashkin, V.; Shcharbin, D.; Bryszewska, M. Dendrimers Show Promise for siRNA and microRNA Therapeutics. Pharmaceutics 2018, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Szwed, A.; el Brahmi, N.; Milowska, K.; Kurowska, J.; Fuentes-Paniagua, E.; Pedziwiatr-Werbicka, E.; Gabryelak, T.; Katir, N.; de la Mata, F.J.; et al. Synthesis, characterization and biological properties of new hybrid carbosilane-viologen-phosphorus dendrimers. RSC Adv. 2015, 5, 25942–25958. [Google Scholar] [CrossRef]
- Pedziwiatr-Werbicka, E.; Milowska, K.; Dzmitruk, V.; Ionov, M.; Shcharbin, D.; Bryszewska, M. Dendrimers and hyperbranched structures for biomedical applications. Eur. Polymer J. 2019, 119, 61–73. [Google Scholar] [CrossRef]
- Pourgholi, F.; Hajivalili, M.; Farhad, J.N.; Kafil, H.S.; Yousefi, M. Nanoparticles: Novel vehicles in treatment of Glioblastoma. Biomed. Pharmacother. 2016, 77, 98–107. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Pala, C.; Cetin-Uyanikgil, E.O.; Sevimli-Gur, C. Synthesis of silica-PAMAM dendrimer nanoparticles as promising carriers in Neuroblastoma cells. Anal. Biochem. 2017, 519, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bernas, T.; Dobrucki, J. Mitochondrial and nonmitochondrial reduction of MTT: Interaction of MTT with TMRE, JC-1, and NAO mitochondrial fluorescent probes. Cytometry 2002, 47, 236–242. [Google Scholar] [CrossRef]
- Bartosz, G. Use of spectroscopic probes for detection of reactive oxygen species. Clin. Chim. Acta. 2006, 368, 53–76. [Google Scholar] [CrossRef] [PubMed]
- Salvioli, S.; Ardizzoni, A.; Franceschi, C.; Cossarizza, A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: Implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997, 411, 77–82. [Google Scholar] [CrossRef]
- Lazniewska, J.; Milowska, K.; Katir, N.; el Kadib, A.; Bryszewska, M.; Majoral, J.P.; Gabryelak, T. Viologen-phosphorus dendrimers exhibit minor toxicity against a murine neuroblastoma cell line. Cell Mol. Biol. Lett. 2013, 18, 459–478. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S.S. The use of lead citrate of high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef]
- Szwed, A.; Milowska, K.; Ionov, M.; Shcharbin, D.; Moreno, S.; Ramirez, R.G.; de la Mata, J.; Majoral, J.P.; Bryszewska, M.; Gabryelak, T. Interaction between dendrimers and regulatory proteins. Comparison of effects of carbosilane and carbosilane-viologen-phosphorus dendrimers. RSC Adv. 2016, 6, 97546–97554. [Google Scholar] [CrossRef]
- Shcharbin, D.; Pedziwiatr-Werbicka, E.; Vcherashniaya, A.; Janaszewska, A.; Marcinkowska, M.; Goska, P.; Klajnert-Maculewicz, B.; Ionov, M.; Abashkin, V.; Ihnatsyeu-Kachan, A.; et al. Binding of poly(amidoamine), carbosilane, phosphorus and hybrid dendrimers to thrombin-Constants and mechanisms. Colloids Surf. B 2017, 155, 11–16. [Google Scholar] [CrossRef]
- Zarros, A.; Johnson, S.A.; Mackenzie-Gray, S.C.; Baillie, G.S. Cytodynamics and endpoint selection for a reliable in vitro assessment of nanoneurotoxicity. Nanomedicine 2015, 11, 407–408. [Google Scholar] [CrossRef]
- Janaszewska, A.; Studzian, M.; Petersen, J.F.; Ficker, M.; Christensen, J.B.; Klajnert-Maculewicz, B. PAMAM dendrimer with 4-carbomethoxypyrrolidone-in vitro assessment of neurotoxicity. Nanomedicine 2015, 11, 409–411. [Google Scholar] [CrossRef]
- Navya, P.N.; Daima, H.K. Rational engineering of physicochemical properties of nanomaterials for biomedical applications with nanotoxicological perspectives. Nano Converg. 2016, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.P.; Byrne, H.J. Polyamidoamine dendrimer nanoparticle cytotoxicity, oxidative stress, caspase activation and inflammatory response: Experimental observation and numerical simulation. Nanomedicine 2013, 9, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Naha, P.C.; Davoren, M.; Lyng, F.M.; Byrne, H.J. Reactive oxygen species (ROS) induced cytokine production and cytotoxicity of PAMAM dendrimers in J774A.1 cells. Toxicol. Appl. Pharmacol. 2010, 246, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Khalid, H.; Mukherjee, S.P.; O’Neill, L.; Byrne, H.J. Structural dependence of in vitro cytotoxicity, oxidative stress and uptake mechanisms of poly (propylene imine) dendritic nanoparticles. J. Appl. Toxicol. 2016, 36, 64–73. [Google Scholar] [CrossRef]
- Kitchens, K.M.; Kolhatkar, R.B.; Swaan, P.W.; Ghandehari, H. Endocytosis inhibitors prevent poly(amidoamine) dendrimer internalization and permeability across Caco-2 cells. Mol. Pharm. 2008, 5, 364–369. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Lyng, F.M.; Garcia, A.; Davoren, M.; Byrne, H.J. Mechanistic studies of in vitro cytotoxicity of poly(amidoamine) dendrimers in mammalian cells, Toxicol. Appl. Pharmacol. 2010, 248, 259–268. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the Abilities of Ambient and Manufactured Nanoparticles to Induce Cellular Toxicity According to an Oxidative Stress Paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef]
- Lazniewska, J.; Milowska, K.; Zablocka, M.; Mignani, S.; Caminade, A.M.; Majoral, J.P.; Bryszewska, M.; Gabryelak, T. Mechanism of cationic phosphorus dendrimer toxicity against murine neural cell lines. Mol. Pharm. 2013, 10, 3484–3496. [Google Scholar] [CrossRef]
- Lazniewska, J.; Janaszewska, A.; Miłowska, K.; Caminade, A.M.; Mignani, S.; Katir, N.; el Kadib, A.; Bryszewska, M.; Majoral, J.P.; Gabryelak, T.; et al. Promising low-toxicity of viologen-phosphorus dendrimers against embryonic mouse hippocampal cells. Molecules 2013, 18, 12222–12240. [Google Scholar] [CrossRef]
- Milowska, K.; Szwed, A.; Mutrynowska, M.; Gomez-Ramirez, R.; de la Mata, F.J.; Gabryelak, T.; Bryszewska, M. Carbosilane dendrimers inhibit α-synuclein fibrillation and prevent cells from rotenone-induced damage. Int. J. Pharm. 2015, 484, 268–275. [Google Scholar] [CrossRef]
- Giovannini, C.; Matarrese, P.; Scazzocchio, B.; Sanchez, M.; Masella, R.; Malorni, W. Mitochondria hyperpolarization is an early event in oxidized low-density lipoprotein-induced apoptosis in Caco-2 intestinal cells. FEBS Lett. 2002, 523, 200–206. [Google Scholar] [CrossRef]
- Sánchez-Alcázar, J.A.; Ault, J.G.; Khodjakov, A.; Schneider, E. Increased mitochondrial cytochrome c levels and mitochondrial hyperpolarization precede camptothecin-induced apoptosis in Jurkat cells. Cell Death Differ. 2000, 7, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Xu, J.; Cheng, D.; Li, X.; Tong, S.; Yan, J.; Li, Q. Anti-proliferative and apoptosis-inducing effects of camptothecin-20(s)-O-(2-pyrazolyl-1) acetic ester in human breast tumor MCF-7 cells. Molecules 2014, 19, 4941–4955. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Rhim, H.S.; Lee, S.; Ko, K.S.; Han, J.; Choi, J.S. Apoptin Gene Delivery by the Functionalized Polyamidoamine Dendrimer Derivatives Induces Cell Death of U87-MG Glioblastoma Cells. J. Pharm. Sci. 2017, 106, 1618–1633. [Google Scholar] [CrossRef]
- Dasgupta, A.; Nomura, M.; Shuck, R.; Yustein, J. Cancer’s Achilles’ Heel: Apoptosis and Necroptosis to the Rescue. Int. J. Mol. Sci. 2016, 18, 23. [Google Scholar] [CrossRef]
- Toné, S.; Sugimoto, K.; Tanda, K.; Suda, T.; Uehira, K.; Kanouchi, H.; Samejima, K.; Minatogawa, Y.; Earnshaw, W.C. Three Distinct Stages of Apoptotic Nuclear Condensation Revealed by Time-Lapse Imaging, Biochemical and Electron Microscopy Analysis of Cell-Free Apoptosis. Exp. Cell Res. 2007, 313, 3635–3644. [Google Scholar] [CrossRef]
- Parimi, S.; Barnes, T.J.; Callen, D.F.; Prestidge, C.A. Mechanistic insight into cell growth, internalization, and cytotoxicity of PAMAM dendrimers. Biomacromolecules 2010, 11, 382–389. [Google Scholar] [CrossRef]
- Kitchens, K.M.; Foraker, A.B.; Kolhatkar, R.B.; Swaan, P.W.; Ghandehari, H. Endocytosis and interaction of poly (amidoamine) dendrimers with Caco-2 cells. Pharm. Res. 2007, 24, 2138–2145. [Google Scholar] [CrossRef]
- Ionov, M.; Wróbel, D.; Gardikis, K.; Hatziantoniou, S.; Demetzos, C.; Majoral, J.P.; Klajnert, B.; Bryszewska, M. Effect of phosphorus dendrimers on DMPC lipid membranes. Chem. Phys. Lipids 2012, 165, 408–413. [Google Scholar] [CrossRef]
- Albertazzi, L.; Serresi, M.; Albanese, A.; Beltram, F. Dendrimer internalization and intracellular trafficking in living cells. Mol. Pharm. 2010, 7, 680–688. [Google Scholar] [CrossRef]
- Albertazzi, L.; Fernandez-Villamarin, M.; Riguera, R.; Fernandez-Megia, E. Peripheral functionalization of dendrimers regulates internalization and intracellular trafficking in living cells. Bioconjug. Chem. 2012, 23, 1059–1068. [Google Scholar] [CrossRef]
- Solarska-Ściuk, K.; Gajewska, A.; Glińska, S.; Studzian, M.; Michlewska, S.; Balcerzak, Ł.; Skolimowski, J.; Kolago, B.; Bartosz, G. Intracellular transport of nanodiamond particles in human endothelial and epithelial cells. Chem. Biol. Interact. 2014, 219, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Shcharbin, D.; Shcharbina, N.; Dzmitruk, V.; Pedziwiatr-Werbicka, E.; Ionov, M.; Mignani, S.; de la Mata, F.J.; Gómez, R.; Muñoz-Fernández, M.A.; Majoral, J.-P.; et al. Dendrimer-Protein Interactions versus Dendrimer-Based Nanomedicine. Colloids Surf. B 2017, 152, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Shakhbazau, A.; Shcharbin, D.; Isayenka, I.; Goncharova, N.; Sevyaryn, I.; Kosmacheva, S.; Potapnev, M.; Ionov, M.; Gabara, B.; Bryszewska, M. Use of polyamidoamine dendrimers to engineer BDNF-producing human mesenchymal stem cells. Mol. Biol. Rep. 2010, 37, 2003–2008. [Google Scholar] [CrossRef]
- Shcharbin, D.; Mazur, J.; Szwedzka, M.; Wasiak, M.; Palecz, B.; Przybyszewska, M.; Zaborski, M.; Bryszewska, M. Interaction between PAMAM 4.5 dendrimer, cadmium and bovine serum albumin: A study using equilibrium dialysis, isothermal titration calorimetry, zeta-potential and fluorescence. Colloids Surf. B 2007, 58, 286–289. [Google Scholar] [CrossRef]




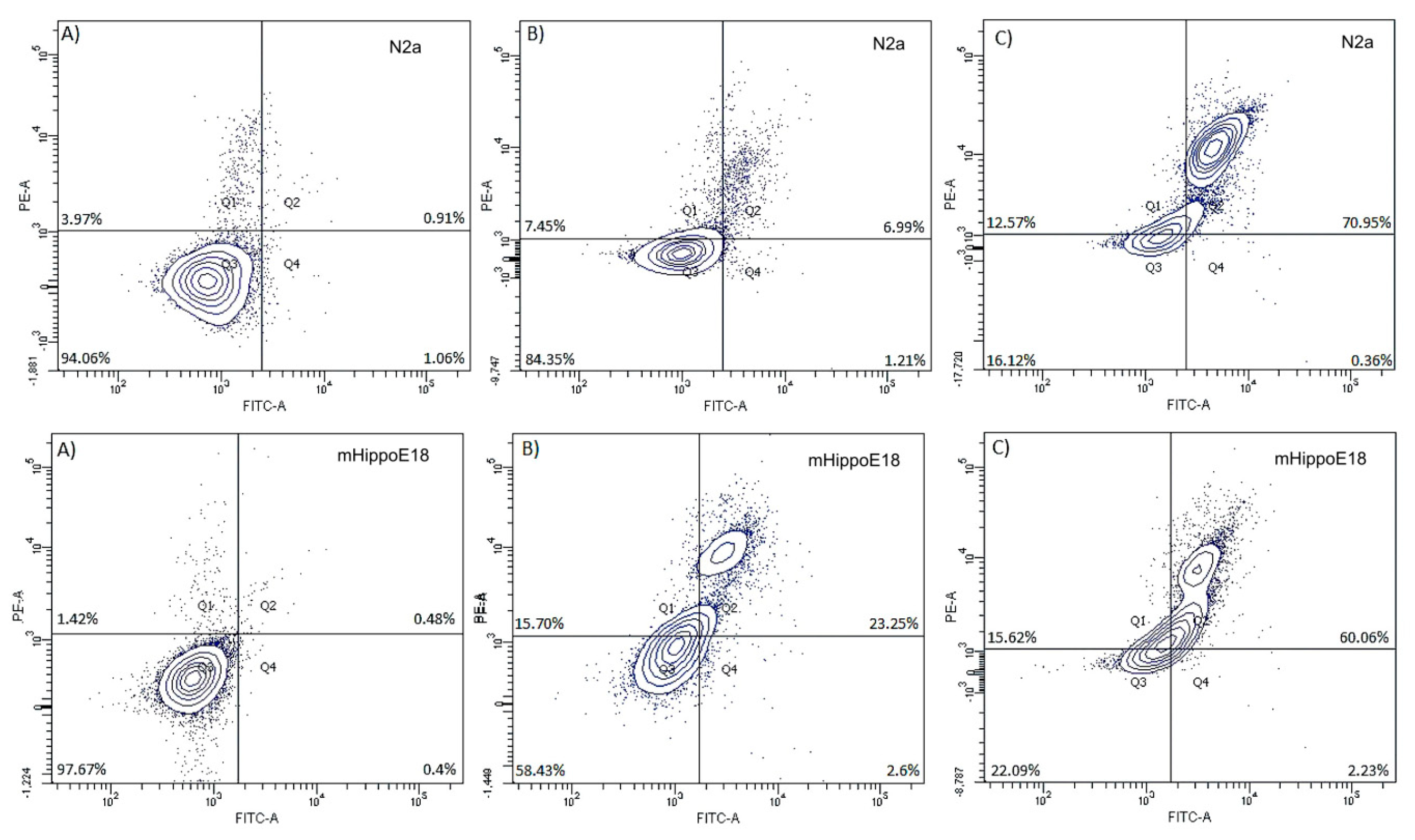

| mHippoE-18 | N2a | |||||
|---|---|---|---|---|---|---|
| Control | SMT1 | SMT2 | Control | SMT1 | SMT2 | |
| Healthy | 98.49 ± 0.85 | 95.89 ± 2.69 | 49.81 ± 6.34 *↓ | 98.37 ± 1.2 | 83.01 ± 7.87 *#↓ | 28.57 ± 1.59 *#↓ |
| Necrotic | 0.39 ± 0.88 | 0.54 ± 1.20 | 2.56 ± 7.69 | 0.20 ± 0.44 | 0.33 ± 0.8 | 1.56 ± 3.13 |
| Early apoptotic | 0.39 ± 0.87 | 2.55 ± 2.36 | 45.41 ± 13.04 *↑ | 0.39 ± 0.88 | 15.95 ± 7.7 *#↑ | 68.68 ± 13.39 *↑ |
| Late apoptotic | 0.72 ± 0.99 | 1.03 ± 1.41 | 2.22 ± 6.67 | 1.04 ± 1.49 | 0.67 ± 1.03 | 1.19 ± 1.34 |
| mHippoE-18 | N2a | |||||
|---|---|---|---|---|---|---|
| Control | SMT1 | SMT2 | Control | SMT1 | SMT2 | |
| Healthy | 94.06 ± 1.00 | 77.16 ± 5.76 *↓ | 18.92 ± 3.21 *↑ | 97.67 ± 1.03 | 61.63 ± 5.13 *#↓ | 16.08 ± 4.18 *↓ |
| Necrotic | 3.97 ± 0.76 | 12.48 ± 5.08 *↑ | 16.52 ± 3.98 *↑ | 1.42 ± 0.52 | 15.07 ± 1.51 *↑ | 14.61 ± 1.38 *↑ |
| Early apoptotic | 1.06 ± 0.93 | 1.54 ± 0.45 | 0.73 ± 0.48 | 0.44 ± 0.15 | 2.55 ± 0.39 | 1.74 ± 0.52 |
| Late apoptotic | 0.91 ± 0.42 | 8.82 ± 1.62 *↑ | 63.84 ± 7.62 *↑ | 0.48 ± 0.29 | 20.75 ± 5.37 *#↑ | 67.58 ± 5.46 *↑ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwed, A.; Miłowska, K.; Michlewska, S.; Moreno, S.; Shcharbin, D.; Gomez-Ramirez, R.; de la Mata, F.J.; Majoral, J.-P.; Bryszewska, M.; Gabryelak, T. Generation Dependent Effects and Entrance to Mitochondria of Hybrid Dendrimers on Normal and Cancer Neuronal Cells In Vitro. Biomolecules 2020, 10, 427. https://doi.org/10.3390/biom10030427
Szwed A, Miłowska K, Michlewska S, Moreno S, Shcharbin D, Gomez-Ramirez R, de la Mata FJ, Majoral J-P, Bryszewska M, Gabryelak T. Generation Dependent Effects and Entrance to Mitochondria of Hybrid Dendrimers on Normal and Cancer Neuronal Cells In Vitro. Biomolecules. 2020; 10(3):427. https://doi.org/10.3390/biom10030427
Chicago/Turabian StyleSzwed, Aleksandra, Katarzyna Miłowska, Sylwia Michlewska, Silvia Moreno, Dzmitry Shcharbin, Rafael Gomez-Ramirez, Francisco Javier de la Mata, Jean-Pierre Majoral, Maria Bryszewska, and Teresa Gabryelak. 2020. "Generation Dependent Effects and Entrance to Mitochondria of Hybrid Dendrimers on Normal and Cancer Neuronal Cells In Vitro" Biomolecules 10, no. 3: 427. https://doi.org/10.3390/biom10030427
APA StyleSzwed, A., Miłowska, K., Michlewska, S., Moreno, S., Shcharbin, D., Gomez-Ramirez, R., de la Mata, F. J., Majoral, J.-P., Bryszewska, M., & Gabryelak, T. (2020). Generation Dependent Effects and Entrance to Mitochondria of Hybrid Dendrimers on Normal and Cancer Neuronal Cells In Vitro. Biomolecules, 10(3), 427. https://doi.org/10.3390/biom10030427








