Acetylcholinesterase: The “Hub” for Neurodegenerative Diseases and Chemical Weapons Convention
Abstract
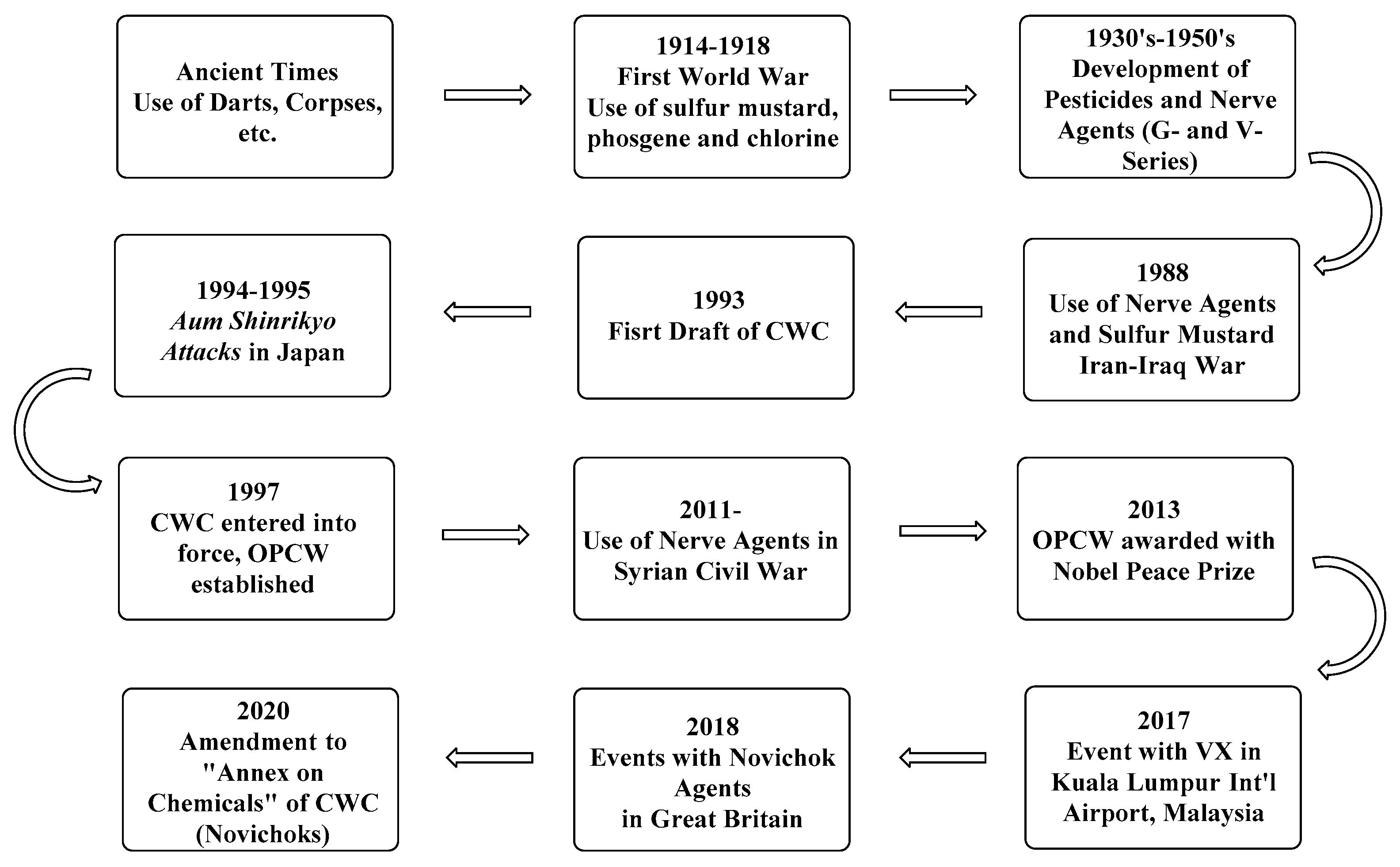
1. Introduction
2. The Chemical Weapons Convention
3. Cholinesterases
4. The Inhibition of Acetylcholinesterase
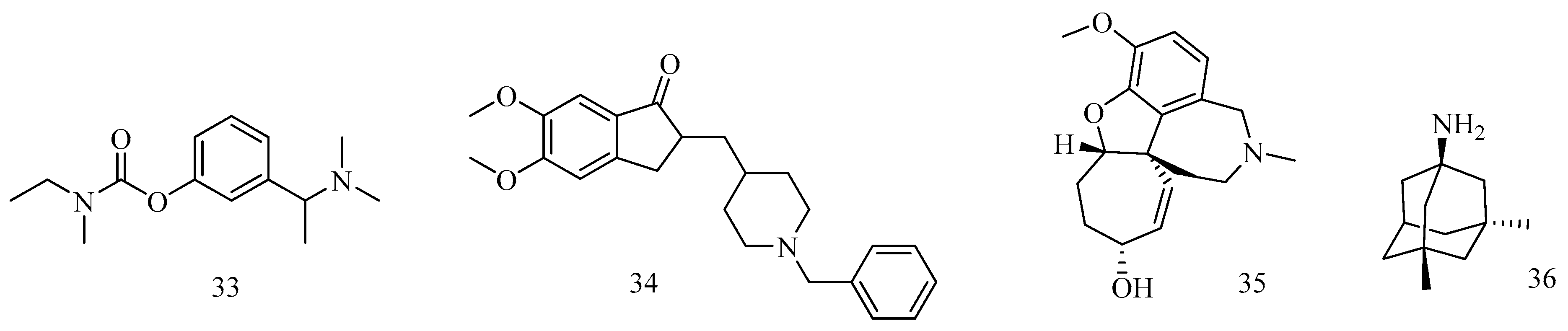
5. Reversible Inhibition of AChE: A Tool for Treatment of AD
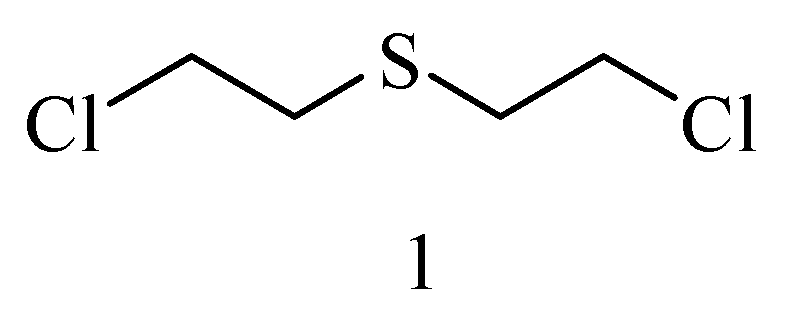
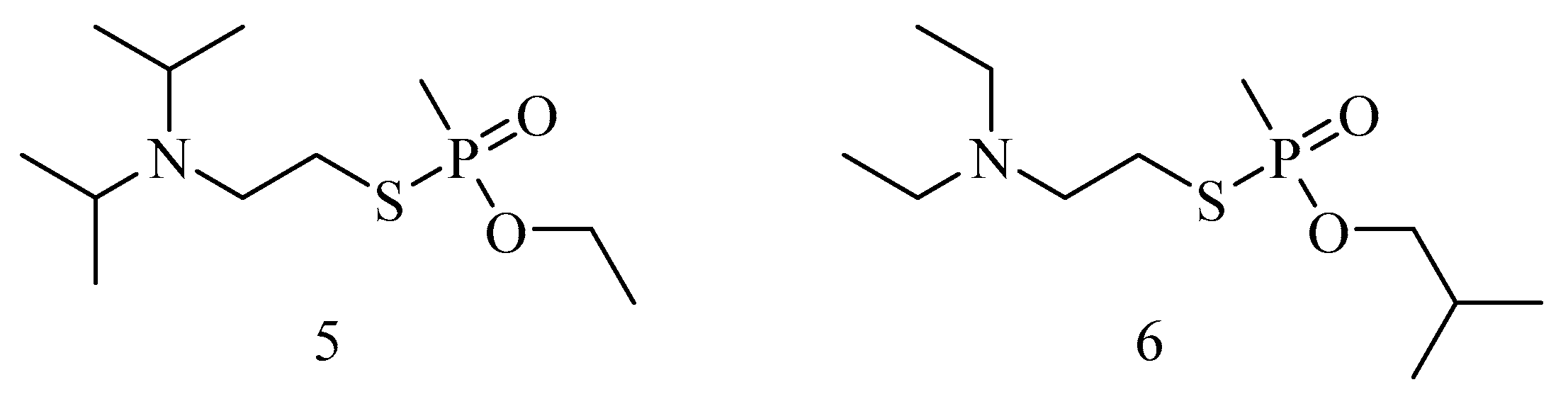
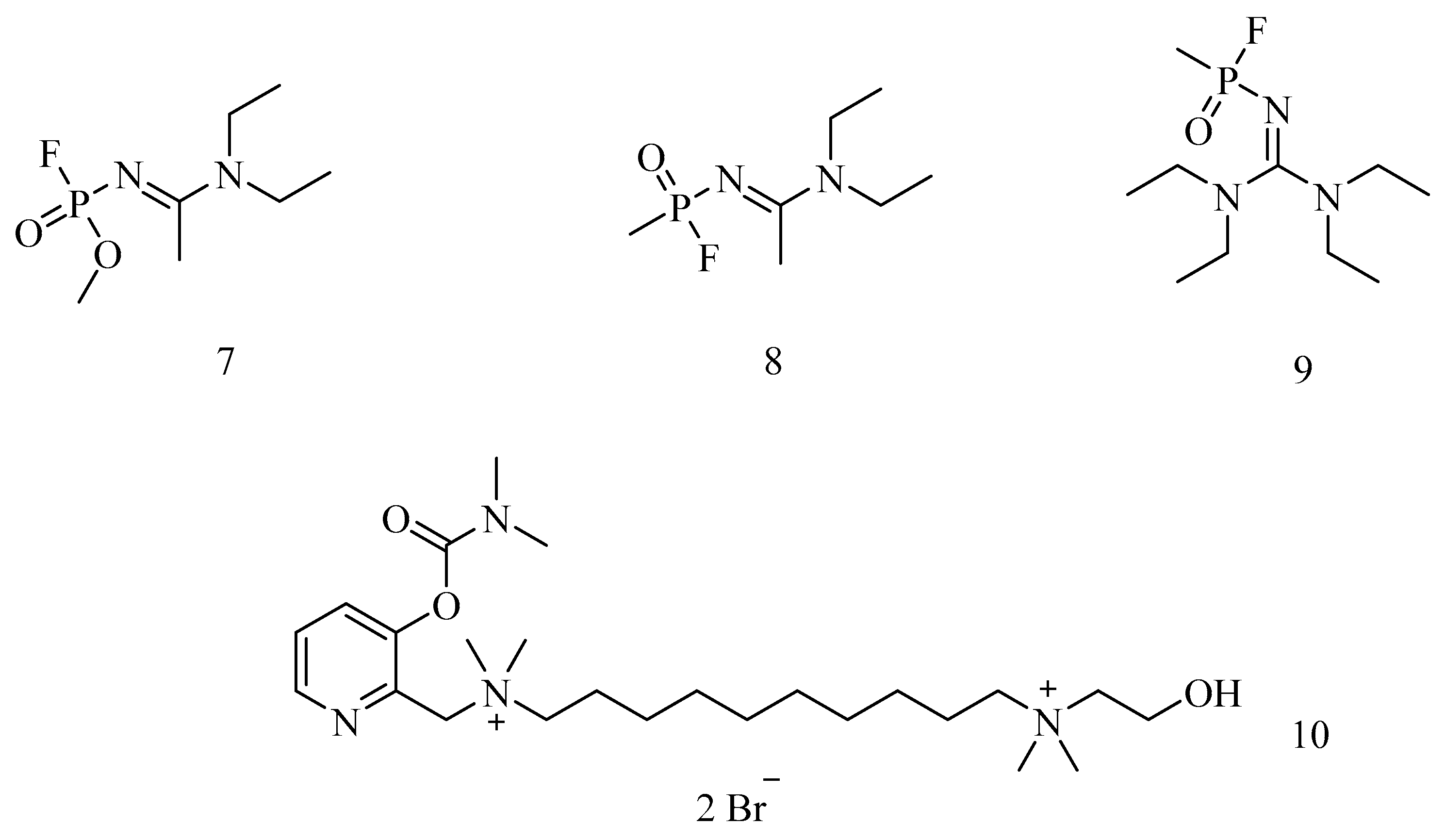
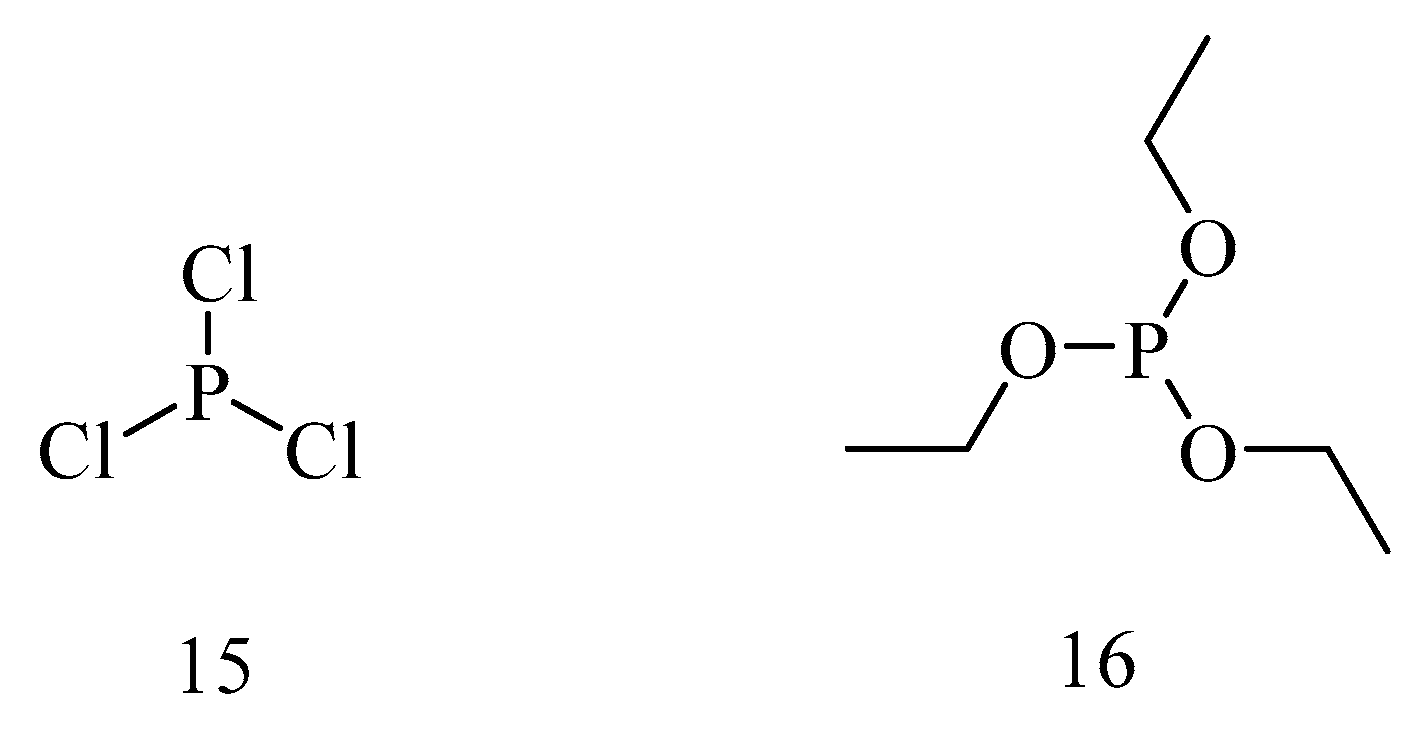
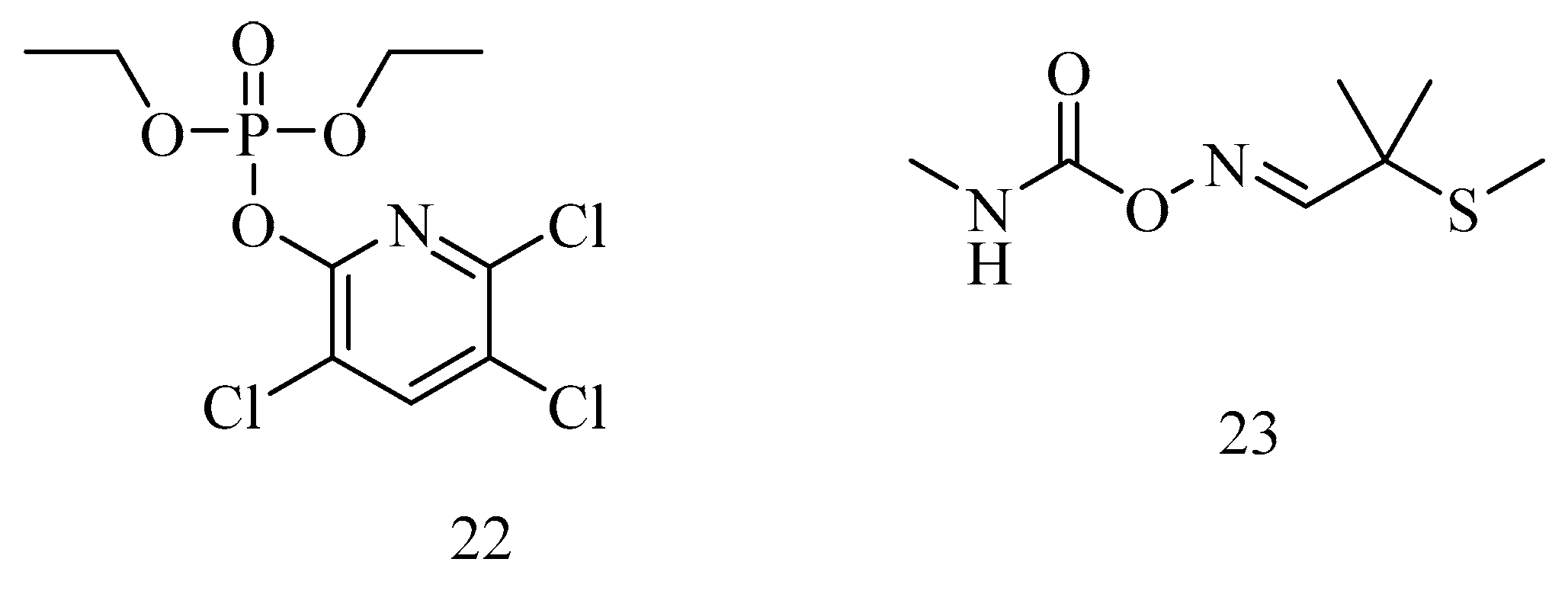
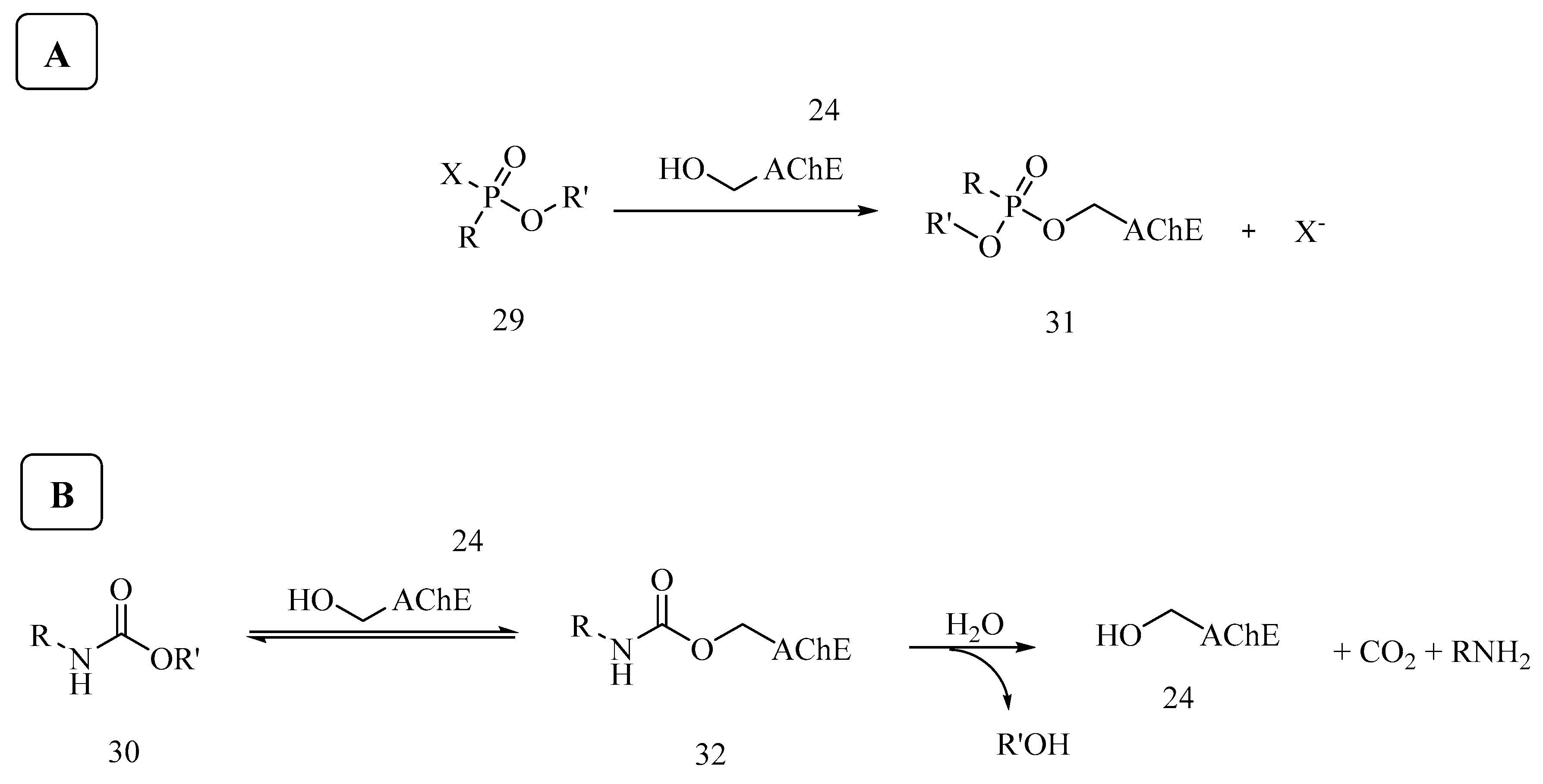
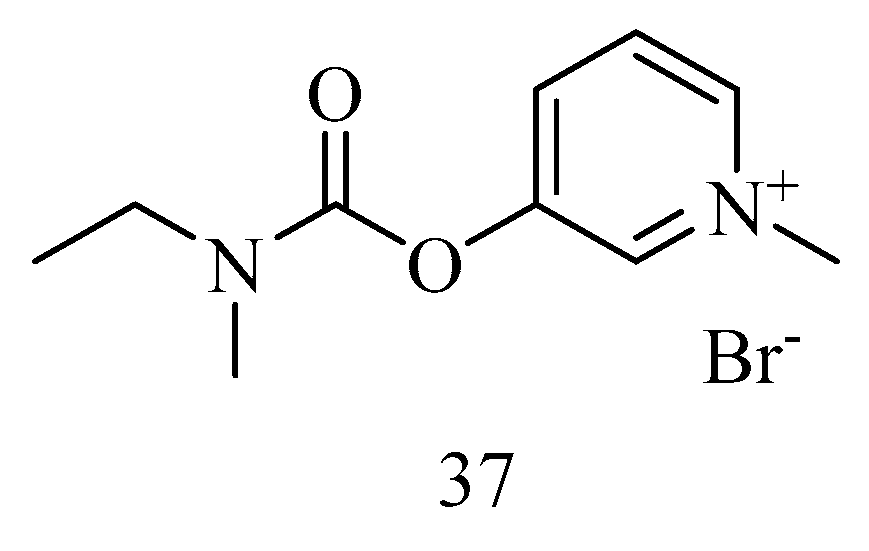
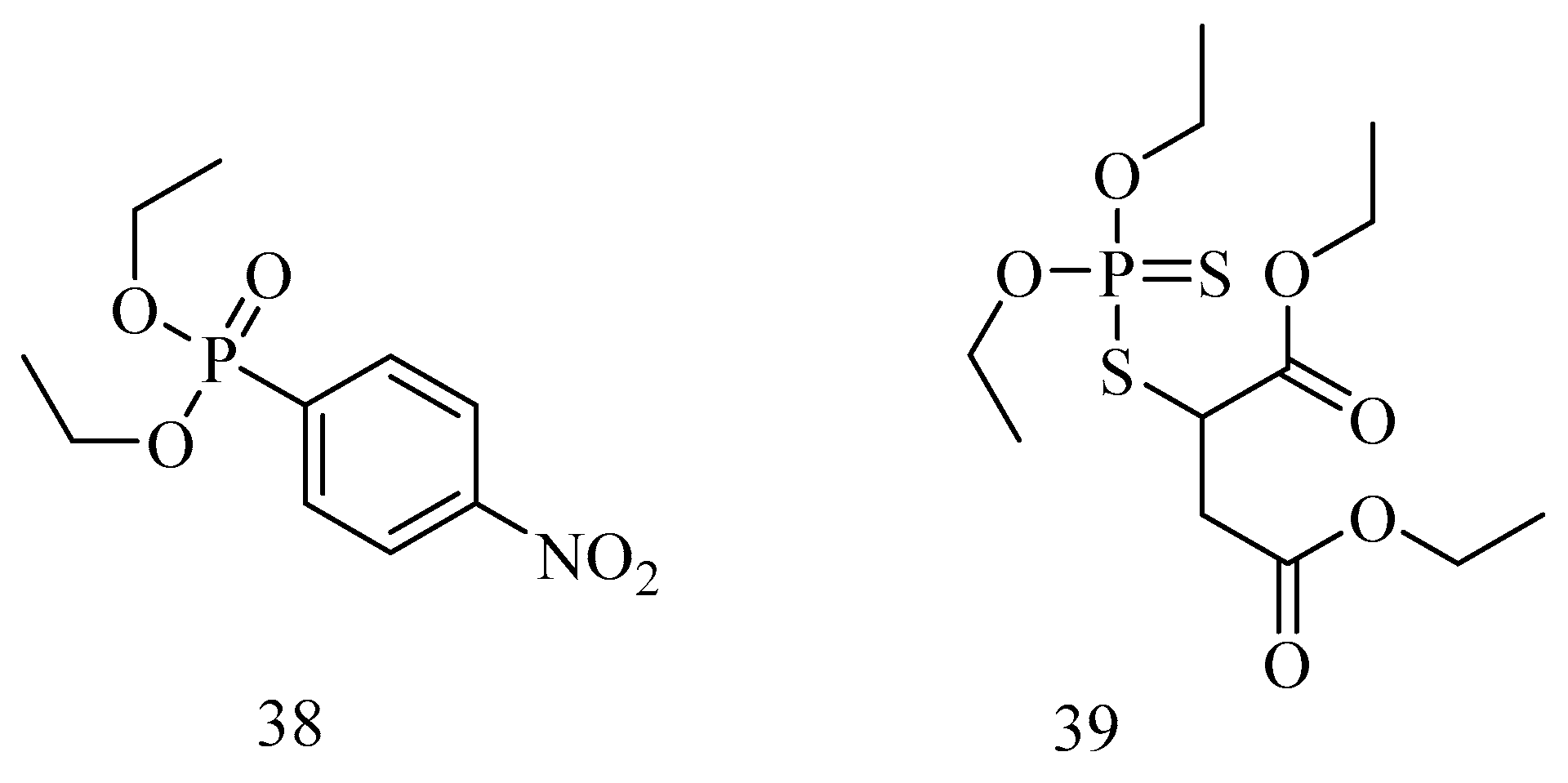
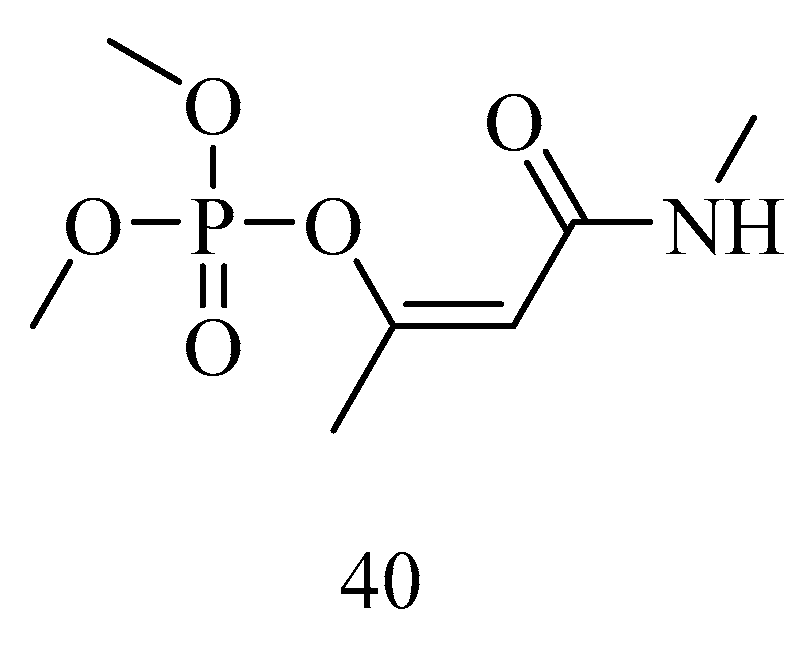
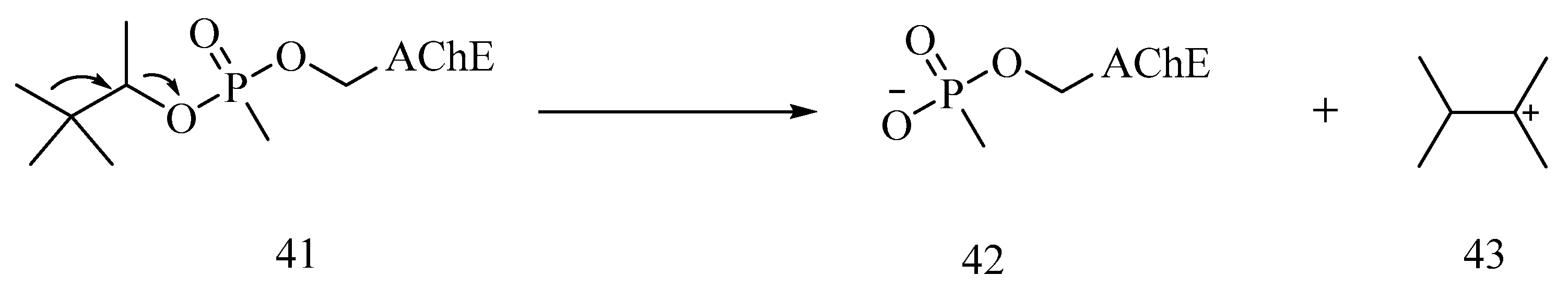
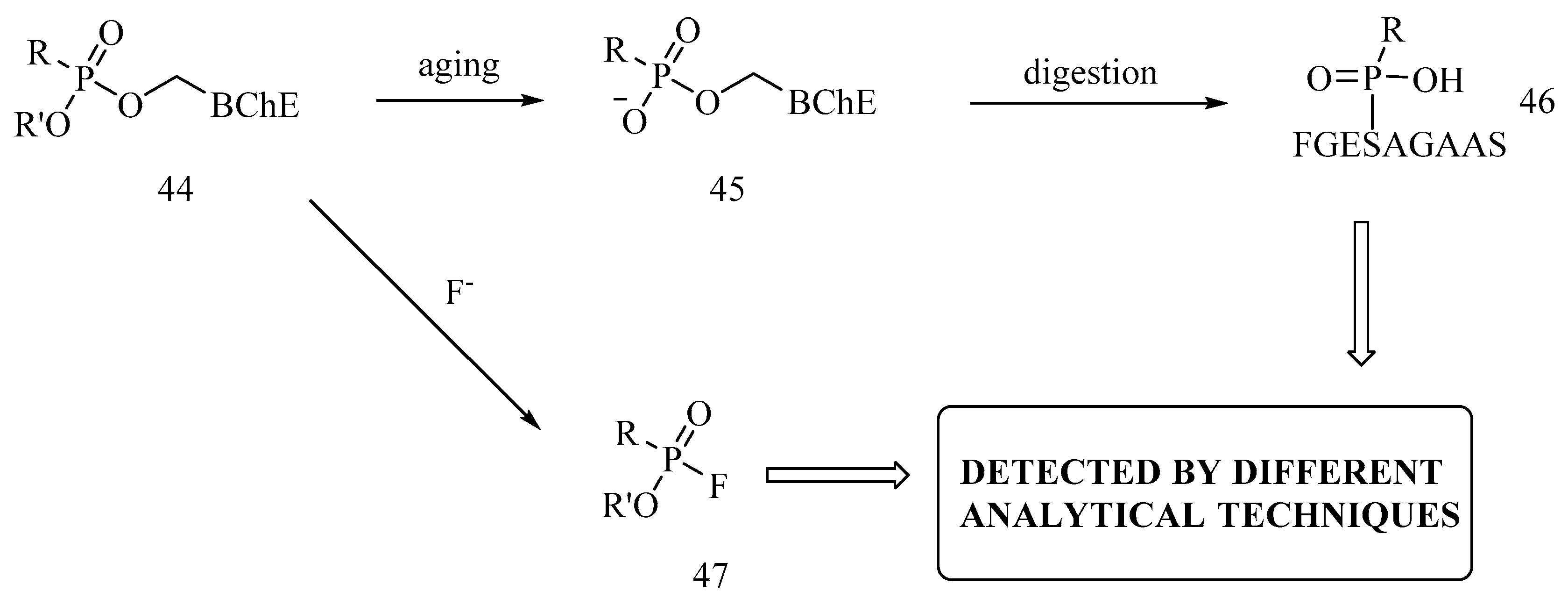
6. Irreversible Inhibition of AChE: The Chemistry of Nerve Agents
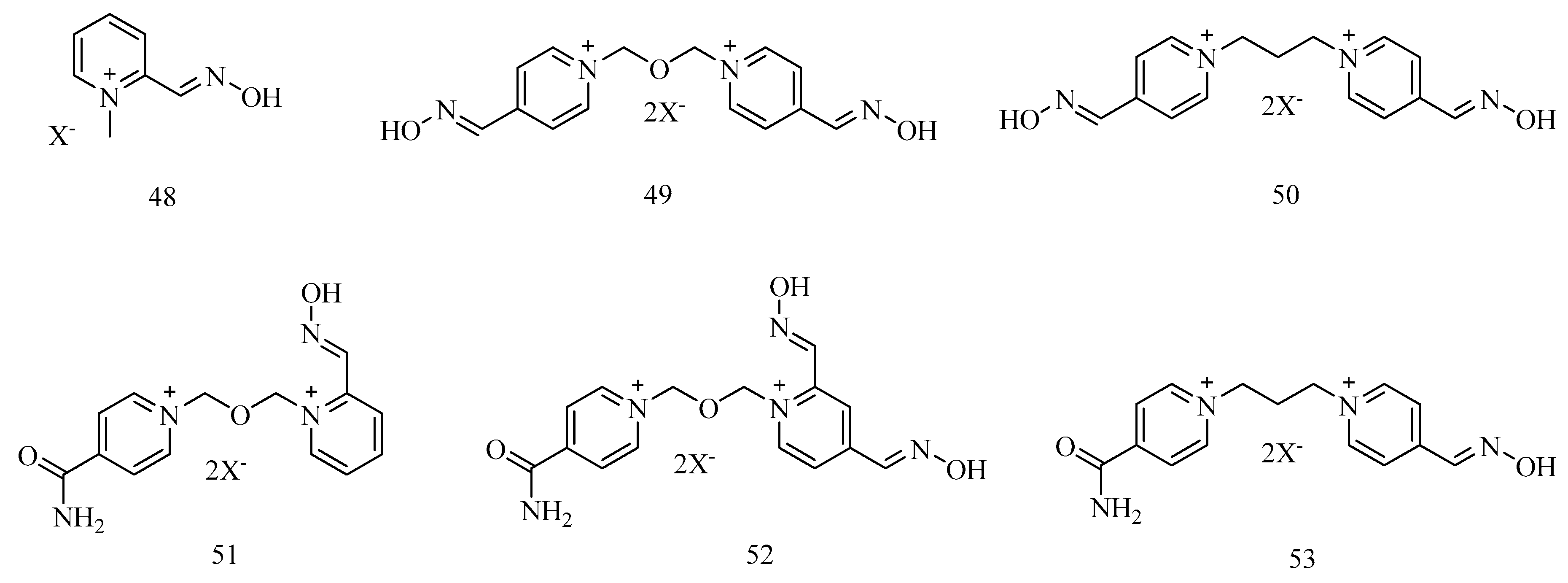
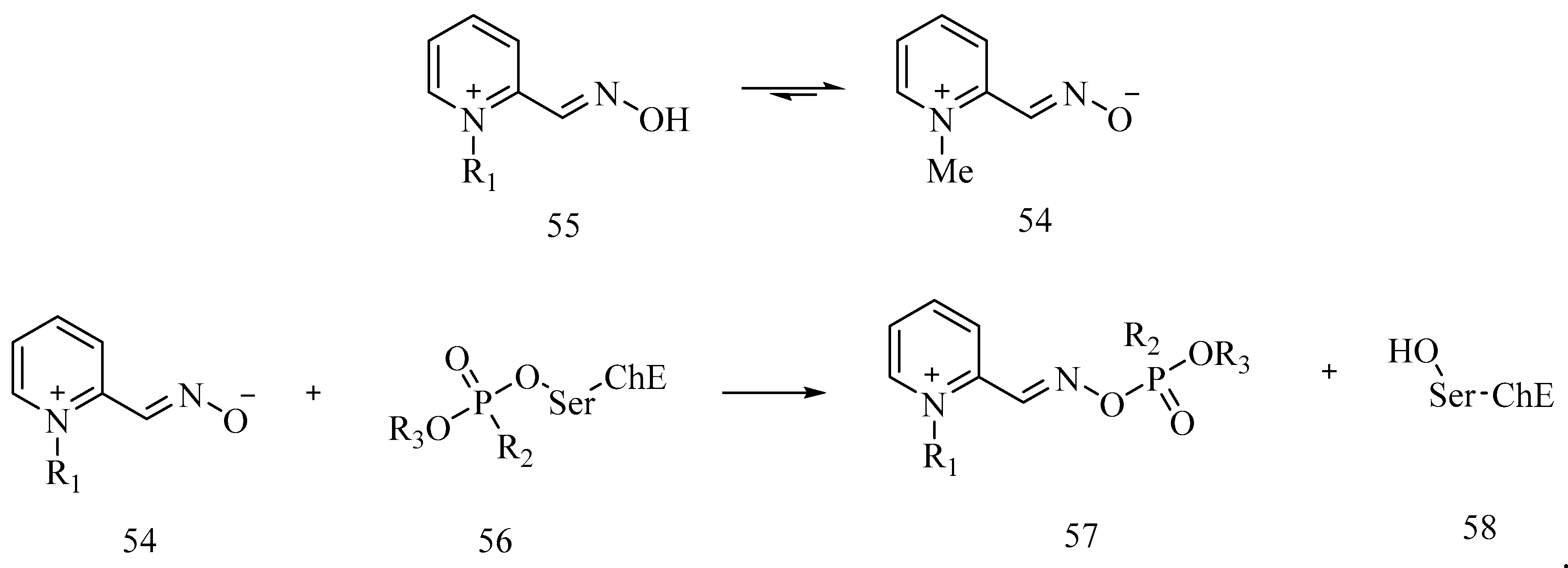
7. Rescuing Cholinesterases: Antidotes towards Nerve Agents
8. Final Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- OPCW. Available online: www.opcw.org (accessed on 3 January 2020).
- Organisation for the Prohibition of Chemical Weapons—OPCW. Available online: https://www.opcw.org/about-us/history (accessed on 3 January 2020).
- Chemical Weapons Convention—CWC. Available online: https://www.opcw.org/chemical-weapons-convention (accessed on 3 January 2020).
- Cavalcante, S.F.A.; Simas, A.B.C.; Kuča, K. Nerve Agents’ Surrogates: Invaluable Tools for Development of Acetylcholinesterase Reactivators. Curr. Org. Chem. 2019, 23, 1539–1559. [Google Scholar] [CrossRef]
- Darling, R.G.; Noste, R.E. Ciottone’s Disaster Medicine; Ciottone, G.R., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2016; pp. 489–498. [Google Scholar]
- Nepovimova, E.; Kuča, K. The history of poisoning: From ancient times until modern era. Arch. Toxicol. 2019, 93, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Vale, A.; Marrs, T.C.; Rice, P. Chemical terrorism and nerve agents. Medicine 2016, 44, 106–108. [Google Scholar] [CrossRef]
- Delfino, R.T.; Ribeiro, T.S.; Figueroa-Villar, J.D. Organophosphorus Compounds as Chemical Warfare Agents: A Review. J. Braz. Chem. Soc. 2009, 20, 407–428. [Google Scholar] [CrossRef]
- Greenfield, R.A.; Brown, B.R.; Hutchins, J.B.; Iandolo, J.J.; Jackson, R.; Slater, L.N.; Bronze, M.S. Microbiological, biological, and chemical weapons of warfare and terrorism. Am. J. Med. Sci. 2002, 323, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J. Weapons of Mass Destruction: Is is all about Chemistry. J. Chem. Ed. 2009, 86, 1377–1381. [Google Scholar] [CrossRef]
- Constanzi, S.; Machado, J.H.; Mitchell, M. Nerve Agents: What They Are, How They Work, How to Counter Them. ACS Chem. Neurosci. 2018, 9, 873–885. [Google Scholar] [CrossRef]
- Tammelin, L.E. Dialkoxy-phosphorylthiocholines, alkoxy-methyl-phosphorylthiocholines and analogous choline esters. Syntheses, pKa of tertiary homologues and cholinesterase inhibition. Acta Chem. Scand. 1957, 11, 1340–1349. [Google Scholar] [CrossRef]
- Tammelin, L.E. Methyl-fluoro-phosphorylcholines. Two synthetic cholinergic drugs and their tertiary homologues. Acta Chem. Scand. 1957, 11, 859–865. [Google Scholar] [CrossRef][Green Version]
- Makhaeva, G.F.; Filonenko, I.V.; Yankovskaya, V.L.; Fomicheva, S.B.; Malygin, V.V. Comparative studies of O,O-dialkyl-O-chloromethylchloroformimino phosphates: Interaction with neuropathy target esterase and acetylcholinesterase. Neurotoxicology 1998, 19, 623–628. [Google Scholar]
- Rozengart, E.V.; Basova, N.E.; Moralev, S.N.; Lushchekina, S.V.; Masson, P.; Varfolomeev, S.D. Research on cholinesterases in the Soviet Union and Russia: A historical perspective. Chem. Biol. Interact. 2013, 203, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Macilawain, C. Study proves Iraq used nerve gas. Nature 1993, 363, 3. [Google Scholar] [CrossRef] [PubMed]
- BBC. Available online: http://news.bbc.co.uk/onthisday/hi/dates/stories/march/16/newsid_4304000/4304853.stm (accessed on 3 January 2020).
- United Nations. Available online: http://www.sciencediplomacy.org/perspective/2015/intersection-science-and-chemical-disarmament (accessed on 3 January 2020).
- Yanagisawa, N. The nerve agent sarin: History, clinical manifestations, and treatment. Brain Nerve 2014, 66, 561–569. [Google Scholar] [PubMed]
- Yanagisawa, N.; Morita, H.; Nakajima, T. Sarin Experiences in Japan: Acute Toxicity and Long-term Effects. J. Neurol. Sci. 2006, 249, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Takatori, T.; Matsuda, Y.; Nakajima, M.; Iwase, H.; Iwadate, K. Definitive for Evidence for the Acute Sarin Poisoning Diagnosis in Tokyo Subway. Toxicol. Appl. Pharm. 1997, 144, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Greaves, I.; Hunt, P. Responding to Terrorism: A Medical Handbook; Churchill Livingstone: London, UK, 2011. [Google Scholar]
- Evison, D.; Hinsley, D.; Rice, P. Chemical Weapons. BMJ 2002, 324, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Riddle, J.R.; Brown, M.; Smith, T.; Ritchie, E.C.; Brix, K.A.; Romano, J. Chemical warfare and the Gulf War: A review of the impact on Gulf Veteran’s health. Mil. Med. 2003, 168, 606–613. [Google Scholar] [CrossRef]
- Gooch, E.E. Chemistry and Warfare: A general studies course. J. Chem. Ed. 2002, 79, 820–821. [Google Scholar] [CrossRef]
- Jang, Y.J.; Kim, K.; Tsay, O.G.; Atwood, D.A.; Churchill, D.G. Destruction and Detection of Chemical Warfare Agents. Chem. Rev. 2015, 115, PR1–PR76. [Google Scholar] [CrossRef]
- Organisation for the Prohibition of Chemical Weapons—OPCW. Available online: https://www.opcw.org/sites/default/files/documents/EC/87/en/ec87nat14_e_.pdf (accessed on 3 March 2020).
- Arms Control Association. ChemicalWeapons: Frequently Asked Questions. Available online: https://www.armscontrol.org/factsheets/Chemical-Weapons-Frequently-Asked-Questions (accessed on 6 March 2020).
- Nozaki, H.; Aikawa, N.; Fujishima, S.; Suzuki, M.; Shinozawa, Y.; Hori, S.; Nogawa, W. A case of VX poisoning and the difference from sarin. Lancet 1995, 346, 698–699. [Google Scholar] [CrossRef]
- Raveh, L.; Eisenkraft, A.; Weissman, B.A. Caramiphen edisylate: An optimal antidote against organophosphate poisoning. Toxicology 2014, 325, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. Syrian gas attack reinforces need for better anti-sarin drugs. Nat. Med. 2013, 19, 1194–1195. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J. As Syria Crisis Mounts, Scientist Looks Back at Last Major Chemical Attack. Science 2013, 341, 1051. [Google Scholar] [CrossRef] [PubMed]
- Patrick, K.; Stanbrook, M.; Flegel, K. Lest We Forget: Why the Use of Chemical Weapons Must not Go Unchallenged. Can. Med. Assoc. J. 2013, 185, 1299. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Science Mag. Available online: https://www.sciencemag.org/news/2013/08/syria-crisis-mounts-scientist-looks-back-25-years-after-investigating-halabja-gas (accessed on 3 January 2020).
- Enserink, M.U.N. Taps Special Labs to Investigate Syrian Attack. Science 2013, 341, 1050–1051. [Google Scholar] [CrossRef] [PubMed]
- Vogel, L. WHO releases guidelines for treating chemical warfare victims after possible Syria attacks. Can. Med. Assoc. J. 2013, 185, E665. [Google Scholar] [CrossRef]
- Gulland, A. Lack of atropine in Syria hampers treatment after gas attacks. BMJ 2013, 347, f5413. [Google Scholar] [CrossRef]
- Asai, Y.; Arnold, J.L. Terrorism in Japan. Prehospital Disaster Med. 2003, 18, 106. [Google Scholar] [CrossRef]
- OPCW. Available online: https://www.opcw.org/news/article/statement-from-opcw-spokesperson-in-response-to-media-queries-regarding-alleged-use-of-nerve-agent-vx-in-malaysia/ (accessed on 3 January 2020).
- Sputnik News. Available online: https://sputniknews.com/asia/201703031051219306-opcw-kim-jong-nam-probe/ (accessed on 3 January 2020).
- Channels New Asia. Available online: http://www.channelnewsasia.com/news/asiapacific/malaysia-to-fully-cooperate-with-opcw-on-vx-probe-ministry/3564532.html (accessed on 3 January 2020).
- Yle Uutiset. Available online: http://yle.fi/uutiset/3-9503798 (accessed on 3 January 2020).
- BBC. Available online: http://www.bbc.com/news/world-europe-43835774 (accessed on 3 January 2020).
- Organisation for the Prohibition of Chemical Weapons—OPCW. SAB Director-General’s Request to the Scientific Advisory Board to Provide Advice on New Types of Nerve Agents. 2 May 2018. Available online: https://www.opcw.org/sites/default/files/documents/S_series/2018/en/s-1621-2018_e_pdf (accessed on 3 March 2020).
- Nepovimova, E.; Kuča, K. Chemical warfare agent NOVICHOK—Mini-review of available data. Food Chem. Toxicol. 2018, 121, 343–350. [Google Scholar] [CrossRef]
- França, T.C.C.; Kitagawa, D.A.S.; Cavalcante, S.F.A.; da Silva, J.A.V.; Nepovimova, E.; Kuča, K. Novichoks: The Dangerous Forth Generation of Chemical Weapons. Int. J. Mol. Sci. 2019, 20, 1222. [Google Scholar] [CrossRef]
- US Department of Commerce—Bureau of Industry and Security. Impact of Proposed Additions to the ‘‘Annex on Chemicals’’ to the Chemical Weapons Convention (CWC) on Legitimate Commercial Chemical, Biotechnology, and Pharmaceutical Activities Involving ‘‘Schedule 1′’ Chemicals (Including Schedule 1 Chemicals Produced as Intermediates). Available online: https://www.cwc.gov/84%20FR%2040389%20I%20NOI%20on%20Schedule%201%20Novichoks%20-%20Impact%20proposed%20CWC%20additions%208-14-19.pdf (accessed on 3 January 2020).
- Organisation for the Prohibition of Chemical Weapons—OPCW. Note by the Technical Secretariat: Consolidated Text of Adopted Changes to Schedule 1 of the Annex on Chemicals to the Chemical Weapons Convention. Available online: https://www.opcw.org/sites/default/files/documents/2019/12/s-1820-2019%28e%29.pdf (accessed on 3 January 2020).
- Sommer, H.Z.; Wicks, G.E., Jr. Chemical Agents. U.S. Patent 4241212, 23 December 1980. [Google Scholar]
- Ellison, H.D. Handbook of Chemical and Biological Warfare Agents; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Tucker, J. War of Nerves: Chemical Warfare from World War I to Al-Qaeda; Anchor: New York, NY, USA, 2007. [Google Scholar]
- Schwenk, M. Chemical warfare agents. Classes and targets. Toxicol. Lett. 2017, 293, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Soltaninejad, K.; Shadnia, S. Basic and Clinical Toxicology of Organophosphorus Compounds; Balali-Mood, M., Abdollahi, M., Eds.; Springer: London, UK, 2014. [Google Scholar]
- Moyer, R.A.; Sidell, F.R.; Salem, H. Encyclopedia of Toxicology; Wexler, P., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2014; pp. 483–488. [Google Scholar]
- Talabani, J.M.; Ali, A.I.; Kadir, A.M.; Rashid, R.; Samin, F.; Greenwood, D.; Hay, A.W.M. Long-term health effects of chemical warfare agents on children following a single heavy exposure. Hum. Exp. Toxicol. 2018, 37, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Worek, F.; Wille, T.; Koller, M.; Thiermann, H. Toxicology of organophosphorus compounds in view of an increasing terrorist threat. Arch. Toxicol. 2016, 90, 2131–2145. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, S.; Bhattacharyya, R.; Banerjee, D. Acute organophosphorus poisoning. Clin. Chim. Acta 2014, 431, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Quinn, D.M. Acetylcholinesterase: Enzyme Structure, Reaction Dynamics, and Virtual Transition States. Chem. Rev. 1987, 87, 955–979. [Google Scholar] [CrossRef]
- Chatonnet, A.; Lockridge, O. Comparison of Butyrylcholinestarse and Acetylcholinesterase. Biochem. J. 1989, 260, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Redman, A.M.G.; Jiang, X.; Lockridge, O.; Doctor, B.P. Differences in Active Site Gorge Dimensions of Cholinesterases Revealed by Binding of Inhibitors to Human Butyrylcholinesterase. Chem. Biol. Interact. 1999, 119–120, 61–69. [Google Scholar] [CrossRef]
- Taylor, P. The Cholinesterases. J. Biol. Chem. 1991, 266, 4025–4028. [Google Scholar]
- Taylor, P.; Radić, Z. The Cholinesterases: From Genes to Proteins. Annu. Rev. Pharmacol. Toxicol. 1994, 34, 281–320. [Google Scholar] [CrossRef]
- Sussman, J.L.; Silman, I. Acetylcholinesterase: Structure and use as a model for specific cation-protein interactions. Curr. Opin. Struct. Biol. 1992, 2, 721–729. [Google Scholar] [CrossRef]
- Soreq, H.; Seidman, S. Acetylcholinesterase—New Roles for an Old Actor. Nat. Rev. 2001, 2, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Silman, I.; Sussman, J.L. Acetylcholinesterase: “Classical” and “Non-classical” Functions and Pharmacology. Curr. Opin. Pharmacol. 2005, 5, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Dvir, H.; Silman, I.; Harel, M.; Rosenberry, T.L.; Sussman, J.L. Acetylcholinesterase: From 3D structure to function. Chem. Biol. Interact. 2010, 187, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Lockridge, O. Review of human butyrylcholinesterase structure, function, genetic variants, history of use in the clinic, and potential therapeutic uses. Pharmacol. Ther. 2015, 148, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Nobel Prize Foundation. Available online: http://www.nobelprize.org/nobel_prizes/peace/laureates/2013/opcw-facts.html (accessed on 3 March 2020).
- Black, R.M.; Harrison, J.M. The Chemistry of Organophosphorus Compounds, Vol 4, Ter- and Quinquephosphorus Acids and Their Derivatives; Hartley, F.R., Ed.; John Wiley & Sons: Chichester, UK, 1996; pp. 781–840. [Google Scholar]
- Mundy, J.L.; Harrison, J.M.; Watts, P.; Timperley, C.M. Isotopically labelled phosphorus compounds: Some deuterated methyl and ethyl derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 1847–1857. [Google Scholar] [CrossRef]
- Timperley, C.M. Best Synthetic Methods, 1st ed.; Organophosphorus (V); Chemistry Academic Press: Massachusetts, MA, USA, 2014. [Google Scholar]
- Ledgard, J. The Preparatory Manual of Chemical Warfare Agents, 3rd ed.; UVKCHEM: Puyallup, UK, 2006. [Google Scholar]
- Tajti, Á.; Keglevich, G. The importance of organophosphorus compounds as biologically active agents. In Organophosphorus Chemistry—Novel Developments; Keglevich, G., Ed.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2018; Chapter 3; pp. 53–65. [Google Scholar]
- Mattes, C.E.; Lynch, T.J.; Singh, A.; Bradley, R.M.; Kellaris, P.A.; Brady, R.O.; Dretchen, K.L. Therapeutic use of butyrylcholinesterase for cocaine intoxication. Toxicol. Appl. Pharmacol. 1997, 145, 372–380. [Google Scholar] [CrossRef]
- Carmona, G.N.; Jufer, R.A.; Goldberg, S.R.; Gorelick, D.A.; Greig, N.H.; Yu, Q.S.; Cone, E.J.; Schindler, C.W. Butyrylcholinesterase accelerates cocaine metabolism: In vitro and in vivo effects in nonhuman primates and humans. Drug Metab. Dispos. 2000, 28, 367–371. [Google Scholar]
- Brimijoin, S.; Gao, Y.; Geng, L.; Chen, V.P. Treating Cocaine Addiction, Obesity, and Emotional Disorders by Viral Gene Transfer of Butyrylcholinesterase. Front. Pharmacol. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Murthy, V.; Brimijoin, S. Cocaine and Butyrylcholinesterase Gene Therapy. In The Neuroscience of Cocaine—Mechanisms and Treatment; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2017; Chapter 68; pp. 673–678. [Google Scholar]
- Masson, P.; Lockridge, O. Butyrylcholinesterase for protection from organophosphorus poisons: Catalytic complexities and hysteretic behavior. Arch. Biochem. Biophys. 2010, 494, 107–120. [Google Scholar] [CrossRef]
- Zhang, P.; Jain, P.; Tsao, C.; Sinclair, A.; Sun, F.; Hung, H.-C.; Bai, T.; Wu, K.; Jiang, S. Butyrylcholinesterase nanocapsule as a long circulating bioscavenger with reduced immune response. J. Control. Release 2016, 230, 73–78. [Google Scholar] [CrossRef]
- Rice, H.; Mann, T.M.; Armstrong, S.J.; Price, M.E.; Green, A.C.; Tattersall, J.E.H. The potential role of bioscavenger in the medical management of nerve-agent poisoned casualties. Chem. Biol. Interact. 2016, 259, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Lushchekina, S.V.; Schopfer, L.M.; Grigorenko, B.L.; Nemukhin, A.V.; Varfolomeev, S.D.; Lockridge, O.; Masson, P. Optimization of Cholinesterase-Based Catalytic Bioscavengers Against Organophosphorus Agents. Front. Pharmacol. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nachon, F.; Brazzolotto, X.; Trovaslet, M.; Masson, P. Progress in the development of enzyme-based nerve agent bioscavengers. Chem. Biol. Interact. 2013, 206, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Cerasoli, D.M.; Griffiths, E.M.; Doctor, B.P.; Saxena, A.; Fedorko, J.M.; Greig, N.H.; Yu, Q.S.; Huang, Y.; Wilgus, H.; Karatzas, C.N.; et al. In vitro and in vivo characterization of recombinant human butyrylcholinesterase (Protexia) as a potential nerve agent bioscavenger. Chem. Biol. Interact. 2005, 157–158, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Musilek, K.; Holas, O.; Horova, A.; Pohanka, M.; Zdarova-Karasova, J.; Jun, D.; Kuca, K. Pesticides in the Modern World—Effects of Pesticides Exposure; Stoytcheva, M., Ed.; InTech: London, UK, 2011; Available online: http://www.intechopen.com/books/pesticides-in-the-modern-worldeffects-of-pesticides-exposure/progress-in-antidotes-acetylcholinesterase-reactivators-againstorganophosphorus-pesticides (accessed on 3 March 2020).
- Thomas, E.A.; Bornstein, J.C. Inhibitory cotransmission or after-hyperpolarizing potentials can regulate firing in recurrent networks with excitatory metabotropic transmission. Neuroscience 2003, 120, 333–351. [Google Scholar] [CrossRef]
- Shafferman, A.; Kronman, C.; Flashner, Y.; Leitner, M.; Grosfeld, H.; Ordentlich, A.; Gozes, Y.; Cohen, S.; Ariel, N.; Barak, D.; et al. Mutagenesis of Human Acetylcholinesterase. J. Biol. Chem. 1992, 25, 17640–17648. [Google Scholar]
- Lockridge, O. Structure of human serum cholinesterase. Bioessays 1988, 9, 125–128. [Google Scholar] [CrossRef]
- Vellom, D.C.; Radic, Z.; Li, Y.; Pickering, N.A.; Camp, S.; Taylor, P. Amino acid residues controlling acetylcholinesterase and butyrylcholinesterase specificity. Biochemistry 1993, 32, 12–17. [Google Scholar] [CrossRef]
- Hörnberg, A.; Tunemalm, A.K.; Ekström, F. Crystal structures of acetylcholinesterase in complex with organophosphorus compounds suggest that the acyl pocket modulates the aging reaction by precluding the formation of the trigonal bipyramidal transition state. Biochemistry 2007, 46, 4815–4825. [Google Scholar] [CrossRef]
- Bartling, A.; Worek, F.; Szinicz, L.; Thiermann, H. Enzyme-kinetic investigation of different sarin analogues reacting with human acetylcholinesterase and butyrylcholinesterase. Toxicology 2007, 233, 166–172. [Google Scholar] [CrossRef]
- Nicolet, Y.; Lockridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal Structure of Human Butyrylcholinesterase and of Its Complexes with Substrate and Products. Biol. Chem. 2003, 278, 41141–41147. [Google Scholar] [CrossRef] [PubMed]
- Bencsura, A.; Enyedy, I.Y.; Kovach, I.M. Probing the Active Site of Acetylcholinesterase by Molecular Dynamics of Its Phosphonate Ester Adducts. J. Am. Chem. Soc. 1996, 118, 8531–8541. [Google Scholar] [CrossRef]
- Ballard, C.; Gauthier, S.; Corbett, A.; Brayne, C.; Aarsland, D.; Jones, E. Alzheimer’s disease. Lancet 2011, 377, 1019–1031. [Google Scholar] [CrossRef]
- Okumura, T.; Seto, Y.; Fuse, A. Countermeasures against chemical terrorism in Japan. Forensic Sci. Int. 2013, 227, 2–6. [Google Scholar] [CrossRef]
- Tu, A.T. Aum Shinrikyo’s chemical and biological weapons: More than sarin. Forensic Sci. Rev. 2014, 26, 115–120. [Google Scholar] [PubMed]
- Hardacre, H. Aum Shinrikyo and the Japanese Media. JPRI Working Paper No. 19. 1996. Available online: http://www.jpri.org/publications/workingpapers/wp19.html (accessed on 3 March 2020).
- Hroudová, J.; Singh, N.; Fišar, Z.; Ghosh, K.K. Progress in drug development for Alzheimer’s disease: An overview in relation to mitochondrial energy metabolism. Eur. J. Med. Chem. 2016, 121, 774–784. [Google Scholar] [CrossRef]
- Seto, Y. The Sarin Gas Attack in Japan and the Related Forensic Investigation. OPCW Synth. 2001, June, 14–17. Available online: https://www.opcw.org/news/article/the-sarin-gas-attack-in-japan-and-the-related-forensic-investigation (accessed on 20 January 2020).
- Gorecki, L.; Korabecny, J.; Musilek, K.; Malinak, D.; Nepovimova, E.; Dolezal, R.; Jun, D.; Soukup, O.; Kuča, K. SAR study to find optimal cholinesterase reactivator against organophosphorous nerve agents and pesticides. Arch. Toxicol. 2016, 90, 2831–2859. [Google Scholar] [CrossRef]
- Pohanka, M. Cholinesterases, a target of Pharmacology and Toxicology. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2011, 155, 219–230. [Google Scholar] [CrossRef]
- Pohanka, M. Acetylcholinesterase inhibitors: A patent review (2008–present). Expert Opin. Ther. Pat. 2012, 1–16. [Google Scholar] [CrossRef]
- Franjesevic, A.J.; Sillart, S.B.; Beck, J.M.; Vyas, S.; Callam, C.S.; Hadad, C.M. Ressurrectionand reactivation of acetylcholinesterase and butyrylcholinesterase. Chem. Eur. J. 2019, 25, 5337–5371. [Google Scholar] [CrossRef] [PubMed]
- Tõugu, V. Acetylcholinesterase: Mechanism of Catalysis and Inhibition. Curr. Med. Chem. 2001, 1, 155–170. [Google Scholar] [CrossRef]
- Greenfield, S.; Vaux, D.J. Parkinson’s disease, Alzheimer’s disease and motor neurone disease: Identifying a common mechanism. Neuroscience 2002, 113, 485–492. [Google Scholar] [CrossRef]
- Brinton, R.D.; Yamazaki, R.S. Advances and Challenges in the Prevention and Treatment of Alzheimer’s Disease. Pharm. Res. 1998, 15, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Lauderback, C.M. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: Potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic. Biol. Med. 2002, 32, 1050–1060. [Google Scholar] [CrossRef]
- Blennow, K.; de Leon, M.J.; Zetterberg, K. Alzheimer’s disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef]
- Scarpinia, E.; Schelternsa, P.; Feldman, H. Treatment of Alzheimer’s disease; current status and new perspectives. Lancet Neurol. 2003, 2, 539–547. [Google Scholar] [CrossRef]
- Rösler, M.; Anand, R.; Cicin-Sain, A.; Gauthier, S.; Agid, Y.; Dal-Bianco, P.; Stähelin, H.B.; Hartman, R.; Gharabawi, M. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: International randomised controlled trial. BMJ 1999, 318, 633–638. [Google Scholar] [CrossRef]
- Fifer, E.K. Drugs affecting cholinergic neurotransmission. In Foye’s Medicinal Chemistry, 6th ed.; Lemke, T.L., Williams, D.A., Eds.; Lippincott Williams & Wilkins, Baltimore: Philadelphia, PA, USA, 2008; Chapter 12; pp. 361–391. [Google Scholar]
- Pohanka, M. Inhibitors of acetylcholinesterase and butyrylcholinesterase meet immunity. Int. J. Mol. Sci. 2014, 15, 9809–9825. [Google Scholar] [CrossRef]
- Glenner, G.G.; Wong, C.W. Alzheimer’s Disease: Initial Report Of The Purification And Characterization Of A Novel Cerebrovascular Amyloid Protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef]
- Kihara, T.; Shimohama, S. Alzheimer’s disease and acetylcholine receptors. Acta Neurobiol. Exp. 2004, 64, 99–105. [Google Scholar]
- Scott, L.J.; Goa, K.L. Galantamine: A review of its use in Alzheimer’s disease. Drugs 2000, 60, 1095–1122. [Google Scholar] [CrossRef] [PubMed]
- Woodruff-Pak, D.S.; Vogel, R.W.; Wenk, G.L. Galantamine: Effect on nicotinic receptor binding, acetylcholinesterase inhibition, and learning. Proc. Natl. Acad. Sci. USA 2001, 98, 2089–2094. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, E.X.; Pereira, E.F.R.; Aracava, Y.; Fawcett, W.P.; Oliveira, M.; Randall, W.R.; Adler, M. Effective countermeasure against poisoning by organophosphorus insecticides and nerve agents. Proc. Natl. Acad. Sci. USA 2006, 103, 13220–13225. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Chen, H.S.; Zhang, D.; Lipton, S.A. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J. Neurosci. 2010, 30, 11246–11250. [Google Scholar] [CrossRef]
- Robinson, D.M.; Keating, G.M. Memantine: A review of its use in Alzheimer’s disease. Drugs 2006, 66, 1515–1534. [Google Scholar] [CrossRef]
- Jann, M.W. Rivastigmine, a New-Generation Cholinesterase Inhibitor for the Treatment of Alzheimer’s Disease. Pharmacotherapy 2000, 20, 1–12. [Google Scholar] [CrossRef]
- Khoury, R.; Rajamanickam, J.; Grossberg, G.T. An update on the safety of current therapies for Alzheimer’s disease: Focus on rivastigmine. Ther. Adv. Drug Saf. 2018, 9, 171–178. [Google Scholar] [CrossRef]
- Sun, X.; Jin, L.; Ling, P. Review of drugs for Alzheimer’s disease. Drug Discov. Ther. 2012, 6, 285–290. [Google Scholar] [CrossRef]
- Lao, K.; Ji, N.; Zhang, X.; Qiao, W.; Tang, Z.; Gou, X. Drug development for Alzheimer’s disease: Review. J. Drug Target 2019, 27, 164–173. [Google Scholar] [CrossRef]
- Hung, S.Y.; Fu, W.M. Drug candidates in clinical trials for Alzheimer’s disease. J. Biomed. Sci. 2017, 24, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer’s disease drug development pipeline: 2019. Alzheimers Dement. 2019, 5, 272–293. [Google Scholar] [CrossRef] [PubMed]
- Cazarim, M.S.; Moriguti, J.C.; Ogunjimi, A.T.; Pereira, L.R.L. Perspectives for treating Alzheimer’s disease: A review on promising pharmacological substances. Sao Paulo Med. J. 2016, 134, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, D.; Ghezzi, L.; Scarpini, E. Immunotherapy against amyloid pathology in Alzheimer’s disease. J. Neurol. Sci. 2013, 333, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D. Alzheimer’s disease and amyloid: Culprit or coincidence? Int. Rev. Neurobiol. 2012, 102, 277–316. [Google Scholar] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Ningthoujam, D.S.; Mukherjee, S.; Devi, L.J.; Singh, E.S.; Tamreihao, K.; Khunjamayum, R.; Banerjee, S.; Mukhopadhyay, D. In vitro degradation of β-amyloid fibrils by microbial keratinase. Alzheimers Dement. 2019, 5, 154–163. [Google Scholar] [CrossRef]
- Guzior, N.; Bajda, M.; Skrok, M.; Kurpiewsk, K.; Lewiński, K.; Brus, B.; Pišlar, A.; Kos, J.; Gobec, S.; Malawska, B. Development of multifunctional, heterodimeric isoindoline-1,3-dione derivatives as cholinesterase and β-amyloid aggregation inhibitors with neuroprotective properties. Eur. J. Med. Chem. 2015, 92, 738–749. [Google Scholar] [CrossRef]
- Johnson, G.; Moore, S.W. The peripheral anionic site of acetylcholinesterase: Structure, functions and potential role in rational drug design. Curr. Pharm. Des. 2006, 12, 217–225. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Alvarez, A.; Pérez, C.A.; Moreno, R.D.; Vicente, M.; Linker, C.; Casanueva, O.I.; Soto, C.; Garrido, J. Acetylcholinesterase Accelerates Assembly of Amyloid-β-Peptides into Alzheimer’s Fibrils: Possible Role of the Peripheral Site of the Enzyme. Neuron 1996, 16, 881–891. [Google Scholar] [CrossRef]
- Das, B.; Yan, R. Role of BACE1 in Alzheimer’s synaptic function. Transl. Neurodegener. 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Coimbra, J.R.M.; Marques, D.F.F.; Baptista, S.J.; Pereira, C.M.F.; Moreira, P.I.; Dinis, T.C.P.; Santos, A.E.; Salvador, J.A.R. Highlights in BACE1 Inhibitors for Alzheimer’s Disease Treatment. Front. Chem. 2018, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Yan, R. A Close Look at BACE1 Inhibitors for Alzheimer’s Disease Treatment. CNS Drugs 2019, 33, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, Z.; Wang, R.; Zhang, X.; Zhang, S.; Wu, Y.; Staufenbiel, M.; Cai, F.; Song, W. Amyloid-β protein (Aβ) Glu11 is the major β-secretase site of β-site amyloid-β precursor protein-cleaving enzyme 1 (BACE1), and shifting the cleavage site to Aβ Asp1 contributes to Alzheimer pathogenesis. Eur. J. Neurosci. 2013, 37, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R. BACE1: The beta-secretase enzyme in Alzheimer’s disease. J. Mol. Neurosci. 2004, 23, 105–114. [Google Scholar] [CrossRef]
- Cole, S.L.; Vassar, R. The Alzheimer’s disease β-secretase enzyme, BACE1. Mol. Neurodegener. 2007, 2, 1–25. [Google Scholar] [CrossRef]
- Egan, M.F.; Kost, J.; Voss, T.; Mukai, Y.; Aisen, P.S.; Cummings, J.L.; Tariot, P.N.; Vellas, B.; van Dyck, C.H.; Boada, M.; et al. Randomized Trial of Verubecestat for Prodromal Alzheimer’s Disease. N. Engl. J. Med. 2019, 380, 1408–1420. [Google Scholar] [CrossRef]
- Doggrell, S.A. Lessons that can be learnt from the failure of verubecestat in Alzheimer’s disease. Expert Opin. Pharmacother. 2019, 20, 2095–2099. [Google Scholar] [CrossRef]
- Mdawar, B.; Ghossoub, E.; Khoury, R. Selective serotonin reuptake inhibitors and Alzheimer’s disease. Neural Regen. Res. 2020, 15, 41–46. [Google Scholar]
- Elsworthy, R.J.; Aldred, S. Depression in Alzheimer’s Disease: An Alternative Role for Selective Serotonin Reuptake Inhibitors? J. Alzheimers Dis. 2019, 69, 651–661. [Google Scholar] [CrossRef]
- Sepehry, A.A.; Lee, P.E.; Hsiung, G.Y.; Beattie, B.L.; Jacova, C. Effect of selective serotonin reuptake inhibitors in Alzheimer’s disease with comorbid depression: A meta-analysis of depression and cognitive outcomes. Drugs Aging 2012, 29, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, P.P.; Lian, Y.J.; Liu, H.B.; Kang, J.S. The effect of selective serotonin reuptake inhibitors on cognitive function in patients with Alzheimer’s disease and vascular dementia: Focusing on fluoxetine with long follow-up periods. Signal Transduct. Target. Ther. 2019, 4, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Kargbo, R.B. Treatment of Alzheimer’s by PROTAC-Tau Protein Degradation. ACS Med. Chem. Lett. 2019, 10, 699–700. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou, M.; Li, J.; Zhang, B.; Wang, Z.; Shaabani, S.; Ter Brake, F.; Essa, K.; Dömling, A. PROTACs- a game-changing technology. Expert Opin. Drug Discov. 2019, 14, 1255–1268. [Google Scholar] [CrossRef]
- Kumar, V. Potential medicinal plants for CNS disorders: An overview. Phytother. Res. 2006, 20, 1023–1035. [Google Scholar] [CrossRef]
- White, R.F.; Steele, L.; O’Callaghan, J.P.; Sullivan, K.; Binns, J.H.; Golomb, B.A.; Bloom, F.E.; Bunker, J.A.; Crawford, F.; Graves, J.C. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex 2016, 74, 449–475. [Google Scholar] [CrossRef]
- Macht, V.A.; Woodruff, J.L.; Grillo, C.A.; Wood, C.S.; Wilson, M.A.; Reagan, L.P. Pathophysiology in a model of Gulf War Illness: Contributions of pyridostigmine bromide and stress. Psychoneuroendocrinology 2018, 96, 195–202. [Google Scholar] [CrossRef]
- Shen, Z.-X. Pyridostigmine bromide and Gulf War syndrome. Med. Hypotheses 1998, 51, 235–237. [Google Scholar] [CrossRef]
- Wagner, M.J.; Promes, S. (Eds.) Last Minute Emergency Medicine: A Concise Review for the Specialty Boards; McGraw-Hill Medical: New York, NY, USA, 2007. [Google Scholar]
- Wright, L.K.M.; Lee, R.B.; Vincelli, N.M.; Whalley, C.E.; Lumley, L.A. Female rats are less susceptible during puberty to the lethal effects of percutaneous exposure to VX. Toxicol. Lett. 2016, 241, 167–174. [Google Scholar] [CrossRef]
- Zhuang, Q.; Young, A.; Callam, C.S.; McElroy, C.A.; Ekici, O.D.; Yoder, R.J.; Hadad, C.M. Efforts towards treatment against aging organophosphorus-inhibited acetylcholinesterase. Ann. N. Y. Acad. Sci. 2016, 1374, 94–104. [Google Scholar] [CrossRef]
- Cavalcante, S.F.A.; Kitagawa, D.A.S.; Rodrigues, R.B.; Bernardo, L.B.; da Silva, T.N.; dos Santos, W.V.; Correa, A.B.A.; de Almeida, J.S.F.D.; França, T.C.C.; Kuča, K.; et al. Synthesis and in vitro evaluation of neutral aryloximes as reactivators of Electrophorus eel acetylcholinesterase inhibited by NEMP, a VX surrogate. Chem. Biol. Interact. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, D.A.S.; Cavalcante, S.F.A.; de Paula, R.L.; Rodrigues, R.B.; Bernardo, L.B.; da Silva, M.C.J.; da Silva, T.N.; dos Santos, W.V.; Granjeiro, J.M.; de Almeida, J.S.F.D.; et al. In Vitro Evaluation of Neutral Aryloximes as Reactivators for Electrophorus eel Acetylcholinesterase Inhibited by Paraoxon. Biomolecules 2019, 9, 583. [Google Scholar] [CrossRef] [PubMed]
- Benson, S.W. Bond Energies. J. Chem. Ed. 1965, 42, 502–518. [Google Scholar] [CrossRef]
- Reuters. Available online: www.reuters.com/article/india-children-idUSL4N0FO18520130718 (accessed on 3 January 2020).
- BBC. Available online: http://www.bbc.com/news/world-asia-23390972 (accessed on 3 January 2020).
- Stokstad, E. Pesticides Under Fire for Risks to Pollinators. Science 2013, 340, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, M.; Casida, J.E. Neonicotinoid insecticide toxicology: Mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 24–268. [Google Scholar] [CrossRef] [PubMed]
- Milatovic, D.; Jokanovic, M. Pyridinium Oximes as Cholinesterase Reactivators in the Treatment of OP Poisoning. In Handbook of Toxicology of Chemical Warfare Agents; Gupta, R.C., Ed.; Elsevier: Amsterdam, Netherlands, 2009; Chapter 65; pp. 985–994. [Google Scholar]
- Kuča, K.; Musilek, K.; Jun, D.; Bajgar, J.; Kassa, J. Novel Oximes. In Elsevier Handbook of Toxicology of Chemical Warfare Agents; Gupta, R.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; Chapter 66; pp. 997–1021. [Google Scholar]
- Worek, F.; Thiermann, H.; Wille, T. Oximes in organophosphate poisoning: 60 years of hope and despair. Chem. Biol. Interact. 2016, 259, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.S.; Prates, A.; Alves, S.R.; Oliveira-Silva, J.J.; Riehl, C.A.S.; Figueroa-Villar, J.D. The Effect of Neutral Oximes on the Reactivation of Human Acetylcholinesterase Inhibited with Paraoxon. J. Braz. Chem. Soc. 2012, 23, 1216–1225. [Google Scholar] [CrossRef]
- Mumford, H.; Docx, C.J.; Price, M.E.; Green, A.C.; Tattersall, J.E.H.; Armstrong, S.J. Human plasma-derived BuChE as a stoichiometric bioscavenger for treatment of nerve agent poisoning. Chem. Biol. Interact. 2013, 203, 160–166. [Google Scholar] [CrossRef]
- Ilyushin, D.G.; Smirnov, I.V.; Belogurov, A.A., Jr.; Dyachenko, I.A.; Zharmukhamedova, T.I.; Novozhilova, T.I.; Bychikhin, E.A.; Serebryakova, M.V.; Kharybin, O.N.; Murashev, A.N.; et al. Chemical polysialylation of human recombinant butyrylcholinesterase delivers a long-acting bioscavenger for nerve agents in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, 1243–1248. [Google Scholar] [CrossRef]
- Valiyaveettil, M.; Alamneh, Y.; Rezk, P.; Biggemann, L.; Perkins, M.W.; Scieuto, A.M.; Doctor, B.P.; Nambiar, M.P. Protective efficacy of catalytic bioscavenger, paraoxonase 1 against sarin and soman exposure in guinea pigs. Biochem. Pharmacol. 2011, 81, 800–809. [Google Scholar] [CrossRef]
- Trovaslet-Leroy, M.; Musilova, L.; Renault, F.; Brazzolotto, X.; Misik, J.; Novotny, L.; Froment, M.T.; Gillon, E.; Loiodice, M.; Verdier, L.; et al. Organophosphate hydrolases as catalytic bioscavengers of organophosphorus nerve agents. Toxicol Lett. 2011, 206, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Black, R.M.; Clarke, R.J.; Read, R.W.; Reid, M.T.J. Application of gas chromatography-mass spectrometry and gas chromatography-tandem mass spectrometry to the analysis of chemical warfare samples, found to contain residues of the nerve agent sarin, sulphur mustard and their degradation products. J. Chromatogr. A 1994, 662, 301–321. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, Y.H. Liquid chromatography-tandem mass spectrometry determination of the degradation products of V-type nerve agents, N,N-dialkylaminoethanesulfonic acids. J. Anal. Chem. 2015, 70, 1001–1007. [Google Scholar] [CrossRef]
- Kientz, C.E. Chromatography and mass spectrometry of chemical warfare agents, toxins and related compounds: State of the art and future prospects. J. Chromatogr. A 1998, 814, 1–23. [Google Scholar] [CrossRef]
- Koller, M.; Becker, C.; Thiermann, H.; Worek, F. GC-MS and LC-MS analysis of nerve agents in body fluids: Intra-laboratory verification test using spiked plasma and urine samples. J. Chromatogr. B 2010, 878, 1226–1233. [Google Scholar] [CrossRef]
- Mesilaakso, M. (Ed.) Chemical Weapons Convention Chemicals Analysis: Sample Collection, Preparation and Analytical Methods; Wiley: Chichester, UK, 2015. [Google Scholar]
- Vanninen, P. (Ed.) Recommended Operating Procedures for Analysis in the Verification of Chemical Disarmament; The Ministry of Foreign Affairs of Finland, University of Helsinki: Helsinki, Finland, 2011.
- Black, R.M.; Muir, B. Derivatisation reactions in the chromatographic analysis of chemical warfare agents and their degradation products. J. Chromatogr. A 2003, 1000, 253–281. [Google Scholar] [CrossRef]
- Read, M.W.; Black, R.M. Rapid screening procedures for the hydrolysis products of chemical warfare agents using positive and negative ion liquid chromatography-mass spectrometry with atmospheric pressure chemical ionisation. J. Chromatogr. A 1999, 862, 169–177. [Google Scholar] [CrossRef]
- Driskell, W.J.; Shih, M.; Needham, L.L.; Barr, D.B. Quantitation of organophosphorus nerve agent metabolites in human urine using isotope dilution gas chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2002, 26, 6–10. [Google Scholar] [CrossRef]
- Sega, G.A.; Tomkins, B.A.; Griest, W.H. Analysis of methylphosphonic acid, ethyl methylphosphonic acid and isopropyl methylphosphonic acid at low microgram per liter levels in groundwater. J. Chromatogr. A 1997, 790, 143–152. [Google Scholar] [CrossRef]
- Noort, D.; Hulst, A.G.; Platenburg, D.H.J.M.; Polhuijs, M.; Benschop, H.P. Quantitative analysis of O-isopropyl methylphosphonic acid in serum samples of Japanese citizens allegedly exposed to sarin: Estimation of internal dosage. Arch. Toxicol. 1998, 72, 671–675. [Google Scholar] [CrossRef]
- Noort, D.; Benschop, H.P.; Black, R.M. Biomonitoring of exposure to chemical warfare agents: A review. Toxicol. Appl. Pharmacol. 2002, 184, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Mathews, T.P.; Carter, M.D.; Johnson, D.; Isenberg, S.L.; Graham, L.A.; Thomas, J.D.; Johnson, R.C. High-Confidence Qualitative Identification of Organophosphorus Nerve Agent Adducts to Human Butyrylcholinesterase. Anal. Chem. 2017, 89, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Bielmann, A.; Curty, C.; Bochet, C.G. Solid-Phase Synthesis of the Aged-Nonapeptide-Nerve-Agent Adduct of Butyrylcholinesterase as Reference Materials for Analytical Verification. Helv. Chim. Acta 2019, 100, e1700198. [Google Scholar] [CrossRef]
- Jokanovic, M.; Prostran, M. Pyridinium oximes as cholinesterase reactivators. Structure-activity relationship and efficacy in the treatment of poisoning with organophosphorus compounds. Curr. Med. Chem. 2009, 16, 2177–2188. [Google Scholar] [CrossRef]
- Wilhelm, C.M.; Snider, T.H.; Babin, M.C.; Jett, D.A.; Platoff, G.E., Jr.; Yeung, D.T. A comprehensive evaluation of the efficacy of leading oximes therapies in guinea pigs exposed to organophosphorus chemical warfare agents or pesticides. Toxicol. Appl. Pharmacol. 2014, 281, 254–265. [Google Scholar] [CrossRef]
- Marrs, T.C.; Rice, P.; Vale, J.A. The role of oximes in the treatment of nerve agent poisoning in civilian casualties. Toxicol. Rev. 2006, 25, 297–323. [Google Scholar] [CrossRef]
- Bajgar, J.; Fusek, J.; Kassa, J.; Kuča, K.; Jun, D. Pharmacological Prophylaxis Against Nerve Agent Poisoning: Experimental Studies and Practical Implication. In Handbook of Toxicology of Chemical Warfare Agents; Gupta, R.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; Chapter 64; pp. 677–684. [Google Scholar]
- Cannard, K.J. The acute treatment of nerve agent exposure. Neurol. Sci. 2006, 249, 86–94. [Google Scholar] [CrossRef]
- Yokoyama, K. Our recent experiences with sarin poisoning cases in Japan and pesticide users with references to some selected chemicals. Neurotoxicology 2007, 28, 364–373. [Google Scholar] [CrossRef]
- Worek, F.; Wille, T.; Koller, M.; Thiermann, H. Structural requirements for effective oximes—Evaluation of kinetic in vitro data with phosphylated human AChE and structurally different oximes. Chem. Biol. Interact. 2013, 203, 125–128. [Google Scholar] [CrossRef]
- Kuča, K.; Jun, D.; Mušilek, K. Structural Requirements of Acetylcholinesterase Reactivators. Mini Rev. Med. Chem. 2006, 6, 269–277. [Google Scholar] [CrossRef]
- de Jong, L.P.A.; Verhagen, M.A.A.; Langenberg, J.P.; Hagedorn, I.; Löffler, M. The bispyridinium-dioxime HLö-7: A potent reactivator for acetylcholinesterase inhibited by the stereoisomers of tabun and soman. Biochem. Pharmacol. 1989, 38, 633–640. [Google Scholar] [CrossRef]
- Eyer, P.; Hagedorn, I.; Klimmek, R.; Lippstreu, P.; Löffler, M.; Oldiges, H.; Spöhrer, U.; Steidl, I.; Szinicz, L.; Worek, F. HLö-7 dimethanesulfonate, a potent bispyridinium-dioxime against anticholinesterases. Arch. Toxicol. 1992, 66, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Cabal, J.; Kuča, K.; Kassa, J. Specification of the Structure of Oximes Able to Reactivate Tabun-Inhibited Acetylcholinesterase. Basic Clin. Pharmacol. Toxicol. 2004, 95, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Jokanović, M. Structure-activity relationship and efficacy of pyridinium oximes in the treatment of poisoning with organophosphorus compounds: A review of recent data. Curr. Top. Med. Chem. 2012, 12, 1775–1789. [Google Scholar] [CrossRef]
- Aroniadou-Anderjaska, V.; Figueiredo, T.H.; Apland, J.P.; Prager, E.M.; Pidoplichko, V.I.; Miller, S.L.; Braga, M.F.M. Countermeasures Against Chemical Threats Long-term neuropathological and behavioral impairments after exposure to nerve agents. Ann. N. Y. Acad. Sci. 2006, 1374, 17–28. [Google Scholar] [CrossRef]
- Aroniadou-Anderjaska, V.; Figueiredo, T.H.; Apland, J.P.; Qashu, F.; Braga, M.F.M. Primary brain targets of nerve agents the role of the amygdala in comparison to the hippocampus. Neurotoxicology 2009, 30, 772–776. [Google Scholar] [CrossRef]
- Moshiri, M.; Darchini-Maragheh, E.; Balali-Mood, M. Advances in toxicology and medical treatment of chemical warfare nerve agents. DARU 2012, 20, 1–24. [Google Scholar] [CrossRef]
- Čolović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Antonijevic, B.; Stojiljkovic, M.P. Unequal Efficacy of Pyridinium Oximes in Acute Organophosphate Poisoning. Clin. Med. Res. 2007, 5, 71–82. [Google Scholar] [CrossRef]
- Wiener, S.W.; Hoffman, R.S. Nerve Agents: A Comprehensive Review. J. Intensive Care Med. 2004, 19, 22–37. [Google Scholar] [CrossRef]
- McDonough, J.H.; Van Shura, K.E.; LaMont, J.C.; McMonagle, J.D.; Shih, T.-M. Comparison of the Intramuscular, Intranasal or Sublingual Routes of Midazolam Administration for the Control of Soman-Induced Seizures. Basic Clin. Pharmacol. Toxicol. 2009, 104, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.D.; Reddy, D.S. Midazolam as an anticonvulsant antidote for organophosphate intoxication—A pharmacotherapeutic appraisal. Epilepsia 2015, 56, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Kapoora, M.; Cloyd, J.C.; Siegelad, R.A. A review of intranasal formulations for the treatment of seizure emergencies. J. Control. Release 2016, 237, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Candiotti, K. A primer on nerve agents: What the emergency responder, anesthesiologist, and intensivist needs to know. Can. J. Anesth. 2017, 64, 1059–1070. [Google Scholar] [CrossRef]
- Benfield, J.; Musto, A. Intranasal Therapy to Stop Status Epilepticus in Prehospital Settings. Drugs R D 2018, 18, 7–17. [Google Scholar] [CrossRef]

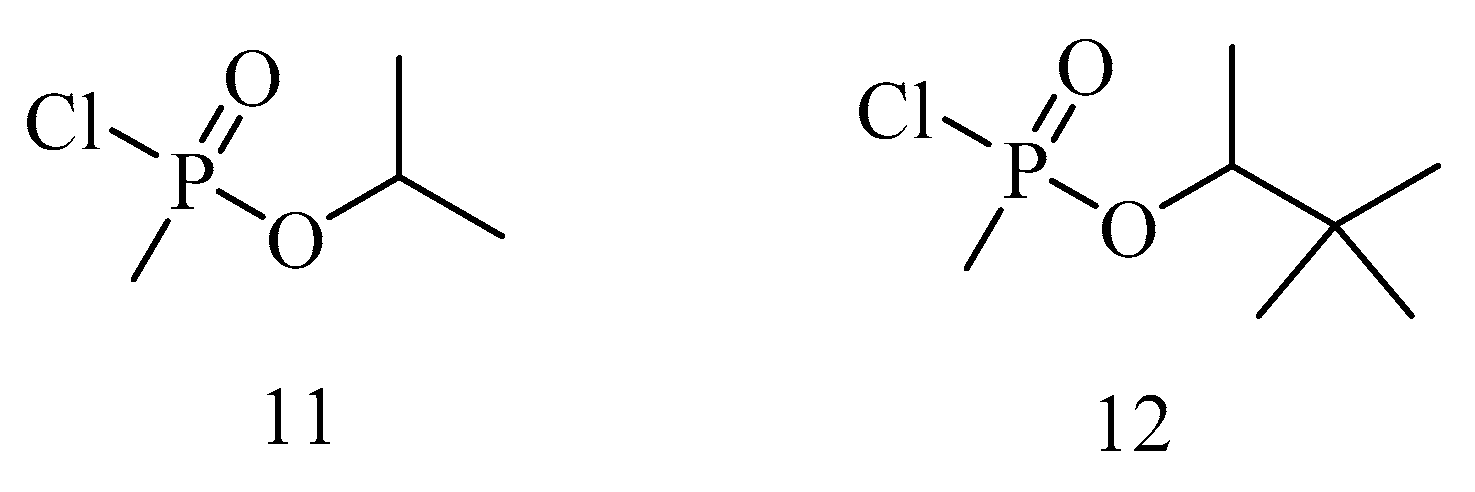





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalcante, S.F.d.A.; Simas, A.B.C.; Barcellos, M.C.; de Oliveira, V.G.M.; Sousa, R.B.; Cabral, P.A.d.M.; Kuča, K.; França, T.C.C. Acetylcholinesterase: The “Hub” for Neurodegenerative Diseases and Chemical Weapons Convention. Biomolecules 2020, 10, 414. https://doi.org/10.3390/biom10030414
Cavalcante SFdA, Simas ABC, Barcellos MC, de Oliveira VGM, Sousa RB, Cabral PAdM, Kuča K, França TCC. Acetylcholinesterase: The “Hub” for Neurodegenerative Diseases and Chemical Weapons Convention. Biomolecules. 2020; 10(3):414. https://doi.org/10.3390/biom10030414
Chicago/Turabian StyleCavalcante, Samir F. de A., Alessandro B. C. Simas, Marcos C. Barcellos, Victor G. M. de Oliveira, Roberto B. Sousa, Paulo A. de M. Cabral, Kamil Kuča, and Tanos C. C. França. 2020. "Acetylcholinesterase: The “Hub” for Neurodegenerative Diseases and Chemical Weapons Convention" Biomolecules 10, no. 3: 414. https://doi.org/10.3390/biom10030414
APA StyleCavalcante, S. F. d. A., Simas, A. B. C., Barcellos, M. C., de Oliveira, V. G. M., Sousa, R. B., Cabral, P. A. d. M., Kuča, K., & França, T. C. C. (2020). Acetylcholinesterase: The “Hub” for Neurodegenerative Diseases and Chemical Weapons Convention. Biomolecules, 10(3), 414. https://doi.org/10.3390/biom10030414





