Hydroxylamine Analogue of Agmatine: Magic Bullet for Arginine Decarboxylase
Abstract
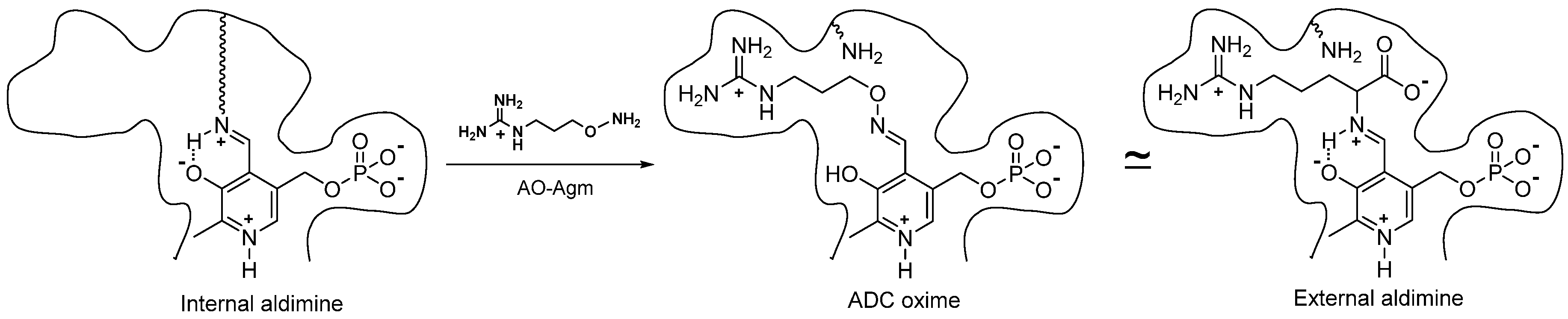
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Partially Purified ADC and ODC
2.3. E. coli ADC and ODC Activity Assays
2.4. Ultrafiltration of ADC~PLP=AO-Agm Complex
2.5. Assay of Mouse Kidney ODC Activity
2.6. Cell Culture
2.7. Fungal Growth Inhibition
2.8. Determination of Polyamine Content in the Fungi
3. Results
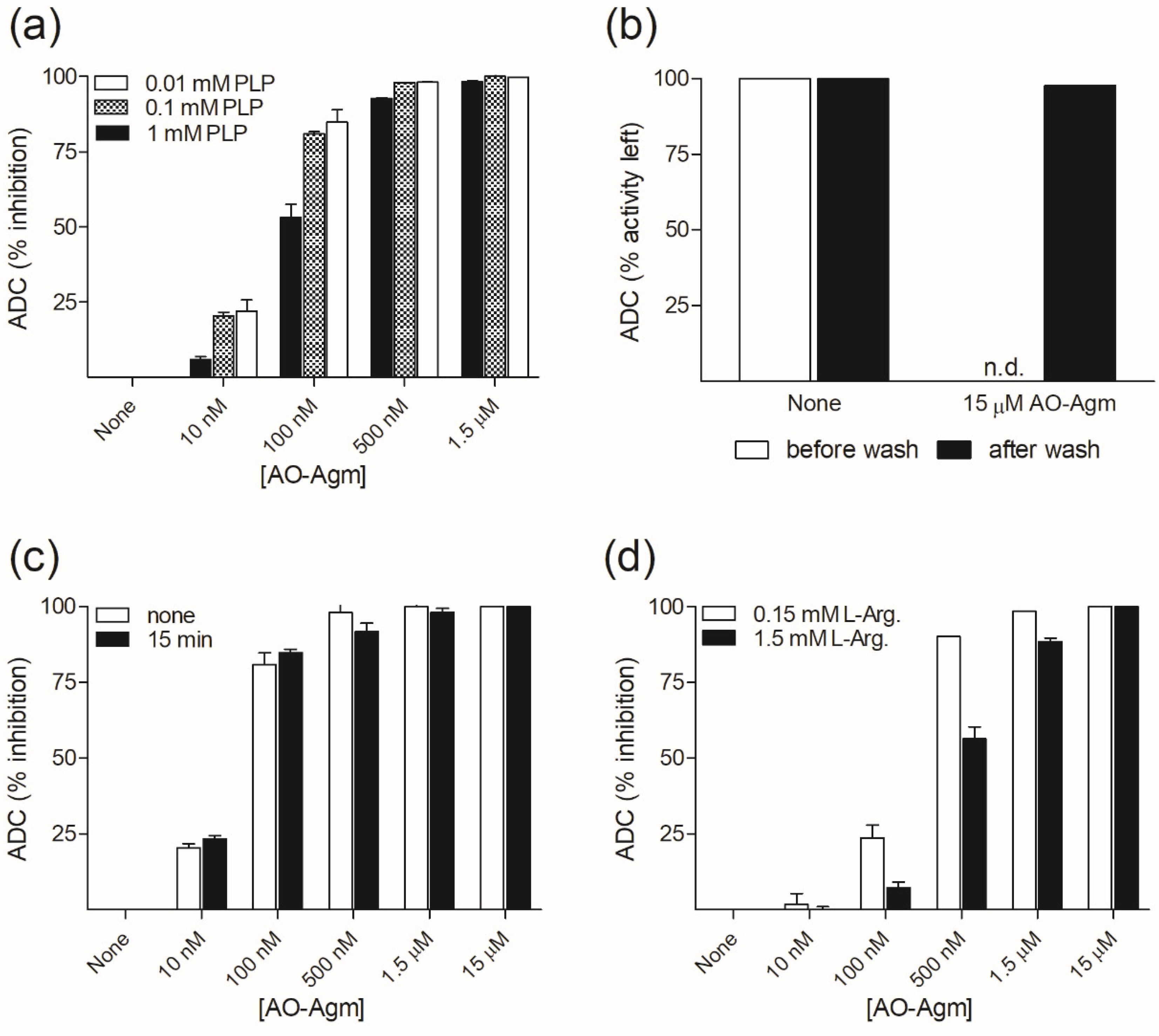
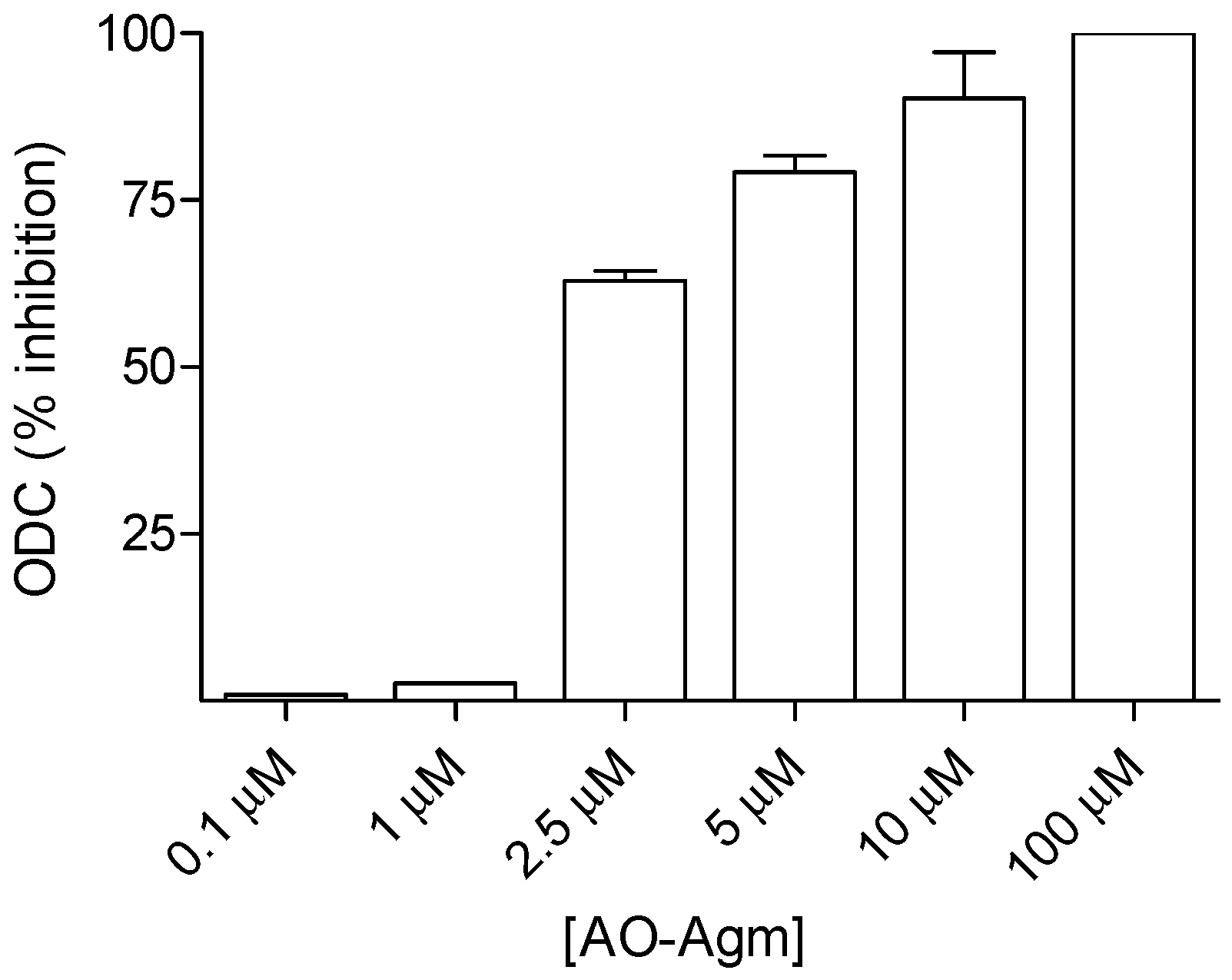
3.1. Inhibition of E. coli ADC and ODC by AO-Agm
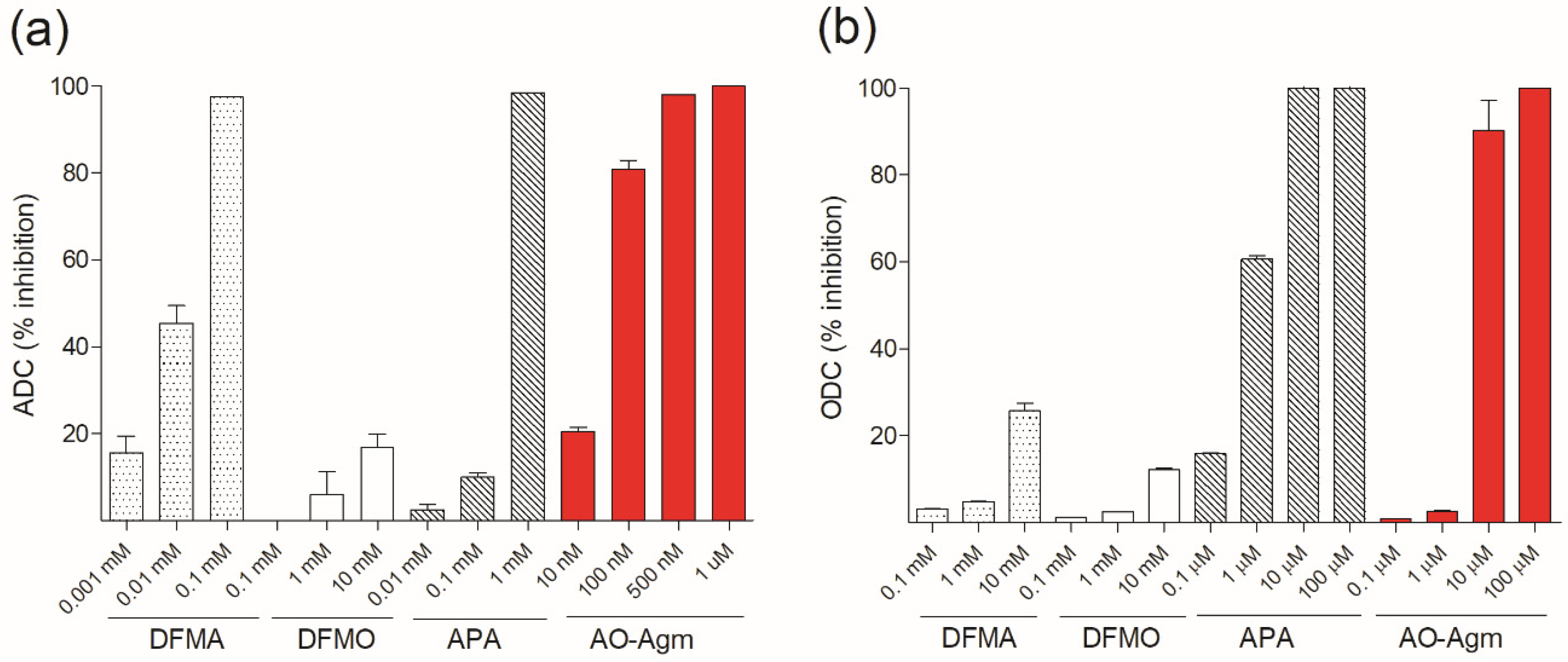
3.2. Inhibition of E. coli ADC and ODC by DFMA, DFMO and APA
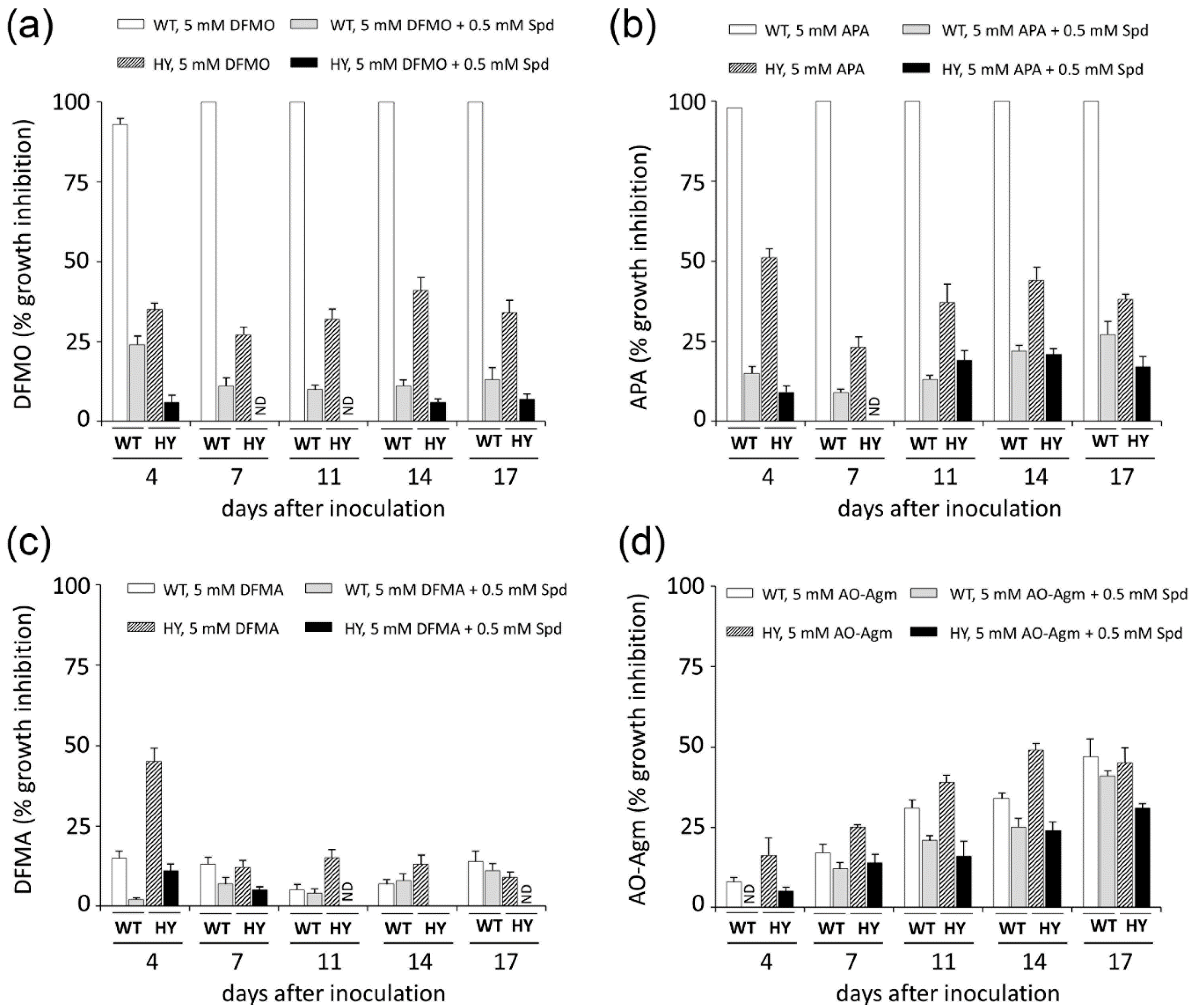
3.3. Inhibition of Mammalian and Fungal Cell Growth by AO-Agm
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pegg, A.E. Functions of polyamines in mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef] [PubMed]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining mysteries of molecular biology: The role of polyamines in the cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.J. Polyamine function in archaea and bacteria. J. Biol. Chem. 2018, 293, 18693–18701. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A.; Murray Stewart, T.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Ramani, D.; De Bandt, J.P.; Cynober, L. Aliphatic polyamines in physiology and diseases. Clin. Nut. 2014, 33, 14–22. [Google Scholar] [CrossRef]
- Mounce, B.C.; Olsen, M.E.; Vignuzzi, M.; Connor, J.H. Polyamines and their role in virus infection. Microbiol. Mol. Biol. Rev. 2017, 81, e00029-17. [Google Scholar] [CrossRef]
- Alhonen, L.; Uimari, A.; Pietilä, M.; Hyvönen, M.T.; Pirinen, E.; Keinänen, T.A. Transgenic animals modelling polyamine metabolism related diseases. Essays Biochem. 2009, 46, 125–144. [Google Scholar]
- Wallace, H.M.; Fraser, A.V. Inhibitors of polyamine metabolism: Review article. Amino Acids 2004, 26, 353–365. [Google Scholar] [CrossRef]
- Casero, R.A.; Woster, P.M. Recent advances in the development of polyamine analogues as antitumor agents. J. Med. Chem. 2009, 52, 4551–4573. [Google Scholar] [CrossRef]
- Khomutov, M.; Hyvönen, M.T.; Simonian, A.; Formanovsky, A.A.; Mikhura, I.V.; Chizhov, A.O.; Kochetkov, S.N.; Alhonen, L.; Vepsäläinen, J.; Keinänen, T.A.; et al. Unforeseen possibilities to investigate the regulation of polyamine metabolism revealed by novel C-methylated spermine derivatives. J. Med. Chem. 2019, 62, 11335–11347. [Google Scholar] [CrossRef]
- Keinänen, T.A.; Hyvönen, M.T.; Alhonen, L.; Vepsäläinen, J.; Khomutov, A.R. Selective regulation of polyamine metabolism with methylated polyamine analogues. Amino Acids 2014, 46, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Seiler, N. Thirty years of polyamine-related approaches to cancer therapy. Retrospect and prospect. Part 1. Selective enzyme inhibitors. Curr. Drug Targets 2003, 4, 537–564. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, B.W.; Bey, P.; Danzin, C.; Jung, M.J.; Casara, P.; Vevert, J.P. Catalytic irreversible inhibition of mammalian ornithine decarboxylase (E.C.4.1.1.17) by substrate and product analogues. J. Am. Chem. Soc. 1978, 100, 2551–2553. [Google Scholar] [CrossRef]
- Burri, C.C.; Brun, R. Eflornithine for the treatment of human African trypanosomiasis. Parasitol. Res. 2003, 90, S49–S52. [Google Scholar] [CrossRef] [PubMed]
- Gerner, E.W.; Bruckheimer, E.; Cohen, A. Cancer pharmacoprevention: Targeting polyamine metabolism to manage risk factors for colon cancer. J. Biol. Chem. 2018, 293, 18770–18778. [Google Scholar] [CrossRef] [PubMed]
- Rajam, M.V.; Weinstein, L.H.; Galston, A.W. Prevention of a plant disease by specific inhibition of fungal polyamine biosynthesis. Proc. Natl. Acad. Sci. USA. 1985, 82, 6874–6878. [Google Scholar] [CrossRef]
- Khomutov, R.M.; Denisova, G.F.; Khomutov, A.R.; Belostotskaia, K.M.; Shlosman, R.B.; Artamonova, E.Y. Aminooxypropylamine—An effective inhibitor of ornithine decarboxylase in vitro and in vivo. Bioorg. Khim. 1985, 11, 1574–1576. [Google Scholar]
- Stanek, J.; Frei, J.; Mett, H.; Schneider, P.; Regenass, U. 2-Substituted 3-(aminooxy)propanamines as inhibitors of ornithine decarboxylase: synthesis and biological activity. J. Med. Chem. 1992, 35, 1339–1344. [Google Scholar] [CrossRef]
- Milovica, V.; Turchanowa, L.; Khomutov, A.R.; Khomutov, R.M.; Caspary, W.F.; Stein, J. Hydroxylamine-containing inhibitors of polyamine biosynthesis and impairment of colon cancer cell growth. Biochem. Pharmacol. 2001, 61, 199–206. [Google Scholar] [CrossRef]
- DasGupta, R.; Krause-Ihle, T.; Bergmann, B.; Muller, I.B.; Khomutov, A.R.; Muller, S.; Walter, R.D.; Luersen, K. 3-Aminooxy-1-aminopropane and derivatives have an antiproliferative effect on cultured Plasmodium falciparum by decreasing intracellular polyamine concentrations. Antimicrob. Agents Chemother. 2005, 49, 2857–2864. [Google Scholar] [CrossRef]
- Singh, S.; Mukherjee, A.; Khomutov, A.R.; Persson, L.; Heby, O.; Chatterjee, M.; Madhubala, R. Antileishmanial effect of 3-aminooxy-1-aminopropane is due to polyamine depletion. Antimicrob. Agents Chemother. 2007, 51, 528–534. [Google Scholar] [CrossRef][Green Version]
- Khomutov, A.R.; Dzavakhia, V.G.; Voinova, T.M.; Ermolinsky, B.S.; Khomutov, R.M. Aminooxy analogue of putrescine inhibits polyketide biosynthetic pathway of natural products. Bioorg. Khim. 1989, 15, 707–709. [Google Scholar]
- Morris, S.M., Jr. Recent advances in arginine metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 45–51. [Google Scholar] [CrossRef]
- Forouhar, F.; Lew, S.; Seetharaman, J.; Xiao, R.; Acton, T.B.; Montelione, G.T.; Tong, L. Structures of bacterial biosynthetic arginine decarboxylases. Acta Cryst. 2010, F66, 1562–1566. [Google Scholar] [CrossRef]
- Lin, J.; Smith, M.P.; Chapin, K.C.; Baik, H.S.; Bennett, G.N.; Foster, J.W. Mechanisms of acid resistance in enterohemorrhagic Escherichia coli. Appl. Environ. Microbiol. 1996, 62, 3094–3100. [Google Scholar] [CrossRef]
- Bitonti, A.J.; Casara, P.J.; McCann, P.P.; Bey, P. Catalytic irreversible inhibition of bacterial and plant arginine decarboxylase activities by novel substrate and product analogues. Biochem. J. 1987, 242, 69–74. [Google Scholar] [CrossRef]
- Khan, A.J.; Minocha, S.C. Biosynthetic arginine decarboxylase in phytopathogenic fungi. Life Sci. 1989, 44, 1215–1222. [Google Scholar] [CrossRef]
- Gadkari, T.V.; Cortes, N.; Madrasi, K.; Tsoukias, N.M.; Joshi, M.S. Agmatine induced NO dependent rat mesenteric artery relaxation and its impairment in salt-sensitive hypertension. Nitric Oxide 2013, 35, 65–71. [Google Scholar] [CrossRef]
- Yarlett, N.; Waters, W.R.; Harp, J.A.; Wannemuehler, M.J.; Morada, M.; Bellcastro, J.; Upton, S.J.; Marton, L.J.; Frydman, B.J. Activities of D,L-alpha-difluoromethylarginine and polyamine analogues against Cryptosporidium parvum infection in a T-cell receptor alpha-deficient mouse model. Antimicrob. Agents Chemother. 2007, 51, 1234–1239. [Google Scholar] [CrossRef]
- Alam, M.; Srivastava, A.; Dutta, A.; Sau, A.K. Biochemical and biophysical studies of Helicobacter pylori arginine decarboxylase, an enzyme important for acid adaptation in host. IUBMB Life 2018, 70, 658–669. [Google Scholar] [CrossRef]
- Simonian, A.R.; Grigorenko, N.A.; Vepsäläinen, J.; Khomutov, A.R. New charge-deficient agmatine analogs. Russ. J. Bioorg. Chem. 2005, 31, 583–587. [Google Scholar] [CrossRef]
- Khomutov, A.R.; Khomutov, R.M. Synthesis of putrescine and spermidine aminooxyanalogues. Bioorg. Khim. 1989, 15, 698–703. [Google Scholar]
- Sun, X.; Song, W.; Liu, L. Enzymatic production of agmatine by recombinant arginine decarboxylase. J. Mol. Catal. B Enzym. 2015, 121, 1–8. [Google Scholar] [CrossRef]
- Hyvönen, T.; Keinänen, T.A.; Khomutov, A.R.; Khomutov, R.M.; Eloranta, T.O. Monitoring of the uptake and metabolism of aminooxy analogues of polyamines in cultured cells by high-performance liquid chromatography. J. Chromatogr. 1992, 574, 17–21. [Google Scholar] [CrossRef]
- Jänne, J.; Williams-Ashman, H.G. On the purification of L-ornithine decarboxylase from rat prostate and effects of thiol compounds on the enzyme. J. Biol. Chem. 1971, 246, 1725–1732. [Google Scholar]
- Newton, G.G.; Abraham, E.P. Isolation of cephalosporin C, a penicillin-like antibiotic containing D-alpha-aminoadipic acid. Biochem. J. 1956, 62, 651–658. [Google Scholar]
- Bartoshevich, Y.; Novak, M.; Domratcheva, A.; Skryabin, K. Method of cephalosporin C biosynthesis by using new Acremonium chrysogenum strain, RNCM No F-4081D. Russian Federation Patent No 2426793, 20 August 2011. [Google Scholar]
- Zhgun, A.A.; Ivanova, M.A.; Domracheva, A.G.; Novak, M.I.; Elidarov, M.A.; Skryabin, K.G.; Bartoshevich, Y.E. Genetic transformation of the mycelium fungi Acremonium chrysogenum. Appl. Biochem. Microbiol. 2008, 44, 600–607. [Google Scholar] [CrossRef]
- Dumina, M.V.; Zhgun, A.A.; Domratcheva, A.G.; Novak, M.I.; Eldarov, M.A. Chromosomal polymorphism of Acremonium chrysogenum strains producing cephalosporin C. Russ. J. Genet. 2012, 48, 778–784. [Google Scholar] [CrossRef]
- Dumina, M.V.; Zhgun, A.A.; Kerpichnikov, I.V.; Domracheva, A.G.; Novak, M.I.; Valiachmetov, A.Y.; Knorre, D.A.; Severin, F.F.; Eldarov, M.A.; Bartoshevich, Y.E. Functional analysis of MFS protein CefT involved in the transport of beta-lactam antibiotics in Acremonium chrysogenum and Saccharomyces cerevisiae. Appl. Biochem. Microbiol. 2013, 49, 368–377. [Google Scholar] [CrossRef]
- Zhgun, A.A.; Nuraeva, G.K.; Dumina, M.V.; Voinova, T.M.; Dzhavakhiya, V.V.; Eldarov, M.A. 1,3-Diaminopropane and spermidine upregulate lovastatin production and expression of lovastatin biosynthetic genes in Aspergillus terreus via LaeA regulation. Appl. Biochem. Microbiol. 2019, 55, 244–255. [Google Scholar] [CrossRef]
- Dumina, M.V.; Zhgun, A.A.; Novak, M.I.; Domratcheva, A.G.; Petukhov, D.V.; Dzhavakhiya, V.V.; Elidarov, M.A.; Bartoshevich, I.E. Comparative gene expression profiling reveals key changes in expression levels of cephalosporin C biosynthesis and transport genes between low and high-producing strains of Acremonium chrysogenum. World J. Microbiol. Biotechnol. 2014, 30, 2933–2941. [Google Scholar] [CrossRef] [PubMed]
- Minocha, R.; Long, S. Simultaneous separation and quantitation of amino acids and polyamines of forest tree tissues and cell cultures within a single high-performance liquid chromatography run using dansyl derivatization. J. Chromatogr. A 2004, 1035, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.H.; Morris, D.R. Biosynthetic arginine decarboxylase from Escherichia coli. J. Biol. Chem. 1973, 248, 1685–1697. [Google Scholar]
- Seely, J.E.; Pösö, H.; Pegg, A.E. Purification of ornithine decarboxylase from kidneys of androgen-treated mice. Biochemistry 1982, 21, 3394–3399. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, A.; Dawson, P.E. Rapid oxime and hydrazone ligations with aromatic aldehydes for biomolecular labeling. Bioconjug. Chem. 2008, 19, 2543–2548. [Google Scholar] [CrossRef]
- Kallio, A.; McCann, P.P. Difluoromethylornithine irreversibly inactivates ornithine decarboxylase of Pseudomonas aeruginosa, but does not inhibit the enzymes of Escherichia coli. Biochem. J. 1981, 200, 69–75. [Google Scholar] [CrossRef]
- Preeti; Tapas, S.; Kumar, P.; Madhubala, R.; Tomar, S. Structural insight into DFMO resistant ornithine decarboxylase from Entamoeba histolytica: An inkling to adaptive evolution. PLoS ONE 2013, 8, e53397. [Google Scholar] [CrossRef]
- Coleman, C.S.; Hu, G.; Pegg, A.E. Putrescine biosynthesis in mammalian tissues. Biochem. J. 2004, 379, 849–855. [Google Scholar] [CrossRef]
- Keinänen, T.A.; Hyvönen, T.; Pankaskie, M.C.; Vepsäläinen, J.J.; Eloranta, T.O. Derivatives of 1-aminooxy-3-aminopropane as polyamine antimetabolites: Stability and effects on BHK21/C13 cells. J. Biochem. 1994, 116, 1056–1062. [Google Scholar] [CrossRef]
- Slocum, R.D.; Bitonti, A.J.; McCann, P.P.; Feirert, R.P. DL-alpha-difluoromethyl[3,4-3H]arginine metabolism in tobacco and mammalian cells. Inhibition of ornithine decarboxylase activity after arginase-mediated hydrolysis of DL-alpha-difluoromethylarginine to DL-alpha-difluoromethylornithine. Biochem. J. 1988, 255, 197–202. [Google Scholar] [CrossRef]
- Sashchenko, L.P.; Severin, E.S.; Khomutov, R.M. On the inhibition of L-glutamic acid decarboxylase by derivatives of hydroxylamine and related compounds. Biokhimiia (Moscow) 1968, 33, 142–147. [Google Scholar]
- Liu, W.; Peterson, P.E.; Carter, R.J.; Zhou, X.; Langston, J.A.; Fisher, A.J.; Toney, M.D. Crystal structures of unbound and aminooxyacetate-bound Escherichia coli gamma-aminobutyrate aminotransferase. Biochemistry 2004, 43, 10896–10905. [Google Scholar] [CrossRef] [PubMed]
- Markovic-Housley, Z.; Schirmer, T.; Hohenester, E.; Khomutov, A.R.; Khomutov, R.M.; Karpeisky, M.Y.; Sandmeier, E.; Christen, P.; Jansonius, J.N. Crystal structure and solution studies of oxime adducts of mitochondrial aspartate aminotransferase. Eur. J. Biochem. 1996, 236, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Capitani, G.; Eliot, A.C.; Gut, H.; Khomutov, R.M.; Kirsch, J.F.; Grutter, M.G. Structure of 1-aminocyclopropane-1-carboxylate synthase in complex with an amino-oxy analogue of the substrate: Implications for substrate binding. Biochim. Biophys. Acta 2003, 1647, 55–60. [Google Scholar] [CrossRef]
- Gabibov, A.G.; Shuster, A.M.; Khomutov, A.R.; Kostina, M.B.; Khurs, E.N.; Goriachenkova, E.V.; Khomutov, R.M. Cystathionase: Catalytic Activity of the Expression Products of cDNA Fragments, and Specific Inhibition of the Native Enzyme and the Fusion Protein by Substrate like O-substituted hydroxylamines. Dokl. Russ. Acad. Sci. 1996, 350, 405–407. [Google Scholar]
- Castro-Oropeza, R.; Pino-Ángeles, A.; Khomutov, M.A.; Urdiales, J.L.; Moya-García, A.A.; Vepsäläinen, J.; Persson, L.; Sarabia, F.; Khomutov, A.; Sánchez-Jiménez, F. Aminooxy analogue of histamine is an efficient inhibitor of mammalian L-histidine decarboxylase: combined in silico and experimental evidences. Amino Acids 2014, 46, 621–631. [Google Scholar] [CrossRef]
- Artamonova, E.Y.; Zavalova, L.L.; Khomutov, R.M.; Khomutov, A.R. Irreversible inhibition of S-adenosylmethionine decarboxylase by hydroxylamine-containing analogues of decarboxylated S-adenosylmethionine. Bioorg. Khim. 1986, 12, 206–212. [Google Scholar]
- Pegg, A.E.; Jones, D.B.; Secrist, J.A., III. Effect of inhibitors of S-adenosylmethionine decarboxylase on polyamine content and growth of L1210 cells. Biochemistry 1988, 27, 1408–1415. [Google Scholar] [CrossRef]
- McCloskey, D.E.; Bale, S.; Secrist, J.A., 3rd; Tiwari, A.; Moss, T.H., 3rd; Valiyaveettil, J.; Brooks, W.H.; Guida, W.C.; Pegg, A.E.; Ealick, S.E. New insights into the design of inhibitors of human S-adenosylmethionine decarboxylase: studies of adenine C8 substitution in structural analogues of S-adenosylmethionine. J. Med. Chem. 2009, 52, 1388–1407. [Google Scholar] [CrossRef]
- Murray Stewart, T.; Dunston, T.T.; Woster, P.M.; Casero, R.A., Jr. Polyamine catabolism and oxidative damage. J. Biol. Chem. 2018, 293, 18736–18745. [Google Scholar] [CrossRef]
- Ha, H.C.; Sirisoma, N.S.; Kuppusamy, P.; Zweier, J.L.; Woster, P.A.; Casero, R.A., Jr. The natural polyamine spermine functions directly as a free radical scavenger. Proc. Natl. Acad. Sci. USA 1998, 95, 11140–11143. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-Y.; Su, G.-C.; Huang, W.-Y.; Ko, M.-Y.; Yeh, H.-Y.; Chang, G.-D.; Lin, S.-J.; Chi, P. Promotion of homology-directed DNA repair by polyamines. Nat. Commun. 2019, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; García-Estrada, C.; Kosalková, K.; Ullán, R.V.; Albillos, S.M.; Martín, J.-F. The inducers 1,3-diaminopropane and spermidine produce a drastic increase in the expression of the penicillin biosynthetic genes for prolonged time, mediated by the laeA regulator. Fungal Genet. Biol. 2012, 49, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Zhgun, A.A.; Nuraeva, G.K.; Eldarov, M.A. The role of LaeA and LovE regulators in lovastatin biosynthesis with exogenous polyamines in Aspergillus terreus. Appl. Biochem. Microbiol. 2019, 55, 542–552. [Google Scholar] [CrossRef]


| Treatment | Growth | Put | Spd | Spm |
|---|---|---|---|---|
| (Fold) | (pmol/mg Protein) | |||
| Control | 1.00 ± 0.01 | 1395 ± 164 | 6959 ± 354 | 3492 ± 175 |
| AO-Agm (10 μM) | 0.96 ± 0.02 | 1515 ± 98 | 7237 ± 73 | 3782 ± 55 |
| AO-Agm (100 μM) | 0.96 ± 0.04 | 1447 ± 43 | 7047 ± 155 | 3682 ± 152 |
| AO-Agm (1 mM) | 0.72 ± 0.04 *** | 1074 ± 30 * | 3609 ± 124 *** | 3795 ± 98 |
| SC | 1.00 ± 0.04 | 1258 ± 157 | 6483 ± 282 | 3582 ± 146 |
| SC+AO-Agm (10 μM) | 0.94 ± 0.03 | 922 ± 60 * | 6405 ± 61 | 3903 ± 72 |
| SC+AO-Agm (100 μM) | 0.97 ± 0.02 | 954 ± 115 * | 6280 ± 122 | 3621 ± 87 |
| SC+AO-Agm (1 mM) | 0.45 ± 0.01*** | n.d. *** | 1074 ± 159 *** | 2389 ± 105 *** |
| Strain | Addition | Put | Spd | Spm |
|---|---|---|---|---|
| (pmol/mg Dry Weight) | ||||
| WT | Control | <10 | 670 ± 110 | 220 ± 30 |
| 5 mM DFMO | <10 | 140 ± 10 | 170 ± 20 | |
| 0.5 mM Spd | <10 | 12,200 ± 2180 ### | 70 ± 10 ### | |
| 5 mM DFMO + 0.5 mM Spd | <10 | 11,530 ± 3690 ### | 90 ± 30 ### | |
| HY | Control | <10 | 3460 ± 520 *** | 990 ± 50 *** |
| 5 mM DFMO | <10 | 410 ± 60 | 424 ± 39 ### | |
| 0.5 mM Spd | <10 | 39,180 ± 4840 ### | 330 ± 10 ### | |
| 5 mM DFMO + 0.5 mM Spd | <10 | 46,630 ± 6700 ### | 340 ± 56 ### | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyvönen, M.T.; Keinänen, T.A.; Nuraeva, G.K.; Yanvarev, D.V.; Khomutov, M.; Khurs, E.N.; Kochetkov, S.N.; Vepsäläinen, J.; Zhgun, A.A.; Khomutov, A.R. Hydroxylamine Analogue of Agmatine: Magic Bullet for Arginine Decarboxylase. Biomolecules 2020, 10, 406. https://doi.org/10.3390/biom10030406
Hyvönen MT, Keinänen TA, Nuraeva GK, Yanvarev DV, Khomutov M, Khurs EN, Kochetkov SN, Vepsäläinen J, Zhgun AA, Khomutov AR. Hydroxylamine Analogue of Agmatine: Magic Bullet for Arginine Decarboxylase. Biomolecules. 2020; 10(3):406. https://doi.org/10.3390/biom10030406
Chicago/Turabian StyleHyvönen, Mervi T., Tuomo A. Keinänen, Gulgina K. Nuraeva, Dmitry V. Yanvarev, Maxim Khomutov, Elena N. Khurs, Sergey N. Kochetkov, Jouko Vepsäläinen, Alexander A. Zhgun, and Alex R. Khomutov. 2020. "Hydroxylamine Analogue of Agmatine: Magic Bullet for Arginine Decarboxylase" Biomolecules 10, no. 3: 406. https://doi.org/10.3390/biom10030406
APA StyleHyvönen, M. T., Keinänen, T. A., Nuraeva, G. K., Yanvarev, D. V., Khomutov, M., Khurs, E. N., Kochetkov, S. N., Vepsäläinen, J., Zhgun, A. A., & Khomutov, A. R. (2020). Hydroxylamine Analogue of Agmatine: Magic Bullet for Arginine Decarboxylase. Biomolecules, 10(3), 406. https://doi.org/10.3390/biom10030406






