Hear, Hear for Notch: Control of Cell Fates in the Inner Ear by Notch Signaling
Abstract
1. Introduction
2. The First Steps in Ear Induction—How Notch Signals Regulate the Size of the Otic Placode
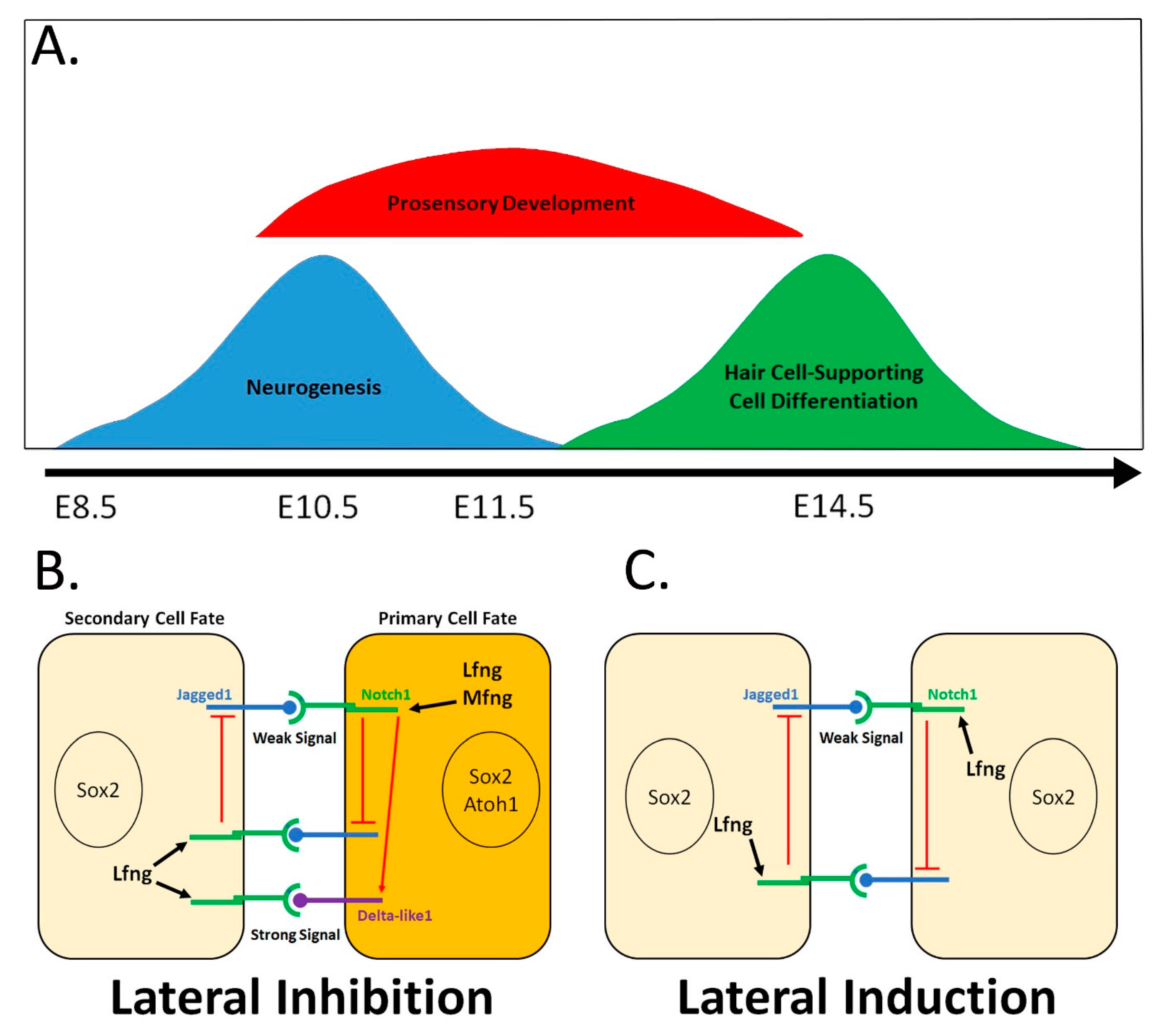
3. Notch Signaling Regulates Neurosensory Fate in the Inner Ear
4. Notch-Mediated Lateral Induction Plays A Key Maintenance Role in Prosensory Domain Development
5. How Is Lateral Induction Initiated and Terminated?
6. Unresolved Questions in Notch-Mediated Lateral Induction
7. Drawing A Line in the Shifting Sands: Notch Signaling Plays a Role in Boundary Formation in the Developing Cochlea
8. A Future for Notch? Notch Signaling In the Inner Ear after Sensory Cell Development
| Notch Receptors and Ligands | Type of Mutation | Phenotype | Reference |
|---|---|---|---|
| Notch1 | Inner ear-specific knockout with Foxg1-Cre or Pax2-Cre | Many cochlear supporting cells (with the exception of inner pillar cells) convert to ectopic inner and outer hair cells | [61,82] |
| Jag1 | Inner ear-specific knockout with Foxg1-Cre | Severe loss of semicircular canals and small or absent vestibular sensory organs. Cochlea has either reduced or absent outer hair cells and supernumerary inner hair cells. | [55,56] |
| Jag1 | Headturner allele; ENU-induced mutation (G289D) | Truncated anterior and/or posterior semicircular canals, loss of some outer hair cells, supernumerary inner hair cells. | [91] |
| Jag1 | Ozzy allele; ENU-induced mutation (W167R) | Variably truncated semicircular canals | [105] |
| Jag1 | Slalom allele; ENU-induced mutation (P269S) | Truncated anterior and/or posterior semicircular canals, loss of some outer hair cells, supernumerary inner hair cells. | [91] |
| Jag1 | Nodder allele; ENU-induced mutation (H268Q) | Vestibular defects (head nodding) | [106] |
| Jag2 | Null mutant | Supernumerary inner and outer hair cells and inner phalangeal cells. | [82,107] |
| Dll1 | Inner ear-specific knockout with Foxg1-Cre | Supernumerary inner and outer hair cells and a small increase in supporting cells | [55] |
| Dll3 | Null mutant | Despite expression in hair cells, no hair cell phenotype | [108] |
| Notch Transcriptional Co-Activators | Type of Mutation | Phenotype | Reference |
| RBPJk | Inner ear-specific knockout with Foxg1-Cre or Pax2-Cre | Severe loss of semicircular canals and small or absent vestibular sensory organs. Cochlea shows evidence of supernumerary inner hair cells but mice die before this becomes patent | [71,109] |
| MAML1-3 | Activation of dnMAML allele with Pax2-Cre | Supernumerary inner hair cells and inner phalangeal cells. | [79] |
| Notch Modifying Enzymes | Type of Mutation | Phenotype | Reference |
| Pofut1 | Inner ear-specific knockout with Pax2-Cre | Supernumerary inner and outer hair cells and inner phalangeal cells. | [79] |
| Lfng | Null mutant | Single mutants have no cochlear phenotype; double mutants have supernumerary inner hair cells and inner phalangeal cells. | [79] |
| Mfng | Null mutant | ||
| Lfng; Mfng | Null mutant | ||
| Lfng; Jag2 | Null mutants | The Lfng mutant allele rescues the Jag2 mutant phenotype in the inner hair cell region but not the outer hair cell region | [110] |
| Notch Downstream Targets | Type of Mutation | Phenotype | Reference |
| Hes1 | Null mutant | Increasing severity of supernumerary inner and outer hair cells with increasing combinations of multiple mutant alleles; Hes1;Hes5;Hey1 triple mutants having the most severe phenotype [102] | [87,111,112,113,114,115] |
| Hes5 | Null mutant | ||
| Hey1 | Null mutant | ||
| HeyL | Null mutant | ||
| Hey2 | Null mutant | No significant phenotype in null; however pharmacological inhibition of Notch signaling in Hey2 mutants causes inner pillar cells to convert to hair cells. | [114] |
9. Conclusions
Funding
Conflicts of Interest
References
- Alsina, B.; Giraldez, F.; Pujades, C. Patterning and cell fate in ear development. Int. J. Dev. Biol. 2009, 53, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Bok, J.; Chang, W.; Wu, D.K. Patterning and morphogenesis of the vertebrate inner ear. Int. J. Dev. Biol. 2007, 51, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, T.T. Development of the inner ear. Curr. Opin. Genet. Dev. 2015, 32, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, T.; Groves, A.K.; Martin, K. The first steps towards hearing: Mechanisms of otic placode induction. Int. J. Dev. Biol. 2007, 51, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Streit, A. The preplacodal region: An ectodermal domain with multipotential progenitors that contribute to sense organs and cranial sensory ganglia. Int. J. Dev. Biol. 2007, 51, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Groves, A.K.; Bronner-Fraser, M. Competence, specification and commitment in otic placode induction. Development 2000, 127, 3489–3499. [Google Scholar]
- Edlund, R.K.; Birol, O.; Groves, A.K. The role of foxi family transcription factors in the development of the ear and jaw. Curr. Top. Dev. Biol. 2015, 111, 461–495. [Google Scholar] [CrossRef]
- Groves, A.K.; LaBonne, C. Setting appropriate boundaries: Fate, patterning and competence at the neural plate border. Dev. Biol. 2014, 389, 2–12. [Google Scholar] [CrossRef]
- Singh, S.; Groves, A.K. The molecular basis of craniofacial placode development. Wiley Interdiscip Rev. Dev. Biol. 2016, 5, 363–376. [Google Scholar] [CrossRef]
- Birol, O.; Ohyama, T.; Edlund, R.K.; Drakou, K.; Georgiades, P.; Groves, A.K. The mouse Foxi3 transcription factor is necessary for the development of posterior placodes. Dev. Biol. 2016, 409, 139–151. [Google Scholar] [CrossRef]
- Ohyama, T.; Groves, A.K. Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis 2004, 38, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Sai, X.; Ladher, R.K. Early steps in inner ear development: Induction and morphogenesis of the otic placode. Front. Pharmacol. 2015, 6, 19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wright, T.J.; Mansour, S.L. FGF signaling in ear development and innervation. Curr. Top. Dev Biol 2003, 57, 225–259. [Google Scholar] [CrossRef] [PubMed]
- Urness, L.D.; Wang, X.; Shibata, S.; Ohyama, T.; Mansour, S.L. Fgf10 is required for specification of non-sensory regions of the cochlear epithelium. Dev. Biol. 2015, 400, 59–71. [Google Scholar] [CrossRef]
- Urness, L.D.; Wang, X.; Doan, H.; Shumway, N.; Noyes, C.A.; Gutierrez-Magana, E.; Lu, R.; Mansour, S.L. Spatial and temporal inhibition of FGFR2b ligands reveals continuous requirements and novel targets in mouse inner ear morphogenesis. Development 2018, 145. [Google Scholar] [CrossRef]
- Ohyama, T.; Mohamed, O.A.; Taketo, M.M.; Dufort, D.; Groves, A.K. Wnt signals mediate a fate decision between otic placode and epidermis. Development 2006, 133, 865–875. [Google Scholar] [CrossRef]
- Jayasena, C.S.; Ohyama, T.; Segil, N.; Groves, A.K. Notch signaling augments the canonical Wnt pathway to specify the size of the otic placode. Development 2008, 135, 2251–2261. [Google Scholar] [CrossRef]
- Estrach, S.; Ambler, C.A.; Lo Celso, C.; Hozumi, K.; Watt, F.M. Jagged 1 is a beta-catenin target gene required for ectopic hair follicle formation in adult epidermis. Development 2006, 133, 4427–4438. [Google Scholar] [CrossRef]
- Ambler, C.A.; Watt, F.M. Expression of Notch pathway genes in mammalian epidermis and modulation by beta-catenin. Dev. Dyn. 2007, 236, 1595–1601. [Google Scholar] [CrossRef]
- Groves, A.K.; Fekete, D.M. Shaping sound in space: The regulation of inner ear patterning. Development 2012, 139, 245–257. [Google Scholar] [CrossRef]
- Raft, S.; Groves, A.K. Segregating neural and mechanosensory fates in the developing ear: Patterning, signaling, and transcriptional control. Cell Tissue Res. 2015, 359, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Raft, S.; Nowotschin, S.; Liao, J.; Morrow, B.E. Suppression of neural fate and control of inner ear morphogenesis by Tbx1. Development 2004, 131, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Raft, S.; Koundakjian, E.J.; Quinones, H.; Jayasena, C.S.; Goodrich, L.V.; Johnson, J.E.; Segil, N.; Groves, A.K. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development 2007, 134, 4405–4415. [Google Scholar] [CrossRef] [PubMed]
- Adam, J.; Myat, A.; Le Roux, I.; Eddison, M.; Henrique, D.; Ish-Horowicz, D.; Lewis, J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: Parallels with Drosophila sense-organ development. Development 1998, 125, 4645–4654. [Google Scholar] [PubMed]
- Morsli, H.; Choo, D.; Ryan, A.; Johnson, R.; Wu, D.K. Development of the mouse inner ear and origin of its sensory organs. J. Neurosci. 1998, 18, 3327–3335. [Google Scholar] [CrossRef] [PubMed]
- Abelló, G.; Khatri, S.; Giráldez, F.; Alsina, B. Early regionalization of the otic placode and its regulation by the Notch signaling pathway. Mech. Dev. 2007, 124, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.H.; Pauley, S.; Jahan, I.; Beisel, K.W.; Millen, K.J.; Fritzsch, B. Lmx1a is required for segregation of sensory epithelia and normal ear histogenesis and morphogenesis. Cell Tissue Res. 2008, 334, 339–358. [Google Scholar] [CrossRef]
- Koundakjian, E.J.; Appler, J.L.; Goodrich, L.V. Auditory neurons make stereotyped wiring decisions before maturation of their targets. J. Neurosci. 2007, 27, 14078–14088. [Google Scholar] [CrossRef]
- Henrique, D.; Adam, J.; Myat, A.; Chitnis, A.; Lewis, J.; Ish-Horowicz, D. Expression of a Delta homologue in prospective neurons in the chick. Nature 1995, 375, 787–790. [Google Scholar] [CrossRef]
- Daudet, N.; Ariza-McNaughton, L.; Lewis, J. Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. Development 2007, 134, 2369–2378. [Google Scholar] [CrossRef]
- Itoh, M.; Kim, C.-H.; Palardy, G.; Oda, T.; Jiang, Y.-J.; Maust, D.; Yeo, S.-Y.; Lorick, K.; Wright, G.J.; Ariza-McNaughton, L.; et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev. Cell 2003, 4, 67–82. [Google Scholar] [CrossRef]
- Haddon, C.; Jiang, Y.J.; Smithers, L.; Lewis, J. Delta-Notch signalling and the patterning of sensory cell differentiation in the zebrafish ear: Evidence from the mind bomb mutant. Development 1998, 125, 4637–4644. [Google Scholar] [PubMed]
- Fode, C.; Ma, Q.; Casarosa, S.; Ang, S.L.; Anderson, D.J.; Guillemot, F. A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes Dev. 2000, 14, 67–80. [Google Scholar] [PubMed]
- Gowan, K.; Helms, A.W.; Hunsaker, T.L.; Collisson, T.; Ebert, P.J.; Odom, R.; Johnson, J.E. Crossinhibitory activities of Ngn1 and Math1 allow specification of distinct dorsal interneurons. Neuron 2001, 31, 219–232. [Google Scholar] [CrossRef]
- Riccomagno, M.M.; Takada, S.; Epstein, D.J. Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev. 2005, 19, 1612–1623. [Google Scholar] [CrossRef]
- Riccomagno, M.M.; Martinu, L.; Mulheisen, M.; Wu, D.K.; Epstein, D.J. Specification of the mammalian cochlea is dependent on Sonic hedgehog. Genes Dev. 2002, 16, 2365–2378. [Google Scholar] [CrossRef]
- Basch, M.L.; Brown, R.M.; Jen, H.-I.; Groves, A.K. Where hearing starts: The development of the mammalian cochlea. J. Anat. 2016, 228, 233–254. [Google Scholar] [CrossRef]
- Huang, Y.; Hill, J.; Yatteau, A.; Wong, L.; Jiang, T.; Petrovic, J.; Gan, L.; Dong, L.; Wu, D.K. Reciprocal negative regulation between lmx1a and lmo4 is required for inner ear formation. J. Neurosci. 2018, 38, 5429–5440. [Google Scholar] [CrossRef]
- Koo, S.K.; Hill, J.K.; Hwang, C.H.; Lin, Z.S.; Millen, K.J.; Wu, D.K. Lmx1a maintains proper neurogenic, sensory, and non-sensory domains in the mammalian inner ear. Dev. Biol. 2009, 333, 14–25. [Google Scholar] [CrossRef]
- Wu, D.K.; Nunes, F.D.; Choo, D. Axial specification for sensory organs versus non-sensory structures of the chicken inner ear. Development 1998, 125, 11–20. [Google Scholar]
- Chang, W.; Brigande, J.V.; Fekete, D.M.; Wu, D.K. The development of semicircular canals in the inner ear: Role of FGFs in sensory cristae. Development 2004, 131, 4201–4211. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.K.; Oh, S.H. Sensory organ generation in the chick inner ear. J. Neurosci. 1996, 16, 6454–6462. [Google Scholar] [CrossRef] [PubMed]
- Abelló, G.; Khatri, S.; Radosevic, M.; Scotting, P.J.; Giráldez, F.; Alsina, B. Independent regulation of Sox3 and Lmx1b by FGF and BMP signaling influences the neurogenic and non-neurogenic domains in the chick otic placode. Dev. Biol. 2010, 339, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.; Kamaid, A.; Alsina, B.; Giraldez, F. Differential expression of Sox2 and Sox3 in neuronal and sensory progenitors of the developing inner ear of the chick. J. Comp. Neurol. 2007, 503, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.; Abelló, G.; Petrovic, J.; Giraldez, F. Patterning and cell fate in the inner ear: A case for Notch in the chicken embryo. Dev. Growth Differ. 2013, 55, 96–112. [Google Scholar] [CrossRef]
- Neves, J.; Uchikawa, M.; Bigas, A.; Giraldez, F. The prosensory function of Sox2 in the chicken inner ear relies on the direct regulation of Atoh1. PLoS ONE 2012, 7, e30871. [Google Scholar] [CrossRef]
- Neves, J.; Vachkov, I.; Giraldez, F. Sox2 regulation of hair cell development: Incoherence makes sense. Hear. Res. 2013, 297, 20–29. [Google Scholar] [CrossRef]
- Neves, J.; Parada, C.; Chamizo, M.; Giráldez, F. Jagged 1 regulates the restriction of Sox2 expression in the developing chicken inner ear: A mechanism for sensory organ specification. Development 2011, 138, 735–744. [Google Scholar] [CrossRef]
- Mann, Z.F.; Gálvez, H.; Pedreno, D.; Chen, Z.; Chrysostomou, E.; Żak, M.; Kang, M.; Canden, E.; Daudet, N. Shaping of inner ear sensory organs through antagonistic interactions between Notch signalling and Lmx1a. Elife 2017, 6. [Google Scholar] [CrossRef]
- Gu, R.; Brown, R.M.; Hsu, C.-W.; Cai, T.; Crowder, A.L.; Piazza, V.G.; Vadakkan, T.J.; Dickinson, M.E.; Groves, A.K. Lineage tracing of Sox2-expressing progenitor cells in the mouse inner ear reveals a broad contribution to non-sensory tissues and insights into the origin of the organ of Corti. Dev. Biol. 2016, 414, 72–84. [Google Scholar] [CrossRef]
- Steevens, A.R.; Glatzer, J.C.; Kellogg, C.C.; Low, W.C.; Santi, P.A.; Kiernan, A.E. SOX2 is required for inner ear growth and cochlear nonsensory formation before sensory development. Development 2019, 146. [Google Scholar] [CrossRef] [PubMed]
- Skene, P.J.; Henikoff, S. An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. Elife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, E.C.; Campbell, S.; Taylor, R.R.; Forge, A.; Hume, C.R. Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. J. Assoc Res. Otolaryngol 2008, 9, 65–89. [Google Scholar] [CrossRef] [PubMed]
- Lindsell, C.E.; Boulter, J.; diSibio, G.; Gossler, A.; Weinmaster, G. Expression patterns of Jagged, Delta1, Notch1, Notch2, and Notch3 genes identify ligand-receptor pairs that may function in neural development. Mol. Cell. Neurosci. 1996, 8, 14–27. [Google Scholar] [CrossRef]
- Kiernan, A.E.; Xu, J.; Gridley, T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006, 2, e4. [Google Scholar] [CrossRef]
- Brooker, R.; Hozumi, K.; Lewis, J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development 2006, 133, 1277–1286. [Google Scholar] [CrossRef]
- Eddison, M.; Le Roux, I.; Lewis, J. Notch signaling in the development of the inner ear: Lessons from Drosophila. Proc. Natl. Acad. Sci. USA 2000, 97, 11692–11699. [Google Scholar] [CrossRef]
- Hartman, B.H.; Reh, T.A.; Bermingham-McDonogh, O. Notch signaling specifies prosensory domains via lateral induction in the developing mammalian inner ear. Proc. Natl. Acad. Sci. USA 2010, 107, 15792–15797. [Google Scholar] [CrossRef]
- Pan, W.; Jin, Y.; Chen, J.; Rottier, R.J.; Steel, K.P.; Kiernan, A.E. Ectopic expression of activated notch or SOX2 reveals similar and unique roles in the development of the sensory cell progenitors in the mammalian inner ear. J. Neurosci. 2013, 33, 16146–16157. [Google Scholar] [CrossRef]
- Liu, Z.; Brunskill, E.; Varnum-Finney, B.; Zhang, C.; Zhang, A.; Jay, P.Y.; Bernstein, I.; Morimoto, M.; Kopan, R. The intracellular domains of Notch1 and Notch2 are functionally equivalent during development and carcinogenesis. Development 2015, 142, 2452–2463. [Google Scholar] [CrossRef]
- Kiernan, A.E.; Cordes, R.; Kopan, R.; Gossler, A.; Gridley, T. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development 2005, 132, 4353–4362. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, J.; Formosa-Jordan, P.; Luna-Escalante, J.C.; Abelló, G.; Ibañes, M.; Neves, J.; Giraldez, F. Ligand-dependent Notch signaling strength orchestrates lateral induction and lateral inhibition in the developing inner ear. Development 2014, 141, 2313–2324. [Google Scholar] [CrossRef] [PubMed]
- Rana, N.A.; Haltiwanger, R.S. Fringe benefits: Functional and structural impacts of O-glycosylation on the extracellular domain of Notch receptors. Curr. Opin. Struct. Biol. 2011, 21, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Sjöqvist, M.; Andersson, E.R. Do as I say, Not(ch) as I do: Lateral control of cell fate. Dev. Biol. 2019, 447, 58–70. [Google Scholar] [CrossRef]
- Brückner, K.; Perez, L.; Clausen, H.; Cohen, S. Glycosyltransferase activity of Fringe modulates Notch-Delta interactions. Nature 2000, 406, 411–415. [Google Scholar] [CrossRef]
- Tan, J.B.; Xu, K.; Cretegny, K.; Visan, I.; Yuan, J.S.; Egan, S.E.; Guidos, C.J. Lunatic and manic fringe cooperatively enhance marginal zone B cell precursor competition for delta-like 1 in splenic endothelial niches. Immunity 2009, 30, 254–263. [Google Scholar] [CrossRef]
- Hicks, C.; Johnston, S.H.; diSibio, G.; Collazo, A.; Vogt, T.F.; Weinmaster, G. Fringe differentially modulates Jagged1 and Delta1 signalling through Notch1 and Notch2. Nat. Cell Biol. 2000, 2, 515–520. [Google Scholar] [CrossRef]
- Handford, P.A.; Korona, B.; Suckling, R.; Redfield, C.; Lea, S.M. Structural Insights into Notch Receptor-Ligand Interactions. Adv. Exp. Med. Biol. 2018, 1066, 33–46. [Google Scholar] [CrossRef]
- Takeuchi, H.; Haltiwanger, R.S. Significance of glycosylation in Notch signaling. Biochem. Biophys. Res. Commun. 2014, 453, 235–242. [Google Scholar] [CrossRef]
- LeBon, L.; Lee, T.V.; Sprinzak, D.; Jafar-Nejad, H.; Elowitz, M.B. Fringe proteins modulate Notch-ligand cis and trans interactions to specify signaling states. Elife 2014, 3, e02950. [Google Scholar] [CrossRef]
- Basch, M.L.; Ohyama, T.; Segil, N.; Groves, A.K. Canonical Notch signaling is not necessary for prosensory induction in the mouse cochlea: Insights from a conditional mutant of RBPjkappa. J. Neurosci. 2011, 31, 8046–8058. [Google Scholar] [CrossRef]
- Fritzsch, B. The Water-to-Land Transition: Evolution of the Tetrapod Basilar Papilla, Middle Ear, and Auditory Nuclei. In The Evolutionary Biology of Hearing; Webster, D.B., Popper, A.N., Fay, R.R., Eds.; Springer: New York, NY, USA, 1992; pp. 351–375. [Google Scholar]
- Fritzsch, B.; Jahan, I.; Pan, N.; Kersigo, J.; Duncan, J.; Kopecky, B. Dissecting the molecular basis of organ of Corti development: Where are we now? Hear. Res. 2011, 276, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Fritzsch, B. The ear of Latimeria chalumnae revisited. Zoology (Jena) 2003, 106, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Beurg, M.; Tan, X.; Fettiplace, R. A prestin motor in chicken auditory hair cells: Active force generation in a nonmammalian species. Neuron 2013, 79, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.A. Evolutionary paths to mammalian cochleae. J. Assoc Res. Otolaryngol 2012, 13, 733–743. [Google Scholar] [CrossRef]
- Chen, P.; Segil, N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development 1999, 126, 1581–1590. [Google Scholar]
- Lee, Y.-S.; Liu, F.; Segil, N. A morphogenetic wave of p27Kip1 transcription directs cell cycle exit during organ of Corti development. Development 2006, 133, 2817–2826. [Google Scholar] [CrossRef]
- Chen, P.; Johnson, J.E.; Zoghbi, H.Y.; Segil, N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development 2002, 129, 2495–2505. [Google Scholar]
- Lumpkin, E.A.; Collisson, T.; Parab, P.; Omer-Abdalla, A.; Haeberle, H.; Chen, P.; Doetzlhofer, A.; White, P.; Groves, A.; Segil, N.; et al. Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr Patterns 2003, 3, 389–395. [Google Scholar] [CrossRef]
- Ohyama, T.; Basch, M.L.; Mishina, Y.; Lyons, K.M.; Segil, N.; Groves, A.K. BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J. Neurosci. 2010, 30, 15044–15051. [Google Scholar] [CrossRef]
- Basch, M.L.; Brown, R.M.; Jen, H.-I.; Semerci, F.; Depreux, F.; Edlund, R.K.; Zhang, H.; Norton, C.R.; Gridley, T.; Cole, S.E.; et al. Fine-tuning of Notch signaling sets the boundary of the organ of Corti and establishes sensory cell fates. Elife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Mizutari, K.; Fujioka, M.; Hosoya, M.; Bramhall, N.; Okano, H.J.; Okano, H.; Edge, A.S.B. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron 2013, 77, 58–69. [Google Scholar] [CrossRef]
- Radosevic, M.; Fargas, L.; Alsina, B. The role of her4 in inner ear development and its relationship with proneural genes and Notch signalling. PLoS ONE 2014, 9, e109860. [Google Scholar] [CrossRef] [PubMed]
- Abdolazimi, Y.; Stojanova, Z.; Segil, N. Selection of cell fate in the organ of Corti involves the integration of Hes/Hey signaling at the Atoh1 promoter. Development 2016, 143, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, N.A.; Hassan, B.A.; Price, S.D.; Vollrath, M.A.; Ben-Arie, N.; Eatock, R.A.; Bellen, H.J.; Lysakowski, A.; Zoghbi, H.Y. Math1: An essential gene for the generation of inner ear hair cells. Science 1999, 284, 1837–1841. [Google Scholar] [CrossRef]
- Zheng, J.L.; Shou, J.; Guillemot, F.; Kageyama, R.; Gao, W.Q. Hes1 is a negative regulator of inner ear hair cell differentiation. Development 2000, 127, 4551–4560. [Google Scholar]
- Cai, T.; Seymour, M.L.; Zhang, H.; Pereira, F.A.; Groves, A.K. Conditional deletion of Atoh1 reveals distinct critical periods for survival and function of hair cells in the organ of Corti. J. Neurosci. 2013, 33, 10110–10122. [Google Scholar] [CrossRef]
- Kiernan, A.E.; Pelling, A.L.; Leung, K.K.H.; Tang, A.S.P.; Bell, D.M.; Tease, C.; Lovell-Badge, R.; Steel, K.P.; Cheah, K.S.E. Sox2 is required for sensory organ development in the mammalian inner ear. Nature 2005, 434, 1031–1035. [Google Scholar] [CrossRef]
- Maass, J.C.; Gu, R.; Basch, M.L.; Waldhaus, J.; Lopez, E.M.; Xia, A.; Oghalai, J.S.; Heller, S.; Groves, A.K. Changes in the regulation of the Notch signaling pathway are temporally correlated with regenerative failure in the mouse cochlea. Front. Cell Neurosci. 2015, 9, 110. [Google Scholar] [CrossRef]
- Kiernan, A.E.; Ahituv, N.; Fuchs, H.; Balling, R.; Avraham, K.B.; Steel, K.P.; Hrabé de Angelis, M. The Notch ligand Jagged1 is required for inner ear sensory development. Proc. Natl. Acad. Sci. USA 2001, 98, 3873–3878. [Google Scholar] [CrossRef]
- Golub, J.S.; Tong, L.; Ngyuen, T.B.; Hume, C.R.; Palmiter, R.D.; Rubel, E.W.; Stone, J.S. Hair cell replacement in adult mouse utricles after targeted ablation of hair cells with diphtheria toxin. J. Neurosci. 2012, 32, 15093–15105. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.; Zhang, S.; He, Z.; Tang, M.; Chai, R. Role of Wnt and Notch signaling in regulating hair cell regeneration in the cochlea. Front. Med. 2016, 10, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Samarajeewa, A.; Jacques, B.E.; Dabdoub, A. Therapeutic potential of wnt and notch signaling and epigenetic regulation in mammalian sensory hair cell regeneration. Mol. Ther. 2019, 27, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Owen, T.; Fang, J.; Srinivasan, R.S.; Zuo, J. In vivo Notch reactivation in differentiating cochlear hair cells induces Sox2 and Prox1 expression but does not disrupt hair cell maturation. Dev. Dyn. 2012, 241, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Savoy-Burke, G.; Gilels, F.A.; Pan, W.; Pratt, D.; Que, J.; Gan, L.; White, P.M.; Kiernan, A.E. Activated notch causes deafness by promoting a supporting cell phenotype in developing auditory hair cells. PLoS ONE 2014, 9, e108160. [Google Scholar] [CrossRef] [PubMed]
- McGovern, M.M.; Zhou, L.; Randle, M.R.; Cox, B.C. Spontaneous hair cell regeneration is prevented by increased notch signaling in supporting cells. Front. Cell Neurosci. 2018, 12, 120. [Google Scholar] [CrossRef]
- Żak, M.; Klis, S.F.L.; Grolman, W. The Wnt and Notch signalling pathways in the developing cochlea: Formation of hair cells and induction of regenerative potential. Int. J. Dev. Neurosci. 2015, 47, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Lin, C.; Guo, L.; Wu, J.; Chen, Y.; Chai, R.; Li, W.; Li, H. Extensive supporting cell proliferation and mitotic hair cell generation by in vivo genetic reprogramming in the neonatal mouse cochlea. J. Neurosci. 2016, 36, 8734–8745. [Google Scholar] [CrossRef]
- Ni, W.; Zeng, S.; Li, W.; Chen, Y.; Zhang, S.; Tang, M.; Sun, S.; Chai, R.; Li, H. Wnt activation followed by Notch inhibition promotes mitotic hair cell regeneration in the postnatal mouse cochlea. Oncotarget 2016, 7, 66754–66768. [Google Scholar] [CrossRef]
- Li, W.; Wu, J.; Yang, J.; Sun, S.; Chai, R.; Chen, Z.-Y.; Li, H. Notch inhibition induces mitotically generated hair cells in mammalian cochleae via activating the Wnt pathway. Proc. Natl. Acad. Sci. USA 2015, 112, 166–171. [Google Scholar] [CrossRef]
- Haapasalo, A.; Kovacs, D.M. The many substrates of presenilin/γ-secretase. J. Alzheimers Dis. 2011, 25, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Li, W.; Huang, M.; Quan, Y.-Z.; Scheffer, D.; Tian, C.; Tao, Y.; Liu, X.; Hochedlinger, K.; Indzhykulian, A.A.; et al. Renewed proliferation in adult mouse cochlea and regeneration of hair cells. Nat. Commun. 2019, 10, 5530. [Google Scholar] [CrossRef] [PubMed]
- Jen, H.-I.; Hill, M.C.; Tao, L.; Sheng, K.; Cao, W.; Zhang, H.; Yu, H.V.; Llamas, J.; Zong, C.; Martin, J.F.; et al. Transcriptomic and epigenetic regulation of hair cell regeneration in the mouse utricle and its potentiation by Atoh1. Elife 2019, 8. [Google Scholar] [CrossRef]
- Vrijens, K.; Thys, S.; De Jeu, M.T.; Postnov, A.A.; Pfister, M.; Cox, L.; Zwijsen, A.; Van Hoof, V.; Mueller, M.; De Clerck, N.M.; et al. A Jag1 vestibular mouse mutant, displays characteristics of Alagille syndrome. Neurobiol. Dis. 2006, 24, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Hansson, E.M.; Lanner, F.; Das, D.; Mutvei, A.; Marklund, U.; Ericson, J.; Farnebo, F.; Stumm, G.; Stenmark, H.; Andersson, E.R.; et al. Control of Notch-ligand endocytosis by ligand-receptor interaction. J. Cell Sci. 2010, 123, 2931–2942. [Google Scholar] [CrossRef] [PubMed]
- Lanford, P.J.; Lan, Y.; Jiang, R.; Lindsell, C.; Weinmaster, G.; Gridley, T.; Kelley, M.W. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat. Genet. 1999, 21, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Hartman, B.H.; Hayashi, T.; Nelson, B.R.; Bermingham-McDonogh, O.; Reh, T.A. Dll3 is expressed in developing hair cells in the mammalian cochlea. Dev. Dyn. 2007, 236, 2875–2883. [Google Scholar] [CrossRef]
- Yamamoto, N.; Chang, W.; Kelley, M.W. Rbpj regulates development of prosensory cells in the mammalian inner ear. Dev. Biol. 2011, 353, 367–379. [Google Scholar] [CrossRef][Green Version]
- Zhang, N.; Martin, G.V.; Kelley, M.W.; Gridley, T. A mutation in the Lunatic fringe gene suppresses the effects of a Jagged2 mutation on inner hair cell development in the cochlea. Curr. Biol. 2000, 10, 659–662. [Google Scholar] [CrossRef]
- Zine, A.; Aubert, A.; Qiu, J.; Therianos, S.; Guillemot, F.; Kageyama, R.; de Ribaupierre, F. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J. Neurosci. 2001, 21, 4712–4720. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mark, S.; Radde-Gallwitz, K.; Schlisner, R.; Chin, M.T.; Chen, P. Hey2 functions in parallel with Hes1 and Hes5 for mammalian auditory sensory organ development. BMC Dev. Biol. 2008, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Tateya, T.; Imayoshi, I.; Tateya, I.; Ito, J.; Kageyama, R. Cooperative functions of Hes/Hey genes in auditory hair cell and supporting cell development. Dev. Biol. 2011, 352, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Doetzlhofer, A.; Basch, M.L.; Ohyama, T.; Gessler, M.; Groves, A.K.; Segil, N. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev. Cell 2009, 16, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Kokubo, H.; Hartman, B.H.; Ray, C.A.; Reh, T.A.; Bermingham-McDonogh, O. Hesr1 and Hesr2 may act as early effectors of Notch signaling in the developing cochlea. Dev. Biol. 2008, 316, 87–99. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, R.; Groves, A.K. Hear, Hear for Notch: Control of Cell Fates in the Inner Ear by Notch Signaling. Biomolecules 2020, 10, 370. https://doi.org/10.3390/biom10030370
Brown R, Groves AK. Hear, Hear for Notch: Control of Cell Fates in the Inner Ear by Notch Signaling. Biomolecules. 2020; 10(3):370. https://doi.org/10.3390/biom10030370
Chicago/Turabian StyleBrown, Rogers, and Andrew K. Groves. 2020. "Hear, Hear for Notch: Control of Cell Fates in the Inner Ear by Notch Signaling" Biomolecules 10, no. 3: 370. https://doi.org/10.3390/biom10030370
APA StyleBrown, R., & Groves, A. K. (2020). Hear, Hear for Notch: Control of Cell Fates in the Inner Ear by Notch Signaling. Biomolecules, 10(3), 370. https://doi.org/10.3390/biom10030370





