Untargetted Metabolomic Exploration of the Mycobacterium tuberculosis Stress Response to Cinnamon Essential Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Mycobacterial Strain and Culture Conditions
2.1.1. Inoculum Preparation
2.1.2. Bacterial Culture Conditions
2.2. Liquid Chromatography–Mass Spectrometry (HPLC–ESI–QTOF–MS) Analysis
2.2.1. Metabolite Extraction
2.2.2. Chromatographic Conditions
2.2.3. Mass Spectrometry Conditions
2.2.4. Data Processing
3. Results
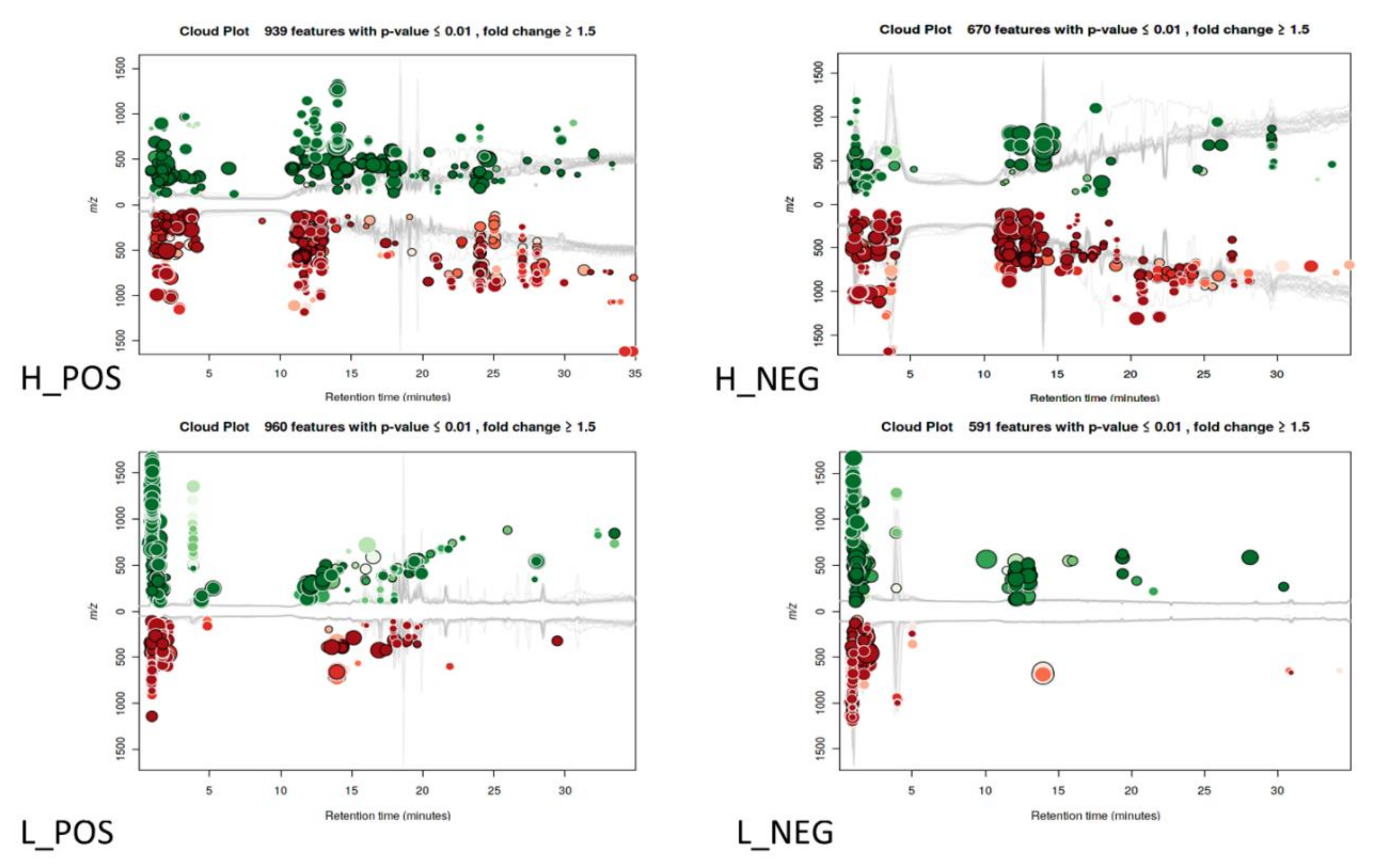
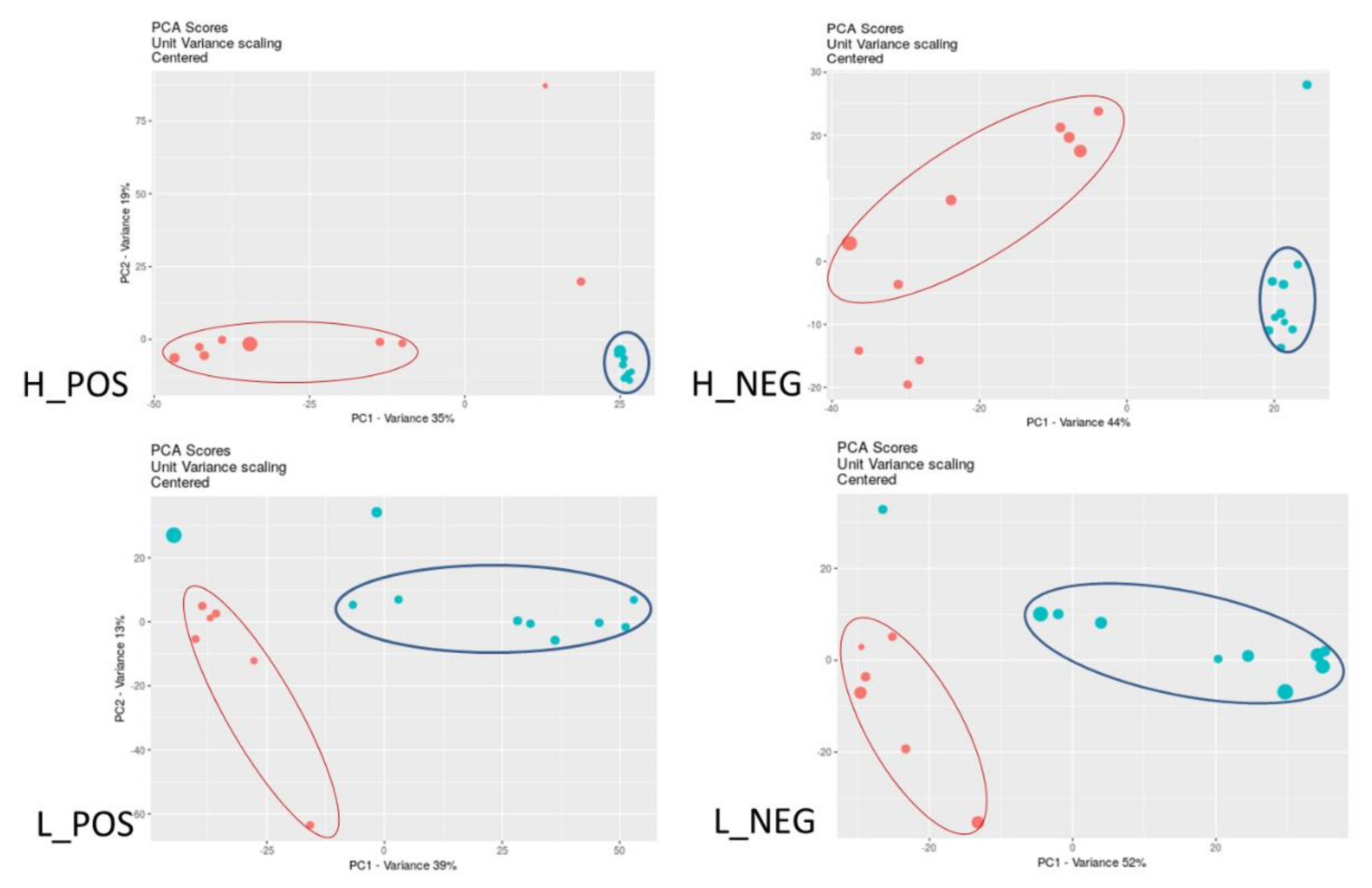
3.1. Changes in Metabolic Profiles
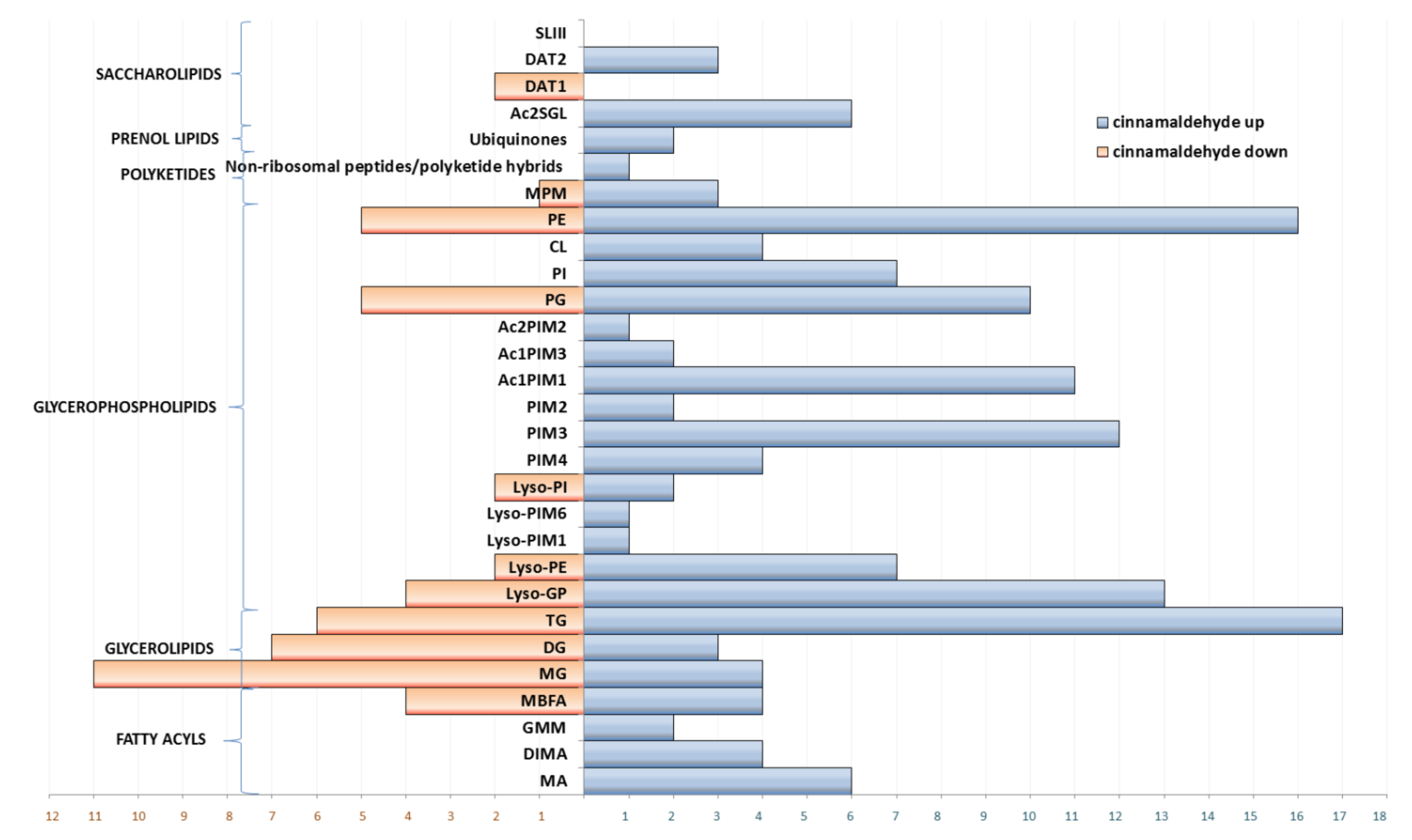
3.2. Dysregulation of Lipids under Essential Oil/Cinnamaldehyde Treatment
3.2.1. Fatty Acyls
3.2.2. Glycerolipids
3.2.3. Glycerophospholipids
3.2.4. Polyketides and Prenol Lipids
3.2.5. Saccharolipids
3.3. Changes upon the Influence of DMSO
3.4. Essential Oil/Cinnamaldehyde vs. DMSO
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cueto, M.; Brown, T.M.; Fee, E. The World Health Organization; Cambridge University Press (CUP): Cambridge, UK, 2019. [Google Scholar]
- Layre, E.; Sweet, L.; Hong, S.; Madigan, C.A.; Desjardins, D.; Young, D.C.; Cheng, T.Y.; Annand, J.W.; Kim, K.; Shamputa, I.C.; et al. A Comparative Lipidomics Platform for Chemotaxonomic Analysis of Mycobacterium tuberculosis. Chem. Biol. 2011, 18, 1537–1549. [Google Scholar] [CrossRef]
- Lau, S.K.; Lam, C.W.; Curreem, S.O.T.; Lee, K.C.; Lau, C.C.Y.; Chow, F.W.N.; Ngan, A.H.Y.; To, K.K.W.; Chan, J.F.W.; Hung, I.F.N.; et al. Identification of specific metabolites in culture supernatant of Mycobacterium tuberculosis using metabolomics: Exploration of potential biomarkers. Emerg. Microbes Infect. 2015, 4, e6–e10. [Google Scholar] [CrossRef]
- Raghunandanan, S.; Jose, L.; Gopinath, V.; Kumar, R.A. Comparative label-free lipidomic analysis of Mycobacterium tuberculosis during dormancy and reactivation. Sci. Rep. 2019, 9, 3660. [Google Scholar] [CrossRef]
- Eoh, H. Metabolomics: A window into the adaptive physiology of Mycobacterium tuberculosis. Tuberculosis 2014, 94, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Gengenbacher, M.; McEwen, G.; Mollenkopf, H.-J.; Zamboni, N.; Kaufmann, S.H.E.; Sauer, U. Integration of Metabolomics and Transcriptomics Reveals a Complex Diet of Mycobacterium tuberculosis during Early Macrophage Infection. mSystems 2017, 2, e00057-17. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Hameed, S.; Sabareesh, V.; Kumar, P.; Singh, S.; Fatima, Z. Investigations into Isoniazid Treated Mycobacterium tuberculosis by Electrospray Mass Spectrometry Reveals New Insights into Its Lipid Composition. J. Pathog. 2018, 2018, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, M.; Szappanos, B.; Buchieri, M.V.; Trauner, A.; Piazza, I.; Picotti, P.; Gagneux, S.; Borrell, S.; Gicquel, B.; Lelievre, J.; et al. High-throughput metabolomic analysis predicts mode of action of uncharacterized antimicrobial compounds. Sci. Transl. Med. 2018, 10, eaal3973. [Google Scholar] [CrossRef]
- Tuyiringire, N.; Tusubira, D.; Munyampundu, J.-P.; Tolo, C.U.; Muvunyi, C.; Ogwang, P.E. Application of metabolomics to drug discovery and understanding the mechanisms of action of medicinal plants with anti-tuberculosis activity. Clin. Transl. Med. 2018, 7, 29. [Google Scholar] [CrossRef]
- Nandakumar, M.; Nathan, C.; Rhee, K.Y. Isocitrate lyase mediates broad antibiotic tolerance in Mycobacterium tuberculosis. Nat. Commun. 2014, 5, 4306. [Google Scholar] [CrossRef]
- Du Preez, I.; Loots, D.T. Altered Fatty Acid Metabolism Due to Rifampicin-Resistance Conferring Mutations in the rpoB Gene of Mycobacterium tuberculosis: Mapping the Potential of Pharmaco-metabolomics for Global Health and Personalized Medicine. OMICS: A J. Integr. Biol. 2012, 16, 596–603. [Google Scholar] [CrossRef]
- Loots, D.T. New insights into the survival mechanisms of rifampicin-resistantMycobacterium tuberculosis. J. Antimicrob. Chemother. 2015, 71, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, N.; Shah, R.R.; Layre, E.; Young, D.; Ford, C.; Murray, M.B.; Fortune, S.M.; Moody, D.B. Rifampin Resistance Mutations Are Associated with Broad Chemical Remodeling of Mycobacterium tuberculosis*. J. Biol. Chem. 2016, 291, 14248–14256. [Google Scholar] [CrossRef] [PubMed]
- Markley, J.L.; Brüschweiler, R.; Edison, A.; Eghbalnia, H.R.; Powers, R.; Raftery, D.; Wishart, D.S. The future of NMR-based metabolomics. Curr. Opin. Biotechnol. 2017, 43, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Agin, A.; Heintz, D.; Ruhland, E.; De La Barca, J.C.; Zumsteg, J.; Moal, V.; Gauchez, A.; Namer, I. Metabolomics—An overview. From basic principles to potential biomarkers (part 1). Médecine Nucléaire 2016, 40, 4–10. [Google Scholar] [CrossRef]
- Fan, T.W.-M.; Lorkiewicz, P.K.; Sellers, K.; Moseley, H.; Higashi, R.M.; Lane, A.N. Stable isotope-resolved metabolomics and applications for drug development. Pharmacol. Ther. 2011, 133, 366–391. [Google Scholar] [CrossRef]
- Fillet, M.; Frederich, M. The emergence of metabolomics as a key discipline in the drug discovery process. Drug Discov. Today Technol. 2015, 13, 19–24. [Google Scholar] [CrossRef]
- Rabinowitz, J.D.; Purdy, J.G.; Vastag, L.; Shenk, T.; Koyuncu, E. Metabolomics in drug target discovery. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 235–246. [Google Scholar] [CrossRef]
- Zhou, B.; Xiao, J.F.; Tuli, L.; Ressom, H. LC-MS-based metabolomics. Mol. BioSyst. 2011, 8, 470–481. [Google Scholar] [CrossRef]
- Ashakirin, S.N.; Tripathy, M.; Patil, U.K.; Majeed, A.B.A. Chemistry and bioactivity of cinnamaldehyde: A natural molecule of medicinal importance. Int. J. Pharm. Pharm. Res. 2017, 8, 2333–2340. [Google Scholar]
- Sawicki, R.; Golus, J.; Przekora-Kuśmierz, A.; Ludwiczuk, A.; Sieniawska, E.; Ginalska, G. Antimycobacterial Activity of Cinnamaldehyde in a Mycobacterium tuberculosis(H37Ra) Model. Molecules 2018, 23, 2381. [Google Scholar] [CrossRef]
- Ochoa, S.A.; Nevárez-Moorillón, G.V.; Sánchez-Torres, L.; Villanueva-García, M.; Sánchez-Ramírez, B.E.; Rodríguez-Valdez, L.M.; Rivera-Chavira, B. Quantitative structure-activity relationship of molecules constituent of different essential oils with antimycobacterial activity against Mycobacterium tuberculosis and Mycobacterium bovis. BMC Complement. Altern. Med. 2015, 15, 332. [Google Scholar]
- Mota, A.P.P.; Campelo, T.A.; Frota, C.C. Evaluation of the antimicrobial activity of Cinnamomum zeylanicum essential oil and trans-Cinnamaldehyde against resistant Mycobacterium tuberculosis. Biosci. J. 2019, 35, 296–306. [Google Scholar] [CrossRef]
- Powers, R.; Halouska, S.; Zhang, B.; Gaupp, R.; Lei, S.; Snell, E.; Fenton, R.J.; Barletta, R.G.; Somerville, G.A. Revisiting Protocols for the NMR Analysis of Bacterial Metabolomes. J. Integr. OMICS 2013, 3, 120–137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crellin, P.K.; Luo, C.Y.; Morita, Y.S. Metabolism of Plasma Membrane Lipids in Mycobacteria and Corynebacteria. In Lipid Metabolism; IntechOpen: London, UK, 2013. [Google Scholar]
- Bhatt, A.; Molle, V.; Besra, G.; Jacobs, W.R.; Kremer, L. TheMycobacterium tuberculosisFAS-II condensing enzymes: Their role in mycolic acid biosynthesis, acid-fastness, pathogenesis and in future drug development. Mol. Microbiol. 2007, 64, 1442–1454. [Google Scholar] [CrossRef]
- Astarie-Dequeker, C.; Le Guyader, L.; Malaga, W.; Seaphanh, F.-K.; Chalut, C.; Lopez, A.; Guilhot, C. Phthiocerol Dimycocerosates of M. tuberculosis Participate in Macrophage Invasion by Inducing Changes in the Organization of Plasma Membrane Lipids. PLOS Pathog. 2009, 5, e1000289. [Google Scholar] [CrossRef]
- Fujiwara, N. Distribution, Characterization of Mycobacterial Glycolipids and Host Responses. In Glycosylation; IntechOpen: London, UK, 2012. [Google Scholar]
- Garton, N.J.; Christensen, H.; Minnikin, D.E.; Adegbola, R.A.; Barer, M.R. Intracellular lipophilic inclusions of mycobacteria in vitro and in sputum. Microbiol. 2002, 148, 2951–2958. [Google Scholar] [CrossRef]
- Hammond, R.J.H.; Baron, V.O.; Oravcova, K.; Lipworth, S.; Gillespie, S.H. Phenotypic resistance in mycobacteria: Is it because I am old or fat that I resist you? J. Antimicrob. Chemother. 2015, 70, 2823–2827. [Google Scholar] [CrossRef]
- Shi, L.; Sohaskey, C.D.; Pheiffer, C.; Pfeiffer, C.; Datta, P.; Parks, M.; McFadden, J.; North, R.J.; Gennaro, M.L. Carbon flux rerouting during Mycobacterium tuberculosis growth arrest. Mol. Microbiol. 2010, 78, 1199–1215. [Google Scholar] [CrossRef]
- Santucci, P.; Johansen, M.D.; Point, V.; Poncin, I.; Viljoen, A.; Cavalier, J.-F.; Kremer, L.; Canaan, S. Nitrogen deprivation induces triacylglycerol accumulation, drug tolerance and hypervirulence in mycobacteria. Sci. Rep. 2019, 9, 8667. [Google Scholar] [CrossRef]
- Schlame, M. Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. J. Lipid Res. 2007, 49, 1607–1620. [Google Scholar] [CrossRef]
- Jackson, M.; Crick, D.; Brennan, P.J. Phosphatidylinositol Is an Essential Phospholipid of Mycobacteria. J. Biol. Chem. 2000, 275, 30092–30099. [Google Scholar] [CrossRef] [PubMed]
- Bansal-Mutalik, R.; Nikaido, H. Mycobacterial outer membrane is a lipid bilayer and the inner membrane is unusually rich in diacylphosphatidylinositol dimannosides. Proc. Natl. Acad. Sci. USA 2014, 111, 4958–4963. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, I.; Sugita, M. Mycoketide: A CD1c-Presented Antigen with Important Implications in Mycobacterial Infection. Clin. Dev. Immunol. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Fontes, F.L.; Gonzalez-Juarrero, M.; McNeil, M.R.; Crans, D.C.; Jackson, M.; Crick, D. Partial Saturation of Menaquinone in Mycobacterium tuberculosis: Function and Essentiality of a Novel Reductase, MenJ. ACS Central Sci. 2015, 1, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Sogi, K.M. Investigation into the Biosynthesis and Function of a Sulfomenaquinone from Mycobacterium tuberculosis; University of California: Berkeley, CA, USA, 2012. [Google Scholar]
- Gonzalo-Asensio, J.; Maia, C.; Ferrer, N.L.; Barilone, N.; Laval, F.; Soto, C.Y.; Winter, N.; Daffé, M.; Gicquel, B.; Martin, C.; et al. The Virulence-associated Two-component PhoP-PhoR System Controls the Biosynthesis of Polyketide-derived Lipids inMycobacterium tuberculosis. J. Biol. Chem. 2005, 281, 1313–1316. [Google Scholar] [CrossRef]
- Chesne-Seck, M.L.; Barilone, N.; Boudou, F.; Gonzalo-Asensio, J.; Kolattukudy, P.E.; Martin, C.; Cole, S.T.; Gicquel, B.; Gopaul, D.; Jackson, M. A Point Mutation in the Two-Component Regulator PhoP-PhoR Accounts for the Absence of Polyketide-Derived Acyltrehaloses but Not That of Phthiocerol Dimycocerosates in Mycobacterium tuberculosis H37Ra. J. Bacteriol. 2007, 190, 1329–1334. [Google Scholar] [CrossRef]
- Besra, G.; Bolton, R.C.; McNeil, M.R.; Ridell, M.; Simpson, K.E.; Glushka, J.; Van Halbeek, H.; Brennan, P.J.; Minnikin, D.E. Structural elucidation of a novel family of acyltrehaloses from Mycobacterium tuberculosis. Biochem. 1992, 31, 9832–9837. [Google Scholar] [CrossRef]
- Dyrda, G.; Boniewska-Bernacka, E.; Man, D.; Barchiewicz, K.; Słota, R. The effect of organic solvents on selected microorganisms and model liposome membrane. Mol. Biol. Rep. 2019, 46, 3225–3232. [Google Scholar] [CrossRef]
- Jacob, S.W.; De La Torre, J.C. Dimethyl Sulfoxide (DMSO) in Trauma and Disease; Informa UK Limited: Colchester, UK, 2015. [Google Scholar]
- Gurtovenko, A.; Anwar, J. Modulating the Structure and Properties of Cell Membranes: The Molecular Mechanism of Action of Dimethyl Sulfoxide. J. Phys. Chem. B 2007, 111, 10453–10460. [Google Scholar] [CrossRef]
- Schrader, A.M.; Cheng, C.-Y.; Israelachvili, J.N.; Han, S. Communication: Contrasting effects of glycerol and DMSO on lipid membrane surface hydration dynamics and forces. J. Chem. Phys. 2016, 145, 041101. [Google Scholar] [CrossRef]
- Forsberg, E.M.; Huan, T.; Rinehart, D.; Benton, H.P.; Warth, B.; Hilmers, B.; Siuzdak, G. Data processing, multi-omic pathway mapping, and metabolite activity analysis using XCMS Online. Nat. Protoc. 2018, 13, 633–651. [Google Scholar] [CrossRef] [PubMed]
- Minato, Y.; Thiede, J.M.; Kordus, S.; McKlveen, E.J.; Turman, B.J.; Baughn, A.D. Mycobacterium tuberculosis Folate Metabolism and the Mechanistic Basis for para-Aminosalicylic Acid Susceptibility and Resistance. Antimicrob. Agents Chemother. 2015, 59, 5097–5106. [Google Scholar] [CrossRef] [PubMed]
- Boissier, F.; Bardou, F.; Guillet, V.; Uttenweiler, S.; Daffé, M.; Quémard, A.; Mourey, L. Further Insight into S-Adenosylmethionine-dependent Methyltransferases: Structural characterization of hma, an enzyme essential for the biosynthesis of oxygenated mycolic acids in Mycobacterium tuberculosis. J. Biol. Chem. 2006, 281, 4434–4445. [Google Scholar] [CrossRef]
- Camacho, L.R.; Constant, P.; Raynaud, C.; Lanéelle, M.-A.; Triccas, J.A.; Gicquel, B.; Daffé, M.; Guilhot, C. Analysis of the Phthiocerol Dimycocerosate Locus of Mycobacterium tuberculosis: Evidence that this lipid is involved in the cell wall permeability barrier. J. Biol. Chem. 2001, 276, 19845–19854. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Matsumura, T.; Ato, M.; Hamasaki, M.; Nishiuchi, Y.; Murakami, Y.; Maeda, Y.; Yoshimori, T.; Matsumoto, S.; Kobayashi, K.; et al. Critical roles forlipomannan and lipoarabinomannan in cell wall integrity of mycobacteria and pathogenesis of tuberculosis. mBio 2013, 4, e00472-12. [Google Scholar] [CrossRef] [PubMed]
- Salaemae, W.; Azhar, A.; Booker, G.W.; Polyak, S. Biotin biosynthesis in Mycobacterium tuberculosis: Physiology, biochemistry and molecular intervention. Protein Cell 2011, 2, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Klotzsche, M.; Wilson, D.J.; Boshoff, H.I.; Eoh, H.; Manjunatha, U.; Blumenthal, A.; Rhee, K.; Barry, I.C.; Aldrich, C.C.; et al. Evaluating the Sensitivity of Mycobacterium tuberculosis to Biotin Deprivation Using Regulated Gene Expression. PLOS Pathog. 2011, 7, e1002264. [Google Scholar]
- Jirapanjawat, T.; Ney, B.; Taylor, M.C.; Warden, A.C.; Afroze, S.; Russell, R.J.; Lee, B.M.; Jackson, C.J.; Oakeshott, J.; Pandey, G.; et al. The Redox Cofactor F420 Protects Mycobacteria from Diverse Antimicrobial Compounds and Mediates a Reductive Detoxification System. Appl. Environ. Microbiol. 2016, 82, 6810–6818. [Google Scholar] [CrossRef]
- Cocchiara, J.; Letizia, C.; Lalko, J.; Lapczynski, A.; Api, A. Fragrance material review on cinnamaldehyde. Food Chem. Toxicol. 2005, 43, 867–923. [Google Scholar] [CrossRef]
| KEGG Pathway Name and Metabolites | Total | Hits (Fold Change) | p-Value <0.05 |
|---|---|---|---|
| tetrahydrofolate biosynthesis: | 2 | 2 | 0.012 |
| 7,8-dihydrofolate | (42.4 up) | ||
| 7,8-dihydropteroate | (9.7 up) | ||
| tRNA charging: | 4 | 2 | 0.03 |
| L-histidine | (3.2 down) | ||
| L-tyrosine | (3.2 down) | ||
| factor 420 biosynthesis: | 2 | 2 | 0.03863 |
| 5-amino-6-(D-ribitylamino)uracil | (9.1 down) | ||
| 5,10-methylene-tetrahydromethanopterin | (162.3 up ) | ||
| biotin biosynthesis from 8-amino-7-oxononanoate I: | 2 | 2 | 0.03863 |
| S-adenosyl-4-methylthio-2-oxobutanoate | (4.2 down) | ||
| 8-amino-7-oxononanoate | (12.8 down) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sieniawska, E.; Sawicki, R.; Golus, J.; Georgiev, M.I. Untargetted Metabolomic Exploration of the Mycobacterium tuberculosis Stress Response to Cinnamon Essential Oil. Biomolecules 2020, 10, 357. https://doi.org/10.3390/biom10030357
Sieniawska E, Sawicki R, Golus J, Georgiev MI. Untargetted Metabolomic Exploration of the Mycobacterium tuberculosis Stress Response to Cinnamon Essential Oil. Biomolecules. 2020; 10(3):357. https://doi.org/10.3390/biom10030357
Chicago/Turabian StyleSieniawska, Elwira, Rafał Sawicki, Joanna Golus, and Milen I. Georgiev. 2020. "Untargetted Metabolomic Exploration of the Mycobacterium tuberculosis Stress Response to Cinnamon Essential Oil" Biomolecules 10, no. 3: 357. https://doi.org/10.3390/biom10030357
APA StyleSieniawska, E., Sawicki, R., Golus, J., & Georgiev, M. I. (2020). Untargetted Metabolomic Exploration of the Mycobacterium tuberculosis Stress Response to Cinnamon Essential Oil. Biomolecules, 10(3), 357. https://doi.org/10.3390/biom10030357







