The Stimulatory Effect of Purine-Type Cytokinins on Proliferation and Polyphenolic Compound Accumulation in Shoot Culture of Salvia viridis
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Shoot Proliferation and Biomass Accumulation
2.3. Determination of Polyphenolic Compounds
2.4. Statistical Analysis
3. Results
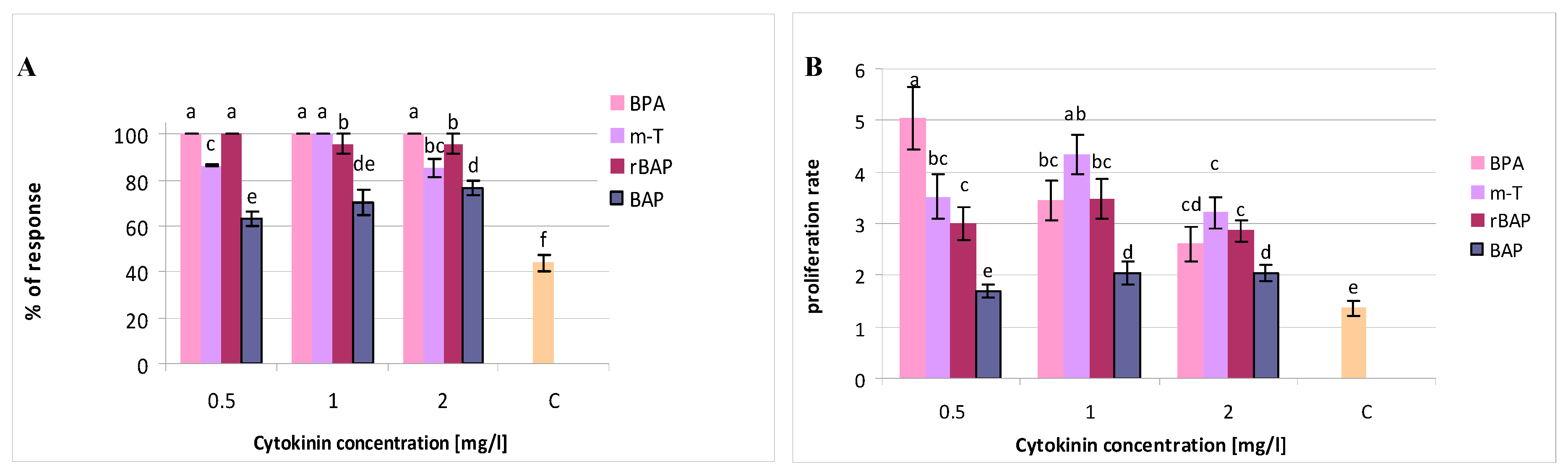
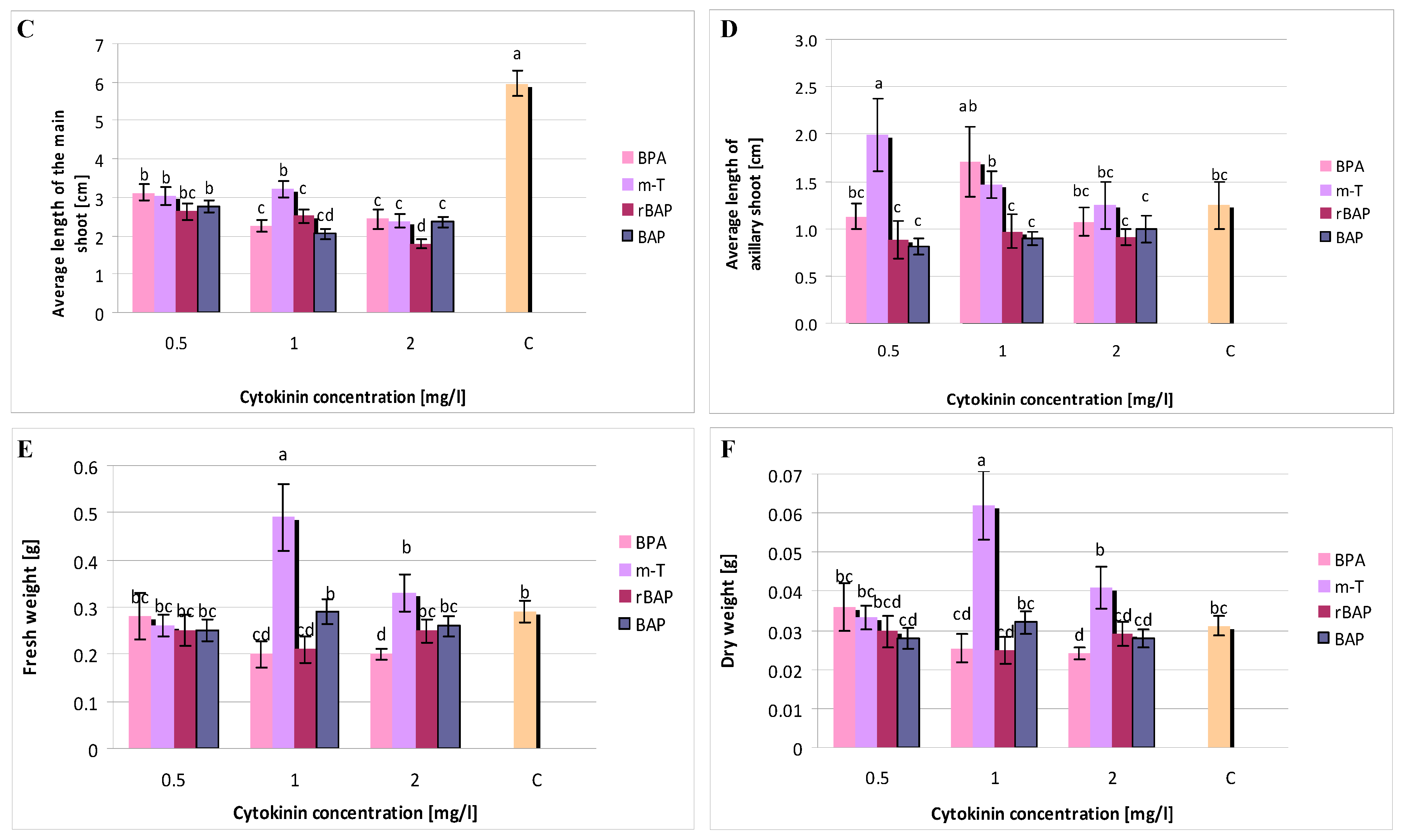
3.1. Effect of Purine-Type Cytokinins on Shoot Propagation and Biomass Accumulation
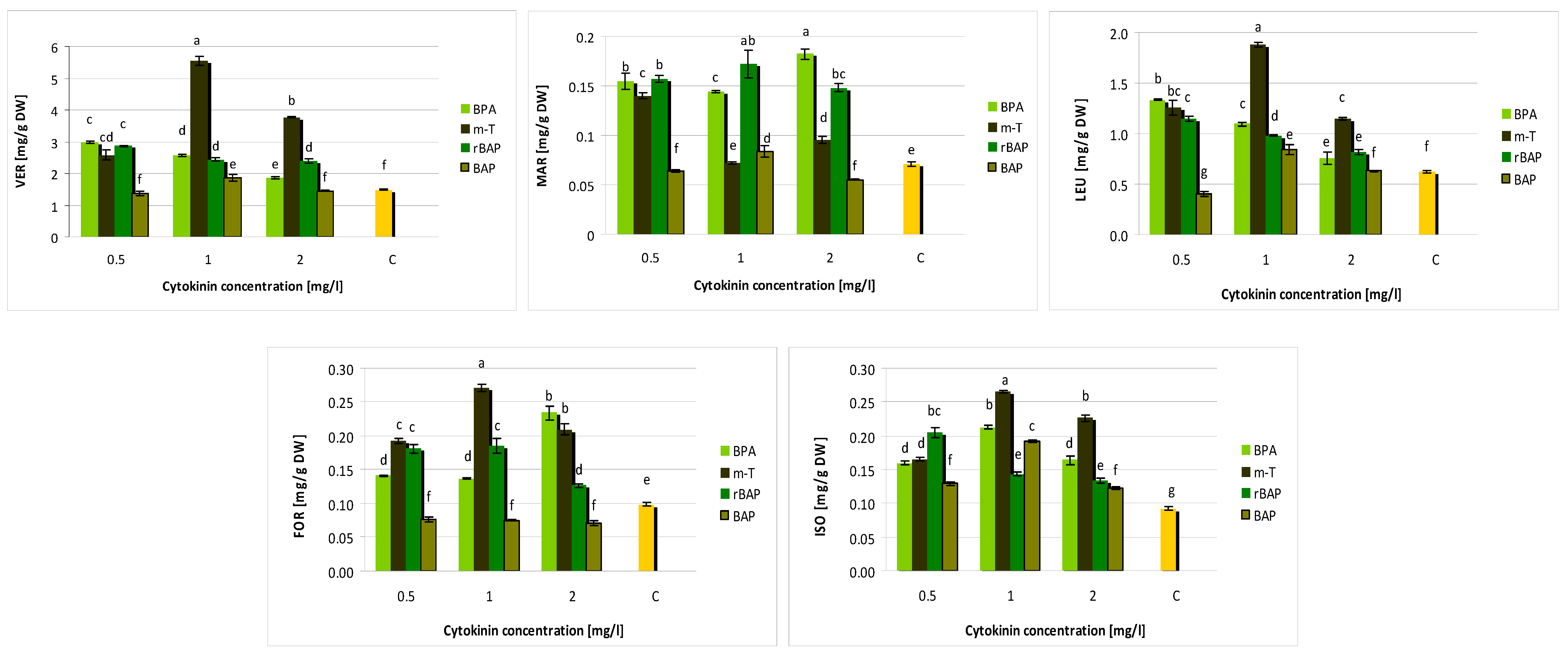
3.2. Effect of Purine-Type Cytokinins on Metabolite Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yayli, N.; Cansu, T.B.; Yilmaz, N.; Yasar, A.; Cetin, M.M.; Yayli, N. Constituents of the essential oil from the flower, leaf and stem of Salvia viridis L. grown in turkey. Asian J. Chem. 2010, 22, 3439–3446. [Google Scholar]
- Rungsimakan, S.; Rowan, M.G. Terpenoids, flavonoids and caffeic acid derivatives from Salvia viridis L. cvar. Blue Jeans. Phytochemistry 2014, 108, 177–188. [Google Scholar] [CrossRef]
- Naghibi, F.; Mosaddegh, M.; Motamed, M.M.; Ghorbani, A. Labiatae family in folk medicine in Iran: From ethnobotany to pharmacology. Iran. J. Pharm. Res. 2005, 4, 63–79. [Google Scholar]
- Grzegorczyk-Karolak, I.; Kiss, A. Determination of the phenolic profile and antioxidant properties of Salvia viridis L. shoots: A comparison of aqueous and hydroethanolic extracts. Molecules 2018, 23, 1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Lisiecki, P.; Kiss, A. Accumulation of phenolic compounds in different in vitro cultures of Salvia viridis L. and their antioxidant and antimicrobial potential. Phytochem. Lett. 2019, 30, 324–332. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Aslam Gondal, T.; Imran, A.; Shahbaz, M.; Muhammad Amir, R.; Wasim Sajid, M.; Batool Qaisrani, T.; Atif, M.; Hussain, G.; et al. Therapeutic potential of rosmarinic acid: A comprehensive review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef] [Green Version]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio) synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef]
- Echeverrigaray, S.; Carrer, R.P.; Andrade, L.B. Micropropagation of Salvia guaranitica Benth. through axillary shoot proliferation. Braz. Arch. Biol. Technol. 2010, 53, 883–888. [Google Scholar] [CrossRef] [Green Version]
- Misic, D.; Grubisic, D.; Konjevic, R. Micropropagation of Salvia brachyodon through nodal explants. Biol. Plant. 2006, 50, 473–476. [Google Scholar] [CrossRef]
- Skała, E.; Wysokinska, H. In vitro regeneration of Salvia nemorosa L. from shoot tips and leaf explants. In Vitro Cell. Dev. Biol. Plant. 2004, 40, 596–602. [Google Scholar]
- Naser, A.A.; Fawzia, M.J.; Nabila, S.K.; Rida, A.S. Micropropagation and accumulation of essential oils in wild sage (Salvia fruticosa Mill.). Sci. Hortic. 2004, 100, 193–202. [Google Scholar]
- Makunga, N.P.; Van Staden, J. An efficient system for the production of clonal plantlets of the medicinally important aromatic plant: Salvia africana-lutea L. Plant. Cell Tiss. Organ. Cult. 2008, 92, 63–72. [Google Scholar] [CrossRef]
- Dowom, S.A.; Abrishamchi, P.; Radjabian, T.; Salami, S.A. Enhanced phenolic acids production in regenerated shoot cultures of Salvia virgata Jacq. After elicitation with Ag+ ions, methyl jasmonate and yeast extract. Ind. Crops Prod. 2017, 103, 81–88. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Skała, E.; Kiss, A.K. Hairy root cultures of Salvia viridis L. for production of polyphenolic compounds. Ind. Crops Prod. 2018, 117, 235–244. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.J. Plant Propagation by Tissue Culture: Vol. 1: The Background, 3rd ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 175–226. [Google Scholar]
- Van Staden, J.; Zazimalova, E.; George, E.F. Plant growth regulators II: Cytokinins, their analogues and antagonists. In Plant Propagation by Tissue Culture; George, E.F., Hall, M.A., De Klerk, G.J., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 1, pp. 205–226. [Google Scholar]
- Chalupa, V. In vitro propagation of Tilia platyphyllos by axillary shoot proliferation and somatic embryogenesis. J. For. Sci. 2003, 49, 537–543. [Google Scholar] [CrossRef] [Green Version]
- Hamdy, M.A.A.; Hattori, K. In vitro micropropagation of (Vicia faba L.) cultivars ‘Waza Soramame and Cairo 241’ by nodal explants proliferation and somatic embryogenesis. Biotechnology 2006, 5, 32–37. [Google Scholar]
- Werbrouck, S.P.O.; Strnad, M.; Van Onckelen, H.A.; Debergh, P.C. Meta-topolin, an alternative to benzyladenine in tissue culture in tissue culture? Physiol. Plant. 1996, 98, 291–297. [Google Scholar] [CrossRef]
- Strnad, M.; Hanuš, J.; Vaněk, T.; Kamínek, M.; Ballantine, J.A.; Fussell, B.; Hanke, D.E. Meta-topolin, a highly active aromatic cytokinin from poplar leaves (Populus× canadensis Moench., cv. Robusta). Phytochemistry 1997, 45, 213–218. [Google Scholar] [CrossRef]
- Strnad, M. The aromatic cytokinins. Physiol. Plant. 1997, 101, 674–688. [Google Scholar] [CrossRef]
- Bairu, M.W.; Stirk, W.A.; Dolezal, K.; Van Staden, J. The role of topolins in micropropagation and somaclonal variation of banana cultivars ‘Williams’ and ‘Grand Naine’ (Musa spp. AAA). Plant. Cell Tiss. Organ. Cult. 2008, 95, 373–379. [Google Scholar] [CrossRef]
- Mok, M.C.; Martin, R.C.; Dobrev, P.I.; Vanková, R.; Shing-Ho, P.; Yonekura-Sakakibara, K.; Sakakibara, H.; Mok, D.W.S. Topolins and hydroxylated thidiazuron derivatives are substrates of cytokinin O-glucosyltransferase with position specificity related to receptor recognition. Plant. Physiol. 2005, 137, 1057–1066. [Google Scholar] [CrossRef] [Green Version]
- Moyo, M.; Finnie, J.F.; Van Staden, J. Topolins in Pelargonium sidoides micropropagation: Do the new brooms really sweep cleaner? Plant. Cell Tiss. Organ. Cult. 2012, 110, 319–327. [Google Scholar] [CrossRef]
- Aremu, A.O.; Bairu, M.W.; Szucova, L.; Dolezal, K.; Finnie, J.F.; Van Staden, J. Shoot and root proliferation in ‘Williams’ banana: Are the topolins better cytokinins? Plant. Cell Tiss. Organ. Cult. 2012, 111, 209–218. [Google Scholar] [CrossRef]
- Gentile, A.; Gutiérrez, M.J.; Martinez, J.; Frattarelli, A.; Nota, P.; Caboni, E. Effect of meta-Topolin on micropropagation and adventitious shoot regeneration in Prunus rootstocks. Plant. Cell Tiss. Organ. Cult. 2014, 118, 373–381. [Google Scholar] [CrossRef]
- Koszeghi, S.; Bereczki, C.; Balog, A.; Benedek, K. Comparing the effects of benzyladenine and meta-topolin on sweet basil (Ocimum basilicum) micropropagation. Not. Sci. Biol. 2014, 6, 422–427. [Google Scholar] [CrossRef] [Green Version]
- Weremczuk-Jeżyna, I.; Skała, E.; Kuźma, Ł.; Kiss, A.K.; Grzegorczyk-Karolak, I. The effect of purine-type cytokinin on the proliferation and production of phenolic compounds in transformed shoots of Dracocephalum forrestii. J. Biotechnol. 2019, 306, 125–133. [Google Scholar] [CrossRef]
- Wojtania, A. Effect of meta-topolin in vitro propagation of Pelargonium x hortorum and Pelargonium x hederaefolium cultivars. Acta Soc. Bot. Pol. 2010, 79, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Grzegorczyk, I.; Bilichowski, I.; Mikiciuk-Olasik, E.; Wysokinska, H. In vitro cultures of Salvia officinalis L. as a source of antioxidant compounds. Acta Soc. Bot. Pol. 2005, 74, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Weremczuk-Jeżyna, I.; Kuźma, Ł.; Kiss, A.K.; Grzegorczyk-Karolak, I. Effect of cytokinins on shoots proliferation and rosmarinic and salvianolic acid B production in shoot culture of Dracocephalum forrestii WW Smith. Acta Physiol. Plant. 2018, 40, 189. [Google Scholar] [CrossRef] [Green Version]
- Santos-Gomes, P.C.; Seabra, R.M.; Andrade, P.B.; Fernandes-Ferreira, M. Phenolic antioxidant compounds produced by in vitro shoots of sage (Salvia officinalis L.). Plant. Sci. 2002, 162, 981–987. [Google Scholar] [CrossRef]
- Aremu, A.O.; Bairu, M.W.; Szüčová, L.; Doležal, K.; Finnie, J.F.; Van Staden, J. Assessment of the role of meta-topolins on in vitro produced phenolics and acclimatization competence of micropropagated ‘Williams’ banana. Acta Physiol. Plant. 2012, 34, 2265–2273. [Google Scholar] [CrossRef]
- Amoo, S.O.; Aremu, A.O.; Van Staden, J. Shoot proliferation and rooting treatments influence secondary metabolite production and antioxidant activity in tissue culture-derived Aloe arborescens grown ex vitro. Plant. Growth Regul. 2013, 70, 115–122. [Google Scholar] [CrossRef]
- Aremu, A.O.; Gruz, J.; Šubrtová, M.; Szüčová, L.; Doležal, K.; Bairu, M.W.; Finnie, J.F.; Van Staden, J. Antioxidant and phenolic acid profiles of tissue cultured and acclimatized Merwilla plumbea plantlets in relation to the applied cytokinins. J. Plant. Physiol. 2013, 170, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Wysokińska, H. The effect of cytokinins on shoot proliferation, secondary metabolite production and antioxidant potential in shoot cultures of Scutellaria alpina. Plant. Cell Tiss. Organ. Cult. 2015, 122, 699–708. [Google Scholar] [CrossRef] [Green Version]
- Karalija, E.; Zeljković, S.Ć.; Tarkowski, P.; Muratović, E.; Parić, A. The effect of cytokinins on growth, phenolics, antioxidant and antimicrobial potential in liquid agitated shoot cultures of Knautia sarajevensis. Plant. Cell Tiss. Organ. Cult. 2017, 131, 347–357. [Google Scholar] [CrossRef]
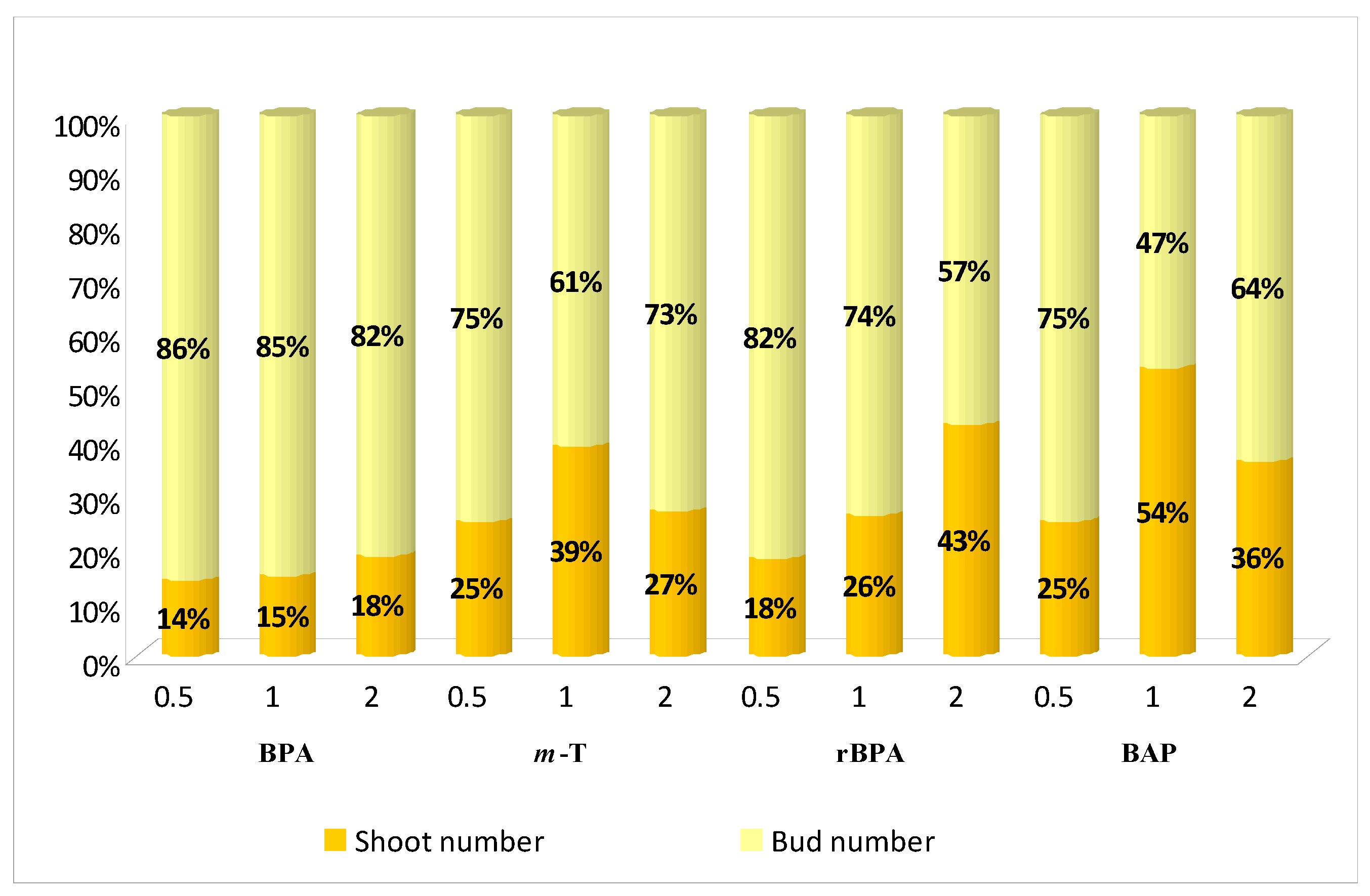

| Cytokinin Type and Content | TPC | TPA | TP | TPA/TP Ratio |
|---|---|---|---|---|
| C | 10.06 ± 0.10 | 7.69 ± 0.07 | 2.37 ± 0.03 | 3.2 |
| BPA 0.5 | 17.39 ± 0.56 | 12.62 ± 0.50 | 4.77 ± 0.06 | 2.7 |
| BPA 1 | 16.66 ± 0.17 | 12.53 ± 0.12 | 4.13 ± 0.06 | 3.0 |
| BPA 2 | 18.66 ± 0.27 | 15.47 ± 0.17 | 3.19 ± 0.10 | 4.8 |
| m-T 0.5 | 14.80 ± 0.81 | 10.67 ± 0.58 | 4.12 ± 0.24 | 2.6 |
| m-T 1 | 16.67 ± 0.25 | 8.64 ± 0.07 | 8.03 ± 0.18 | 1.1 |
| m-T 2 | 15.94 ± 0.16 | 10.49 ± 0.11 | 5.45 ± 0.05 | 1.9 |
| rBAP 0.5 | 17.01 ±0.11 | 12.45 ± 0.08 | 4.55 ± 0.04 | 2.7 |
| rBAP 1 | 17.65 ± 0.24 | 13.74 ± 0.17 | 3.91 ± 0.08 | 3.5 |
| rBAP 2 | 14.74 ± 0.45 | 11.14 ± 0.36 | 3.61 ± 0.10 | 3.1 |
| BAP 0.5 | 9.63 ± 0.38 | 7.69 ± 0.07 | 2.04 ± 0.09 | 3.7 |
| BAP 1 | 13.62 ± 0.78 | 10.56 ± 0.62 | 3.05 ± 0.16 | 3.5 |
| BAP 2 | 8.50 ± 0.06 | 6.16 ± 0.05 | 2.33 ± 0.02 | 2.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzegorczyk-Karolak, I.; Hnatuszko-Konka, K.; Zarzycka, M.; Kuźma, Ł. The Stimulatory Effect of Purine-Type Cytokinins on Proliferation and Polyphenolic Compound Accumulation in Shoot Culture of Salvia viridis. Biomolecules 2020, 10, 178. https://doi.org/10.3390/biom10020178
Grzegorczyk-Karolak I, Hnatuszko-Konka K, Zarzycka M, Kuźma Ł. The Stimulatory Effect of Purine-Type Cytokinins on Proliferation and Polyphenolic Compound Accumulation in Shoot Culture of Salvia viridis. Biomolecules. 2020; 10(2):178. https://doi.org/10.3390/biom10020178
Chicago/Turabian StyleGrzegorczyk-Karolak, Izabela, Katarzyna Hnatuszko-Konka, Mariola Zarzycka, and Łukasz Kuźma. 2020. "The Stimulatory Effect of Purine-Type Cytokinins on Proliferation and Polyphenolic Compound Accumulation in Shoot Culture of Salvia viridis" Biomolecules 10, no. 2: 178. https://doi.org/10.3390/biom10020178
APA StyleGrzegorczyk-Karolak, I., Hnatuszko-Konka, K., Zarzycka, M., & Kuźma, Ł. (2020). The Stimulatory Effect of Purine-Type Cytokinins on Proliferation and Polyphenolic Compound Accumulation in Shoot Culture of Salvia viridis. Biomolecules, 10(2), 178. https://doi.org/10.3390/biom10020178






