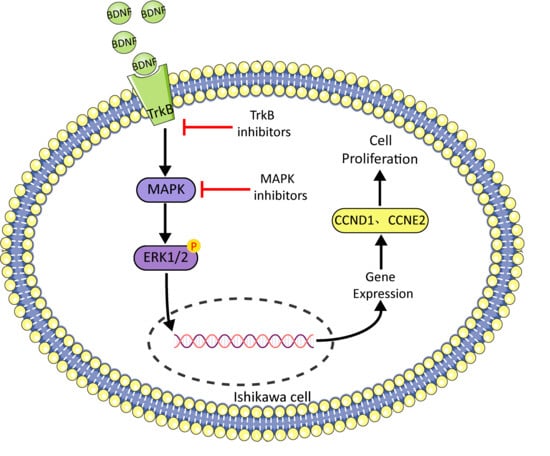
Brain-Derived Neurotrophic Factor Regulates Ishikawa Cell Proliferation through the TrkB-ERK1/2 Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Transfection with Small Interfering RNA
2.3. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction Analysis
2.4. Western Blot Analysis
2.5. Cell Viability Assays
2.6. Colony Formation Assay
2.7. Cellular Immunofluorescence
2.8. Statistical Analysis
3. Results
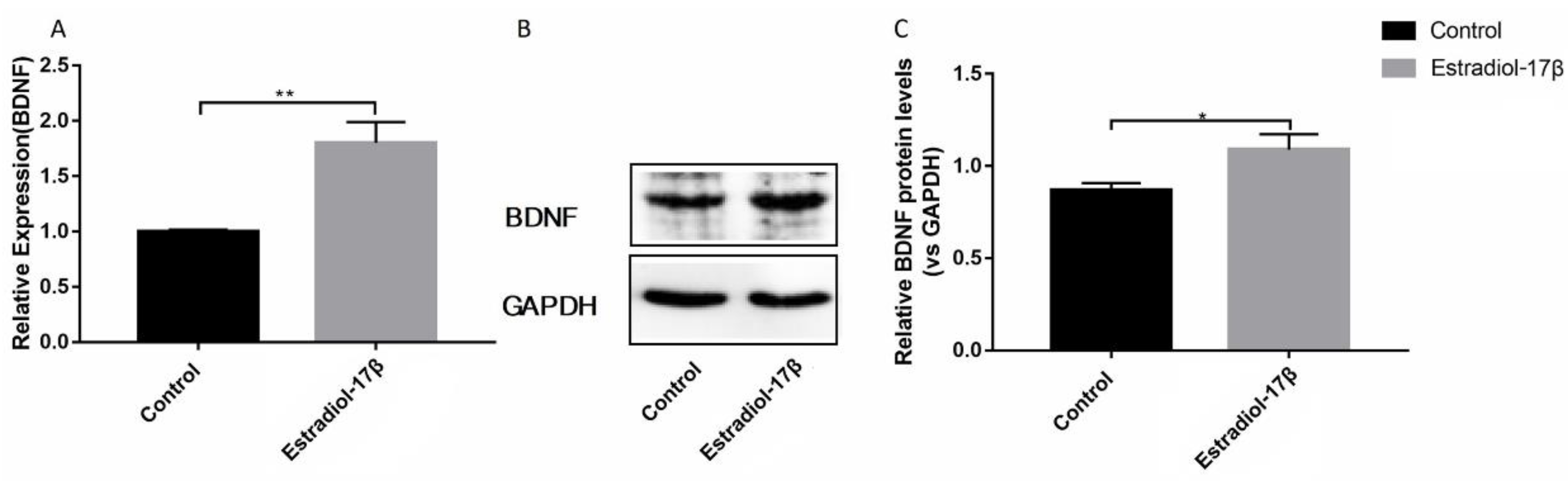
3.1. E2 promotes the Synthesis of BDNF Protein in Ishikawa Cells
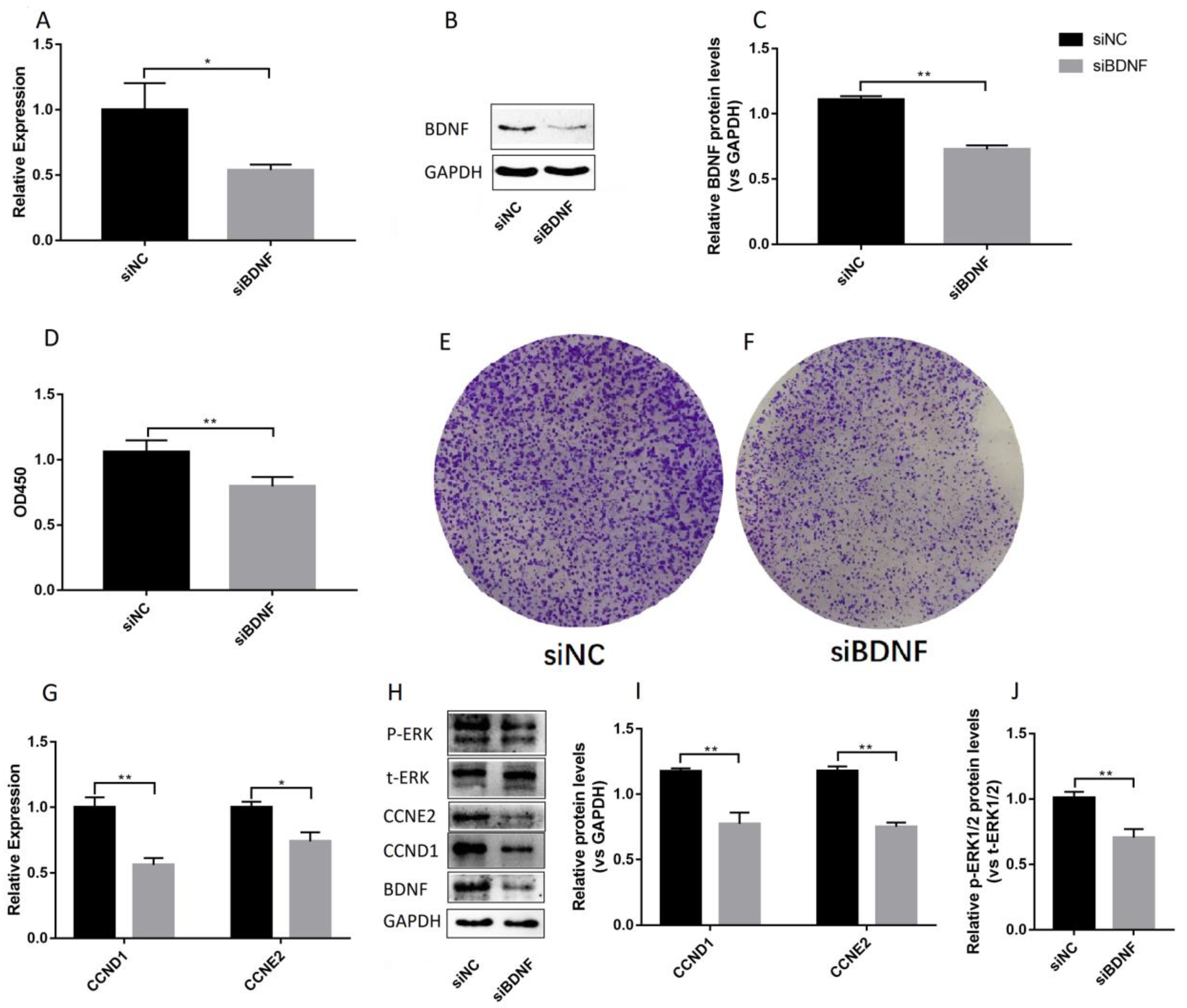
3.2. Effects of BDNF on Proliferation of Ishikawa Cells
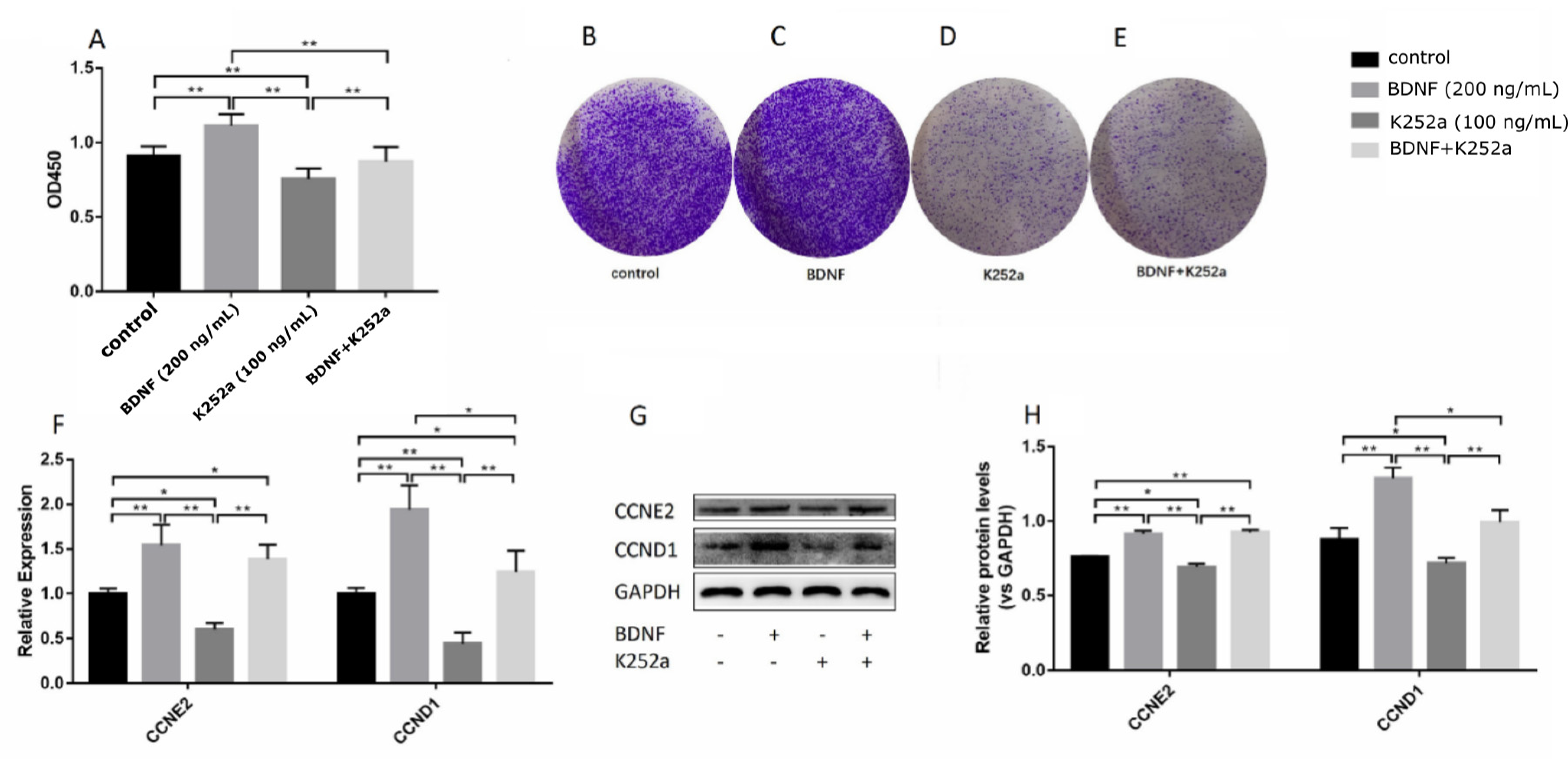
3.3. BDNF Promotes the Proliferation of Ishikawa Cells via TrkB
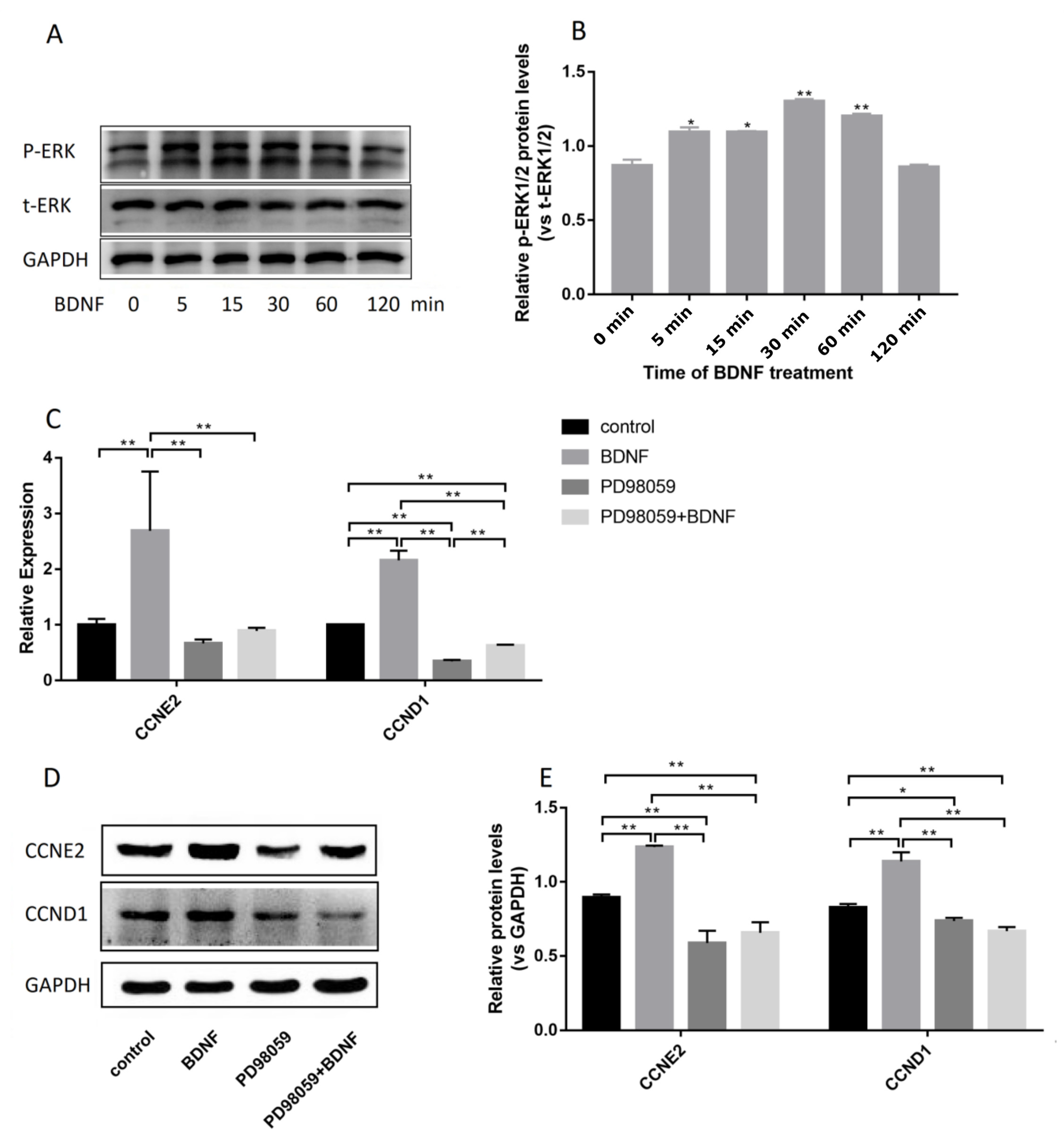
3.4. BDNF Promotes Cell Proliferation through the MAPK/ERK1/2 Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brenner, R.M.; West, N.B.; McClellan, M.C. Estrogen and progestin receptors in the reproductive tract of male and female primates. Biol. Reprod. 1990, 42, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schütz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef]
- Komatsu, N.; Maekawa, T.; Takeuchi, S.; Takahashi, S. Epidermal growth factor and transforming growth factor-α stimulate the proliferation of mouse uterine stromal cells. Zool. Sci. 2003, 20, 639–645. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Colette, S.; Defrère, S.; Lousse, J.-C.; Van Langendonckt, A.; Loumaye, E.; Donnez, J. Evaluation of estrogen treatment in an immunodeficient mouse endometriosis model. Gynecol. Obstet. Investig. 2009, 68, 262–268. [Google Scholar] [CrossRef]
- Kaushal, J.B.; Sankhwar, P.; Kumari, S.; Popli, P.; Shukla, V.; Hussain, M.K.; Hajela, K.; Dwivedi, A. The regulation of hh/gli1 signaling cascade involves gsk3β- mediated mechanism in estrogen-derived endometrial hyperplasia. Entific Rep. 2017, 7, 6557. [Google Scholar] [CrossRef]
- Groothuis, P.; Dassen, H.; Romano, A.; Punyadeera, C. Estrogen and the endometrium: Lessons learned from gene expression profiling in rodents and human. Hum. Reprod. Update 2007, 13, 405–417. [Google Scholar] [CrossRef]
- Ma, W.-G.; Song, H.; Das, S.K.; Paria, B.C.; Dey, S.K. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc. Natl. Acad. Sci. USA 2003, 100, 2963–2968. [Google Scholar] [CrossRef]
- Wang, H.; Dey, S.K. Roadmap to embryo implantation: Clues from mouse models. Nat. Rev. Genet. 2006, 7, 185–199. [Google Scholar] [CrossRef]
- Gelety, T.J.; Buyalos, R.P. The influence of supraphysiologic estradiol levels on human nidation. J. Assist. Reprod. Genet. 1995, 12, 406–412. [Google Scholar] [CrossRef]
- Gunin, A.G.; Emelianov, V.U.; Mironkin, I.U.; Morozov, M.P.; Tolmachev, A.S. Lithium treatment enhances estradiol-induced proliferation and hyperplasia formation in the uterus of mice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 114, 83–91. [Google Scholar] [CrossRef]
- O’Brien, J.E.; Peterson, T.J.; Tong, M.H.; Lee, E.-J.; Pfaff, L.E.; Hewitt, S.C.; Korach, K.S.; Weiss, J.; Jameson, J.L. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor α binding to classical estrogen response elements. J. Biol. Chem. 2006, 281, 26683–26692. [Google Scholar] [CrossRef] [PubMed]
- Lubahn, D.B.; Moyer, J.S.; Golding, T.S.; Couse, J.F.; Korach, K.S.; Smithies, O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc. Natl. Acad. Sci. USA 1993, 90, 11162–11166. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Kim, T.H.; Fazleabas, A.T.; Palomino, W.A.; Ahn, S.H.; Tayade, C.; Schammel, D.P.; Young, S.L.; Jeong, J.W.; Lessey, B.A. Kras activation and over-expression of sirt1/bcl6 contributes to the pathogenesis of endometriosis and progesterone resistance. Sci. Rep. 2017, 7, 6765. [Google Scholar] [CrossRef] [PubMed]
- Huhtinen, K.; Desai, R.; Ståhle, M.; Salminen, A.; Handelsman, D.J.; Perheentupa, A.; Poutanen, M. Endometrial and endometriotic concentrations of estrone and estradiol are determined by local metabolism rather than circulating levels. J. Clin. Endocrinol. Metab. 2012, 97, 4228–4235. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Johnson, G.A. Pig blastocyst–uterine interactions. Differentiation 2014, 87, 52–65. [Google Scholar] [CrossRef]
- Ignar-Trowbridge, D.M.; Nelson, K.G.; Bidwell, M.C.; Curtis, S.W.; Washburn, T.F.; McLachlan, J.A.; Korach, K.S. Coupling of dual signaling pathways: Epidermal growth factor action involves the estrogen receptor. Proc. Natl. Acad. Sci. USA 1992, 89, 4658–4662. [Google Scholar] [CrossRef]
- Gargett, C.E.; Chan, R.W.; Schwab, K.E. Hormone and growth factor signaling in endometrial renewal: Role of stem/progenitor cells. Mol. Cell. Endocrinol. 2008, 288, 22–29. [Google Scholar] [CrossRef]
- Li-Hua, Z.; Li-Hua, S.; Ya-Li, H.; Yue, J.; Hong-Yu, L.; Xiao-Yue, S.; Xiao-Yan, J.; Xin, Z.; Hai-Xiang, S.; Gui-Jun, Y. Pcaf impairs endometrial receptivity and embryo implantation by down-regulating β3-integrin expression via hoxa10 acetylation. J. Clin. Endocrinol. Metab. 2013, 4417–4428. [Google Scholar] [CrossRef]
- Li, H.-W.R.; Li, Y.-X.; Li, T.-T.; Fan, H.; Ng, E.H.-Y.; Yeung, W.S.-B.; Ho, P.-C.; Lee, K.-F. Effect of ulipristal acetate and mifepristone at emergency contraception dose on the embryo-endometrial attachment using an in vitro human trophoblastic spheroid and endometrial cell co-culture model. Hum. Reprod. 2017, 32, 2414–2422. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, D.; Yang, Y.U.; Cui, X.; Sun, J.; Liang, C.; Qin, H.; Yang, X.; Liu, S.; Yan, Q. Microrna-200c impairs uterine receptivity formation by targeting fut4 and α1,3-fucosylation. Cell Death Differ. 2017. [Google Scholar] [CrossRef]
- Hempstead, B.L. Dissecting the diverse actions of pro- and mature neurotrophins. Curr. Alzheimer Res. 2006, 3. [Google Scholar] [CrossRef] [PubMed]
- Ernfors, P.; Kucera, J.; Lee, K.F.; Loring, J.; Jaenisch, R. Studies on the physiological role of brain-derived neurotrophic factor and neurotrophin-3 in knockout mice. Int. J. Dev. Biol. 1995, 39, 799. [Google Scholar] [PubMed]
- Kawamura, K.; Kawamura, N.; Mulders, S.M.; Sollewijn Gelpke, M.D.; Hsueh, A.J. Ovarian brain-derived neurotrophic factor (bdnf) promotes the development of oocytes into preimplantation embryos. Proc. Natl. Acad. Sci. USA 2005, 102, 9206–9211. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Johnson, A.L. Expression and function of brain-derived neurotrophin factor and its receptor, trkb, in ovarian follicles from the domestic hen (gallus gallus domesticus). J. Exp. Biol. 2001, 204, 2087–2095. [Google Scholar]
- Acheson, A.; Conover, J.C.; Fandl, J.P.; DeChiara, T.M.; Russell, M.; Thadani, A.; Squinto, S.P.; Yancopoulos, G.D.; Lindsay, R.M. A bdnf autocrine loop in adult sensory neurons prevents cell death. Nature 1995, 374, 450–453. [Google Scholar] [CrossRef]
- Chen, S.; Wang, F.; Liu, Z.; Zhao, Y.; Jiang, Y.; Chen, L.; Li, C.; Zhou, X. Brain-derived neurotrophic factor promotes proliferation and progesterone synthesis in bovine granulosa cells. J. Cell. Physiol. 2019, 234, 8776–8787. [Google Scholar] [CrossRef]
- Russo, N.; Russo, M.; Daino, D.; Freschi, L.; Fiore, L.; Merlini, S.; Bucci, F.; Santoro, A.; Pluchino, N.; Luisi, S. Evaluation of brain-derived neurotrophic factor in menstrual blood and its identification in human endometrium. Gynecol. Endocrinol. 2012, 28, 492–495. [Google Scholar] [CrossRef]
- Mirshokraei, P.; Hassanpour, H.; Rahnama, A.; Foster, W. Gene expression of bdnf and its receptors, trkb and p75 in the uterus and oviduct of pregnant and non-pregnant ewes. Res. Vet. Sci. 2013, 95, 164–168. [Google Scholar] [CrossRef]
- Bao, W.; Qiu, H.; Yang, T.; Luo, X.; Zhang, H.; Wan, X. Upregulation of trkb promotes epithelial-mesenchymal transition and anoikis resistance in endometrial carcinoma. PLoS ONE 2013, 8, e70616. [Google Scholar] [CrossRef]
- Kawamura, K.; Kawamura, N.; Sato, W.; Fukuda, J.; Kumagai, J.; Tanaka, T. Brain-derived neurotrophic factor promotes implantation and subsequent placental development by stimulating trophoblast cell growth and survival. Endocrinology 2009, 150, 3774–3782. [Google Scholar] [CrossRef]
- Kawamura, N.; Kawamura, K.; Manabe, M.; Tanaka, T. Inhibition of brain-derived neurotrophic factor/tyrosine kinase b signaling suppresses choriocarcinoma cell growth. Endocrinology 2010, 151, 3006–3014. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Kawamura, N.; Fukuda, J.; Kumagai, J.; Hsueh, A.J.W.; Tanaka, T. Regulation of preimplantation embryo development by brain-derived neurotrophic factor. Dev. Biol. 2007, 311, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Solum, D.T.; Handa, R.J. Estrogen regulates the development of brain-derived neurotrophic factor mrna and protein in the rat hippocampus. J. Neuroence Off. J. Soc. Neuroence 2002, 22, 2650–2659. [Google Scholar] [CrossRef]
- Sohrabji, F.; Lewis, D.K. Estrogen-bdnf interactions: Implications for neurodegenerative diseases. Front. Neuroendocrinol. 2006, 27, 404–414. [Google Scholar] [CrossRef]
- Wessels, J.M.; Leyland, N.A.; Agarwal, S.K.; Foster, W.G. Estrogen induced changes in uterine brain-derived neurotrophic factor and its receptors. Hum. Reprod. 2015, 30, 925–936. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Yang, H.; Ma, X. The effect of estrogen on the proliferation of endometrial cancer cells is mediated by errγ through akt and erk1/2. Eur. J. Cancer Prev. 2014, 23, 418–424. [Google Scholar] [CrossRef]
- Tong, W.; Pollard, J.W. Progesterone inhibits estrogen-induced cyclin d1 and cdk4 nuclear translocation, cyclin e-and cyclin a-cdk2 kinase activation, and cell proliferation in uterine epithelial cells in mice. Mol. Cell. Biol. 1999, 19, 2251–2264. [Google Scholar] [CrossRef]
- Doisneau-Sixou, S.; Sergio, C.; Carroll, J.; Hui, R.; Musgrove, E.; Sutherland, R. Estrogen and antiestrogen regulation of cell cycle progression in breast cancer cells. Endocr. Relat. Cancer 2003, 10, 179–186. [Google Scholar] [CrossRef]
- Caldon, C.E.; Sergio, C.M.; Schütte, J.; Boersma, M.N.; Sutherland, R.L.; Carroll, J.S.; Musgrove, E.A. Estrogen regulation of cyclin e2 requires cyclin d1 but not c-myc. Mol. Cell. Biol. 2009, 29, 4623–4639. [Google Scholar] [CrossRef]
- Morgan, D.O. The Cell Cycle: Principles of Control; New Science Press: London, UK, 2007. [Google Scholar]
- Lim, W.; Bae, H.; Bazer, F.W.; Song, G. Brain derived neurotrophic factor improves proliferation of endometrial epithelial cells by inhibition of endoplasmic reticulum stress during early pregnancy. J. Cell. Physiol. 2017, 232, 3641–3651. [Google Scholar] [CrossRef]
- Tapley, P.; Lamballe, F.; Barbacid, M. K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene 1992, 7, 371–381. [Google Scholar] [PubMed]
- Roskoski, R., Jr. Erk1/2 map kinases: Structure, function, and regulation. Pharmacol. Res. 2012, 66, 105–143. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lin, X.; Lim, I.Y.; Chen, L.; Teh, A.L.; MacIsaac, J.L.; Tan, K.H.; Kobor, M.S.; Chong, Y.S.; Gluckman, P.D. Analysis of two birth tissues provides new insights into the epigenetic landscape of neonates born preterm. Clin. Epigenetics 2019, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]


| Name | Sequences |
|---|---|
| Gapdh | F-5ACCCATCACCATCTTCCAGGAG3’ |
| R-5’GAAGGGGCGGAGATGATGAC3’ | |
| Bdnf | F-5’TCTGGAGAGCGTGAATGG3’ |
| R-5’AGGCACTTGACTACTGAGC3’ | |
| CCNE2 | F-5’AAGAGGAAAACTACCCAGGATG3 |
| R-5’ATAATGCAAGGACTGATCCCC3’ | |
| CCND1 | F-5’CCTCGGTGTCCTACTTCAAATG3’ |
| R-5’GCGGTCCAGGTAGTTCATG3’ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, M.; Niu, Q.; Xiang, X.; Yuan, C.; Iqbal, T.; Huang, Y.; Tian, M.; Zhao, Z.; Li, C.; Zhou, X. Brain-Derived Neurotrophic Factor Regulates Ishikawa Cell Proliferation through the TrkB-ERK1/2 Signaling Pathway. Biomolecules 2020, 10, 1645. https://doi.org/10.3390/biom10121645
Cao M, Niu Q, Xiang X, Yuan C, Iqbal T, Huang Y, Tian M, Zhao Z, Li C, Zhou X. Brain-Derived Neurotrophic Factor Regulates Ishikawa Cell Proliferation through the TrkB-ERK1/2 Signaling Pathway. Biomolecules. 2020; 10(12):1645. https://doi.org/10.3390/biom10121645
Chicago/Turabian StyleCao, Maosheng, Qiaoge Niu, XinYu Xiang, Chenfeng Yuan, Tariq Iqbal, Yuwen Huang, Meng Tian, Zijiao Zhao, Chunjin Li, and Xu Zhou. 2020. "Brain-Derived Neurotrophic Factor Regulates Ishikawa Cell Proliferation through the TrkB-ERK1/2 Signaling Pathway" Biomolecules 10, no. 12: 1645. https://doi.org/10.3390/biom10121645
APA StyleCao, M., Niu, Q., Xiang, X., Yuan, C., Iqbal, T., Huang, Y., Tian, M., Zhao, Z., Li, C., & Zhou, X. (2020). Brain-Derived Neurotrophic Factor Regulates Ishikawa Cell Proliferation through the TrkB-ERK1/2 Signaling Pathway. Biomolecules, 10(12), 1645. https://doi.org/10.3390/biom10121645