Autophagy and Intracellular Membrane Trafficking Subversion by Pathogenic Yersinia Species
Abstract
1. Introduction
2. Autophagy
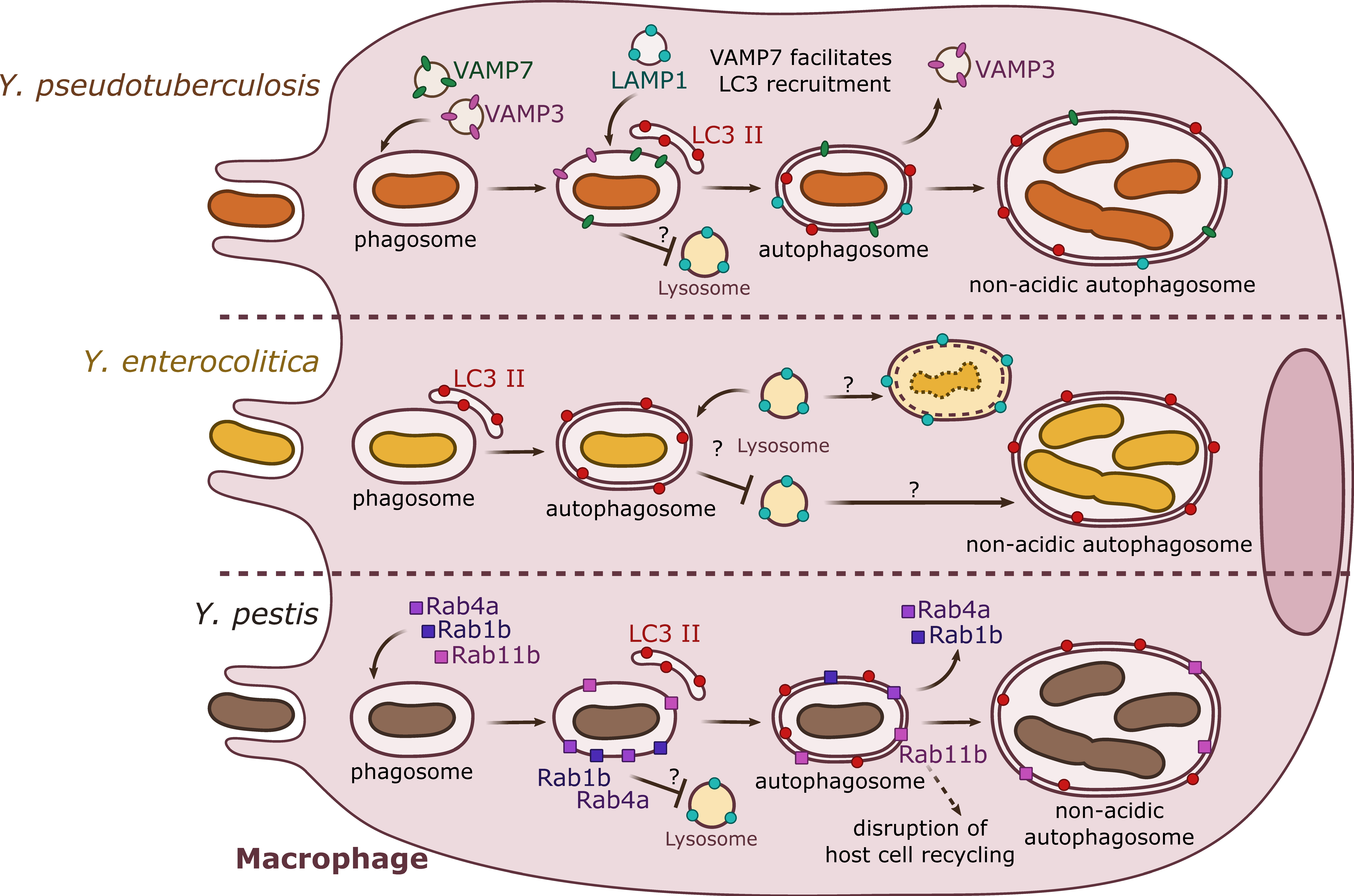
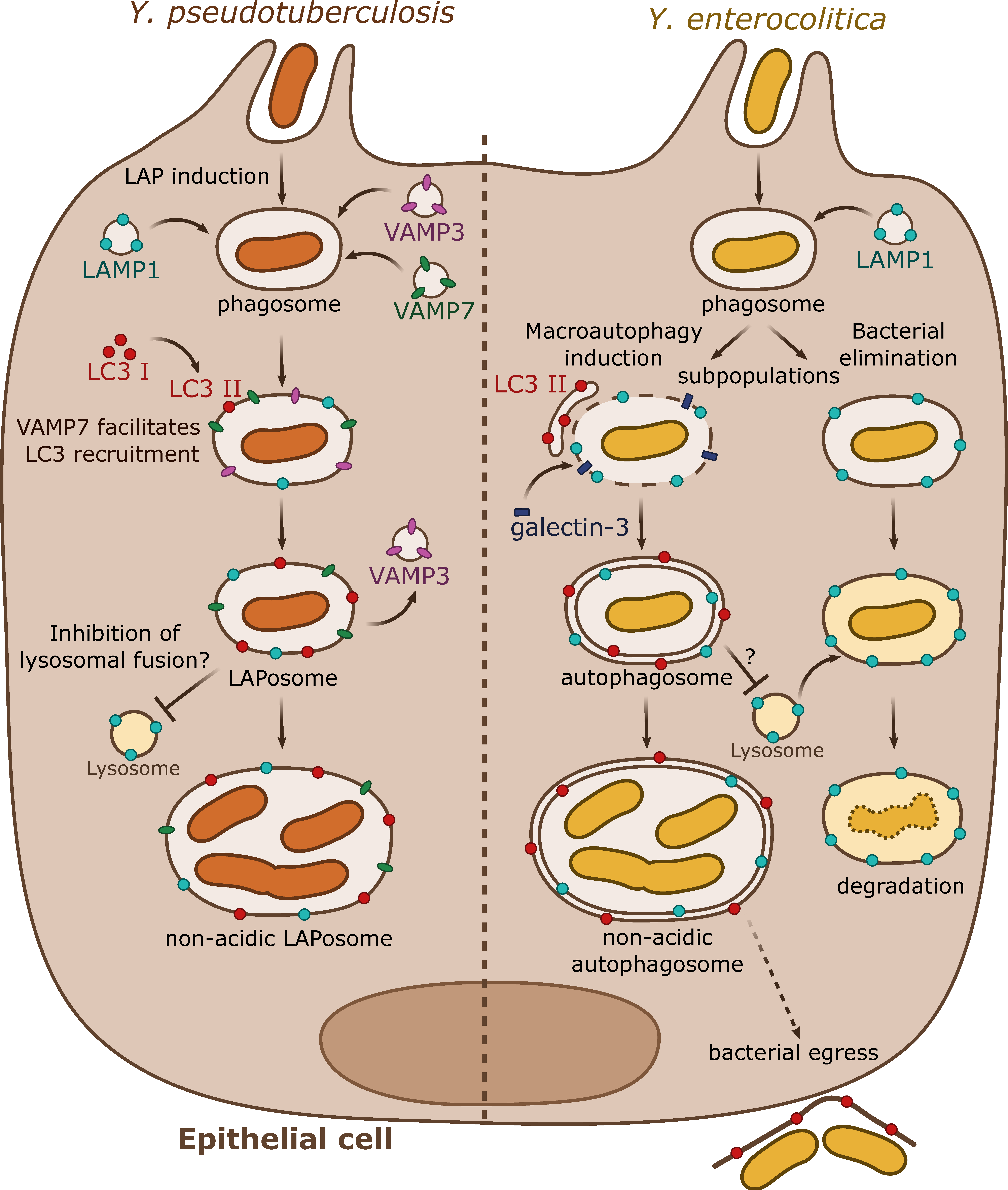
3. Yersinia pseudotuberculosis
4. Yersinia enterocolitica
5. Yersinia pestis
6. Yersinia ruckeri
7. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Naktin, J.; Beavis, K.G. Yersinia enterocolitica and Yersinia pseudotuberculosis. Clin. Lab. Med. 1999, 19, 523–536. [Google Scholar] [CrossRef]
- Demeure, C.E.; Dussurget, O.; Fiol, G.M.; Le Guern, A.-S.; Savin, C.; Pizarro-Cerdá, J. Yersinia pestis and plague: An updated view on evolution, virulence determinants, immune subversion, vaccination, and diagnostics. Genes Immun. 2019, 20, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Pepe, J.C.; Miller, V.L. Yersinia enterocolitica invasin: A primary role in the initiation of infection. Proc. Natl. Acad. Sci. USA 1993, 90, 6473–6477. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.A.; Hirst, B.H.; Jepson, M.A. M-Cell Surface Integrin Expression and Invasin-Mediated Targeting of Yersinia pseudotuberculosis to Mouse Peyer’s Patch M Cells. Infect. Immun. 1998, 66, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Fahlgren, A.; Avican, K.; Westermark, L.; Nordfelth, R.; Fällman, M. Colonization of Cecum Is Important for Development of Persistent Infection by Yersinia pseudotuberculosis. Infect. Immun. 2014, 82, 3471–3482. [Google Scholar] [CrossRef] [PubMed]
- Bottone, E.J. Yersinia enterocolitica: The charisma continues. Clin. Microbiol. Rev. 1997, 10, 257–276. [Google Scholar] [CrossRef]
- Simonet, M.; Riot, B.; Fortineau, N.; Berche, P. Invasin production by Yersinia pestis is abolished by insertion of an IS200-like element within the inv gene. Infect. Immun. 1996, 64, 375–379. [Google Scholar] [CrossRef]
- Sebbane, F.; Gardner, D.; Long, D.; Gowen, B.B.; Hinnebusch, B.J. Kinetics of Disease Progression and Host Response in a Rat Model of Bubonic Plague. Am. J. Pathol. 2005, 166, 1427–1439. [Google Scholar] [CrossRef]
- Cornelis, G.R.; Wolf-Watz, H. The Yersinia Yop virulon: A bacterial system for subverting eukaryotic cells. Mol. Microbiol. 1997, 23, 861–867. [Google Scholar] [CrossRef]
- Cornelis, G.R. The Yersinia Ysc-Yop “type III” weaponry. Nat. Rev. Mol. Cell Biol. 2002, 3, 742–752. [Google Scholar] [CrossRef]
- Isberg, R.R.; Falkow, S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature 1985, 317, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Isberg, R.R.; Voorhis, D.L.; Falkow, S. Identification of invasin: A protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell 1987, 50, 769–778. [Google Scholar] [CrossRef]
- Isberg, R.R.; Leong, J.M. Multiple beta β1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell 1990, 60, 861–871. [Google Scholar] [CrossRef]
- Guinet, F.; Avé, P.; Filali, S.; Huon, C.; Savin, C.; Huerre, M.; Fiette, L.; Carniel, E. Dissociation of Tissue Destruction and Bacterial Expansion during Bubonic Plague. PLoS Pathogens 2015, 11, 1–19. [Google Scholar] [CrossRef]
- Pujol, C.; Bliska, J.B. The Ability To Replicate in Macrophages Is Conserved between Yersinia pestis and Yersinia pseudotuberculosis. Infect. Immun. 2003, 71, 5892–5899. [Google Scholar] [CrossRef] [PubMed]
- Grabenstein, J.P.; Marceau, M.; Pujol, C.; Simonet, M.; Bliska, J.B. The Response Regulator PhoP of Yersinia pseudotuberculosis Is Important for Replication in Macrophages and for Virulence. Infect. Immun. 2004, 72, 4973–4984. [Google Scholar] [CrossRef]
- Kaur, J.; Debnath, J. Autophagy at the crossroads of catabolism and anabolism. Nat. Rev. Mol. Cell Biol. 2015, 16, 461–472. [Google Scholar] [CrossRef]
- Hughes, T.; Rusten, T.E. Origin and evolution of self-consumption: Autophagy. Adv. Exp. Med. Biol. 2007, 607, 111–118. [Google Scholar] [CrossRef]
- Khandia, R.; Dadar, M.; Munjal, A.; Dhama, K.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Iqbal, H.M.N.; Singh, K.P.; Joshi, S.K.; et al. A Comprehensive Review of Autophagy and Its Various Roles in Infectious, Non-Infectious, and Lifestyle Diseases: Current Knowledge and Prospects for Disease Prevention, Novel Drug Design, and Therapy. Cells 2019, 8, 674. [Google Scholar] [CrossRef]
- Nakashima, A.; Aoki, A.; Kusabiraki, T.; Shima, T.; Yoshino, O.; Cheng, S.-B.; Sharma, S.; Saito, S. Role of autophagy in oocytogenesis, embryogenesis, implantation, and pathophysiology of pre-eclampsia. J. Obstet. Gynaecol. Res. 2017, 43, 633–643. [Google Scholar] [CrossRef]
- Shibutani, S.T.; Saitoh, T.; Nowag, H.; Münz, C.; Yoshimori, T. Autophagy and autophagy-related proteins in the immune system. Nat. Immunol. 2015, 16, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Parzych, K.R.; Klionsky, D.J. An Overview of Autophagy: Morphology, Mechanism, and Regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Philips, J.A. LC3-associated phagocytosis: Host defense and microbial response. Curr. Opin. Immunol. 2019, 60, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Ganley, I.G.; Lam, D.H.; Wang, J.; Ding, X.; Chen, S.; Jiang, X. ULK1·ATG13·FIP200 Complex Mediates mTOR Signaling and Is Essential for Autophagy. J. Biol. Chem. 2009, 284, 12297–12305. [Google Scholar] [CrossRef]
- Grimmel, M.; Backhaus, C.; Proikas-Cezanne, T. WIPI-Mediated Autophagy and Longevity. Cells 2015, 4, 202–217. [Google Scholar] [CrossRef]
- Ohsumi, Y.; Mizushima, N. Two ubiquitin-like conjugation systems essential for autophagy. Semin. Cell Dev. Biol. 2004, 15, 231–236. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Zhang, H. Autophagosome maturation: An epic journey from the ER to lysosomes. J. Cell Biol. 2019, 218, 757–770. [Google Scholar] [CrossRef]
- Hayashi, K.; Taura, M.; Iwasaki, A. The interaction between IKKα and LC3 promotes type I interferon production through the TLR9-containing LAPosome. Sci. Signal. 2018, 11, 11–21. [Google Scholar] [CrossRef]
- Martinez, J.; Almendinger, J.; Oberst, A.; Ness, R.; Dillon, C.P.; Fitzgerald, P.; Hengartner, M.O.; Green, D.R. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc. Natl. Acad. Sci. USA 2011, 108, 17396–17401. [Google Scholar] [CrossRef]
- Huang, J.; Canadien, V.; Lam, G.Y.; Steinberg, B.E.; Dinauer, M.C.; Magalhaes, M.A.O.; Glogauer, M.; Grinstein, S.; Brumell, J.H. Activation of antibacterial autophagy by NADPH oxidases. Proc. Natl. Acad. Sci. USA 2009, 106, 6226–6231. [Google Scholar] [CrossRef]
- Herb, M.; Gluschko, A.; Schramm, M. LC3-associated phagocytosis—The highway to hell for phagocytosed microbes. Semin. Cell Dev. Biol. 2020, 101, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, S.R.; Mizushima, N. Monitoring and measuring autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef] [PubMed]
- Weidberg, H.; Shvets, E.; Shpilka, T.; Shimron, F.; Shinder, V.; Elazar, Z. LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J. 2010, 29, 1792–1802. [Google Scholar] [CrossRef] [PubMed]
- Weidberg, H.; Shpilka, T.; Shvets, E.; Abada, A.; Shimron, F.; Elazar, Z. LC3 and GATE-16 N Termini mediate membrane fusion processes required for autophagosome biogenesis. Dev. Cell 2011, 20, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Tsuboyama, K.; Koyama-Honda, I.; Sakamaki, Y.; Koike, M.; Morishita, H.; Mizushima, N. The ATG conjugation systems are important for degradation of the inner autophagosomal membrane. Science 2016, 354, 1036–1041. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Cuervo, A.M.; Ravikumar, B.; Sarkar, S.; Korolchuk, V.I.; Kaushik, S.; Klionsky, D.J. In search of an “autophagomometer”. Autophagy 2009, 5, 585–589. [Google Scholar] [CrossRef]
- Moreau, K.; Lacas-Gervais, S.; Fujita, N.; Sebbane, F.; Yoshimori, T.; Simonet, M.; Lafont, F. Autophagosomes can support Yersinia pseudotuberculosis replication in macrophages. Cell. Microbiol. 2010, 12, 1108–1123. [Google Scholar] [CrossRef]
- Tsukano, H.; Kura, F.; Inoue, S.; Sato, S.; Izumiya, H.; Yasuda, T.; Watanabe, H. Yersinia pseudotuberculosis blocks the phagosomal acidification of B10.A mouse macrophages through the inhibition of vacuolar H+-ATPase activity. Microb. Pathog. 1999, 27, 253–263. [Google Scholar] [CrossRef]
- Ligeon, L.-A.; Moreau, K.; Barois, N.; Bongiovanni, A.; Lacorre, D.-A.; Werkmeister, E.; Proux-Gillardeaux, V.; Galli, T.; Lafont, F. Role of VAMP3 and VAMP7 in the commitment of Yersinia pseudotuberculosis to LC3-associated pathways involving single- or double-membrane vacuoles. Autophagy 2014, 10, 1588–1602. [Google Scholar] [CrossRef]
- Deuretzbacher, A.; Czymmeck, N.; Reimer, R.; Trülzsch, K.; Gaus, K.; Hohenberg, H.; Heesemann, J.; Aepfelbacher, M.; Ruckdeschel, K. β 1 Integrin-Dependent Engulfment of Yersinia enterocolitica by Macrophages Is Coupled to the Activation of Autophagy and Suppressed by Type III Protein Secretion. J. Immunol. 2009, 183, 5847–5860. [Google Scholar] [CrossRef]
- Connor, M.G.; Pulsifer, A.R.; Price, C.T.; Abu Kwaik, Y.; Lawrenz, M.B. Yersinia pestis requires host Rab1b for survival in macrophages. PLoS Pathog 2015, 11, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.J.V.; Schimmeck, H.; Gropengießer, J.; Middendorf, L.; Quitmann, M.; Schneider, C.; Holstermann, B.; Wacker, R.; Heussler, V.; Reimer, R.; et al. Activation of the macroautophagy pathway by Yersinia enterocolitica promotes intracellular multiplication and egress of Yersiniae from epithelial cells. Cell. Microbiol. 2019, 21, 1–18. [Google Scholar] [CrossRef]
- Charnetzky, W.T.; Shuford, W.W. Survival and growth of Yersinia pestis within macrophages and an effect of the loss of the 47-megadalton plasmid on growth in macrophages. Infect. Immun. 1985, 47, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Straley, S.C.; Harmon, P.A. Yersinia pestis grows within phagolysosomes in mouse peritoneal macrophages. Infect. Immun. 1984, 45, 655–659. [Google Scholar] [CrossRef]
- Pujol, C.; Klein, K.A.; Romanov, G.A.; Palmer, L.E.; Cirota, C.; Zhao, Z.; Bliska, J.B. Yersinia pestis can reside in autophagosomes and avoid xenophagy in murine macrophages by preventing vacuole acidification. Infect. Immun. 2009, 77, 2251–2261. [Google Scholar] [CrossRef]
- Huang, J.; Birmingham, C.L.; Shahnazari, S.; Shiu, J.; Zheng, Y.T.; Smith, A.C.; Campellone, K.G.; Heo, W.D.; Gruenheid, S.; Meyer, T.; et al. Antibacterial autophagy occurs at PI(3)P-enriched domains of the endoplasmic reticulum and requires Rab1 GTPase. Autophagy 2011, 7, 17–26. [Google Scholar] [CrossRef]
- Connor, M.G.; Pulsifer, A.R.; Chung, D.; Rouchka, E.C.; Ceresa, B.K.; Lawrenz, M.B. Yersinia pestis Targets the Host Endosome Recycling Pathway during the Biogenesis of the Yersinia-Containing Vacuole To Avoid Killing by Macrophages. mBio 2018, 9, 1–19. [Google Scholar] [CrossRef]
- Kumar, G.; Menanteau-Ledouble, S.; Saleh, M.; El-Matbouli, M. Yersinia ruckeri, the causative agent of enteric redmouth disease in fish. Veter. Res. 2015, 46, 1–10. [Google Scholar] [CrossRef]
- Kawula, T.H.; Lelivelt, M.J.; Orndorff, P.E. Using a new inbred fish model and cultured fish tissue cells to study Aeromonas hydrophila and Yersinia ruckeri pathogenesis. Microb. Pathog. 1996, 20, 119–125. [Google Scholar] [CrossRef]
- Tobback, E.; Decostere, A.; Hermans, K.; Van den Broeck, W.; Haesebrouck, F.; Chiers, K. In vitro markers for virulence in Yersinia ruckeri. J. Fish Dis. 2010, 33, 197–209. [Google Scholar] [CrossRef]
- Menanteau-Ledouble, S.; Lawrence, M.L.; El-Matbouli, M. Invasion and replication of Yersinia ruckeri in fish cell cultures. BMC Veter. Res. 2018, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ryckaert, J.; Bossier, P.; D’Herde, K.; Diez-Fraile, A.; Sorgeloos, P.; Haesebrouck, F.; Pasmans, F. Persistence of Yersinia ruckeri in trout macrophages. Fish. Shellfish. Immunol. 2010, 29, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Oyston, P.C.F.; Dorrell, N.; Williams, K.; Li, S.-R.; Green, M.; Titball, R.W.; Wren, B.W. The Response Regulator PhoP Is Important for Survival under Conditions of Macrophage-Induced Stress and Virulence in Yersinia pestis. Infect. Immun. 2000, 68, 3419–3425. [Google Scholar] [CrossRef] [PubMed]
- Titball, R.W.; Hill, J.; Lawton, D.G.; Brown, K.A. Yersinia pestis and plague. Biochem. Soc. Trans. 2003, 31, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.J.; Lane, M.C.; Wagner, N.J.; Weening, E.H.; Miller, V.L. Dissemination of a Highly Virulent Pathogen: Tracking The Early Events That Define Infection. PLoS Pathog. 2015, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Shannon, J.G.; Bosio, C.F.; Hinnebusch, B.J. Dermal Neutrophil, Macrophage and Dendritic Cell Responses to Yersinia pestis Transmitted by Fleas. PLoS Pathog. 2015, 11, e1004734. [Google Scholar] [CrossRef]
- Gonzalez, R.J.; Miller, V.L. A Deadly Path: Bacterial Spread During Bubonic Plague. Trends. Microbiol. 2016, 24, 239–241. [Google Scholar] [CrossRef]
- Walker, K.A.; Maltez, V.I.; Hall, J.D.; Vitko, N.P.; Miller, V.L. A Phenotype at Last: Essential Role for the Yersinia enterocolitica Ysa Type III Secretion System in a Drosophila melanogaster S2 Cell Model. Infect. Immun. 2013, 81, 2478–2487. [Google Scholar] [CrossRef]
- Liu, T.; Wang, K.-Y.; Wang, J.; Chen, D.-F.; Huang, X.-L.; Ouyang, P.; Geng, Y.; He, Y.; Zhou, Y.; Min, J. Genome Sequence of the Fish Pathogen Yersinia ruckeri SC09 Provides Insights into Niche Adaptation and Pathogenic Mechanism. Int. J. Mol. Sci. 2016, 17, 557. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemarignier, M.; Pizarro-Cerdá, J. Autophagy and Intracellular Membrane Trafficking Subversion by Pathogenic Yersinia Species. Biomolecules 2020, 10, 1637. https://doi.org/10.3390/biom10121637
Lemarignier M, Pizarro-Cerdá J. Autophagy and Intracellular Membrane Trafficking Subversion by Pathogenic Yersinia Species. Biomolecules. 2020; 10(12):1637. https://doi.org/10.3390/biom10121637
Chicago/Turabian StyleLemarignier, Marion, and Javier Pizarro-Cerdá. 2020. "Autophagy and Intracellular Membrane Trafficking Subversion by Pathogenic Yersinia Species" Biomolecules 10, no. 12: 1637. https://doi.org/10.3390/biom10121637
APA StyleLemarignier, M., & Pizarro-Cerdá, J. (2020). Autophagy and Intracellular Membrane Trafficking Subversion by Pathogenic Yersinia Species. Biomolecules, 10(12), 1637. https://doi.org/10.3390/biom10121637



