Clinical Applications of Patient-Specific 3D Printed Models in Cardiovascular Disease: Current Status and Future Directions
Abstract
1. Introduction
2. Steps to Create 3D Segmentation Volume Data for 3D Printing
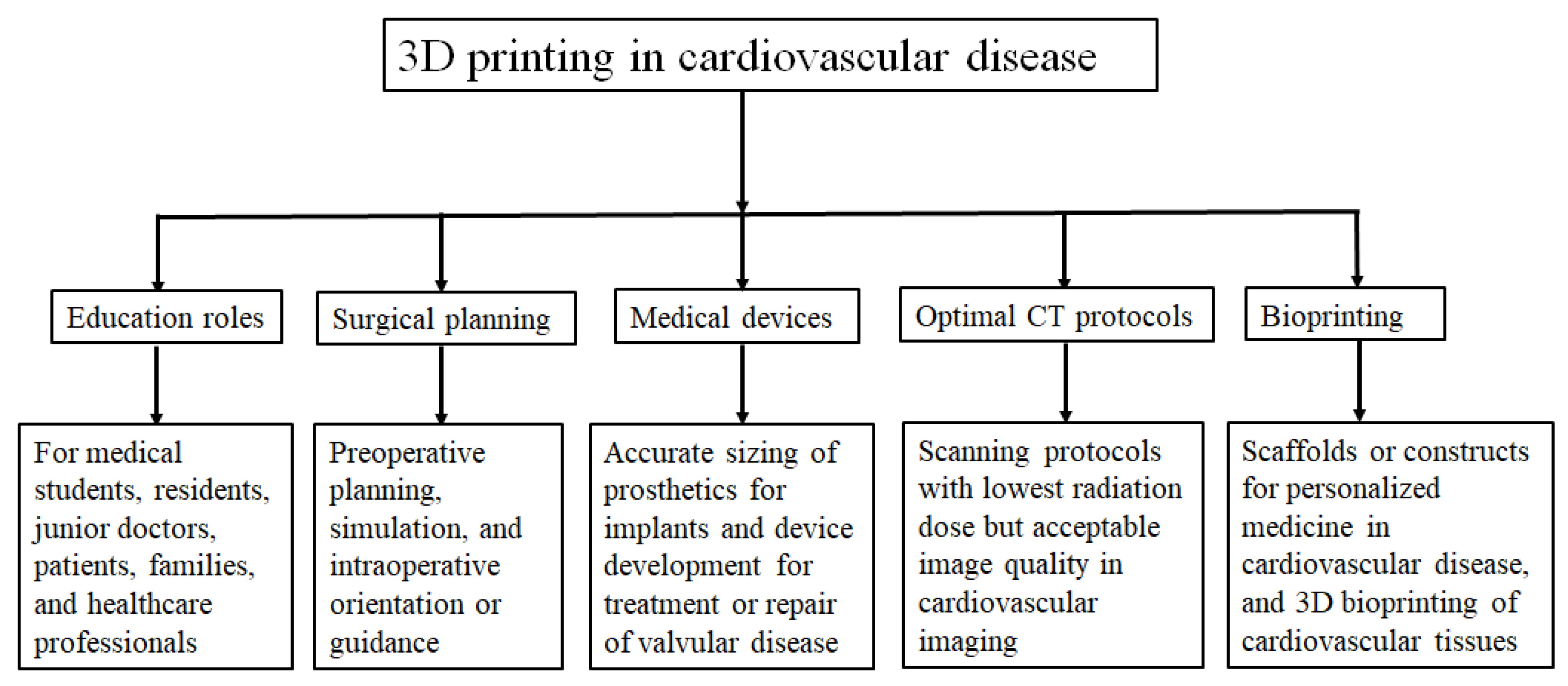
3. Clinical Applications of 3D Printing in Cardiovascular Disease
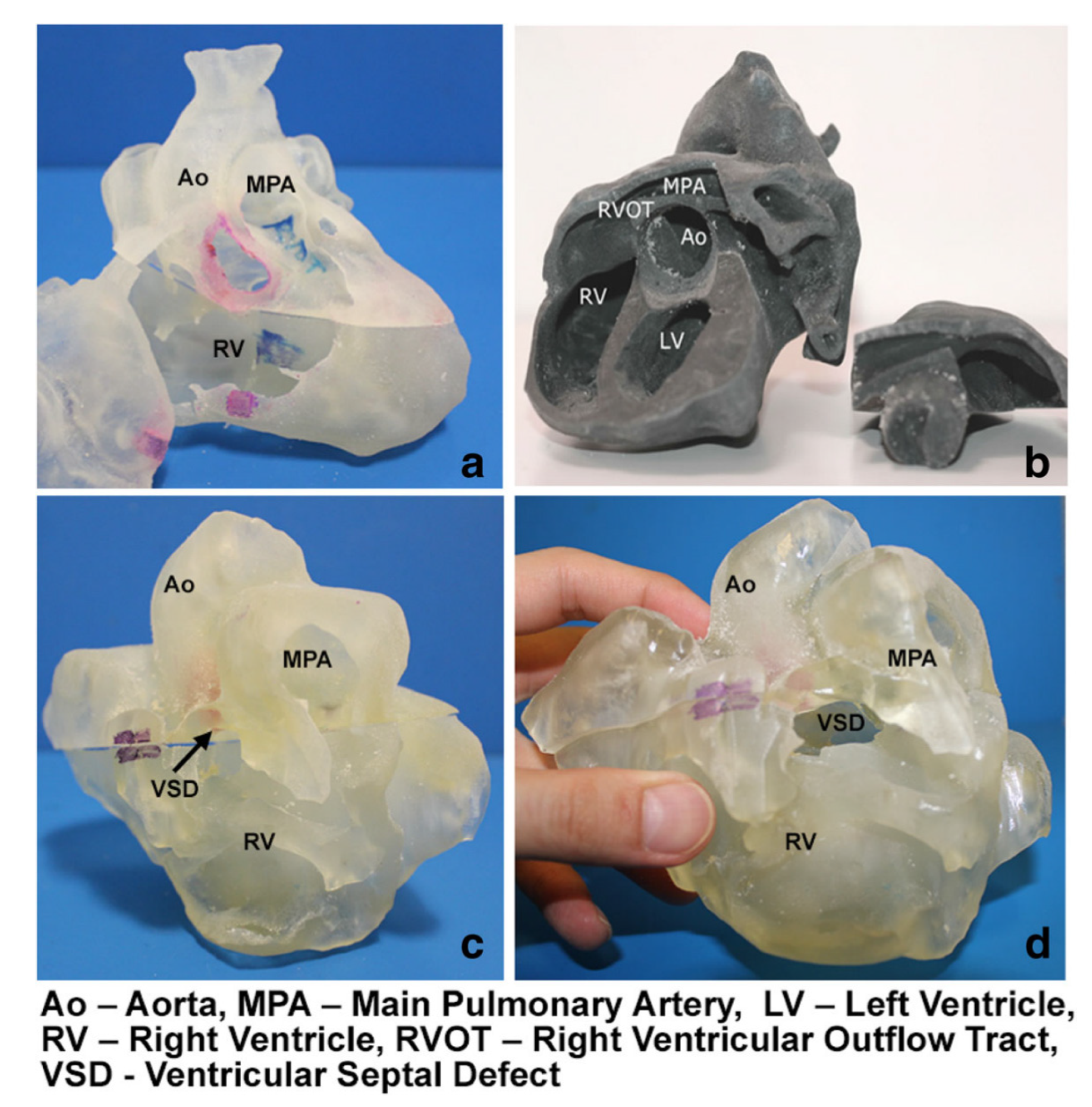
3.1. 3D Printing in Congenital Heart Disease
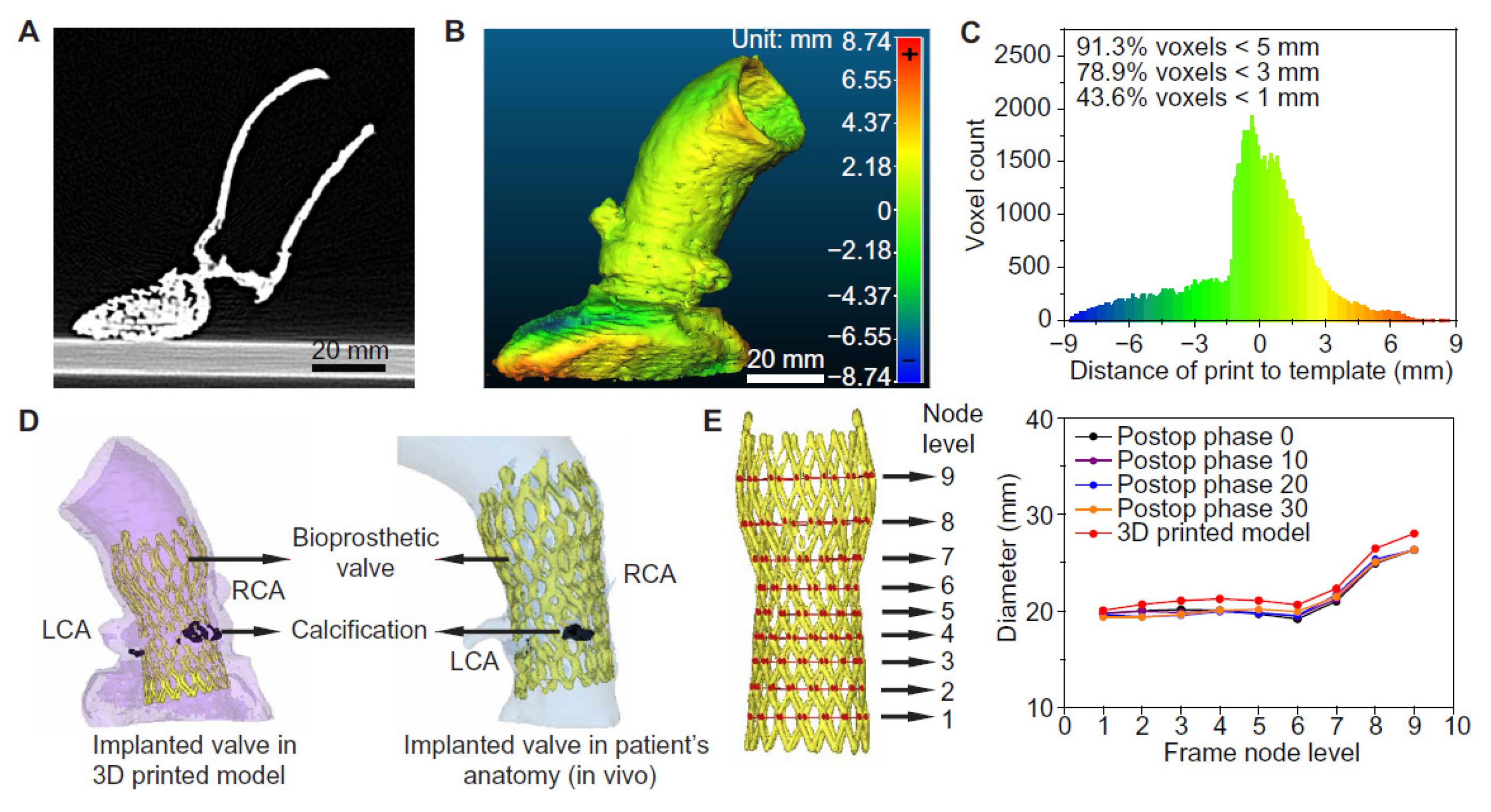
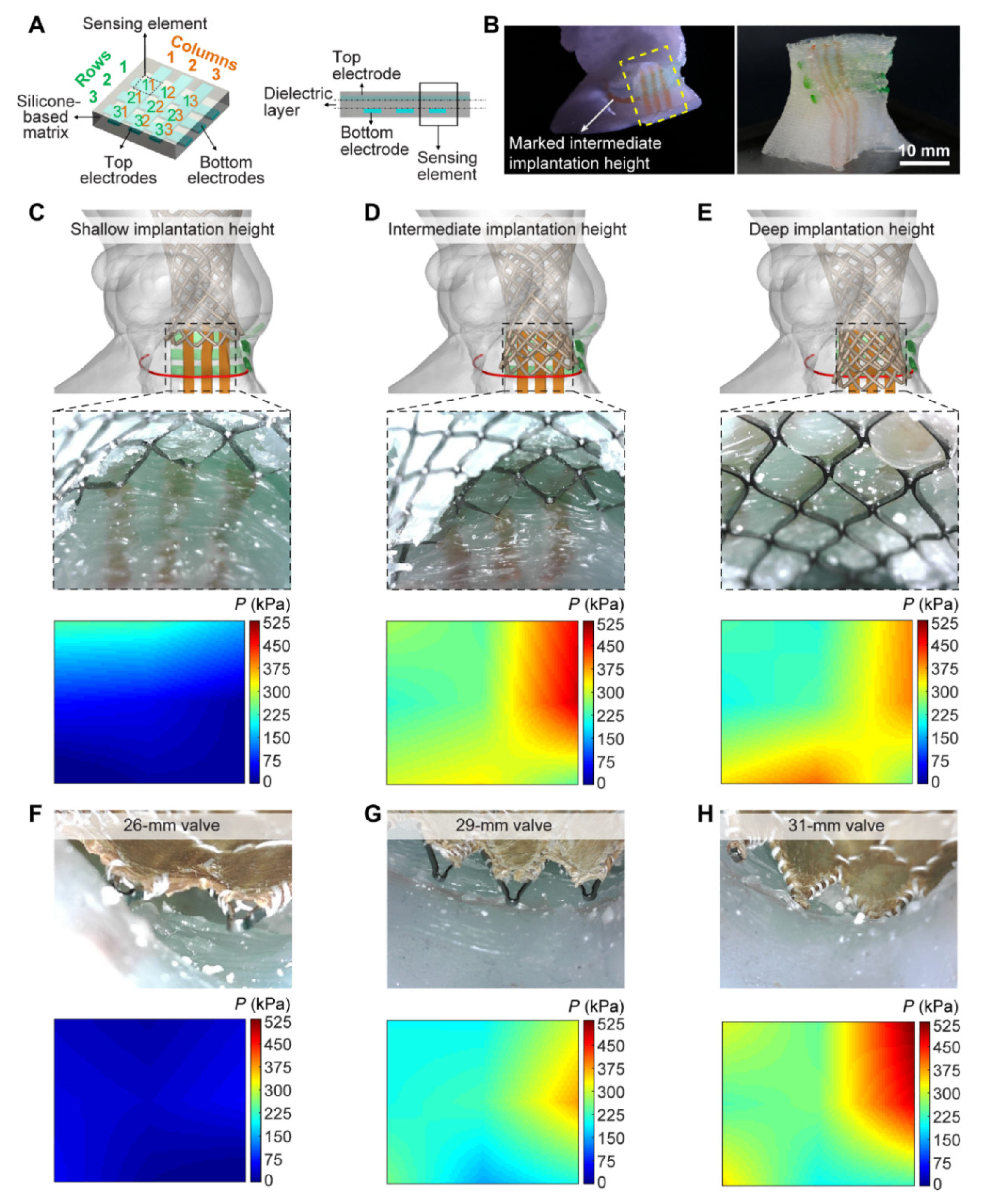
3.2. 3D Printing in Structural Heart Disease and Cardiac Interventions
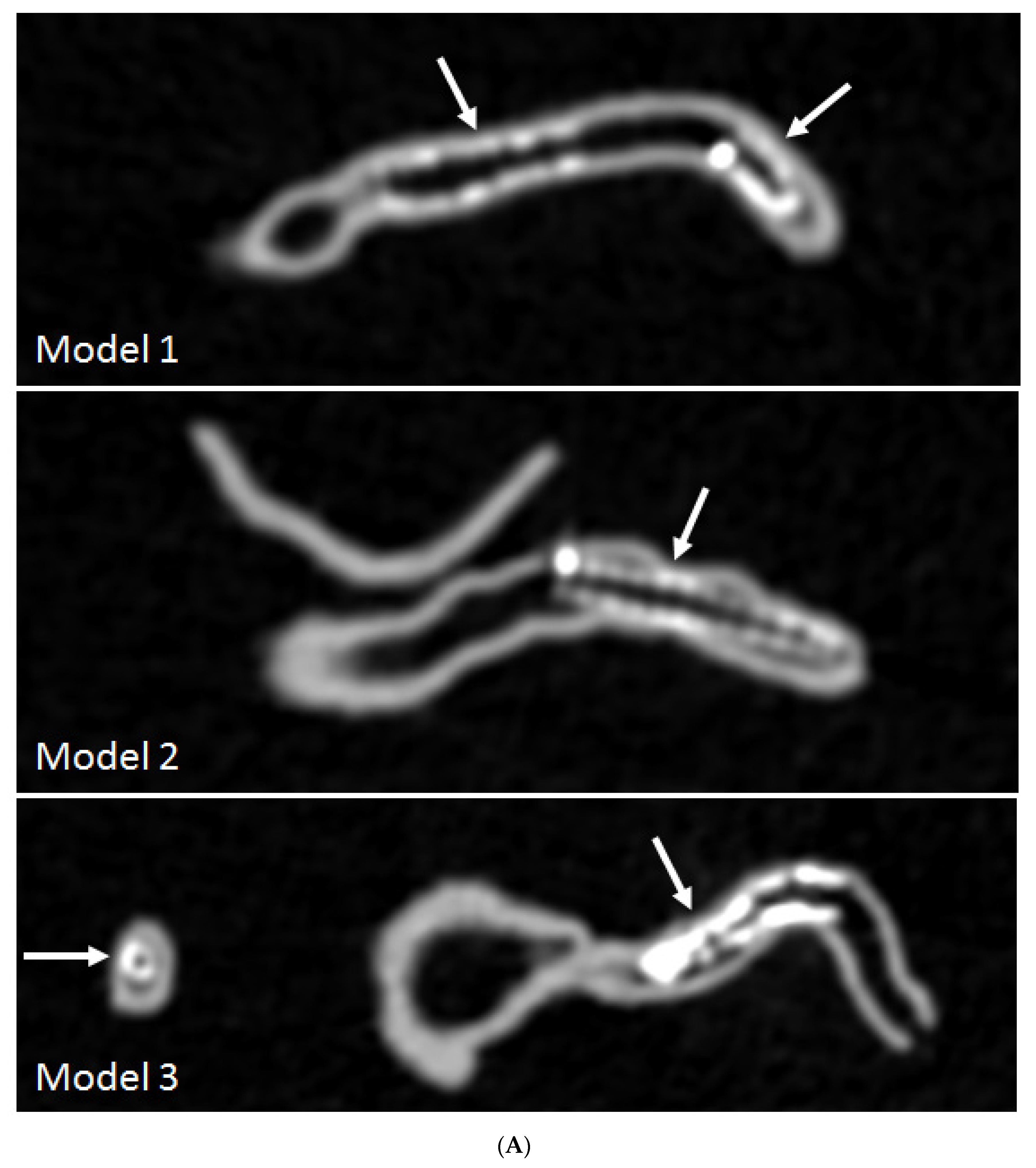
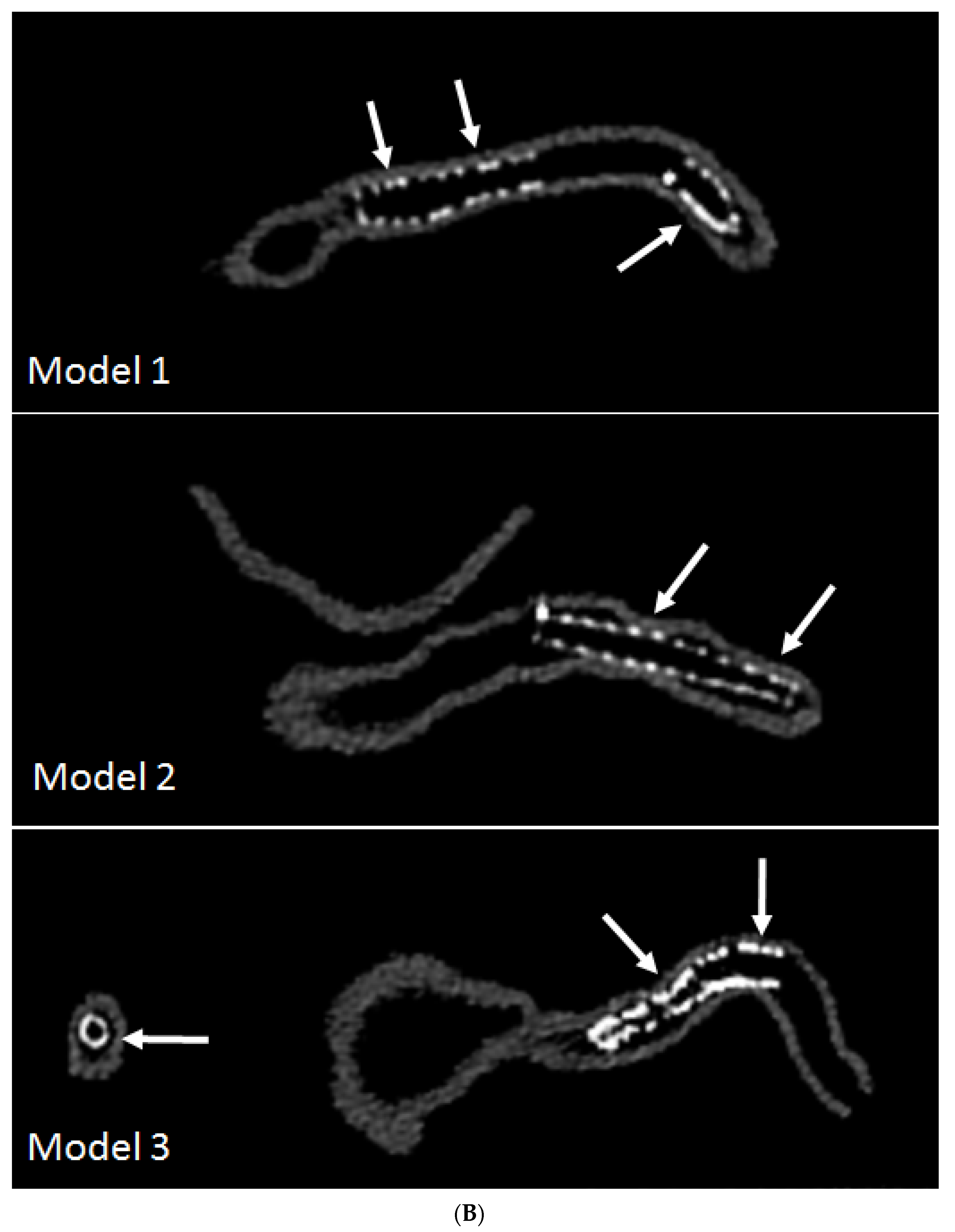
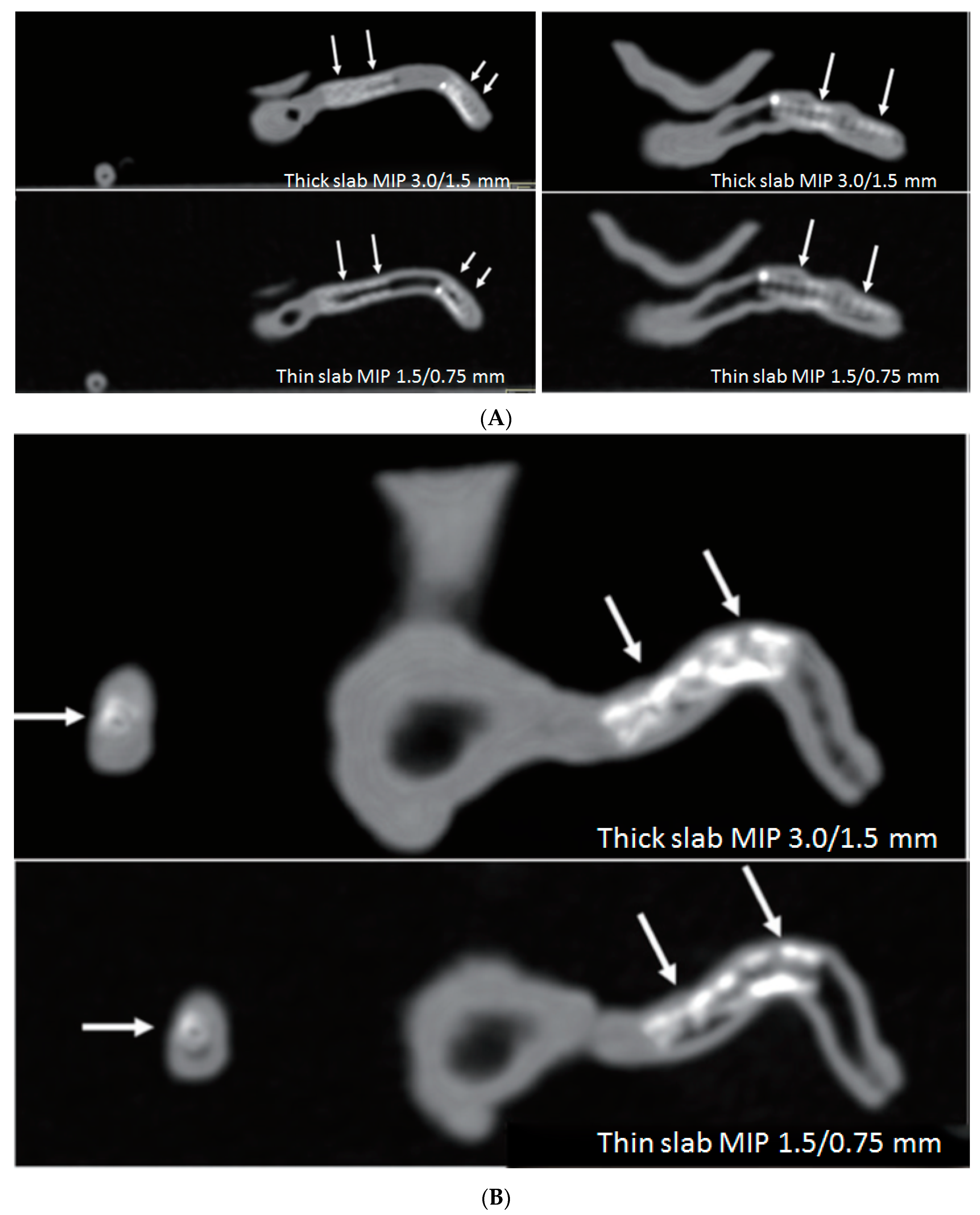
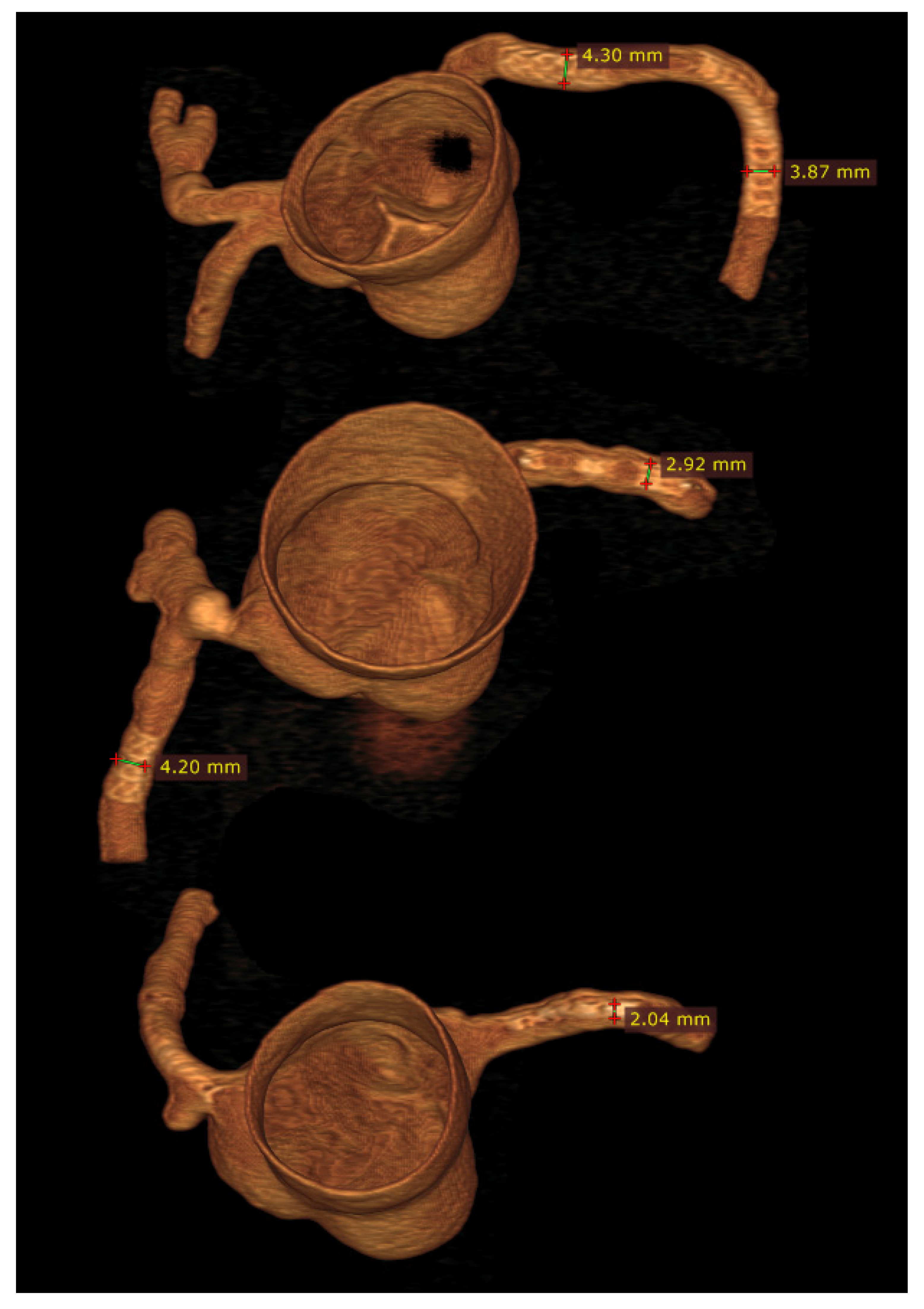
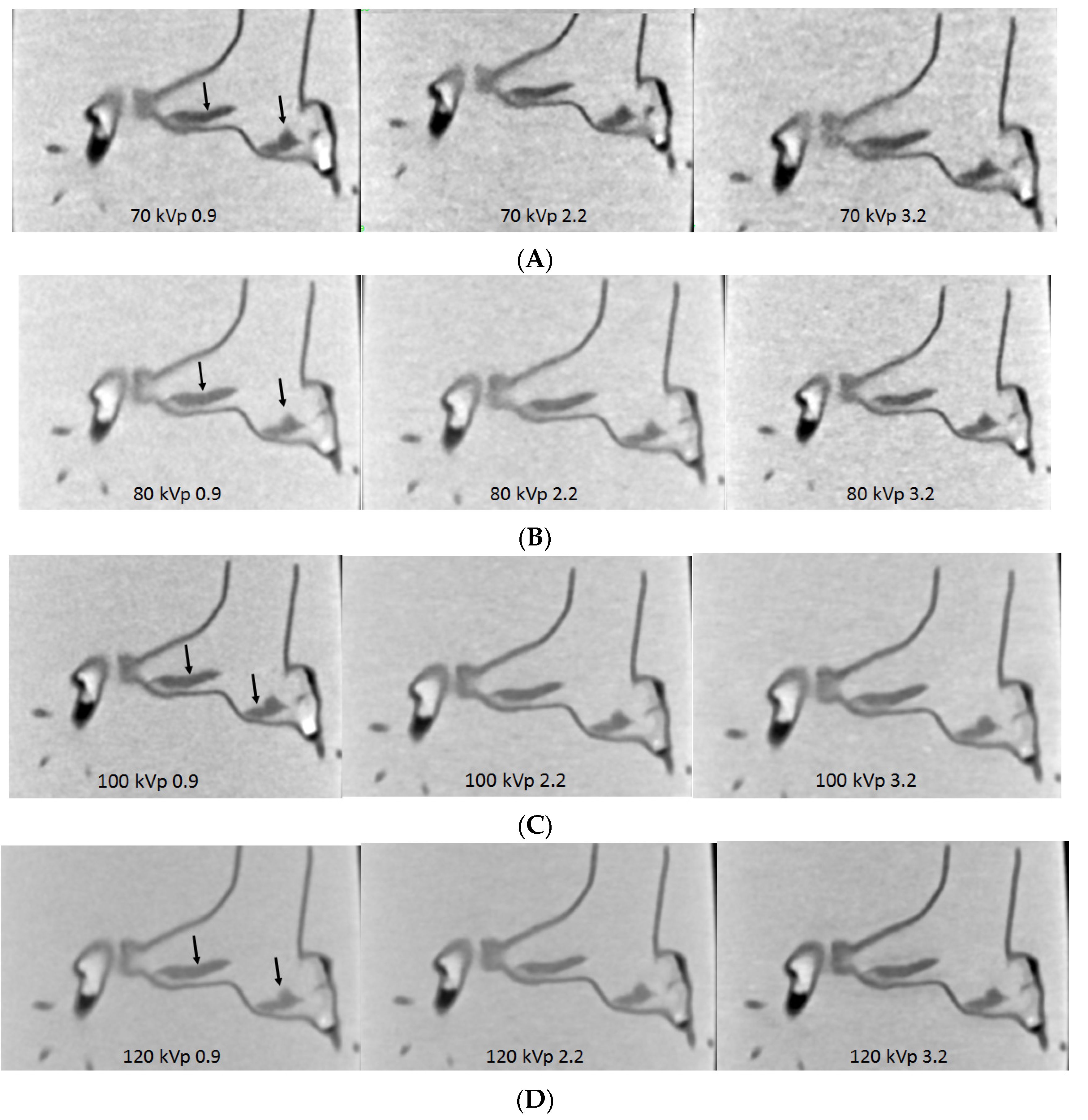
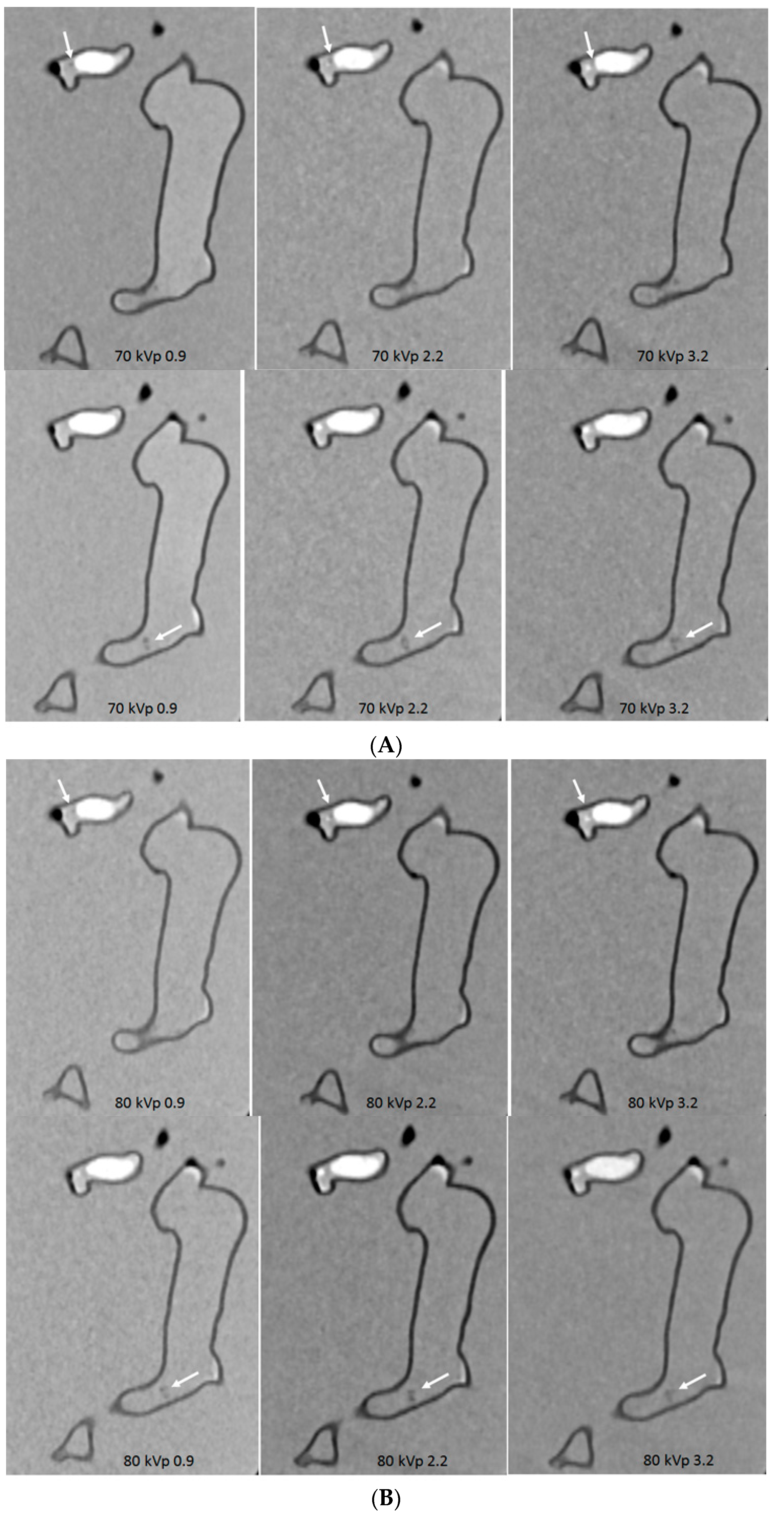
3.3. 3D Printing in Coronary Artery Disease
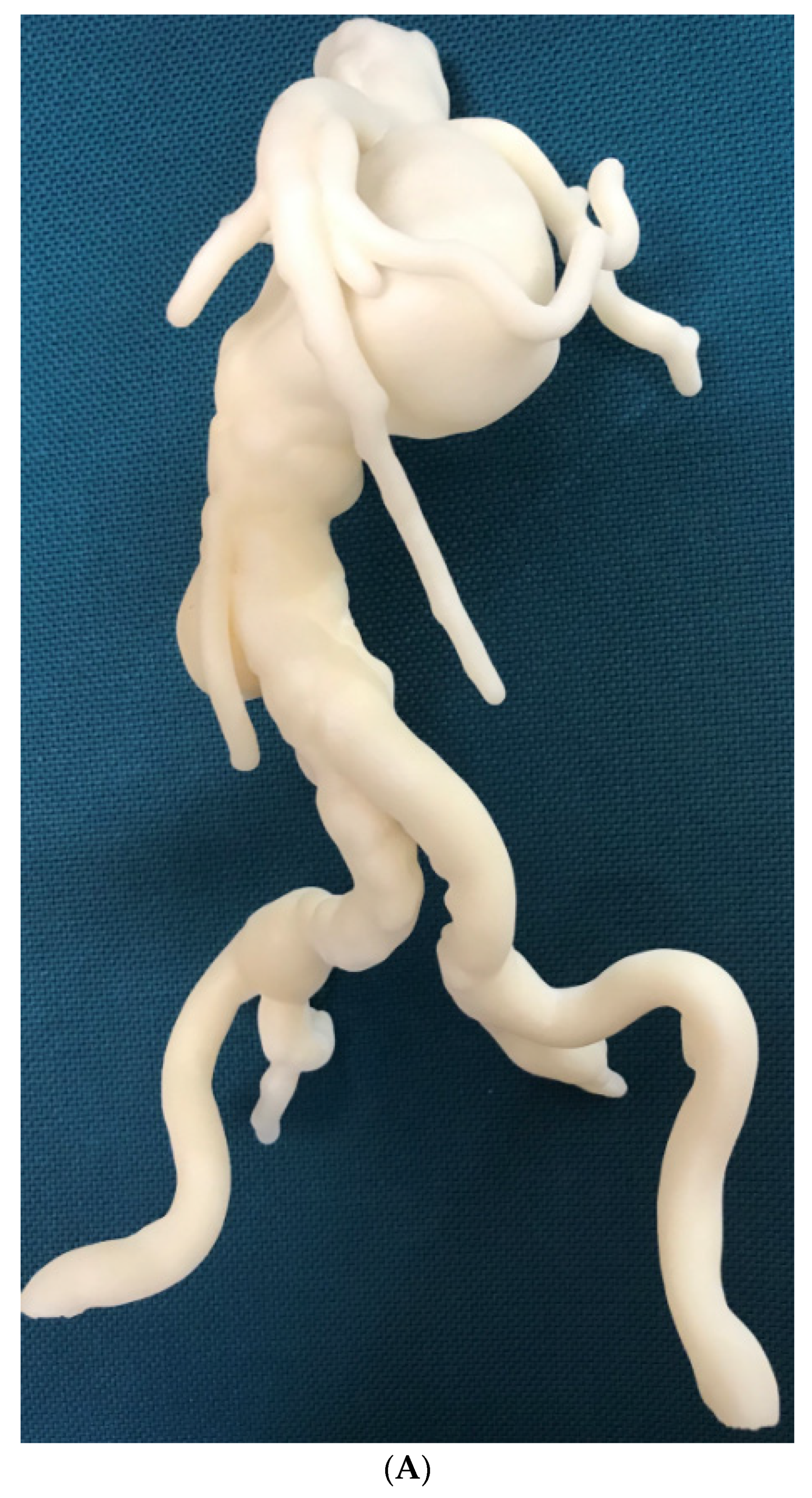
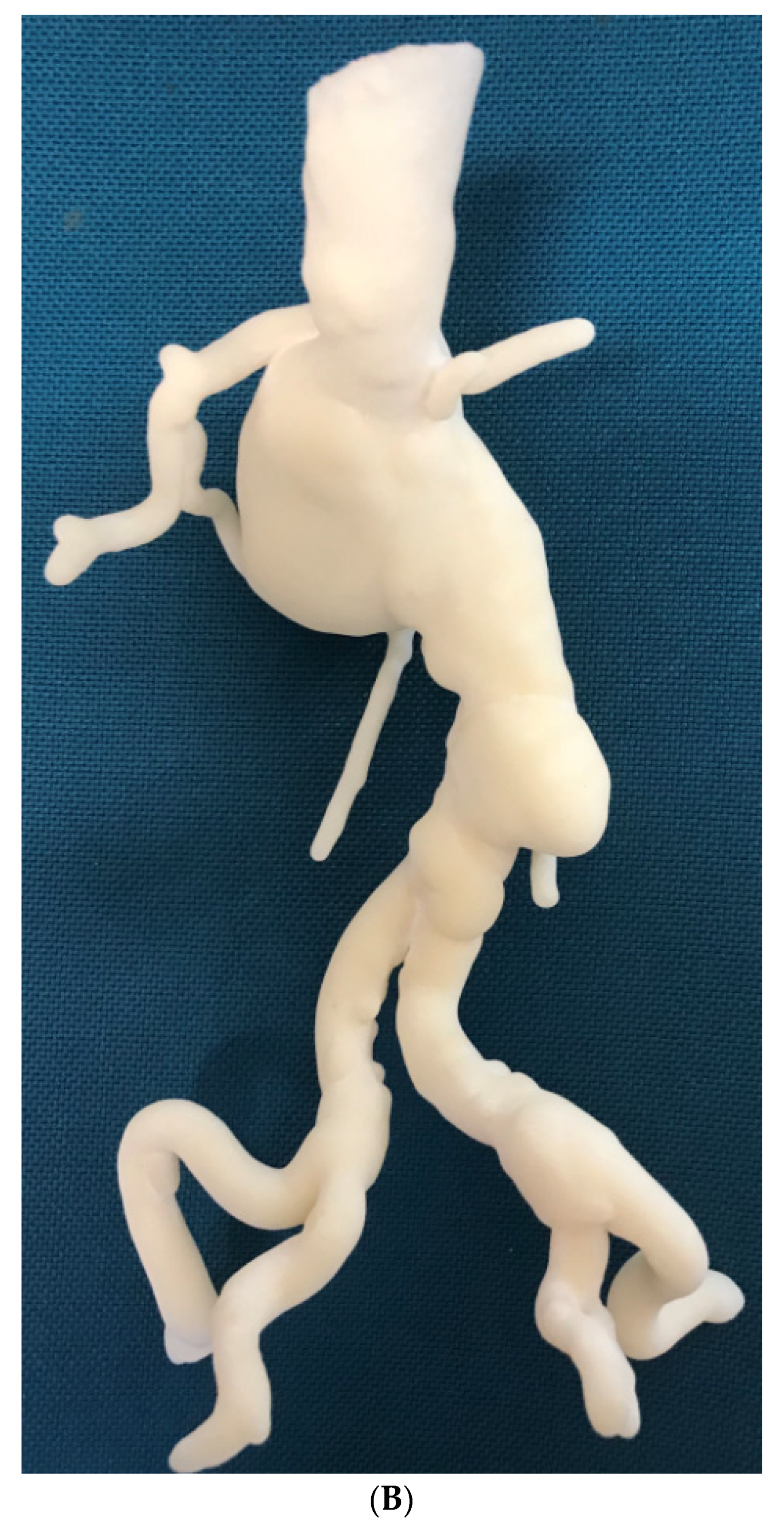
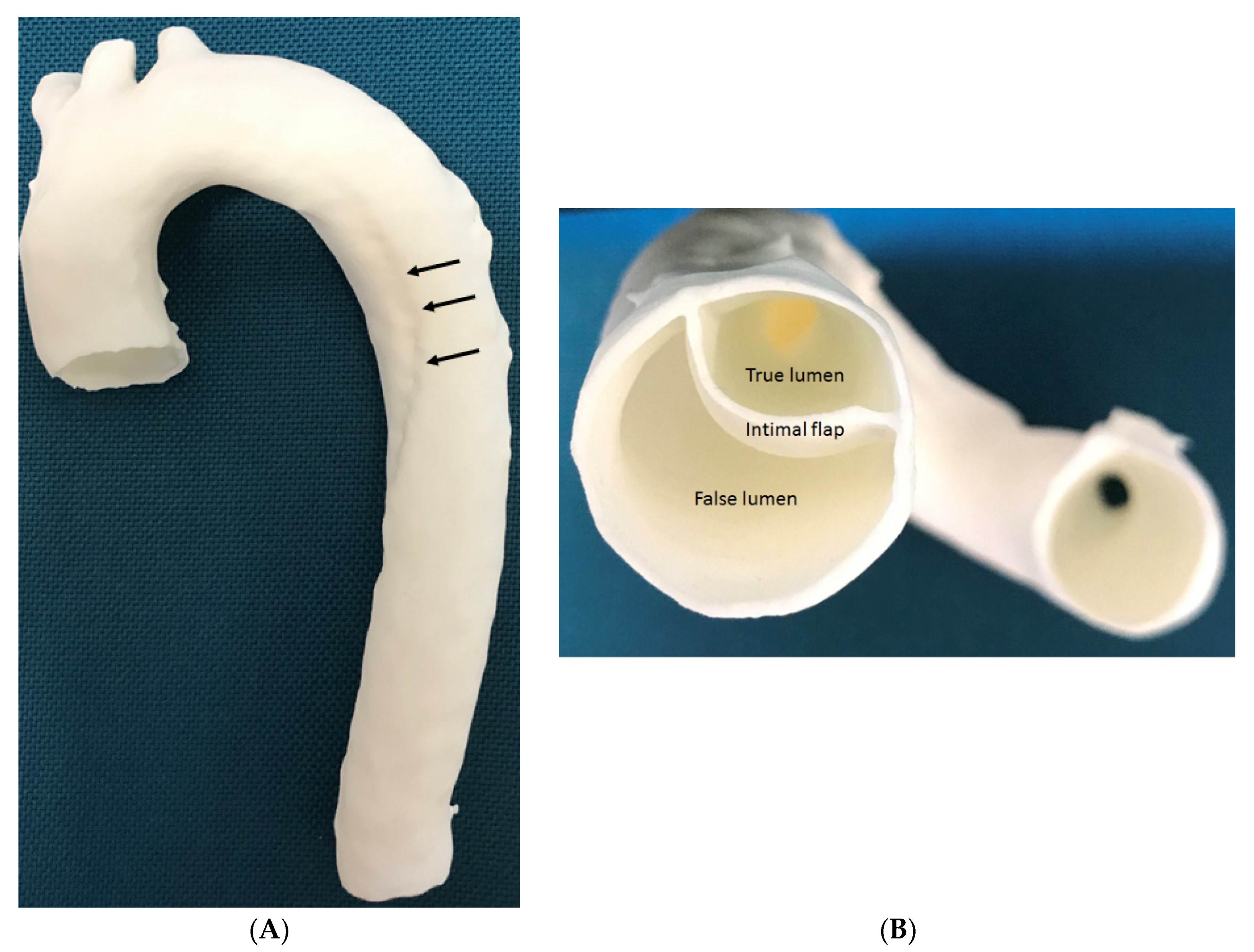
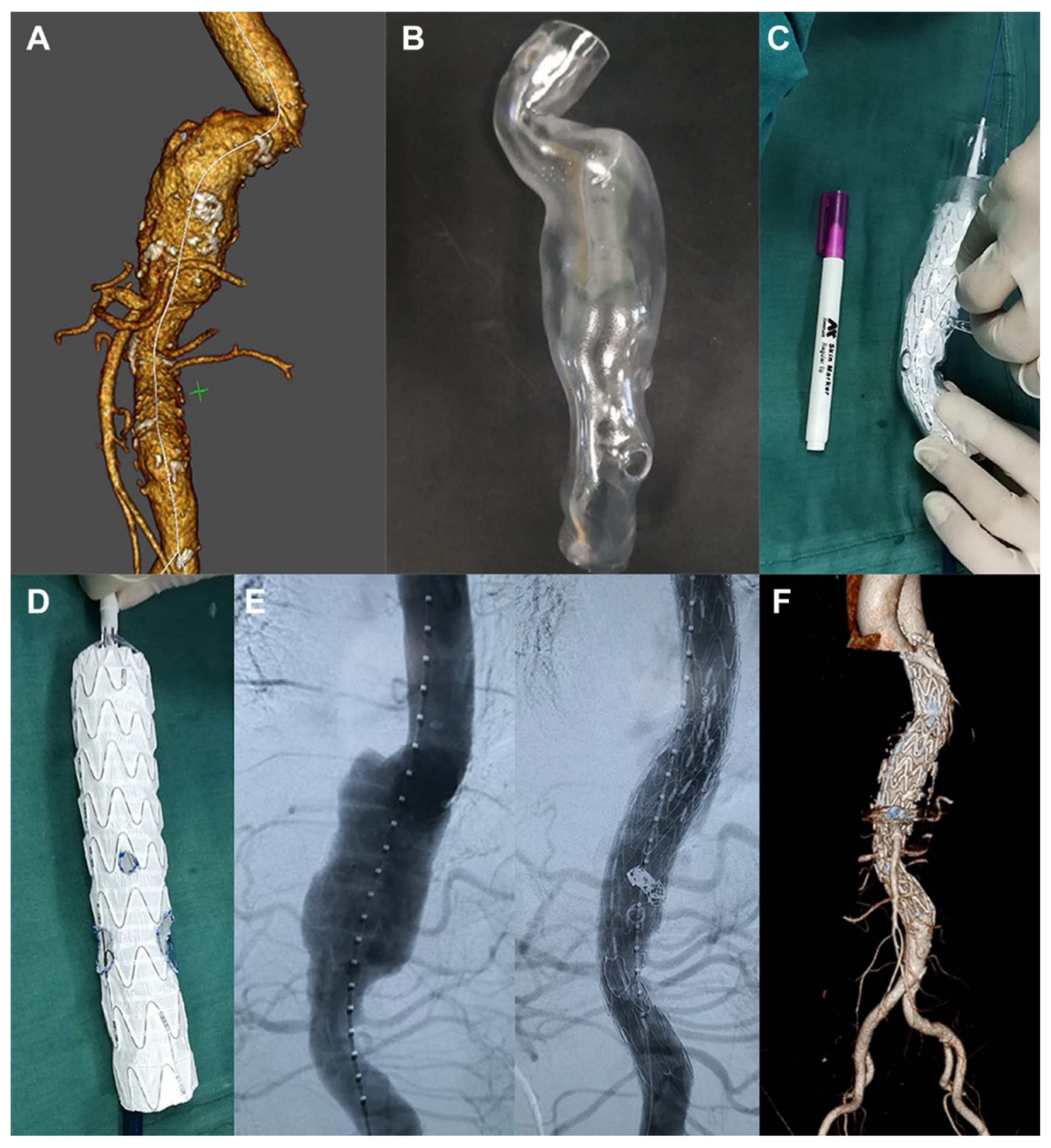
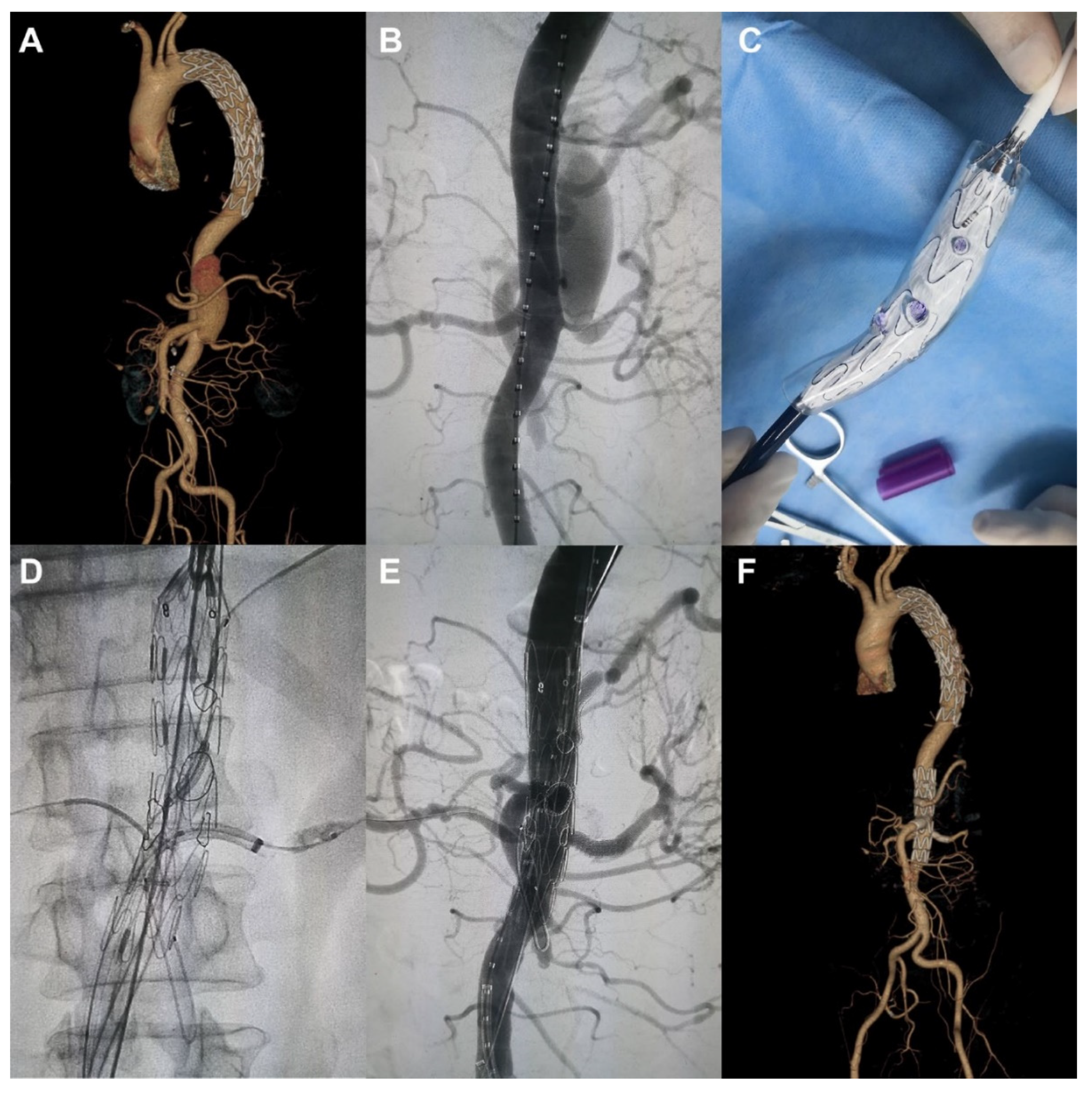
3.4. 3D Printing in Aortic Aneurysm and Aortic Dissection
3.5. 3D Printing in Pulmonary Artery Disease
3.6. 3D Bioprinting in Cardiovascular Disease
4. Limitations and Future Research Directions
5. Summary
Funding
Conflicts of Interest
References
- Kamio, T.; Hayashi, K.; Onda, T.; Takaki, T.; Shibahara, T.; Yakushiji, T.; Shibui, T.; Kato, H. Utilizing a low-cost desktop 3D printer to develop a “one-stop 3D printing lab” for oral and maxillofacial surgery and dentistry fields. 3D Print. Med. 2018, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Speranza, D.; Citro, D.; Padula, F.; Motyl, B.; Marcolin, F.; Cali, M.; Martorelli, M. Additive manufacturing techniques for the reconstruction of 3D fetal faces. Appl. Bionics Biomech. 2017, 2017, 9701762. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.; Plasencia, J.; Richardson, R.; Velez, D.; Nigro, J.J.; Pophal, S.; Frakes, D. 3D printing for congenital heart disease: A single site’s initial three-year experience. 3D Print. Med. 2018, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, A.A.; Steigner, M.L.; George, E.; Barile, M.; Hunsaker, A.R.; Rybicki, F.J.; Mitsouras, D. Cardiothoracic applications of 3-dimensional printing. J. Thorac. Imaging 2016, 31, 253–272. [Google Scholar] [CrossRef]
- Witowski, J.; Wake, N.; Grochowska, A.; Sun, Z.; Budzynski, A.; Major, P.; Popiela, T.J.; Pedziwiatr, M. Investigating accuracy of 3d printed liver models with computed tomography. Quant. Imaging Med. Surg. 2019, 9, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Javan, R.; Herrin, D.; Tangestanipoor, A. Understanding spatially complex segmental and branch anatomy using 3D printing: Liver, lung, prostate, coronary arteries, and circle of Willis. Acad. Radiol. 2016, 23, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Perica, E.; Sun, Z. Patient-specific three-dimensional printing for pre-surgical planning in hepatocellular carcinoma treatment. Quant. Imaging Med. Surg. 2017, 7, 668–677. [Google Scholar] [CrossRef]
- Costello, J.; Olivieri, L.; Krieger, A.; Thabit, O.; Marshall, M.B.; Yoo, S.J.; Kim, P.C.; Jonas, R.A.; Nath, D.S. Utilizing three-dimensional printing technology to assess the feasibility of high-fidelity synthetic ventricular septal defect models for simulation in medical education. World. J. Pediatr. Congenit. Heart Surg. 2014, 5, 421–426. [Google Scholar] [CrossRef]
- Costello, J.P.; Olivieri, L.J.; Su, L.; Krieger, A.; Alfares, F.; Thabit, O.; Marshall, M.B.; Yoo, S.J.; Kim, P.C.; Jonas, R.A.; et al. Incorporating three-dimensional printing into a simulation-based congenital heart disease and critical care training curriculum for resident physicians. Congenit. Heart Dis. 2015, 10, 185–190. [Google Scholar] [CrossRef]
- Javan, R.; Zeman, M. A prototype educational model for hepatobiliary interventions: Unveiling the role of graphic designers in medical 3D printing. J. Digit. Imaging 2018, 31, 133–143. [Google Scholar] [CrossRef]
- Sun, Z.; Lau, I.; Wong, Y.H.; Yeong, C.H. Personalized three-dimensional printed models in congenital heart disease. J. Clin. Med. 2019, 8, 522. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lee, S. A systematic review of 3D printing in cardiovascular and cerebrovascular diseases. Anatol. J. Cardiol. 2017, 17, 423–435. [Google Scholar] [PubMed]
- Lau, I.; Sun, Z. Dimensional accuracy and clinical value of 3D printed models in congenital heart disease: A systematic review and meta-analysis. J. Clin. Med. 2019, 8, 1483. [Google Scholar] [CrossRef] [PubMed]
- Lupulescu, C.; Sun, Z. A systematic review of the clinical value and applications of three-dimensional printing in renal surgery. J. Clin. Med. 2019, 8, 990. [Google Scholar] [CrossRef]
- Valverde, I.; Gomez-Ciriza, G.; Hussain, T.; Suarez-Mejias, C.; Velasco-Forte, M.N.; Byrne, N.; Ordonex, A.; Gonzalez-Calle, A.; Anderson, D.; Hazekamp, M.G.; et al. Three dimensional printed models for surgical planning of complex congenital heart defects: An international multicenter study. Eur. J. Cardio-Thorac. Surg. 2017, 52, 1139–1148. [Google Scholar] [CrossRef]
- White, S.C.; Sedler, J.; Jones, T.W.; Seckeler, M. Utility of three-dimensional models in resident education on simple and complex intracardiac congenital heart defects. Congenit. Heart Dis. 2018, 13, 1045–1049. [Google Scholar] [CrossRef]
- Loke, Y.H.; Harahsheh, A.S.; Krieger, A.; Olivier, L.J. Usage of 3D models of tetralogy of Fallot for medical education: Impact on learning congenital heart disease. BMC Med. Educ. 2017, 7, 54. [Google Scholar] [CrossRef]
- Su, W.; Xiao, Y.; He, S.; Huang, P.; Deng, X. Three-dimensional printing models in congenital heart disease education for medical students: A controlled comparative study. BMC Med. Educ. 2018, 18, 178. [Google Scholar] [CrossRef]
- Smerling, J.; Marboe, C.C.; Lefkowitch, J.H.; Pavilicova, M.; Bacha, E.; Einstein, A.J.; Naka, Y.; Glickstein, J.; Farooqi, K.M. Utility of 3D printed cardiac models for medical student education in congenital heart disease: Across a spectrum of disease severity. Pediatr. Cardiol. 2019, 40, 1258–1265. [Google Scholar] [CrossRef]
- Lim, K.H.; Loo, Z.Y.; Goldie, S.; Adams, J.; McMenamin, P. Use of 3D printed models in medical education: A randomized control trial comparing 3D prints versus cadaveric materials for learning external cardiac anatomy. Anat. Sci. Educ. 2016, 9, 213–221. [Google Scholar] [CrossRef]
- Chepelev, L.; Souza, C.; Althobaity, W.; Miguel, O.; Krishna, S.; Akyuz, E.; Hodgdon, T.; Torres, C.; Wake, N.; Alexander, A.; et al. Preoperative planning and tracheal stent design in thoracic surgery: A primer for the 2017 Radiological Society of North America (RSNA) hands-on course in 3D printing. 3D Print. Med. 2017, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Chepelev, L.; Wake, N.; Ryan, J.; Althobaity, W.; Gupta, A.; Arribas, E.; Santiago, L.; Ballard, D.; Wang, K.C.; Weadock, W.; et al. Radiological Society of North America (RSNA) 3D printing Special Interest Group (SIG): Guidelines for medical 3D printing and appropriateness for clinical scenarios. 3D Print. Med. 2018, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Bagaria, V.; Bhansali, R.; Pawar, P. 3D printing-creating a blueprint for the future of orthopedics: Current concept review and the road ahead! J. Clin. Orthol. Trauma 2018, 9, 207. [Google Scholar] [CrossRef] [PubMed]
- Skelley, N.W.; Hagerty, M.P.; Stannard, J.T.; Feltz, K.P.; Ma, R. Sterility of 3D-printed orthopedic implants using fused deposition modeling. Orthopedics 2020, 43, 46. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Chen, C.; Zhang, Y.; Zheng, W.; Chen, H.; Cai, L. Three-dimensional printing assisted ORIF versus conventional ORIF for tibial plateau fractures: A systematic review and meta-analysis. Int. J. Surg. 2018, 57, 35. [Google Scholar] [CrossRef]
- Anwar, S.; Singh, G.K.; Miller, J.; Sharma, M.; Manning, P.; Billadello, J.J.; Eghtesady, P.; Woodard, P.K. 3D printing is a transformative technology in congenital heart disease. JACC Basic Transl. Sci. 2018, 3, 294–312. [Google Scholar] [CrossRef]
- Perica, E.R.; Sun, Z. A systematic review of three-dimensional printing in liver disease. J. Digit. Imaging 2018, 31, 692–701. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, D. A systematic review of clinical value of three-dimensional printing in renal disease. Quant. Imaging Med. Surg. 2018, 8, 311–325. [Google Scholar] [CrossRef]
- Anwar, S.; Rockefeller, T.; Raptis, D.; Woodard, P.; Eghtesady, P. 3D printing provides a precise approach in the treatment of tetralogy of Fallot, pulmonary atresia with major aortopulmonary collateral arteries. Curr. Treat. Opt. Cardiovasc. Med. 2018, 20, 5. [Google Scholar] [CrossRef]
- Gallo, M.; D’Onofrio, A.; Tarantini, G.; Nocerino, E.; Remondino, F.; Gerosa, G. 3D-printing model for complex aortic transcatheter valve treatment. Int. J. Cardiol. 2016, 210, 139–140. [Google Scholar] [CrossRef]
- Godnell, J.; Pietila, T.; Samuel, B.P.; Kurup, H.K.N.; Haw, M.P.; Vettukattil, J.J. Integration of computed tomography and three-dimensional echocardiography for hybrid three-dimensional printing in congenital heart disease. J. Digit. Imaging 2016, 29, 665–669. [Google Scholar] [CrossRef]
- Ripley, B.; Kelil, T.; Cheezum, M.K.; Goncalves, A.; Di Carli, M.F.; Rybicki, F.J.; Steigner, M.; Mitsouras, D.; Blankstein, R. 3D printing based on cardiac CT assists anatomic visualization prior to transcatheter aortic valve replacement. J. Cardiovasc. Comput. Tomor. 2016, 10, 28–36. [Google Scholar] [CrossRef]
- Ferrari, E.; Gallo, M.; Wang, C.; Zhang, L.; Taramasso, M.; Maisano, F.; Pirelli, L.; Berdajs, D.; von Segesser, L.K. Three-dimensional planning in adult cardiovascular medicine for surgical and transcatheter procedural planning, teaching and technological innovation. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Vettukattil, J.J.; Nijres, B.M.; Gosnell, J.M.; Samuel, B.P.; Haw, M.P. Three-dimensional printing for surgical planning in complex congenital heart disease. J. Card. Surg. 2019, 34, 1363–1369. [Google Scholar] [CrossRef]
- Bhatla, P.; Tretter, J.T.; Chikkabyrappa, S.; Chakravarti, S.; Mosca, R.S. Surgical planning for a complex double-outlet right ventricle using 3D printing. Echocardiography 2017, 34, 802–804. [Google Scholar] [CrossRef]
- Biglino, G.; Koniordou, D.; Gasparini, M.; Capelli, C.; Leaver, L.K.; Khambadkone, S.; Schievano, S.; Taylor, A.M.; Wray, J. Piloting the use of patient-specific cardiac models as a novel tool to facilitate communication during clinical consultations. Pediatr. Cardiol. 2017, 38, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Riggs, K.W.; Dsouza, G.; Broderick, J.T.; Moore, R.A.; Morales, D.L.S. 3D-printed models optimize preoperative planning for pediatric cardiac tumor debulking. Transl. Pediatr. 2018, 7, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Abudayyeh, I.; Gordon, B.; Ansari, M.M.; Jutzy, K.; Stoletniy, L.; Hillard, A. A practical guide to cardiovas.cular 3D printing in clinical practice: Overview and examples. J. Interv. Cardiol. 2018, 31, 375–383. [Google Scholar] [CrossRef]
- Mitsourasy, D.; Liacouras, P.; Imanzadeh, A.; Giannopoulos, A.A.; Cai, T.; Kumamaru, K.K.; George, E.; Wake, N.; Caterson, E.J.; Pomahac, B.; et al. Medical 3D printing for the radiologist. Radiographics 2015, 35, 1965–1988. [Google Scholar] [CrossRef]
- Lau, I.; Sun, Z. Three-dimensional printing in congenital heart disease: A systematic review. J. Med. Radiat. Sci. 2018, 65, 226–236. [Google Scholar] [CrossRef]
- Xu, J.; Tian, Y.; Yin, J.; Wang, J.; Xu, W.; Shi, Z.; Fu, J.; Shu, Q. Utility of three-dimensional printing in preoperative planning for children with anomalous pulmonary venous connection: A single center experience. Quant. Imaging Med. Surg. 2019, 9, 1804–1814. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Y.; Luo, H.; Gao, C.; Zhang, J.; Dai, Y. Is a three-dimensional printing model better than a traditional cardiac model for medical education? A pilot randomized controlled study. Acta Cardiol. Sin. 2017, 33, 664–669. [Google Scholar] [PubMed]
- Biglino, G.; Capelli, C.; Wray, J.; Schievano, S.; Leaver, L.K.; Khambadkone, S.; Giardini, A.; Derrick, G.; Jones, A.; Taylor, A.M. 3D-manufactured patient-specific models of congenital heart defects for communication in clinical practice: Feasibility and acceptability. BMJ Open 2015, 5, e007165. [Google Scholar] [CrossRef] [PubMed]
- Biglino, G.; Capelli, C.; Koniordou, D.; Robertshaw, D.; Leaver, L.K.; Schievano, S.; Taylor, A.M.; Wray, J. Use of 3D models of congenital heart disease as an education tool for cardiac nurses. Congenit. Heart Dis. 2017, 12, 113–118. [Google Scholar] [CrossRef]
- Biglino, G.; Milano, E.G.; Capelli, C.; Wray, J.; Shearn, A.I.; Caputo, M.; Bucciarelli-Ducci, C.; Taylor, A.M.; Schievano, S. Three-dimensional printing in congenital heart disease: Considerations on training and clinical implementation from a teaching session. Int. J. Artif. Organs 2019, 42, 595–599. [Google Scholar] [CrossRef]
- Otton, J.M.; Birbara, N.S.; Hussain, T.; Greil, G.; Foley, T.A.; Pather, N. 3D printing from cardiovascular CT: A practical guide and review. Cardiovasc. Diagn. Ther. 2017, 7, 507–526. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, F.; Cheung, G.S.H.; Chan, A.K.Y.; Wang, D.D.; Lam, Y.Y.; Chow, M.C.W.; Leong, M.C.W.; Kam, K.K.H.; So, K.C.Y.; et al. Device sizing guided by echocardiography-based three-dimensional printing is associated with superior outcome after percutaneous left atrial appendage occlusion. J. Am. Soc. Echocardiogr. 2019, 32, 708–719.e1. [Google Scholar] [CrossRef]
- Hachulla, A.L.; Noble, S.; Guglielmi, G.; Agulleiro, D.; Muller, H.; Vallee, J.P. 3D-printed heart model to guide LAA closure: Useful in clinical practice? Eur. Radiol. 2019, 29, 251–258. [Google Scholar] [CrossRef]
- Hosny, A.; Dilley, J.D.; Kelil, T.; Mathur, M.; Dean, M.N.; Weaver, J.C.; Ripley, B. Pre-procedural fit-testing of TAVR valves using parametric modeling and 3D printing. J. Cardiovasc. Comput. Tomogr. 2019, 13, 21–30. [Google Scholar] [CrossRef]
- Tuncay, V.; van Ooijen, P.M.A. 3D printing for heart valve disease: A systematic review. Eur. Radiol. Exp. 2019, 3, 9. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Qin, T.; Xi, Z.; Sun, L.; Wu, H.; Li, D. 3D printing in adult cardiovascular surgery and interventions: A systematic review. J. Thorac Dis. 2020, 12, 3227–3237. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, C.; Abdel-Razek, O.; Fagan, S.; Pearce, N.; Furey, M.; Harris, S.; Bartellas, M.; Adams, C. Three-dimensional printing for assessment of paravalvular leak in transcatheter aortic valve implantation. J. Cardiothorac. Surg. 2020, 15, 211. [Google Scholar] [CrossRef] [PubMed]
- Haghiashtiani, G.; Qiu, K.; Zhingre Sanchez, J.D.; Fuenning, Z.J.; Nair, P.; Ahlbeeg, S.E.; Iaizzo, P.A.; McAlpine, M.C. 3D printed patient-specific aortic root models with internal sensors for minimally invasive applications. Sci. Adv. 2020, 6, eabb4641. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Walters, H.L.; Kobayashi, D. Utilisation of a three-dimensional printed model for the management of coronary-pulmonary artery fistula from left main coronary artery. Cardiol. Young 2019, 29, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Santos, M.; Oliveira Santos, E.; Marinho, A.V.; Leite, L.; Guardado, J.; Matos, V.; Pego, G.M.; Marques, J.S. Patient-specific 3D printing simulation to guide complex coronary intervention. Rev. Port. Cardiol. 2018, 37, e1–e541. [Google Scholar] [CrossRef]
- Aroney, N.; Lau, K.; Danielle, L.; Burstow, D.; Walters, D. Three-dimensional printing: To guide management of a right coronary artery to left ventricular fistula. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 268. [Google Scholar] [CrossRef]
- Velasco Forte, M.N.; Byrne, N.; Valverde Perez, I.; Bell, A.; Gómez-Ciriza, G.; Krasemann, T.; Sievert, H.; Simpson, J.; Pushparajah, K.; Razavi, R.; et al. 3D printed models in patients with coronary artery fistulae: Anatomical assessment and interventional planning. EuroIntervention 2017, 13, e1080–e1083. [Google Scholar] [CrossRef]
- Lee, M.; Moharem-Elgamal, S.; Beckingham, R.; Hamilton, M.; Manghat, N.; Milano, E.G.; Bucciarelli-Ducci, C.; Caputo, M.; Biglino, G. Evaluating 3D-printed models of coronary anomalies: A survey among clinicians and researchers at a university hospital in the UK. BMJ Open 2019, 9, e025227. [Google Scholar] [CrossRef]
- Abdullah, K.A.; McEntee, M.F.; Reed, W.; Kench, P.L. Development of an organ-specific insert phantom generated using a 3D printer for investigations of cardiac computed tomography protocols. J. Med. Radiat. Sci. 2018, 65, 175–183. [Google Scholar] [CrossRef]
- Sun, Z. Personalized three-dimensional printed coronary artery models for accurate assessment of coronary stenosis using high resolution imaging. Australas. Med. J. 2019, 12, 105–109. [Google Scholar] [CrossRef]
- Sun, Z.; Ng, C.K.; Squelch, A. Synchrotron radiation computed tomography assessment of calcified plaques and coronary stenosis with different slice thicknesses and beam energies on 3D printed coronary models. Quant. Imaging Med. Surg. 2019, 9, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z. 3D printed coronary models offer new opportunities for developing optimal coronary CT angiography protocols in imaging coronary stents. Quant. Imaging Med. Surg. 2019, 9, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Jansen, S. Personalized 3D printed coronary models in coronary stenting. Quant. Imaging Med. Surg. 2019, 9, 1356–1367. [Google Scholar] [CrossRef] [PubMed]
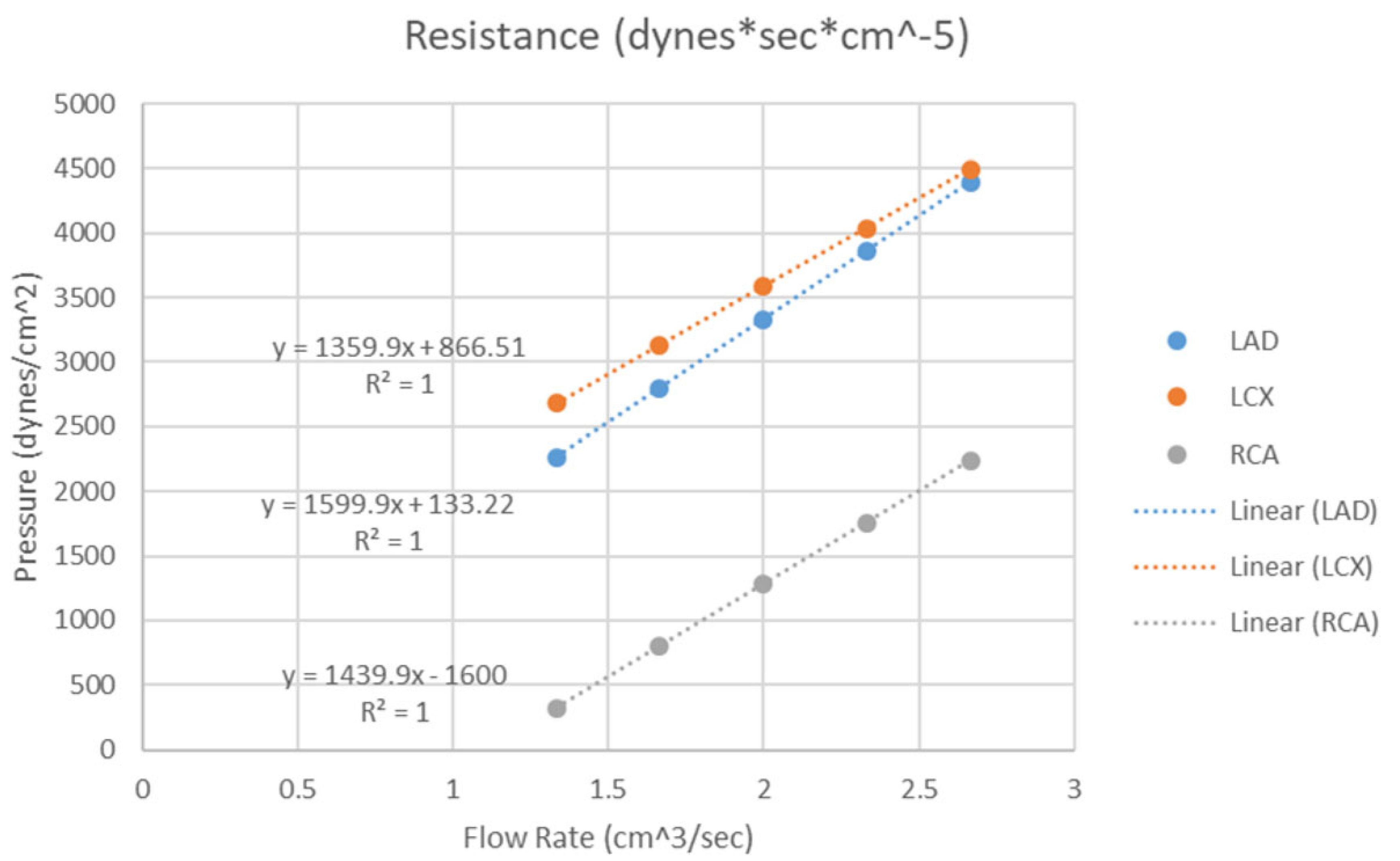
- Sommer, K.N.; Lyer, V.; Kumamaru, K.K.; Rava, R.A.; Ionita, C.N. Method to simulate distal flow resistance in coronary arteries in 3D printed patient specific coronary models. 3D Print. Med. 2020, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Squelch, A.; Sun, Z. Modelling of aortic aneurysm and aortic dissection through 3D printing. J. Med. Radiat. Sci. 2017, 64, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Squelch, A. Patient-specific 3D printed models of aortic aneurysm and aortic dissection. J. Med. Imaging Health Inf. 2017, 7, 886–889. [Google Scholar] [CrossRef]
- Hossien, A.; Gesomino, S.; Maessen, J.; Autschbach, R. The interactive use of multi-dimensional modeling and 3D printing in preplanning of type A aortic dissection. J. Card. Surg. 2016, 31, 441–445. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Zheng, X.; Rong, X.; Zheng, X.; Peng, H.; Silber-Li, Z.; Li, M.; Liu, L. Three-dimensional virtual surgery models for percutaneous coronary intervention (PCI) optimization strategies. Sci. Rep. 2015, 5, 10945. [Google Scholar] [CrossRef]
- Huang, J.; Li, G.; Wang, W.; Wu, K.; Le, T. 3D printing guiding stent graft fenestration: A novel technique for fenestration in endovascular aneurysm repair. Vascular 2017, 25, 442–446. [Google Scholar] [CrossRef]
- Tam, M.D.; Laycock, S.D.; Brown, J.R.; Jakeways, M. 3D printing of an aortic aneurysm to facilitate decision making and device selection for endovascular aneurysm repair in complex neck anatomy. J. Endovasc. Ther. 2013, 20, 863–867. [Google Scholar] [CrossRef]
- Bortman, J.; Mahmood, F.; Schermerhorn, M.; Lo, R.; Swerdlow, N.; Mahmood, F.; Matyal, R. Use of 3-dimensional printing to create patient-specific abdominal aortic aneurysm models for preoperative planning. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1442–1446. [Google Scholar] [CrossRef] [PubMed]
- Mitsuoka, H.; Terai, Y.; Miyano, Y.; Naitou, T.; Tanai, J.; Kawaguchi, S.; Goto, S.; Miura, Y.; Nakai, M.; Yamazaki, F. Preoperative planning for physician-modified endografts using a three-dimensional printer. Ann. Vasc. Dis. 2019, 12, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Rynio, P.; Kazimierczak, A.; Jedrzejczak, T.; Gutowski, P. A 3-dimensional printed aortic arch template to facilitate the creation of physician-modified stent-grafts. J. Endovasc. Ther. 2018, 25, 554–558. [Google Scholar] [CrossRef]
- Rynio, P.; Kazimierczak, A.; Jedrzejczak, T.; Gutowski, P. A 3-dimensional printed aortic arch template to facilitate decision-making regarding the use of an externalized transapical wire during thoracic endovascular aneurysm repair. Ann. Vasc. Surg. 2019, 54, 336.e5–336.e8. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.A.; Chan, Y.C.; Law, Y.; Cheng, S.W.K. The role of three-dimensional printing in contemporary vascular and endovascular surgery: A systematic review. Ann. Vasc. Surg. 2018, 53, 243–254. [Google Scholar] [CrossRef]
- Karkkainen, J.M.; Sandri, G.; Tenorio, E.R.; Alexander, A.; Bjellum, K.; Matsumoto, J.; Morris, J.; Mendes, B.C.; DeMartino, R.R.; Oderich, G.S. Simulation of endovascular aortic repair using 3D printed abdominal aortic aneurysm model and fluid pump. Cardiovasc. Interv. Radiol. 2019, 42, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.H.; Yu, T.; Zhou, M.J.; Liu, C.; Zhou, M.; Jiang, Q.; Liu, C.J.; Li, X.Q.; Liu, Z. Use of 3D printing to guide generation of fenestrations in physian-modified stent-grafts for treatment of thoracoabdominal aortic disease. J. Endovasc. Ther. 2020, 27, L385–L393. [Google Scholar] [CrossRef]
- Olivieri, L.; Krieger, A.; Chen, M.Y.; Kim, P.; Kanter, J.P. 3D heart model guides complex stent angioplasty of pulmonary venous baffle obstruction in a Mustard repair of D-TGA. Int. J. Cardiol. 2014, 172, e297–e298. [Google Scholar] [CrossRef]
- Witowski, J.; Darocha, S.; Kownacki, L.; Pietrasik, A.; Pietura, R.; Banaszkiewicz, M.; Kaminski, J.; Biederman, A.; Torbicki, A.; Kurzyna, M. Augmented reality and three-dimensional printing in percutaneous interventions on pulmonary arteries. Quant. Imaging Med. Surg. 2019, 9, 23–29. [Google Scholar] [CrossRef]
- Schievano, S.; Migliavacca, F.; Coats, L.; Khambadkone, S.; Carminati, M.; Wilson, N.; Deanfield, J.E.; Bonhoeffer, P.; Taylor, A.M. Percutaneous pulmonary valve implantation based on rapid prototyping of right ventricular outflow tract and pulmonary trunk from MR data. Radiology 2007, 242, 490–497. [Google Scholar] [CrossRef]
- Aldosari, S.; Squelch, A.; Sun, Z. Patient-specific 3D printed pulmonary artery model: A preliminary study. Digit. Med. 2017, 3, 170–177. [Google Scholar]
- Sun, Z.; Aldosari, S. Three-dimensional printing in medicine: Opportunities for development of optimal CT scanning protocols. Australas. Med. J. 2018, 11, 529–532. [Google Scholar] [CrossRef]
- Aldosari, S.; Jansen, S.; Sun, Z. Optimization of computed tomography pulmonary angiography protocols using 3D printed model with simulation of pulmonary embolism. Quant. Imaging Med. Surg. 2019, 9, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Aldosari, S.; Jansen, S.; Sun, Z. Patient-specific 3D printed pulmonary artery model with simulation of peripheral pulmonary embolism for developing optimal computed tomography pulmonary angiography protocols. Quant. Imaging Med. Surg. 2019, 9, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Dhanani, J.; Pang, G.; Pincus, J.; Ahern, B.; Goodwin, W.; Cowling, N.; Whitten, G.; Abdul-Aziz, M.H.; Martin, S.; Corke, P.; et al. Increasing ventilator surge capacity in COVID 19 pandemic: Design, manufacture and in vitro-in vivo testing in anaesthetized healthy pigs of a rapid prototyped mechanical ventilator. BMC Res. Notes 2020, 13, 421. [Google Scholar] [CrossRef] [PubMed]
- Lai, B.K.; Erian, J.L.; Pew, S.H.; Echmann, M.S. Emergency open-source three-dimensional printable ventilator circuit splitter and flow regulator during the COVID-19 pandemic. Anesthesiology 2020, 133, 246–248. [Google Scholar] [CrossRef]
- Manero, A.; Smith, P.; Koontz, A.; Dombroski, M.; Sparkman, J.; Courbin, D.; Chi, A. Leveraging 3D printing capacity in times of crisis: Recommendations for COVID-19 distributed manufacturing for medical equipment rapid response. Int. J. Environ. Res. Public Health 2020, 17, 4634. [Google Scholar] [CrossRef]
- Iyengar, K.; Bahl, S.; Vaishya, R.; Vaish, A. Challenges and solutions in meeting up the urgent requirement of ventilators for COVID-19 patients. Diabetes Metab. Syndr. 2020, 14, 499–501. [Google Scholar] [CrossRef]
- Zhang, N.; Xu, Z.; Zhou, L.Y.; Chen, G.; Li, Y.; Yin, H.; Sun, Z. Clinical characteristics and chest CT imaging features of critically ill COVID-19 patients. Eur. Radiol. 2020, 30, 6151–6160. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, N.; Li, Y.; Xu, X. A systematic review of chest imaging findings in COVID-19. Quant. Imaging Med. Surg. 2020, 10, 1058–1079. [Google Scholar] [CrossRef]
- Sun, Z. Diagnostic value of chest CT in coronavirus disease 2019 (COVID-19). Curr. Med. Imaging 2020, 16, 274–275. [Google Scholar] [CrossRef] [PubMed]
- De Smet, K.; De Smet, D.; Rychaert, T.; Laridon, E.; Heremans, B.; Vandenbulcke, R.; Demedts, I.; Bouchaert, B.; Gryspeerdt, S.; Martens, G.A. Diagnostic performance of chest CT for SARS-CoV-2 infection in individuals with or without COVID-19 symptoms. Radiology 2020, 202708, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Sun, Z. Clinical outcomes of COVID-19 in elderly male patients. J. Geriatr. Cardiol. 2020, 17, 243–245. [Google Scholar] [PubMed]
- Frattini, S.; Maccagni, G.; Italia, L.; Metra, M.; Danzi, G.B. Coronavirus disease 2019 and cardiovascular implications. J. Cardiovasc. Med. 2020, 21, 725–732. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Ma, Y.T.; Zhang, J.Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef]
- Alwaqfi, N.R.; Ibrahim, K.S. COVID-19: An update and cardiac involvement. J. Cardiothorac. Surg. 2020, 15, 239. [Google Scholar] [CrossRef]
- Tarfaoui, M.; Nachtane, M.; Goda, I.; Qureshi, Y.; Benyahia, H. 3D printing to support the shortage in personal protective equipment caused by COVID-19 pandemic. Materials 2020, 13, 3339. [Google Scholar] [CrossRef]
- Lewis, P.L.; Green, R.M.; Shah, R.N. 3D-printed gelatin scaffolds of differing pore geometry modulate hepatocyte function and gene expression. Acta Biomater. 2018, 69, 63–70. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, Y.J.; Yong, W.J.; Pati, F.; Shim, J.H.; Kang, K.S.; Kang, I.H.; Park, J.; Cho, D.W. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication 2017, 8, 015007. [Google Scholar] [CrossRef]
- Faulkner-Jones, A.; Fyfe, A.C.; Cornelissen, D.J.; Gardner, J.; King, J.; Courtney, A.; Shu, W. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication 2015, 7, 044102. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Kang, K.; Park, S.A.; Kim, W.D.; Paik, S.S.; Lee, S.H.; Jeong, J.; Choi, D. Generation of multilayered 3D structures of HepG2 cells using a bio-printing technique. Gut Liver 2017, 11, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Alblawi, A.; Ranjani, A.S.; Yasmin, H.; Gupta, S.; Bit, A.; Rahim-Gorji, M. Scaffold-free: A developing technique in field of tissue engineering. Comput. Methods Programs Biomed. 2020, 185, 105148. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater. 2020, 101, 26–42. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482. [Google Scholar] [CrossRef]
- Puluca, N.; Lee, S.; Doppler, S.; Munsterer, A.; DreBen, M.; Krane, M.; Wu, S.M. Bioprinting approaches to engineering vascularized 3D cardiac tissues. Curr. Cardiol. Rep. 2019, 21, 90. [Google Scholar] [CrossRef]
- Tirella, A.; Orsini, A.; Vozzi, G.; Ahluwalia, A. A phase diagram for microfabrication of geometrically controlled hydrogel scaffolds. Biofabrication 2009, 12, 045002. [Google Scholar] [CrossRef]
- Gudapati, H.; Dey, M.; Ozbolat, I. A comprehensive review on droplet-based bioprinting: Past, present and future. Biomaterials 2016, 102, 20–42. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.H.; Kim, S.W.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef]
- Miller, J.S.; Stevens, K.R.; Yang, M.T.; Baker, B.M.; Nguyen, D.-H.T.; Cohen, D.M.; Toro, E.; Chen, A.A.; Galie, P.A.; Yu, X.; et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012, 11, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef] [PubMed]
- Skylar-Scott, M.A.; Gunasekaran, S.; Lewis, J.A. Laser-assisted direct ink writing of planar and 3D metal architectures. Proc. Natl. Acad. Sci. USA 2016, 113, 6137–6142. [Google Scholar] [CrossRef] [PubMed]
- Brandenberg, N.; Lutolf, M.P. In Situ Patterning of Microfluidic Networks in 3D Cell-Laden Hydrogels. Adv. Mater. 2016, 28, 7450–7456. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Park, H.J.; Kim, S.W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H.; et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Redd, M.A.; Zeinstra, N.; Qin, W.; Wei, W.; Martinson, A.; Wang, Y.; Wang, R.K.; Murry, C.E.; Zheng, Y. Patterned human microvascular grafts enable rapid vascularization and increase perfusion in infarcted rat hearts. Nat. Commun. 2019, 10, 584. [Google Scholar] [CrossRef]
- Hynes, W.F.; Pepona, M.; Robertson, C.; Alvarado, J.; Dubbin, K.; Triplett, M.; Adorno, J.J.; Randles, A.; Moya, M.L. Examining metastatic behaviour within 3D bioprinted vasculature for the validation of a 3D computational flow model. Sci. Adv. 2020, 6, eabb3308. [Google Scholar] [CrossRef]
- Illmann, C.F.; Ghadiry-Tavi, R.; Hosking, M.; Harris, K.C. Utility of 3D printed cardiac models in congenital heart disease: A scoping review. Heart 2020, 106, 1631–1637. [Google Scholar] [CrossRef]
- Stepniak, K.; Ursani, A.; Paul, N.; Naguib, H. Novel 3D printing technology for CT phantom coronary arteries with high geometrical accuracy for biomedical imaging applications. Bioprinting 2020, 18, e00074. [Google Scholar] [CrossRef]
- Qiu, T.; Jiang, W.; Yan, P.; Jiao, L.; Wang, X. Development of 3D-printed sulphated chitosan modified bioresorbable stents for coronary artery disease. Front. Bioeng. Biotechnol. 2020, 8, 462. [Google Scholar] [CrossRef]
- Guerra, A.J.; Cano, P.; Rabionet, M.; Puig, T.; Ciurana, J. 3D-printed PCL/PLA composite stents: Towards a new solution to cardiovascular problems. Materials 2018, 11, 1679. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Zhang, J.; Prestwich, G.D. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials 2010, 31, 6173. [Google Scholar] [CrossRef] [PubMed]
- Beyersdorf, F. Three-dimensional bioprinting: New horizon for cardiac surgery. Eur. J. Cardiothorac. Surg. 2014, 46, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Wei, Y.; Hou, W.; Tong, H.; Bai, S. 3D bioprinting technologies for hard tissue and organ engineering. Materials 2016, 9, 802. [Google Scholar] [CrossRef]
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivi, M.; Maiullari, S.; Rainer, A.; Baci, D.; El-Sayed Marei, H.; Seliktar, D.; et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 2018, 8, 13532. [Google Scholar] [CrossRef]
- Duan, B.; Kapetanovic, E.; Hockaday, L.A.; Butcher, J.T. Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater. 2014, 10, 1836. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent advances in bioprinting techniques: Approaches, applications and future prospects. J. Transl. Med. 2016, 14, 271. [Google Scholar] [CrossRef]




| Types of Bioprinters | Biomaterials | Cell Viability/Resolution | Bioprinting Speed | Cost | Advantages | Disadvantages |
|---|---|---|---|---|---|---|
| Inkjet-based bioprinting | Low-viscosity suspension of living cells; biomolecules; growth factors | ~90% 20–100 μm | Fast (<10,000 droplets/s) | Low | Wide availability; low cost; high resolution; high printing speed; ability to introduce concentration gradients in 3D constructs | Poor vertical structure clogging characteristics; thermal and mechanical stress to cells; limited printable materials (liquid only) |
| Pressure-assisted bioprinting | Hydrogel; melt; cells; proteins and ceramic materials; solutions, pastes, or dispersions of low to high viscosity; Poly Lactic-co-Glycolic Acid (PLGA); tricalcium phosphate (TCP); collagen and chitosan; collagenalginate-silica composites coated with HA; and agarose with gelatin | 40–80% 200 μm | Slow | Medium | Numerous materials that can be printed with any dimensions; mild conditions (room temperature); use of cellular spheroids; direct incorporation of cells; and homogenous distribution of cells | Limited mechanical stiffness; critical timing of gelation time; specific matching of the densities of the material and the liquid medium to preserve shapes; low resolution and viability |
| Laser-assisted bioprinting | Hydrogel, media, cells, proteins and ceramic materials of varying viscosity | >95% >20 μm | Medium | High | Nozzle-free, noncontact process; cells are printed with high activity and high resolution; high control of ink droplets and precise delivery | High cost; cumbersome and time consuming; requires a metal film and thus is subject to metallic particle contamination |
| Stereolithography | Light-sensitive polymer materials; curable acrylics and epoxies | >90% ~1.2–200 μm | Fast (<40,000 mm/s) | Low | Solid freeform and nozzle-free technology; highest fabrication accuracy; compatibility with an increasing number of materials; light-sensitive hydrogels can be printed layer-by-layer | Applicable to photopolymers only; lack of biocompatible and biodegradable polymers; harmful effects from residual toxic photo-curing reagents; possibility of harm to DNA and human skin by ultraviolet (UV) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z. Clinical Applications of Patient-Specific 3D Printed Models in Cardiovascular Disease: Current Status and Future Directions. Biomolecules 2020, 10, 1577. https://doi.org/10.3390/biom10111577
Sun Z. Clinical Applications of Patient-Specific 3D Printed Models in Cardiovascular Disease: Current Status and Future Directions. Biomolecules. 2020; 10(11):1577. https://doi.org/10.3390/biom10111577
Chicago/Turabian StyleSun, Zhonghua. 2020. "Clinical Applications of Patient-Specific 3D Printed Models in Cardiovascular Disease: Current Status and Future Directions" Biomolecules 10, no. 11: 1577. https://doi.org/10.3390/biom10111577
APA StyleSun, Z. (2020). Clinical Applications of Patient-Specific 3D Printed Models in Cardiovascular Disease: Current Status and Future Directions. Biomolecules, 10(11), 1577. https://doi.org/10.3390/biom10111577





