Detention in Juvenile Correctional Facilities Is Associated with Higher Platelet Monoamine Oxidase B Activity in Males
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Determination of Platelet MAO-B Activity
2.3. Genotyping of MAOB rs1799836 Polymorphism
2.4. Statistical Analysis
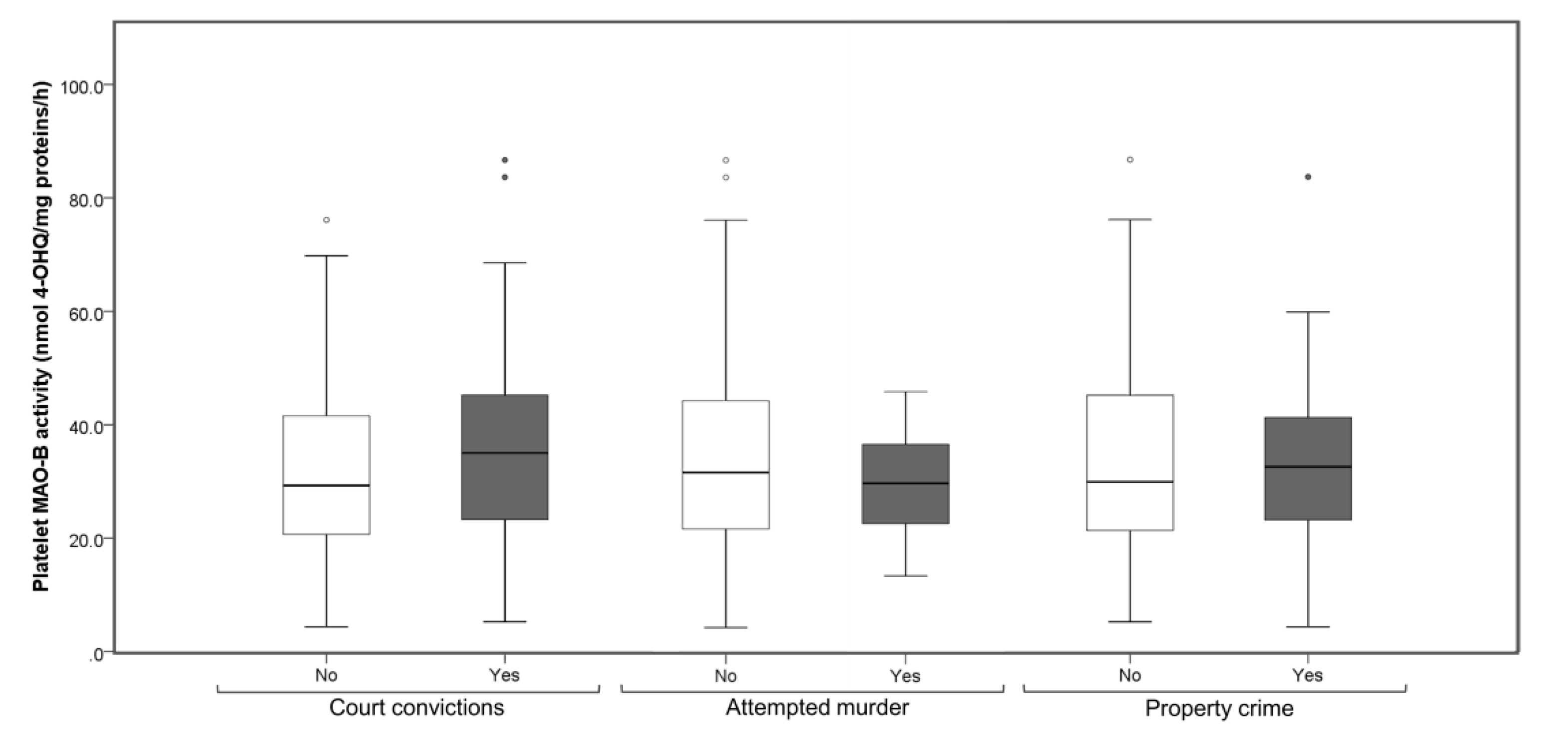
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Spencer, T.J.; Biederman, J.; Mick, E. Attention-deficit/hyperactivity disorder: Diagnosis, lifespan, comorbidities, and neurobiology. J. Pediatric Psychol. 2007, 32, 631–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, J.; Farrington, D.P. Risk factors for conduct disorder and delinquency: Key findings from longitudinal studies. Can. J. Psychiatry 2010, 55, 633–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buitelaar, J.K.; Smeets, K.C.; Herpers, P.; Scheepers, F.; Glennon, J.; Rommelse, N.N.J. Conduct disorders. Eur. Child Adolesc. Psychiatry 2013, 22, S49–S54. [Google Scholar] [CrossRef] [PubMed]
- Eme, R.F. Sex differences in child-onset, life-course-persistent conduct disorder. A review of biological influences. Clin. Psychol. Rev. 2007, 27, 607–627. [Google Scholar] [CrossRef] [PubMed]
- Teplin, L.A.; Abram, K.M.; McClelland, G.M.; Dulcan, M.K.; Mericle, A.A. Psychiatric disorders in youth in juvenile detention. Arch. Gen. Psychiatry 2002, 59, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, J.E.; Dick, D.M. Genetic influences on conduct disorder. Neurosci. Biobehav. Rev. 2018, 91, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Umbach, R.; Raine, A. Biological explanations of criminal behavior. Psychol. Crime Law 2019, 25, 626–640. [Google Scholar] [CrossRef]
- Ruchkin, V.; Koposov, R.A.; Klinteberg, B.; Oreland, L.; Grigorenko, E.L. Platelet MAO-B, personality and psychopathology. J. Abnorm. Psychol. 2005, 114, 477–482. [Google Scholar] [CrossRef]
- Bortolato, M.; Chen, K.; Shih, J.C. Monoamine oxidase inactivation: From pathophysiology to therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 1527–1533. [Google Scholar] [CrossRef] [Green Version]
- Oreland, L. Platelet monoamine oxidase, personality and alcoholism: The rise, fall and resurrection. NeuroToxicology 2004, 25, 79–89. [Google Scholar] [CrossRef]
- Stalenheim, E.G. Long-term validity of biological markers of psychopathy and criminal recidivism: Follow-up 6–8 years after forensic psychiatric investigation. Psychiatry Res. 2004, 121, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Oreland, L.; Hallman, J. The correlation between platelet MAO activity and personality: Short review of findings and a discussion on possible mechanisms. Prog. Brain Res. 1995, 106, 77–84. [Google Scholar] [PubMed]
- Harro, J.; Fischer, K.; Vansteelandt, S.; Harro, M. Both low and high activities of platelet monoamine oxidase increase the probability of becoming a smoker. Eur. Neuropsychopharmacol. 2004, 14, 65–69. [Google Scholar] [CrossRef]
- Bortolato, M.; Godar, S.C.; Davarian, S.; Chen, K.; Shih, J.C. Behavioral disinhibition and reduced anxiety-like behaviors in monoamine oxidase B-deficient mice. Neuropsychopharmacology 2009, 34, 2746–2757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, N.L.; Oreland, L.; Reynolds, C.; McClearn, G.E. Importance of genetic effects for monoamine oxidase activity in thrombocytes in twins reared apart and twins reared together. Psychiatry Res. 1993, 46, 239–251. [Google Scholar] [CrossRef]
- Ho, S.L.; Kapadi, A.L.; Ramsden, D.B.; Williams, A.C. An allelic association study of monoamine oxidase B in Parkinson’s disease. Ann Neurol. 1995, 37, 403–405. [Google Scholar] [CrossRef]
- Netter, P.; Montag, C.; Reuter, M.; Baars, M.; Gallhofer, B. Genetic variation of the MAO B gene is related to shorter reaction times in alcohol dependent patients. J. Addict. Med. Ther. 2015, 3, 1014. [Google Scholar]
- Nedic Erjavec, G.; Nenadic Sviglin, K.; Nikolac Perkovic, M.; Muck-Seler, D.; Jovanovic, T.; Pivac, N. Association of gene polymorphisms encoding dopaminergic system components and platelet MAO-B activity with alcohol dependence and alcohol dependence-related phenotypes. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 54, 321–327. [Google Scholar] [CrossRef]
- Yudofsky, S.C.; Silver, J.M.; Jackson, W.; Endicott, J.; Williams, D. The overt aggression scale for the objective rating of verbal and physical aggression. Am. J. Psychiatry 1986, 143, 35–39. [Google Scholar]
- Forth, A.E.; Kosson, D.S.; Hare, R.D. The Hare PCL: Youth Version; Multi-Health Systems: Toronto, ON, Canada, 2003. [Google Scholar]
- Coccaro, E.F. The Overt Aggression Scale Modified (OAS-M) for clinical trials targeting impulsive aggression and intermittent explosive disorder: Validity, reliability, and correlates. J. Psychiatr. Res. 2020, 124, 50–57. [Google Scholar] [CrossRef]
- APA. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Nikolac Perkovic, M.; Svob Strac, D.; Nedic Erjavec, G.; Uzun, S.; Podobnik, J.; Kozumplik, O.; Vlatkovic, S.; Pivac, N. Monoamine oxidase and agitation in psychiatric patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 69, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Krajl, M. A rapid microfluorimetric determination of monoamine oxidase. Biochem. Pharmacol. 1965, 14, 1684–1686. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef] [Green Version]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Pivac, N.; Knezevic, J.; Mustapic, M.; Dezeljin, M.; Muck-Seler, D.; Kozaric-Kovacic, D.; Balija, M.; Matijevic, T.; Pavelic, J. The lack of association between monoamine oxidase (MAO) intron 13 polymorphism and platelet MAO-B activity among men. Life Sci. 2006, 79, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Pivac, N.; Knezevic, J.; Kozaric-Kovacic, D.; Dezeljin, M.; Mustapic, M.; Rak, D.; Matijevic, T.; Pavelic, J.; Muck-Seler, D. Monoamine oxidase (MAO) intron 13 polymorphism and platelet MAO-B activity in combat-related posttraumatic stress disorder. J. Affect. Disord. 2007, 103, 131–138. [Google Scholar] [CrossRef]
- Svob Strac, D.; Kovacic Petrovic, Z.; Nikolac Perkovic, M.; Umolac, D.; Nedic Erjavec, G.; Pivac, N. Platelet monoamine oxidase type B, MAOB intron 13 and MAOA-uVNTR polymorphism and symptoms of post-traumatic stress disorder. Stress 2016, 19, 362–373. [Google Scholar] [CrossRef]
- Malmberg, K.; Wargelius, H.-L.; Lichtenstein, P.; Oreland, L.; Larsson, J.-O. ADHD and Disruptive behavior scores—Associations with MAO-A and 5-HTT genes and with platelet MAO-B activity in adolescents. BMC Psychiatry 2008, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Gustavson, C.; Wass, C.; Mansson, J.E.; Blennow, K.; Forsman, A.; Anckarsater, H.; Nilsson, T. Platelet monoamine oxidase B activity did not predict destructive personality traits or violent recidivism: A prospective study in male forensic psychiatric examinees. Neuropsychobiology 2010, 6, 87–96. [Google Scholar] [CrossRef]
- Asberg, M. Neurotransmitters and suicidal behavior. The evidence from cerebrospinal fluid studies. Ann. N. Y. Acad. Sci. 1997, 836, 158–181. [Google Scholar] [CrossRef] [PubMed]
- Belfrage, H.; Lidberg, L.; Oreland, L. Platelet monoamine oxidase activity in mentally disordered violent offenders. Acta Psychiatr. Scand. 1992, 85, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Longato-Stadler, E.; Klinteberg, B.A.; Garpenstrand, H.; Oreland, L.; Hallman, J. Personality traits and platelet monoamine oxidase activity in a Swedish male criminal population. Neuropsychobiology 2002, 46, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Alm, P.O.; Alm, M.; Humble, K.; Leppert, J.; Sörensen, S.; Lidberg, L.; Oreland, L. Criminality and platelet monoamine oxidase activity in former juvenile delinquents as adults. Acta Psychiatr. Scand. 1994, 89, 41–45. [Google Scholar] [CrossRef]
- Skondras, M.; Markianos, M.; Botsis, A.; Bistolaki, E.; Christodoulou, G. Platelet monoamine oxidase activity and psychometric correlates in male violent offenders imprisoned for homicide or other violent acts. Eur. Arch. Psychiatry Clin. Neurosci. 2004, 254, 380–386. [Google Scholar] [CrossRef]
- Oreland, L.; Nilsson, K.; Damberg, M.; Hallman, J. Monoamine oxidases: Activities, genotypes and the shaping of behaviour. J. Neural Transm. (Vienna) 2007, 114, 817–822. [Google Scholar] [CrossRef]
- Harro, J.; Oreland, L. The role of MAO in personality and drug use. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 69, 101–111. [Google Scholar] [CrossRef]
- Paaver, M.; Eensoo, D.; Pulver, A.; Harro, J. Adaptive and maladaptive impulsivity, platelet monoamine oxidase (MAO) activity and risk-admitting in different types of risky drivers. Psychopharmacology (Berl) 2006, 186, 32–40. [Google Scholar] [CrossRef]
- Jokinen, J.; Königsson, J.; Moberg, T.; Jönsson, E.G.; Tiihonen, J.; Nordström, P.; Oreland, L.; Åsberg, M. Platelet monoamine oxidase activity and interpersonal violence in male suicide attempters. Psychiatry Res. 2018, 260, 173–176. [Google Scholar] [CrossRef] [Green Version]
- Barnert, E.S.; Abrams, L.S.; Dudovitz, R.; Coker, T.R.; Bath, E.; Tesema, L.; Nelson, B.B.; Biely, C.; Chung, P.J. What is the relationship between incarceration of children and adult health outcomes? Acad. Pediatr. 2019, 19, 342–350. [Google Scholar] [CrossRef]
- Gonçalves, L.C.; Endrass, J.; Astrid Rossegger, A.; Dirkzwager, A.J.E. A longitudinal study of mental health symptoms in young prisoners: Exploring the influence of personal factors and the correctional climate. BMC Psychiatry 2016, 16, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naoi, M.; Riederer, P.; Maruyama, W. Modulation of monoamine oxidase (MAO) expression in neuropsychiatric disorders: Genetic and environmental factors involved in type A MAO expression. J. Neural Transm. 2016, 123, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Bortolato, M.; Shih, J.C. Behavioral outcomes of monoamine oxidase deficiency: Preclinical and clinical evidence. Int. Rev. Neurobiol. 2011, 100, 13–42. [Google Scholar] [PubMed] [Green Version]
- Asor, E.; Ben-Shachar, D. Platelets: A possible glance into brain biological processes in schizophrenia. World J. Psychiatr. 2012, 2, 124–133. [Google Scholar] [CrossRef]
- Camacho, A.; Dimsdale, J.E. Platelets and psychiatry: Lessons learned from old and new studies. Psychosom. Med. 2000, 62, 326–336. [Google Scholar] [CrossRef] [Green Version]
- Yubero-Lahoz, S.; Robledo, P.; Farre, M.; de laTorre, R. Platelet SERT as a peripheral biomarker of serotonergic neurotransmission in the central nervous system. Curr. Med. Chem. 2013, 20, 1382–1396. [Google Scholar] [CrossRef]
- Buchsbaum, M.S.; Coursey, R.D.; Murphy, D.L. The biochemical high-risk paradigm: Behavioral and familial correlates of low platelet monoamine oxidase activity. Science 1976, 194, 339–341. [Google Scholar] [CrossRef]
- Devor, E.J.; Cloninger, C.R.; Hoffman, P.L.; Tabakoff, B. Association of monoamine oxidase (MAO) activity with alcoholism and alcoholic subtypes. Am. J. Med. Genet. 1993, 48, 209–213. [Google Scholar] [CrossRef]
- Schalling, D.; Asberg, M.; Edman, G.; Oreland, L. Markers for vulnerability to psychopathology: Temperament traits associated with platelet MAO activity. Acta Psychiatr. Scand. 1987, 76, 172–182. [Google Scholar] [CrossRef]
- Von Knorring, A.L.; Hallman, J.; von Knorring, L.; Oreland, L. Platelet monoamine oxidase activity in type 1 and type 2 alcoholism. Alcohol Alcohol. 1991, 26, 409–416. [Google Scholar] [CrossRef]
- Stalenheim, E.G.; von Knorring, L.; Oreland, L. Platelet monoamine oxidase activity as a biological marker in a Swedish forensic psychiatric population. Psychiatry Res. 1997, 69, 79–87. [Google Scholar] [CrossRef]
- Stalenheim, E.G. Relationships between attempted suicide, temperamental vulnerability, and violent criminality in a Swedish forensic psychiatric population. Eur. Psychiatry 2001, 16, 386–394. [Google Scholar] [CrossRef]
- Klinteberg, B.A.; Schalling, D.; Edman, G.; Oreland, L.; Asberg, M. Personality correlates of platelet monoamine oxidase (MAO) activity in female and male subjects. Neuropsychobiology 1987, 18, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.O.; Cowley, D.S.; Roy-Byrne, P.P.; Hopfenbeck, J.R. Tridimensional personality traits in sons of alcoholic and nonalcoholic fathers. Alcohol. Clin. Exp. Res. 1996, 20, 445–448. [Google Scholar] [CrossRef]
- Oreland, L.; Garpenstrand, H.; Damberg, M.; Alm, P.O.; Thorell, L.H.; Klinteberg, B.A.; Ekblom, J. The correlation between platelet MAO activity and personality—The effect of smoking and possible mechanisms behind the correlation. Neurobiology (Bp) 1999, 7, 191–203. [Google Scholar]
- Shih, J.C.; Chen, K.; Ridd, M.J. Monoamine oxidase: From genes to behavior. Annu. Rev. Neurosci. 1999, 22, 197–217. [Google Scholar] [CrossRef] [Green Version]
- Verkes, R.J.; Van der Mast, R.C.; Kerkhof, A.J.; Fekkes, D.; Hengeveld, M.W.; Tuyl, J.P.; Van Kempen, G.M. Platelet serotonin, monoamine oxidase activity, and [3H]paroxetine binding related to impulsive suicide attempts and borderline personality disorder. Biol. Psychiatry 1998, 43, 740–746. [Google Scholar] [CrossRef]
- Nedic, G.; Pivac, N.; Hercigonja, D.K.; Jovancevic, M.; Curkovic, K.D.; Muck-Seler, D. Platelet monoamine oxidase activity in children with attention-deficit/hyperactivity disorder. Psychiatry Res. 2010, 175, 252–255. [Google Scholar] [CrossRef]
- Vincent, G.M.; Grisso, T.; Terry, A.; Banks, S. Sex and race differences in mental health symptoms in juvenile justice: The MAYSI-2 national meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry 2008, 47, 282–290. [Google Scholar] [CrossRef]
- Nikolac Perkovic, M.; Nedic Erjavec, G.; Stefulj, J.; Muck-Seler, D.; Pivac, N.; Kocijan Hercigonja, D.; Hranilovic, D.; Curkovic, M.; Dodig-Curkovic, K. Association between the polymorphisms of the selected genes encoding dopaminergic system with ADHD and autism. Psychiatry Res. 2014, 215, 260–261. [Google Scholar] [CrossRef] [Green Version]
- Perfilyeva, A.V.; Bespalova, K.B.; Skvortsova, L.A.; Surdeanu, A.; Garshin, A.A.; Perfilyeva, Y.V.; Khamdiyeva, O.K.; Bekmanov, B.O.; Djansugurova, L.B. No Association between the rs1799836 polymorphism of the monoamine oxidase B gene and the risk of Autism spectrum disorders in the Kazakhstani population. Dis. Markers 2019, 2019, 2846394. [Google Scholar] [CrossRef] [PubMed]
- Antypa, N.; Giegling, I.; Calati, R.; Schneider, B.; Hartmann, A.M.; Friedl, M.; Konte, B.; Lia, L.; De Ronchi, D.; Serretti, A.; et al. MAOA and MAOB polymorphisms and anger-related traits in suicidal participants and controls. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 263, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.L.; Goodwin, F.K.; Ballenger, J.C.; Goyer, P.F.; Major, L.F. Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res. 1979, 1, 131–139. [Google Scholar] [CrossRef]
- Camarena, B.; Fresán, A.; Aguilar, A.; Escamilla, R.; Saracco, R.; Palacios, J.; Tovilla, A.; Nicolini, H. Monoamine oxidase A and B gene polymorphisms and negative and positive symptoms in schizophrenia. ISRN Psychiatry 2012, 2012, 852949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dlugos, A.M.; Palmer, A.A.; de Wit, H. Negative emotionality: Monoamine oxidase B gene variants modulate personality traits in healthy humans. J. Neural Transm. 2009, 116, 1323–1334. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.L.; Li, C.X.; Li, S.B.; Liu, Y.; Hu, L. Association study of monoamine oxidase A/B genes and schizophrenia in Han Chinese. Behav. Brain Funct. 2011, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Pehlivan, S.; Aydin, P.C.; Uysal, M.A.; Ciftci, H.S.; Sever, U.; Yavuz, F.K.; Aydin, N.; Nursal, A.F. Effect of monoamine oxidase B A644G variant on nicotine dependence and/or schizophrenia risk. Arch. Clin. Psychiatry 2019, 46, 21–24. [Google Scholar] [CrossRef] [Green Version]
- Vassos, E.; Collier, D.A.; Fazel, S. Systematic meta-analyses and field synopsis of genetic association studies of violence and aggression. Mol. Psychiatry 2014, 19, 471–477. [Google Scholar] [CrossRef]
- Dick, D.M.; Aliev, F.; Krueger, R.F.; Edwards, A.; Agrawal, A.; Lynskey, M.; Lin, P.; Schuckit, M.; Hesselbrock, V.; Nurnberger, J., Jr.; et al. Genome-wide association study of conduct disorder symptomatology. Mol. Psychiatry 2011, 16, 800–808. [Google Scholar] [CrossRef] [Green Version]
- Jansson, M.; McCarthy, S.; Sullivan, P.F.; Dickman, P.; Andersson, B.; Oreland, L.; Schalling, M.; Pedersen, N.L. MAOA haplotypes associated with thrombocyte-MAO activity. BMC Genet. 2005, 6, 46. [Google Scholar] [CrossRef] [Green Version]
- Costa-Mallen, P.; Kelada, S.N.; Costa, L.G.; Checkoway, H. Characterization of the in vitro transcriptional activity of polymorphic alleles of the human monoamine oxidase-B gene. Neurosci. Lett. 2005, 383, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Jakubauskiene, E.; Janaviciute, V.; Peciuliene, I.; Soderkvist, P.; Kanopka, A. G/A polymorphism in intronic sequence affects the processing of MAO-B gene in patients with Parkinson disease. FEBS Lett. 2012, 586, 3698–3704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garpenstrand, H.; Ekblom, J.; Forslund, K.; Rylander, G.; Oreland, L. Platelet monoamine oxidase activity is related to MAOB intron 13 genotype. J. Neural Transm. 2000, 107, 523–530. [Google Scholar] [CrossRef] [PubMed]

| Scales for Evaluating Dissociative/Aggressive/Delinquent Behavior | MAO-B Activity | Multiple Linear Regression Model | ||||
|---|---|---|---|---|---|---|
| Coefficient | t | p | Corrected R2 | F | p | |
| PCL-YV: total | 0.001 | 0.01 | 0.994 | 0.060 | 4.81 | 0.003 |
| Factor 1 | 0.596 | 1.22 | 0.222 | 0.067 | 5.36 | 0.001 |
| Factor 2 | 0.224 | 0.49 | 0.625 | 0.061 | 4.91 | 0.003 |
| Factor 3 | −0.121 | −0.27 | 0.791 | 0.060 | 4.85 | 0.003 |
| Factor 4 | −0.939 | −1.65 | 0.101 | 0.074 | 5.80 | 0.001 |
| (F1+F2)/(F3+F4) | 0.816 | 0.56 | 0.573 | 0.061 | 4.94 | 0.003 |
| OAS-M: total | 0.100 | 1.39 | 0.168 | 0.070 | 5.52 | 0.001 |
| Verbal aggression | 0.669 | 2.43 | 0.016 | 0.090 | 6.95 | <0.001 |
| Aggression against property | 0.051 | 0.24 | 0.807 | 0.060 | 4.85 | 0.003 |
| Physical aggression against property or objects | 0.201 | 1.27 | 0.206 | 0.068 | 5.40 | 0.001 |
| Physical aggression against self | 0.115 | 0.42 | 0.672 | 0.061 | 4.91 | 0.003 |
| CBCL: total | 0.001 | 0.02 | 0.982 | 0.060 | 4.82 | 0.003 |
| Delinquency (D) | 0.182 | 0.71 | 0.478 | 0.062 | 5.01 | 0.002 |
| Aggression (A) | −0.027 | −0.19 | 0.849 | 0.060 | 4.84 | 0.003 |
| A/D | −0.01 | −0.02 | 0.986 | 0.063 | 4.96 | 0.003 |
| Subjects | MAOB rs1799836 | ||
|---|---|---|---|
| Allele A N (%) | Allele G N (%) | ||
| Healthy controls | 46 (56.8) | 35 (43.2) | |
| Youth with CD | 14 (56.0) | 11 (44.0) | |
| Youth from the juvenile correctional facility | Without CD | 37 (56.9) | 28 (43.1) |
| With CD | 59 (50.9) | 57 (49.1) | |
| Test statistics | Χ2 = 0.96; df = 3; p = 0.810 | ||
| Youth from the Juvenile Correctional Facility | MAOB rs1799836 | χ2 test (df = 1) | |||
|---|---|---|---|---|---|
| Allele A N (%) | Allele G N (%) | χ2 | p | ||
| Court convictions | No | 44 (51.8) | 41 (48.2) | 0.03 | 0.862 |
| Yes | 52 (54.2) | 44 (45.8) | |||
| Attempted murder | No | 81 (50.9) | 78 (49.1) | 1.67 | 0.167 |
| Yes | 15 (68.2) | 7 (31.8) | |||
| Property crime | No | 61 (51.7) | 57 (48.3) | 0.12 | 0.734 |
| Yes | 35 (55.6) | 28 (44.4) | |||
| Scales for Evaluating Dissociative/Aggressive/Delinquent Behavior | MAOB rs1799836 | Multiple Linear Regression Model | ||||
|---|---|---|---|---|---|---|
| Coefficient | t | p | Corrected R2 | F | p | |
| PCL-YV: total | −0.674 | −0.60 | 0.550 | 0.433 | 69.61 | <0.001 |
| Factor 1 | −0.059 | −0.17 | 0.864 | 0.331 | 45.42 | <0.001 |
| Factor 2 | 0.035 | 0.09 | 0.925 | 0.244 | 30.11 | <0.001 |
| Factor 3 | −0.550 | −1.49 | 0.137 | 0.314 | 42.14 | <0.001 |
| Factor 4 | −0.111 | −0.38 | 0.707 | 0.471 | 81.06 | <0.001 |
| (F1+F2)/(F3+F4) | 0.147 | 1.25 | 0.214 | 0.001 | 1.07 | 0.346 |
| OAS-M: total | 0.280 | 0.47 | 0.641 | 0.191 | 22.23 | <0.001 |
| Verbal aggression | 0.739 | 0.91 | 0.363 | 0.137 | 15.30 | <0.001 |
| Aggression against Property | −1.102 | −1.04 | 0.299 | 0.153 | 17.23 | <0.001 |
| Physical aggression against property or objects | −0.244 | −0.34 | 0.698 | 0.072 | 8.01 | <0.001 |
| CBCL: total | −2.762 | −0.92 | 0.358 | 0.328 | 44.83 | <0.001 |
| Delinquency (D) | −0.301 | −0.45 | 0.654 | 0.417 | 65.36 | <0.001 |
| Aggression (A) | −0.297 | −0.25 | 0.805 | 0.393 | 59.15 | <0.001 |
| A/D | 0.251 | 0.77 | 0.444 | −0.008 | 0.30 | 0.743 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podobnik, J.; Nikolac Perkovic, M.; Nedic Erjavec, G.; Dodig Curkovic, K.; Curkovic, M.; Kovac, V.; Svob Strac, D.; Cusek, M.; Bortolato, M.; Pivac, N. Detention in Juvenile Correctional Facilities Is Associated with Higher Platelet Monoamine Oxidase B Activity in Males. Biomolecules 2020, 10, 1555. https://doi.org/10.3390/biom10111555
Podobnik J, Nikolac Perkovic M, Nedic Erjavec G, Dodig Curkovic K, Curkovic M, Kovac V, Svob Strac D, Cusek M, Bortolato M, Pivac N. Detention in Juvenile Correctional Facilities Is Associated with Higher Platelet Monoamine Oxidase B Activity in Males. Biomolecules. 2020; 10(11):1555. https://doi.org/10.3390/biom10111555
Chicago/Turabian StylePodobnik, Josip, Matea Nikolac Perkovic, Gordana Nedic Erjavec, Katarina Dodig Curkovic, Mario Curkovic, Vlatka Kovac, Dubravka Svob Strac, Melita Cusek, Marco Bortolato, and Nela Pivac. 2020. "Detention in Juvenile Correctional Facilities Is Associated with Higher Platelet Monoamine Oxidase B Activity in Males" Biomolecules 10, no. 11: 1555. https://doi.org/10.3390/biom10111555
APA StylePodobnik, J., Nikolac Perkovic, M., Nedic Erjavec, G., Dodig Curkovic, K., Curkovic, M., Kovac, V., Svob Strac, D., Cusek, M., Bortolato, M., & Pivac, N. (2020). Detention in Juvenile Correctional Facilities Is Associated with Higher Platelet Monoamine Oxidase B Activity in Males. Biomolecules, 10(11), 1555. https://doi.org/10.3390/biom10111555







