Secretome Analysis of the Banana Fusarium Wilt Fungi Foc R1 and Foc TR4 Reveals a New Effector OASTL Required for Full Pathogenicity of Foc TR4 in Banana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Pathogen Infection
2.2. Secretory Protein Extraction
2.3. Protein Identification by HPLC-ESI-MS/MS
2.4. Protein Bioinformatics Analysis
2.5. RT-PCR Analysis
2.6. Fungal Vector Construction
2.7. Foc Transformation
2.8. Pathogenicity Assay
3. Results
3.1. Foc R1 and Foc TR4 Infection of Banana
3.2. Identification of Foc R1 and Foc TR4 SPs
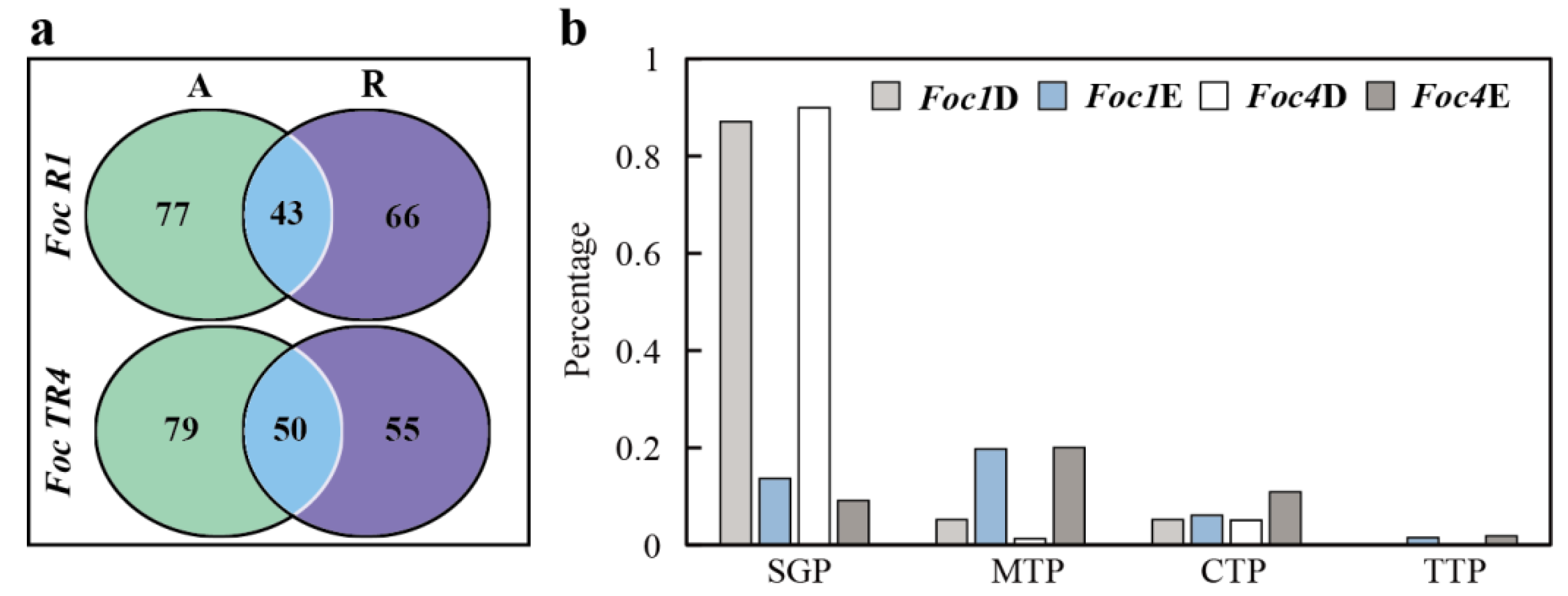
3.3. Analysis of Specific SPs of Foc R1 and Foc TR4 in Culture Alone and with Banana Roots
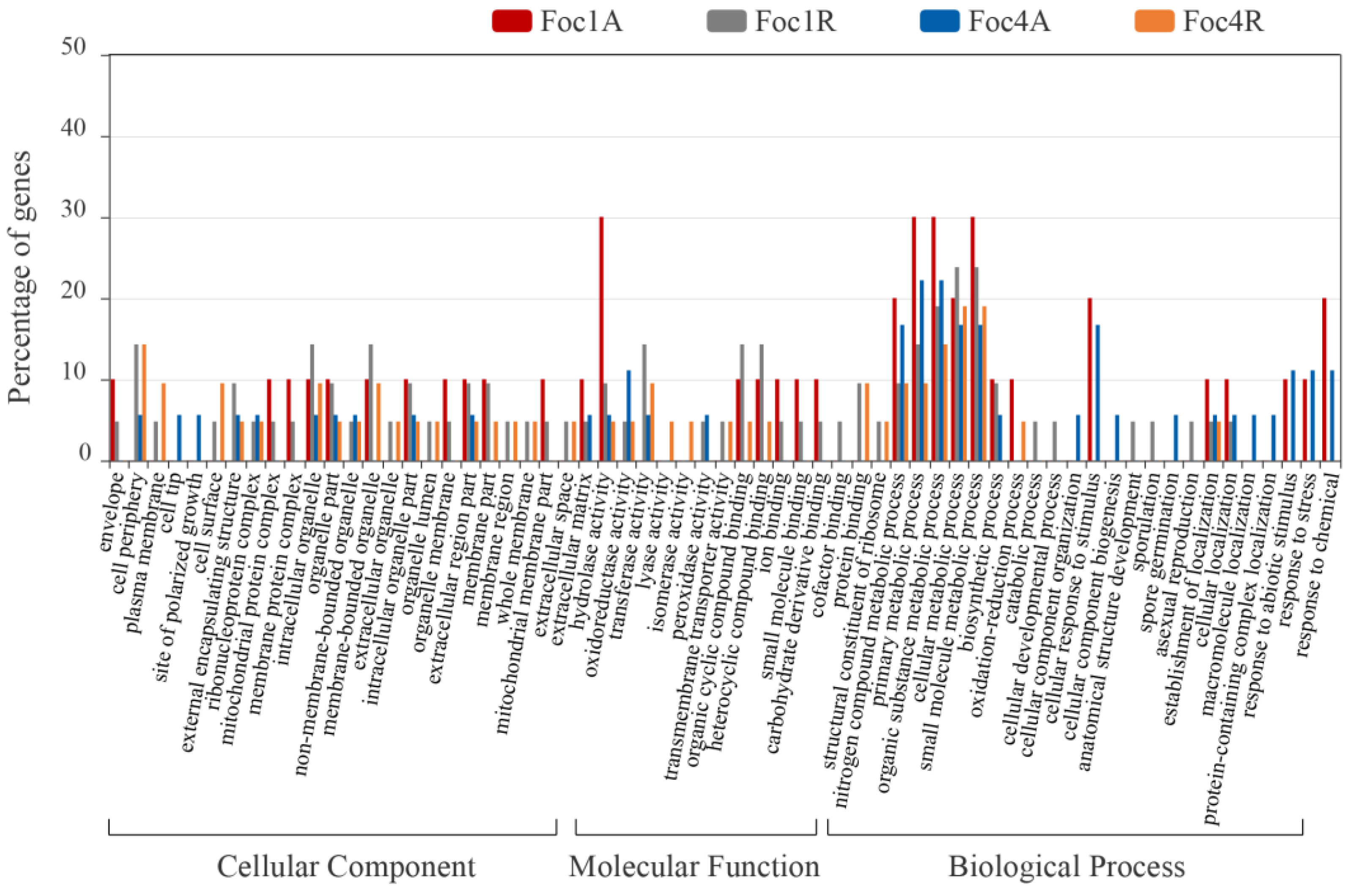
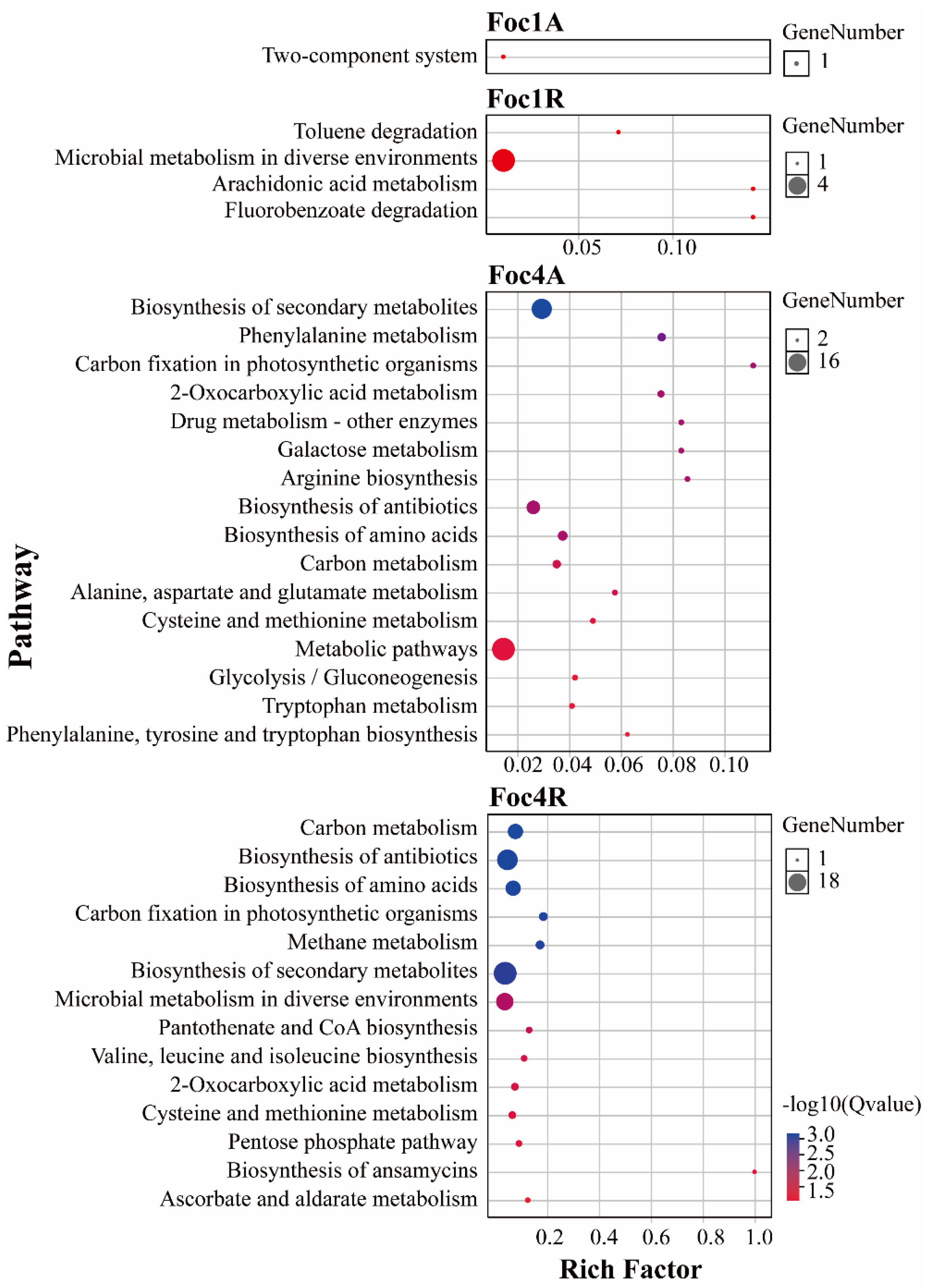
3.4. Analysis of Foc R1- and Foc TR4-Specific SPs
3.5. Knockout of Root-Induced Foc TR4-Specific OASTL Resulted in the Loss of Pathogenicity of Foc TR4 in Banana
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ploetz, R.C. Fusarium wilt of banana is caused by several pathogens referred to as Fusarium oxysporum f. sp. cubense. Phytopathology 2006, 96, 653–656. [Google Scholar] [CrossRef] [Green Version]
- Bubici, G.; Kaushal, M.; Prigigallo, M.; Gómez-Lama Cabanás, C.; Mercado-Blanco, J. Biological control agents against fusarium wilt of banana. Front. Microbiol. 2019, 10, 1290. [Google Scholar] [CrossRef] [Green Version]
- McCotter, S.W.; Horianopoulos, L.C.; Kronstad, J.W. Regulation of the fungal secretome. Curr. Genet. 2016, 62, 533–545. [Google Scholar] [CrossRef]
- Neu, E.; Debener, T. Prediction of the Diplocarpon rosae secretome reveals candidate genes for effectors and virulence factors. Fungal Biol. 2018, 123, 231–239. [Google Scholar] [CrossRef]
- Jin, L.; Ham, J.H.; Hage, R.; Zhao, W.; Soto-Hernández, J.; Lee, S.Y.; Paek, S.M.; Kim, M.G.; Boone, C.; Coplin, D.L.; et al. Direct and indirect targeting of PP2A by conserved bacterial type-III effector proteins. PLoS Pathog. 2016, 12, e1005609. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Shen, C.; Fu, Y.; Xie, J.; Jiang, D.; Li, G.; Cheng, J. A small secreted virulence-related protein is essential for the necrotrophic interactions of sclerotinia sclerotiorum with its host plants. PLoS Pathog. 2016, 12, e1005435. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, Q.; Feng, R.; Li, L.; Ding, L.; Fan, G.; Li, W.; Du, Y.; Zhang, M.; Huang, G.; et al. Negative regulators of plant immunity derived from cinnamyl alcohol dehydrogenases are targeted by multiple Phytophthora Avr3a-like effectors. New Phytol. 2019, 22. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; McLellan, H.; Hughes, R.K.; Boevink, P.C.; Armstrong, M.; Lu, Y.; Banfield, M.; Tian, Z.; Birch, P.R.J. Phytophthora infestans effector SFI3 targets potato UBK to suppress early immune transcriptional responses. New Phytol. 2019, 222, 438–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Altegoer, F.; Steinchen, W.; Binnebesel, L.; Schuhmacher, J.; Glatter, T.; Giammarinaro, P.I.; Djamei, A.; Rensing, S.A.; Reissmann, S.; et al. A kiwellin disarms the metabolic activity of a secreted fungal virulence factor. Nature 2019, 565, 650–653. [Google Scholar] [CrossRef]
- Wang, A.; Pan, L.; Niu, X.; Shu, X.; Yi, X.; Yamamoto, N.; Li, S.; Deng, Q.; Zhu, J.; Liang, Y.; et al. Comparative secretome analysis of different smut fungi and identification of plant cell death-inducing secreted proteins from Tilletia horrida. BMC Plant. Biol. 2019, 19, 360. [Google Scholar] [CrossRef]
- Li, T.; Wu, Y.; Wang, Y.; Gao, H.; Gupta, V.K.; Duan, X.; Qu, H.; Jiang, Y. Secretome profiling reveals virulence-associated proteins of Fusarium proliferatum during interaction with banana fruit. Biomolecules 2019, 9, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurjar, M.S.; Aggarwal, R.; Jogawat, A.; Kulshreshtha, D.; Sharma, S.; Solanke, A.U.; Dubey, H.; Jain, R.K. De novo genome sequencing and secretome analysis of Tilletia indica inciting Karnal bunt of wheat provides pathogenesis-related genes. 3 Biotech. 2019, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Singh, M.; Pandey, D.; Marla, S.; Kumar, A. Secretome analysis identifies potential pathogenicity/virulence factors of Tilletia indica, a quarantined fungal pathogen inciting karnal bunt disease in wheat. Proteomics 2018, 18, e1700473. [Google Scholar] [CrossRef] [PubMed]
- Nogueira-Lopez, G.; Greenwood, D.R.; Middleditch, M.; Winefield, C.; Eaton, C.; Steyaert, J.M.; Mendoza-Mendoza, A. The apoplastic secretome of Trichoderma virens during interaction with maize roots shows an inhibition of plant defence and scavenging oxidative stress secreted proteins. Front. Plant. Sci. 2018, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Holmer, R.; Hontelez, J.; Te Lintel-Hekkert, B.; Marufu, L.; de Zeeuw, T.; Wu, F.; Schijlen, E.; Bisseling, T.; Limpens, E. Host- and stage-dependent secretome of the arbuscular mycorrhizal fungus Rhizophagus irregularis. Plant. J. 2018, 94, 411–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, C.; Wang, M.; Cornejo, O.E.; Jiwan, D.A.; See, D.R.; Chen, X. Secretome characterization and correlation analysis reveal putative pathogenicity mechanisms and identify candidate avirulence genes in the wheat stripe rust fungus Puccinia striiformis f. sp. tritici. Front. Microbiol. 2017, 8, 2394. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, Y.; Li, L.; Zhang, Y.; Yang, X.; Zeng, H.; Shi, M.; Pei, X.; Qiu, D.; Yuan, Q. The novel cerato-platanin-like protein FocCP1 from Fusarium oxysporum triggers an immune response in plants. Int. J. Mol. Sci. 2019, 20, 2849. [Google Scholar] [CrossRef] [Green Version]
- Widinugraheni, S.; Niño-Sánchez, J.; van der Does, H.C.; van Dam, P.; García-Bastidas, F.A.; Subandiyah, S.; Meijer, H.J.G.; Kistler, H.C.; Kema, G.H.J.; Rep, M. A SIX1 homolog in Fusarium oxysporum f. sp. cubense tropical race 4 contributes to virulence towards Cavendish banana. PLoS ONE 2018, 13, e205896. [Google Scholar] [CrossRef]
- An, B.; Hou, X.; Guo, Y.; Zhao, S.; Luo, H.; He, C.; Wang, Q. The effector SIX8 is required for virulence of Fusarium oxysporum f. sp. cubense tropical race 4 to Cavendish banana. Fungal Biol. 2019, 123, 423–430. [Google Scholar] [CrossRef]
- Liu, S.; Wu, B.; Yang, J.; Bi, F.; Dong, T.; Yang, Q.; Hu, C.; Xiang, D.; Chen, H.; Huang, H.; et al. A cerato-platanin family protein FocCP1 is essential for the penetration and virulence of Fusarium oxysporum f. sp. cubense tropical race 4. Int. J. Mol. Sci. 2019, 20, 3785. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Sun, Y.; Tong, Z.; Yang, Q.; Chang, L.; Meng, X.; Wang, L.; Tian, W.; Wang, X. A protein extraction method for low protein concentration solutions compatible with the proteomic analysis of rubber particles. Electrophoresis 2016, 37, 2930–2939. [Google Scholar] [CrossRef] [PubMed]
- Andrie, R.M.; Martinez, J.P.; Ciuffetti, L.M. Development of ToxA and ToxB promoter-driven fluorescent protein expression vectors for use in Filamentous ascomycetes. Mycologia 2005, 97, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, C.P.; Starr, T.L.; Glass, N.L. Plant cell wall-degrading enzymes and their secretion in plant-pathogenic fungi. Annu. Rev. Phytopathol. 2014, 52, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Fiorin, G.L.; Sanchéz-Vallet, A.; Thomazella, D.P.T.; do Prado, P.F.V.; do Nascimento, L.C.; Figueira, A.V.O.; Thomma, B.P.H.J.; Pereira, G.A.G.; Teixeira, P.J.P.L. Suppression of plant immunity by fungal chitinase-like effectors. Curr. Biol. 2018, 28, 3023–3030. [Google Scholar] [CrossRef] [Green Version]
- Fesel, P.H.; Zuccaro, A. β-glucan: Crucial component of the fungal cell wall and elusive MAMP in plants. Fungal Genet. Biol. 2016, 90, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Garfoot, A.L.; Dearing, K.L.; VanSchoiack, A.D.; Wysocki, V.H.; Rappleye, C.A. Eng1 and Exg8 are the major β-glucanases secreted by thefungal pathogen Histoplasma capsulatum. J. Biol. Chem. 2017, 292, 4801–4810. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Li, X.; Wang, X.; Liu, N.; Xu, B.; Peng, Q.; Guo, Z.; Fan, B.; Zhu, C.; Chen, Z. Arabidopsis endoplasmic reticulum-localized UBAC2 proteins interact with PAMP-INDUCED COILED-COIL to regulate pathogen-induced callose deposition and plant immunity. Plant. Cell 2019, 31, 153–171. [Google Scholar] [CrossRef] [Green Version]
- Oide, S.; Bejai, S.; Staal, J.; Guan, N.; Kaliff, M.; Dixelius, C. A novel role of PR2 in abscisic acid (ABA) mediated, pathogen-induced callose deposition in Arabidopsis thaliana. New Phytol. 2013, 200, 1187–1199. [Google Scholar] [CrossRef]
- Gao, J.; Yan, S.; Yu, H.; Zhan, M.; Guan, K.; Wang, Y.; Yang, Z. Sweet sorghum (Sorghum bicolor L.) SbSTOP1 activates the transcription of a β-1,3-glucanase gene to reduce callose deposition under Al toxicity: A novel pathway for Al tolerance in plants. Biosci. Biotechnol. Biochem. 2019, 83, 446–455. [Google Scholar] [CrossRef]
- Samalova, M.; Mélida, H.; Vilaplana, F.; Bulone, V.; Soanes, D.M.; Talbot, N.J.; Gurr, S.J. The β-1,3-glucanosyltransferases (Gels) affect the structure of the rice blast fungal cell wall during appressorium-mediated plant infection. Cell. Microbiol. 2017, 19, e12659. [Google Scholar] [CrossRef] [Green Version]
- Miura, N.; Ueda, M. Evaluation of unconventional protein secretion by Saccharomyces cerevisiae and other fungi. Cells 2018, 7, 128. [Google Scholar] [CrossRef] [Green Version]
- Paper, J.M.; Scott-Craig, J.S.; Adhikari, N.D.; Cuomo, C.A.; Walton, J.D. Comparative proteomics of extracellular proteins in vitro and in planta from the pathogenic fungus Fusarium graminearum. Proteomics 2007, 7, 3171–3183. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yi, X.P.; Peng, M.; Zeng, H.C.; Wang, D.; Li, B.; Tong, Z.; Chang, L.L.; Jin, X.; Wang, X.C. Proteomics of Fusarium oxysporum race 1 and race 4 reveals enzymes involved in carbohydrate metabolism and ion transport that might play important roles in banana Fusarium wilt. PLoS ONE 2014, 9, e113818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nimrichter, L.; de Souza, M.M.; Del Poeta, M.; Nosanchuk, J.D.; Joffe, L.; Tavares Pde, M.; Rodrigues, M.L. Extracellular vesicle-associated transitory cell wall components and their impact on the interaction of fungi with host cells. Front. Microbiol. 2016, 7, 1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Yao, S.H.; Yuan, T.L.; Wang, Y.Z.; Zhang, D.; Tang, W.H. The spatiotemporal control of KatG2 catalase-peroxidase contributes to the invasiveness of Fusarium graminearum in host plants. Mol. Plant. Pathol. 2019, 20, 685–700. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Gold, S.E.; Glenn, A.E. Characterization of two catalase-peroxidase-encoding genes in Fusarium verticillioides reveals differential responses to in vitro versus in planta oxidative challenges. Mol. Plant. Pathol. 2018, 19, 1127–1139. [Google Scholar] [CrossRef] [Green Version]
- Djamei, A.; Schipper, K.; Rabe, F.; Ghosh, A.; Vincon, V.; Kahnt, J.; Osorio, S.; Tohge, T.; Fernie, A.R.; Feussner, I.; et al. Metabolic priming by a secreted fungal effector. Nature 2011, 478, 395–398. [Google Scholar] [CrossRef]
- Dasari, P.; Shopova, I.A.; Stroe, M.; Wartenberg, D.; Martin-Dahse, H.; Beyersdorf, N.; Hortschansky, P.; Dietrich, S.; Cseresnyés, Z.; Figge, M.T.; et al. Aspf2 from Aspergillus fumigatus recruits human immune regulators for immune evasion and cell damage. Front. Immunol. 2018, 9, 1635. [Google Scholar] [CrossRef] [Green Version]
- Ning, M.; Xiu, Y.; Yuan, M.; Bi, J.; Hou, L.; Gu, W.; Wang, W.; Meng, Q. Spiroplasma eriocheiris invasion into Macrobrachium rosenbergii hemocytes is mediated by pathogen enolase and host lipopolysaccharide and β-1, 3-Glucan Binding Protein. Front. Immunol. 2019, 10, 1852. [Google Scholar] [CrossRef] [Green Version]
- Rep, M.; van der Does, H.C.; Meijer, M.; van Wijk, R.; Houterman, P.M.; Dekker, H.L.; de Koster, C.G.; Cornelissen, B.J. A small, cysteine-rich protein secreted by Fusarium oxysporum during colonization of xylem vessels is required for I-3-mediated resistance in tomato. Mol. Microbiol. 2004, 53, 1373–1383. [Google Scholar] [CrossRef]
- Lievens, B.; Houterman, P.M.; Rep, M. Effector gene screening allows unambiguous identification of Fusarium oxysporum f. sp. lycopersici races and discrimination from other formae speciales. FEMS Microbiol. Lett. 2009, 300, 201–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czislowski, E.; Fraser-Smith, S.; Zander, M.; O’Neill, W.T.; Meldrum, R.A.; Tran-Nguyen, L.T.T.; Batley, J.; Aitken, E. Investigation of the diversity of effector genes in the banana pathogen, Fusarium oxysporum f. sp. cubense, reveals evidence of horizontal gene transfer. Mol. Plant. Pathol. 2018, 19, 1155–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, L.C.; Aroca, M.Á.; Laureano-Marín, A.M.; Moreno, I.; García, I.; Gotor, C. Cysteine and cysteine-related signaling pathways in Arabidopsis thaliana. Mol. Plant. 2014, 7, 264–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant. Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.; Yaya, E.E.; Glawischnig, E. The phytoalexins from cultivated and wild crucifers: Chemistry and biology. Nat. Prod. Rep. 2011, 28, 1381–1405. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Masood, A.; Khan, M.I.; Asgher, M.; Fatma, M.; Khan, N.A. Cross-talk between sulfur assimilation and ethylene signaling in plants. Plant. Signal. Behav. 2013, 8, e22478. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Martin, M.C.; Becana, M.; Romero, L.C.; Gotor, C. Knocking out cytosolic cysteine synthesis compromises the antioxidant capacity of the cytosol to maintain discrete concentrations of hydrogen peroxide in Arabidopsis. Plant. Physiol. 2008, 147, 562–572. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, C.; Bermúdez, M.A.; Romero, L.C.; Gotor, C.; Garcia, I. Cysteine homeostasis plays an essential role in plant immunity. New Phytol. 2012, 193, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Hiruma, K.; Fukunaga, S.; Bednarek, P.; Pislewska-Bednarek, M.; Watanabe, S.; Narusaka, Y.; Shirasu, K.; Takano, Y. Glutathione and tryptophan metabolism are required for Arabidopsis immunity during the hypersensitive response to hemibiotrophs. Proc. Natl. Acad. Sci. USA 2013, 110, 9589–9594. [Google Scholar] [CrossRef] [Green Version]



| Accession | Num. of Significant Matches | Description | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F1GA-1 | F1GA-2 | F1GR-1 | F1GR-2 | F4GA-1 | F4GA-2 | F4GR-1 | F4GR-2 | ||
| EMT72692.1 | 11 | 8 | 72 | 21 | O-acetylhomoserine (thiol)-lyase | ||||
| EMT64128.1 | 9 | 11 | 40 | 6 | S-formylglutathione hydrolase | ||||
| EMT70691.1 | 19 | 17 | 20 | 8 | Malate dehydrogenase, mitochondrial | ||||
| EMT64225.1 | 3 | 8 | 3 | 8 | 31 | 4 | Putative beta-glucosidase A | ||
| EMT66678.1 | 19 | 11 | 12 | 19 | 7 | SDO1-like protein C21C3.19 | |||
| EMT68479.1 | 13 | 33 | 2 | Phosphoserine aminotransferase | |||||
| EMT70909.1 | 15 | 27 | 5 | Dihydroxy-acid dehydratase | |||||
| EMT63756.1 | 2 | 27 | 4 | Cys-Gly metallodipeptidase dug1 | |||||
| EMT68383.1 | 11 | 5 | 19 | 7 | hypothetical protein FOC4_g10012800 | ||||
| EMT66833.1 | 10 | 4 | 17 | 8 | Cytochrome c peroxidase, mitochondrial | ||||
| EMT64384.1 | 26 | 2 | hypothetical protein FOC4_g10010883 | ||||||
| EMT68365.1 | 4 | 21 | 5 | 17 | 6 | hypothetical protein FOC4_g10012782 | |||
| EMT60820.1 | 8 | 10 | 5 | 14 | 5 | Putative branched-chain-amino-acid aminotransferase TOXF | |||
| EMT72882.1 | 9 | 2 | 18 | 4 | Putative alpha-N-arabinofuranosidase C | ||||
| EMT73358.1 | 10 | 12 | 12 | Ubiquitin-40S ribosomal protein S27a | |||||
| EMT68567.1 | 16 | 15 | 8 | Serine hydroxymethyltransferase, cytosolic | |||||
| EMT70711.1 | 16 | 19 | 3 | Putative Xaa-Pro aminopeptidase P | |||||
| EMT60464.1 | 4 | 9 | 8 | hypothetical protein FOC4_g10011735 | |||||
| EMT62355.1 | 5 | 3 | 7 | 9 | 4 | Histone H4 | |||
| EMT69138.1 | 7 | 14 | 6 | Cytochrome P450 55A1 | |||||
| EMT61471.1 | 5 | 12 | Aldehyde dehydrogenase | ||||||
| EMT63560.1 | 7 | 6 | 4 | Transketolase | |||||
| EMT69534.1 | 3 | 6 | 3 | 7 | 7 | hypothetical protein FOC4_g10003264 | |||
| EMT73283.1 | 4 | 2 | 3 | 9 | 6 | 2 | Cytochrome c | ||
| EMT61353.1 | 17 | 13 | 2 | hypothetical protein FOC4_g10014408 | |||||
| EMT68171.1 | 3 | 5 | 4 | 6 | Nucleoside diphosphate kinase | ||||
| EMT68727.1 | 13 | 2 | hypothetical protein FOC4_g10000306 | ||||||
| EMT65157.1 | 9 | 4 | 60S ribosomal protein L4-B | ||||||
| EMT60471.1 | 9 | 10 | 2 | Glutathione S-transferase omega-like 2 | |||||
| EMT66944.1 | 2 | 2 | 4 | Aldose reductase A | |||||
| EMT73111.1 | 4 | 6 | 5 | 3 | Coproporphyrinogen-III oxidase | ||||
| EMT62349.1 | 2 | 3 | 2 | 14-3-3 protein like protein | |||||
| EMT66456.1 | 3 | 2 | 4 | Inositol monophosphatase 2 | |||||
| EMT70972.1 | 4 | 2 | Fructose-bisphosphate aldolase | ||||||
| EMT66185.1 | 2 | 3 | Putative oxidoreductase C26H5.09c | ||||||
| EMT65501.1 | 2 | 2 | 40S ribosomal protein S18 | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Peng, C.; Zheng, X.; Chang, L.; Xu, B.; Tong, Z. Secretome Analysis of the Banana Fusarium Wilt Fungi Foc R1 and Foc TR4 Reveals a New Effector OASTL Required for Full Pathogenicity of Foc TR4 in Banana. Biomolecules 2020, 10, 1430. https://doi.org/10.3390/biom10101430
Wang D, Peng C, Zheng X, Chang L, Xu B, Tong Z. Secretome Analysis of the Banana Fusarium Wilt Fungi Foc R1 and Foc TR4 Reveals a New Effector OASTL Required for Full Pathogenicity of Foc TR4 in Banana. Biomolecules. 2020; 10(10):1430. https://doi.org/10.3390/biom10101430
Chicago/Turabian StyleWang, Dan, Cunzhi Peng, Xingmei Zheng, Lili Chang, Bingqiang Xu, and Zheng Tong. 2020. "Secretome Analysis of the Banana Fusarium Wilt Fungi Foc R1 and Foc TR4 Reveals a New Effector OASTL Required for Full Pathogenicity of Foc TR4 in Banana" Biomolecules 10, no. 10: 1430. https://doi.org/10.3390/biom10101430
APA StyleWang, D., Peng, C., Zheng, X., Chang, L., Xu, B., & Tong, Z. (2020). Secretome Analysis of the Banana Fusarium Wilt Fungi Foc R1 and Foc TR4 Reveals a New Effector OASTL Required for Full Pathogenicity of Foc TR4 in Banana. Biomolecules, 10(10), 1430. https://doi.org/10.3390/biom10101430




