Crosstalk between Opioid and Anti-Opioid Systems: An Overview and Its Possible Therapeutic Significance
Abstract
:1. Introduction
2. Opioid System and Reward
3. Opioid System and Drugs of Abuse
4. Anti-Opioids
4.1. MIF-1
4.2. Cholecystokinin
4.3. Nociceptin/Orphanin FQ (N/OFQ)
4.4. NPFF
5. Anti-Opioid Peptides: Potential Therapeutic Interest
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bodnar, R.J. Endogenous opiates and behavior: 2014. Peptides 2016, 75, 18–70. [Google Scholar] [CrossRef] [PubMed]
- Waldhoer, M.; Bartlett, S.E.; Whistler, J.L. Opioid receptors. Annu. Rev. Biochem. 2004, 73, 953–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhawan, B.N.; Cesselin, F.; Raghubir, R.; Reisine, T.; Bradley, P.B.; Portoghese, P.S.; Hamon, M. International Union of Pharmacology. XII. Classification of opioid receptors. Pharmacol. Rev. 1996, 48, 567–592. [Google Scholar] [PubMed]
- Gray, A.C.; Coupar, I.M.; White, P.J. Comparison of opioid receptor distributions in the rat ileum. Life Sci. 2006, 78, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Wittert, G.; Hope, P.; Pyle, D. Tissue distribution of opioid receptor gene expression in the rat. Biochem. Biophys. Res. Commun. 1996, 218, 877–881. [Google Scholar] [CrossRef]
- Dickenson, A.H.; Kieffer, B.L. Opiates: Basic mechanisms. In Text-Book of Pain, 5th ed.; McMahon, S.B., Koltzenburg, M., Eds.; Elsevier: London, UK, 2005; pp. 427–442. [Google Scholar]
- Zöllner, C.; Stein, C. Opioids. Handb. Exp. Pharmacol. 2007, 177, 31–63. [Google Scholar]
- Akil, H.; Owens, C.; Gutstein, H.; Taylor, L.; Curran, E.; Watson, S. Endogenous opioids: Overview and current issues. Drug Alcohol Depend. 1998, 51, 127–140. [Google Scholar] [CrossRef]
- Kieffer, B.L. Recent advances in molecular recognition and signal transduction of active peptides: Receptors for opioid peptides. Cell Mol. Neurobiol. 1995, 15, 615–635. [Google Scholar] [CrossRef]
- Mansour, A.; Khachaturian, H.; Lewis, M.E.; Akil, H.; Watson, S.J. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J. Neurosci. 1987, 7, 2445–2464. [Google Scholar]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of addiction. Neuropsychopharmacology 2010, 35, 217–238. [Google Scholar] [CrossRef] [Green Version]
- Lutz, P.E.; Kieffer, B.L. The multiple facets of opioid receptor function: Implications for addiction. Curr. Opin. Neurobiol. 2013, 23, 473–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shippenberg, T.S.; Zapata, A.; Chefer, V.I. Dynorphin and the pathophysiology of drug addiction. Pharmacol. Ther. 2007, 116, 306–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wee, S.; Koob, G.F. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology 2010, 210, 121–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shippenberg, T.S.; Elmer, G.I. The neurobiology of opiate reinforcement. Crit. Rev. Neurobiol. 1998, 12, 267–303. [Google Scholar] [CrossRef] [PubMed]
- Van Ree, J.M.; Niesink, R.J.; Van Wolfswinkel, L.; Ramsey, N.F.; Kornet, M.M.; Van Furth, W.R.; Vanderschuren, L.J.; Gerrits, M.A.; Van den Berg, C.L. Endogenous opioids and reward. Eur. J. Pharmacol. 2000, 405, 89–101. [Google Scholar] [CrossRef]
- Kieffer, B.L.; Gaveriaux-Ruff, C. Exploring the opioid system by gene knockout. Prog. Neurobiol. 2002, 66, 285–306. [Google Scholar] [CrossRef]
- Shippenberg, T.S.; LeFevour, A.; Chefer, V.I. Targeting endogenous mu- and delta-opioid receptor systems for the treatment of drug addiction. CNS Neurol. Disord. Drug Targets 2008, 7, 442–453. [Google Scholar] [CrossRef] [Green Version]
- Di Chiara, G.; Imperato, A. Ethanol preferentially stimulates dopamine release in the nucleus accumbens of freely moving rats. Eur. J. Pharmacol. 1985, 115, 131–132. [Google Scholar] [CrossRef]
- Fibiger, H.C.; Phillips, A.G. Mesocorticolimbic dopamine systems and reward. Ann. N. Y. Acad. Sci. 1988, 537, 206–215. [Google Scholar] [CrossRef]
- Bechtholt, A.J.; Cunningham, C.L. Ethanol-induced conditioned place preference is expressed through a ventral tegmental area dependent mechanism. Behav. Neurosci. 2005, 119, 213–223. [Google Scholar] [CrossRef]
- Gianoulakis, C. The effect of ethanol on the biosynthesis and regulation of opioid peptides. Experientia 1989, 45, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Herz, A. Endogenous opioid systems and alcohol addiction. Psychopharmacology 1997, 129, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Heyser, C.J.; Roberts, A.J.; Schulteis, G.; Koob, G.F. Central administration of an opiate antagonist decreases oral ethanol self-administration in rats. Alcohol Clin. Exp. Res. 1999, 23, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Hyytia, P.; Kiianmaa, K. Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcohol Clin. Exp. Res. 2001, 25, 25–33. [Google Scholar] [CrossRef] [PubMed]
- June, H.L.; Cummings, R.; Eiler, W.J., 2nd; Foster, K.L.; McKay, P.F.; Seyoum, R.; Garcia, M.; McCane, S.; Grey, C.; Hawkins, S.E.; et al. Central opioid receptors differentially regulate the nalmefene-induced suppression of ethanol-and saccharin-reinforced behaviors in alcohol-preferring (P) rats. Neuropsychopharmacology. 2004, 29, 285–299. [Google Scholar] [CrossRef] [Green Version]
- Margolis, E.B.; Fields, H.L.; Hjelmstad, G.O.; Mitchell, J.M. Delta-opioid receptor expression in the ventral tegmental area protects against elevated alcohol consumption. J. Neurosci. 2008, 28, 12672–12681. [Google Scholar] [CrossRef] [Green Version]
- Contet, C.; Kieffer, B.L.; Befort, K. Mu opioid receptor: A gateway to drug addiction. Curr. Opin. Neurobiol. 2004, 14, 370–378. [Google Scholar] [CrossRef]
- Gianoulakis, C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr. Top. Med. Chem. 2009, 9, 999–1015. [Google Scholar] [CrossRef]
- Koob, G.F. Neuroadaptive mechanisms of addiction: Studies on the extended amygdala. Eur. Neuropsychopharmacol. 2003, 13, 442–452. [Google Scholar] [CrossRef]
- Moldow, R.L.; Fischman, A.J. Cocaine induced secretion of ACTH, beta-endorphin, and corticosterone. Peptides 1987, 8, 819–822. [Google Scholar] [CrossRef]
- Daunais, J.B.; Roberts, D.C.; McGinty, J.F. Cocaine self-administration increases preprodynorphin, but not c-fos, mRNA in rat striatum. Neuroreport 1993, 4, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Daunais, J.B.; McGinty, J.F. Cocaine binges differentially alter striatal preprodynorphin and zif/268 mRNAs. Brain Res. Mol. Brain Res. 1995, 29, 201–210. [Google Scholar] [CrossRef]
- Hurd, Y.L.; Brown, E.E.; Finlay, J.M.; Fibiger, H.C.; Gerfen, C.R. Cocaine self-administration differentially alters mRNA expression of striatal peptides. Brain Res. Mol. Brain Res. 1992, 13, 165–170. [Google Scholar] [CrossRef]
- Sivam, S.P. Cocaine selectively increases striatonigral dynorphin levels by a dopaminergic mechanism. J. Pharmacol. Exp. Ther. 1989, 250, 818–824. [Google Scholar]
- Smiley, P.L.; Johnson, M.; Bush, L.; Gibb, J.W.; Hanson, G.R. Effects of Cocaine on Extrapyramidal and Limbic Dynorphin Systems. J. Pharmacol. Exp. Ther. 1990, 253, 938–943. [Google Scholar] [PubMed]
- Hammer, R.P. Cocaine alters opiate receptor binding in critical brain reward regions. Synapse 1989, 3, 55–60. [Google Scholar] [CrossRef]
- Izenwasser, S.; Heller, B.; Cox, B.M. Continuous cocaine administration enhances mu- but not delta-opioid receptor-mediated inhibition of adenylyl cyclase activity in nucleus accumbens. Eur. J. Pharmacol. 1996, 297, 187–191. [Google Scholar] [CrossRef]
- Schroeder, J.A.; Niculescu, M.; Unterwald, E.M. Cocaine alters mu but not delta or kappa opioid receptor-stimulated in situ [35S]GTPgammaS binding in rat brain. Synapse 2003, 47, 26–32. [Google Scholar] [CrossRef]
- Unterwald, E.M.; Rubenfeld, J.M.; Kreek, M.J. Repeated cocaine administration upregulates κ and µ, but not δ, opioid receptors. Neuroreport 1994, 5, 1613–1616. [Google Scholar] [CrossRef]
- Unterwald, E.M.; Cox, B.M.; Kreek, M.J.; Cote, T.E.; Izenwasser, S. Chronic repeated cocaine administration alters basal and opioid-regulated adenylyl cyclase activity. Synapse 1993, 15, 33–38. [Google Scholar] [CrossRef]
- Heidbreder, C.A.; Goldberg, S.R.; Shippenberg, T.S. The kappa-opioid receptor agonist U-69593 attenuates cocaine-induced behavioral sensitization in the rat. Brain Res. 1993, 616, 335–338. [Google Scholar] [CrossRef]
- Shippenberg, T.S.; LeFevour, A.; Heidbreder, C. kappa-Opioid receptor agonists prevent sensitization to the conditioned rewarding effects of cocaine. J. Pharmacol. Exp. Ther. 1996, 276, 545–554. [Google Scholar] [PubMed]
- Shippenberg, T.S.; Rea, W. Sensitization to the behavioral effects of cocaine: Modulation by dynorphin and kappa-opioid receptor agonists. Pharmacol. Biochem. Behav. 1997, 57, 449–455. [Google Scholar] [CrossRef]
- Zhang, Y.; Butelman, E.R.; Schlussman, S.D.; Ho, A.; Kreek, M.J. Effect of the endogenous kappa opioid agonist dynorphin A(1-17) on cocaine-evoked increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology 2004, 172, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Mathon, D.S.; Lesscher, H.M.; Gerrits, M.A.; Kamal, A.; Pintar, J.E.; Schuller, A.G.; Spruijt, B.M.; Burbach, J.P.; Smidt, M.P.; van Ree, J.M.; et al. Increased GABAergic input to ventral tegmental area dopaminergic neurons associated with decreased cocaine reinforcement in mu-opioid receptor knockout mice. Neuroscience 2005, 130, 359–367. [Google Scholar] [CrossRef]
- Yoo, J.H.; Yang, E.M.; Lee, S.Y.; Loh, H.H.; Ho, I.K.; Jang, C.G. Differential effects of morphine and cocaine on locomotor activity and sensitization in mu-opioid receptor knockout mice. Neurosci. Lett. 2003, 344, 37–40. [Google Scholar] [CrossRef]
- Becker, A.; Grecksch, G.; Kraus, J.; Loh, H.H.; Schroeder, H.; Hollt, V. Rewarding effects of ethanol and cocaine in µ opioid receptor-deficient mice. Naunyn Schmiedebergs. Arch. Pharmacol. 2002, 365, 296–302. [Google Scholar] [CrossRef]
- Hall, F.S.; Goeb, M.; Li, X.F.; Sora, I.; Uhl, G.R. mu-Opioid receptor knockout mice display reduced cocaine conditioned place preference but enhanced sensitization of cocaine-induced locomotion. Brain Res. 2004, 121, 123–130. [Google Scholar]
- Contarino, A.; Kitchener, P.; Vallée, M.; Papaleo, F.; Piazza, P.V. CRF1 receptor-deficiency increases cocaine reward. Neuropharmacology 2017, 117, 41–48. [Google Scholar] [CrossRef]
- Rademacher, D.J.; Steinpreis, R.E. Effects of the selective mu(1)-opioid recep-tor antagonist, naloxonazine, on cocaine-induced conditioned place preferenceand locomotor behavior in rats. Neurosci. Lett. 2002, 332, 159–162. [Google Scholar] [CrossRef]
- Ward, H.G.; Simansky, K.J. Chronic prevention of μ-opioid receptor (MOR) G-protein coupling in the pontine parabrachial nucleus persistently decreases consumption of standard but not palatable food. Psychopharmacology 2006, 187, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Menkens, K.; Bilsky, E.J.; Wild, K.D.; Portoghese, P.S.; Reid, L.D.; Porreca, F. Cocaine place preference is blocked by the delta-opioid receptor antagonist, naltrindole. Eur. J. Pharmacol. 1992, 219, 345–346. [Google Scholar] [CrossRef]
- Kotlinska, J.H.; Gibula-Bruzda, E.; Pachuta, A.; Kunce, D.; Witkowska, E.; Chung, N.N.; Schiller, P.W.; Izdebski, J. Influence of new deltorphin analogues on reinstatement of cocaine-induced conditioned place preference in rats. Behav. Pharmacol. 2010, 21, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Reid, L.D.; Glick, S.D.; Menkens, K.A.; French, E.D.; Bilsky, E.J.; Porreca, F. Cocaine self-administration and naltrindole, a delta-selective opioid antagonist. Neuroreport 1995, 6, 1409–1412. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Mori, T.; Tsuji, M.; Misawa, M.; Nagase, H. The role of delta-opioidreceptor subtypes in cocaine- and methamphetamine-induced place prefer-ences. Life Sci. 1994, 55, L339–L344. [Google Scholar] [CrossRef]
- Jones, D.N.; Bowen, W.D.; Portoghese, P.S.; Holtzman, S.G. Delta-opioid receptor antagonists attenuate motor activity induced by amphetamine but not cocaine. Eur. J. Pharmacol. 1993, 249, 167–177. [Google Scholar] [CrossRef]
- de Vries, T.J.; Babovic-Vuksanovic, D.; Elmer, G.; Shippenberg, T.S. Lack of involvement of delta-opioid receptors in mediating the rewarding effects of cocaine. Psychopharmacology 1995. 120, 442–448.
- Heidbreder, C.; Shoaib, M.; Shippenberg, T.S. Differential role of delta-opioid receptors in the development and expression of behavioral sensitization to cocaine. Eur. J. Pharmacol. 1996, 298, 207–216. [Google Scholar] [CrossRef]
- Shippenberg, T.S.; Heidbreder, C. Sensitization to the conditioned rewarding effects of cocaine: Pharmacological and temporal characteristics. J. Pharmacol. Exp. Ther. 1995, 273, 808–815. [Google Scholar]
- Berrendero, F.; Kieffer, B.L.; Maldonado, R. Attenuation of nicotine-inducedantinociception, rewarding effects, and dependence in mu-opioid receptorknock-out mice. J. Neurosci. 2002, 22, 10935–10940. [Google Scholar] [CrossRef]
- Berrendero, F.; Mendizabal, V.; Robledo, P.; Galeote, L.; Bilkei-Gorzo, A.; Zimmer, A.; Maldonado, R. Nicotine-induced antinociception, rewarding effects, andphysical dependence are decreased in mice lacking the preproenkephalin gene. J. Neurosci. 2005, 25, 1103–1112. [Google Scholar] [CrossRef] [Green Version]
- Trigo, J.M.; Zimmer, A.; Maldonado, R. Nicotine anxiogenic and rewarding effects are decreased in mice lacking beta-endorphin. Neuropharmacology 2009, 56, 1147–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galeote, L.; Berrendero, F.; Bura, S.A.; Zimmer, A.; Maldonado, R. Prodynorphin gene disruption increases the sensitivity to nicotine self-administration in mice. Int. J. Neuropsychopharmacol. 2009, 12, 615–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conte-Devolx, B.; Oliver, C.; Giraud, P.; Gillioz, P.; Castanas, E.; Lissitzky, J.C.; Boudouresque, F.; Millet, Y. Effect of nicotine on in vivo secretion of melanocorticotropic hormones in the rat. Life Sci. 1981, 28, 1067–1073. [Google Scholar] [CrossRef]
- Backon, J. Negative correlation of cigarette smoking and dysmenorrhea: Reduced prostaglandin synthesis due to beta-endorphin, nicotine, or acroleinantagonism. Med. Hypotheses 1989, 28, 213–214. [Google Scholar] [CrossRef]
- del Arbol, J.L.; Munoz, J.R.; Ojeda, L.; Cascales, A.L.; Irles, J.R.; Miranda, M.T.; RuizRequena, M.E.; Aguirre, J.C. Plasma concentrations of beta-endorphinin smokers who consume different numbers of cigarettes per day. Pharmacol. Biochem. Behav. 2000, 67, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, N.A.; Farr, L.A. Beta-endorphin response to an acute pain stimulus. J. Neurosci. Methods 2009, 177, 285–288. [Google Scholar] [CrossRef]
- Spaziante, R.; Merola, B.; Colao, A.; Gargiulo, G.; Cafiero, T.; Irace, C.; Rossi, E.; Oliver, C.; Lombardi, G.; Mazzarella, B.; et al. Beta-endorphin concentrations both in plasma and in cerebrospinal fluid in response to acute painful stimuli. J. Neurosurg. Sci. 1990, 34, 99–106. [Google Scholar]
- Wewers, M.E.; Dhatt, R.K.; Snively, T.A.; Tejwani, G.A. The effect of chronic administration of nicotine on antinociception, opioid receptor binding and met-enkelphalin levels in rats. Brain Res. 1999, 822, 107–113. [Google Scholar] [CrossRef]
- Dhatt, R.K.; Gudehithlu, K.P.; Wemlinger, T.A.; Tejwani, G.A.; Neff, N.H.; Hadji-constantinou, M. Preproenkephalin mRNA and methionine-enkephalin content are increased in mouse striatum after treatment with nicotine. J. Neurochem. 1995, 64, 1878–1883. [Google Scholar] [CrossRef]
- Houdi, A.A.; Dasgupta, R.; Kindy, M.S. Effect of nicotine use and withdrawal on brain preproenkephalin A mRNA. Brain Res. 1998, 799, 257–263. [Google Scholar] [CrossRef]
- Balerio, G.N.; Aso, E.; Berrendero, F.; Murtra, P.; Maldonado, R. Delta9-tetrahydrocannabinol decreases somatic and motivational manifestations of nicotine withdrawal in mice. Eur. J. Neurosci. 2004, 20, 2737–2748. [Google Scholar] [CrossRef] [PubMed]
- Krishnan-Sarin, S.; Rosen, M.I.; O’Malley, S.S. Naloxone challenge in smokers. Preliminary evidence of an opioid component in nicotine dependence. Arch. Gen. Psychiatry 1999, 56, 663–668. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, R.; Valverde, O.; Berrendero, F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006, 29, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Trigo, J.M.; Martin-García, E.; Berrendero, F.; Robledo, P.; Maldonado, R. The endogenous opioid system: A common substrate in drug addiction. Drug Alcohol Depend 2010, 108, 183–194. [Google Scholar] [CrossRef]
- Viganò, D.; Rubino, T.; Parolaro, D. Molecular and cellular basis of cannabinoid and opioid interactions. Pharmacol. Biochem. Behav. 2005, 81, 360–368. [Google Scholar] [CrossRef]
- Pickel, V.M.; Chan, J.; Kash, T.L.; Rodriguez, J.J.; MacKie, K. Compartment-specific localization of cannabinoid 1 (CB1) and mu opioid receptors in rat nucleus accumbens. Neuroscience 2004, 127, 101–112. [Google Scholar] [CrossRef]
- Salio, C.; Fischer, J.; Franzoni, M.F.; Mackie, K.; Kaneko, T.; Conrath, M. CB1-cannabinoid and mu opioid receptor colocalization on postsynaptic target in the rat dorsal horn. Neuroreport 2001, 12, 3689–3692. [Google Scholar] [CrossRef]
- Castañé, A.; Robledo, P.; Matifas, A.; Kieffer, B.L.; Maldonado, R. Cannabinoid withdrawal syndrome is reduced in double mu and delta opioid receptor knock-out mice. Eur. J. Neurosci. 2003, 17, 155–159. [Google Scholar] [CrossRef]
- Ghozland, S.; Matthes, H.W.; Simonin, F.; Filliol, D.; Kieffer, B.L.; Maldonado, R. Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. J. Neurosci. 2002, 22, 1146–1154. [Google Scholar] [CrossRef] [Green Version]
- Justinova, Z.; Tanda, G.; Munzar, P.; Goldberg, S.R. The opioid antagonist naltrexone reduces the reinforcing effects of Delta 9 tetrahydrocannabinol (THC)in squirrel monkeys. Psychopharmacology 2004, 173, 186–194. [Google Scholar]
- Navarro, M.; Carrera, M.R.; Fratta, W.; Valverde, O.; Cossu, G.; Fattore, L.; Chowen, J.A.; Gomez, R.; del Arco, I.; Villanua, M.A.; et al. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J. Neurosci. 2001, 21, 5344–5350. [Google Scholar] [CrossRef]
- Spano, M.S.; Fattore, L.; Cossu, G.; Deiana, S.; Fadda, P.; Fratta, W. CB1 receptor agonist and heroin, but not cocaine, reinstate cannabinoid-seeking behaviour in the rat. Br. J. Pharmacol. 2004, 143, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Chowen, J.; Rocío, A.; Carrera, M.; del Arco, I.; Villanúa, M.A.; Martin, Y.; Roberts, A.J.; Koob, G.F.; de Fonseca, F. R CB1 cannabinoid receptor antagonist-induced opiate withdrawal in morphine-dependent rats. Neuroreport 1998, 9, 3397–3402. [Google Scholar] [CrossRef] [PubMed]
- Kastin, A.J.; Olson, R.D.; Ehrensing, R.H.; Berzas, M.C.; Schally, A.V.; Coy, D.H. MIF-I’s differential actions as an opiate antagonist. Pharmacol. Biochem. Behav. 1979, 11, 721–723. [Google Scholar] [CrossRef]
- Erchegyi, J.; Kastin, A.J.; Zadina, J.E. Isolation of a novel tetrapeptide with opiate and antiopiate activity from human brain cortex: Tyr-Pro-Trp-Gly-NH2 (Tyr-W-MIF-1). Peptides 1992, 13, 623–631. [Google Scholar] [CrossRef]
- Galina, Z.H.; Kastin, A.J. Existence of antiopiate systems as illustrated by MIF-I/Tyr-MIF-I. Minireview. Life Sci. 1986, 39, 2153–2159. [Google Scholar] [CrossRef]
- Zadina, J.E.; Kastin, A.J. Interactions between the antiopiate Tyr-MIF-1 and the mu opiate morphiceptin at their respective binding sites in brain. Peptides 1985, 6, 965–970. [Google Scholar] [CrossRef]
- Zadina, J.E.; Kastin, A.J.; Kersh, D.; Wyatt, A. Tyr-MIF-1 and hemorphin can act as opiate agonists as well as antagonists in the guinea pig ileum. Life Sci. 1992, 51, 869–885. [Google Scholar] [CrossRef]
- Hackler, L.; Kastin, A.J.; Zadina, J.E. Isolation of a novel peptide with a unique binding profile from human brain cortex: Tyr-K-MIF-1 (Tyr-Pro-Lys-Gly-NH2). Peptides 1994, 15, 945–950. [Google Scholar] [CrossRef]
- Malin, D.R.; Zadina, J.E.; Lake, J.R.; Rogillio, R.B.; Leyva, J.E.; Benson, T.M.; Corriere, L.S.; Handunge, B.P.; Kastin, A.J. Tyr-MIF-I precipitates abstinence syndrome in morphine- dependent rats. Brain Res. 1993, 610, 169–171. [Google Scholar] [CrossRef]
- Reed, G.W.; Olson, G.A.; Olson, R.D. The Tyr-MIF-1 family of peptides. Neurosci. Biobehav. Rev. 1994, 18, 519–525. [Google Scholar] [CrossRef]
- Zadina, J.E.; Kastin, A.J. Interaction of Tyr-MIF-I at opiate receptor sites. Pharmacol. Biochem. Behav. 1986, 25, 1303–1305. [Google Scholar] [CrossRef]
- Kastin, A.J.; Stephens, E.; Ehrensing, R.H.; Fischman, A.J. Tyr-MIF- 1 acts as an opiate antagonist in the tail-flick test. Pharmucol. Biochem. Behav. 1984, 21, 937–941. [Google Scholar] [CrossRef]
- Kastin, A.J.; Stephens, E.; Zadina, J.E.; Coy, D.H.; Fischman, A.J. Tyr-MIF-I, identified in brain tissue, and its analogs are active in two models of antinociception. Pharmacol. Biochem. Behav. 1985, 23, 1045–1049. [Google Scholar] [CrossRef]
- Kavaliers, M.; Hirst, M. Inhibitory influences of MIF-1 (PLG) and Tyr-MIF-1 (YPLG) on aggression and defeat-induced analgesia in mice. Peptides 1986, 7, 1007–1010. [Google Scholar] [CrossRef]
- Galina, Z.H.; Kastin, A.J. MIF-1 antagonizes warm-, but not cold-water stress-induced analgesia: Dissociation from immobility. Peptides 1985, 6, 1109–1112. [Google Scholar] [CrossRef]
- Zadina, J.E.; Kastin, A.J.; Ge, L.J.; Gulden, H.; Bungart, K.J. Chronic, but not acute, administration of morphine alters antiopiate (Tyr-MIF-1) binding sites in rat brain. Life Sci. 1989, 44, 555–561. [Google Scholar] [CrossRef]
- Kenakin, T. Agonists, partial agonists, antagonists, inverse agonists and agonist/antagonists? Trends Pharmacol. Sci. 1987, 8, 423–426. [Google Scholar] [CrossRef]
- Pan, W.; Kastin, A.J. From MIF-1 to endomorphin: The Tyr-MIF-1 family of peptides. Peptides 2007, 28, 2411–2434. [Google Scholar] [CrossRef]
- Ehrensing, R.H.; Kastin, A.J.; Michell, G.F. Antagonism of morphine analgesia by prolyl-leucyl-glycinamide (MIF-1) in humans. Pharmacol. Biochem. Behav. 1984, 21, 975–978. [Google Scholar] [CrossRef]
- Kavaliers, M.; Innes, D.G. Sex differences in the effects of Tyr-MIF-1 on morphine- and stress-induced analgesia. Peptides 1992, 13, 1295–1297. [Google Scholar] [CrossRef]
- Tesky, G.C.; Kavaliers, M. Prolyl-leucyl-glycin-amide reduces aggression and blocks defeat-induced opioid analgesia in mice. Peptides 1985, 6, 165–167. [Google Scholar] [CrossRef]
- Ehrensing, R.H.; Michell, G.F.; Kastin, A.J. Similar antagonism of morphine analgesia by MIF-1 and naloxone in Carassius auratus. Pharmacol. Biochem. Behav. 1982, 17, 757–761. [Google Scholar] [CrossRef]
- Yehuda, S.; Kastin, A.J.; Coy, D.H. Antagonistic actions of MIF-I on the hypothermia and hypomotility induced by beta-endorphin or morphine. Int. J. Neurosci. 1980, 11, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.X.; Hu, W.L.; Cong, B.; Ma, C.L.; Ni, Z.Y.; Niu, Z.Q.; Yu, L. Expressions of μ opioid receptor and CCK receptor in rat primary hippocampal neurons and effect of chronic morphine exposure on them. J. Fourth MilMed. Univ. 2007, 28, 1214–1217. [Google Scholar]
- Han, J.S. Cholecystokinin octapeptide (CCK-8): A negative feedback control mechanism for opioid analgesia. Prog. Brain Res. 1995, 105, 263–271. [Google Scholar]
- Han, N.L.; Luo, F.; Bian, Z.P.; Han, J.S. Synergistic effect of cholecystokinin octapeptide and angiotensin II in reversal of morphine induced analgesia in rats. Pain 2000, 85, 465–469. [Google Scholar] [CrossRef]
- Dourish, C.T.; O’Neill, M.F.; Coughlan, J.; Kitchener, S.J.; Hawley, D.; Iversen, S.D. The selective CCK-B receptor antagonist L-365,260 enhances morphine analgesia and prevents morphine tolerance in the rat. Eur. J. Pharmacol. 1990, 176, 35–44. [Google Scholar] [CrossRef]
- Magnuson, D.S.; Sullivan, A.F.; Simonnet, G.; Roques, B.P.; Dickenson, A.H. Differential interactions of cholecystokinin and FLFQPQRF-NH2 with mu and delta opioid antinociception in the rat spinal cord. Neuropeptides 1990, 16, 213–218. [Google Scholar] [CrossRef]
- Watanabe, H.; Nakayama, D.; Ito, K.; Watanabe, C.; Mizoguchi, H.; Fujimura, T.; Murayama, K.; Kawamura, S.; Sato, T.; Sakurada, C.; et al. Tyr-W-MIF-1 analog containing D-Pro2 acts as a selective mu2-opioid receptor antagonist in the mouse. J. Pharmacol. Exp. Ther. 2005, 312, 1075–1081. [Google Scholar] [CrossRef] [Green Version]
- Hebb, A.L.; Poulin, J.F.; Roach, S.P.; Zacharko, R.M.; Drolet, G. Cholecystokin in andendogenous opioid peptides: Interactive influence on pain, cognition, and emotion. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005, 29, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Calo’, G.; Guerrini, R.; Rizzi, A.; Salvadori, S.; Regoli, D. Pharmacology of nociceptin and its receptor: A novel therapeutic target. Br. J. Pharmacol. 2000, 129, 1261–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, J.H.; Xu, W.; Fang, Y.; Mogil, J.S.; Grisel, J.E.; Grandy, D.K.; Han, J.S. Bidirectional modulatory effect of orphanin FQ on morphine-induced analgesia: Antagonism in brain and potentiation in spinal cord of the rat. Br. J. Pharmacol. 1997, 120, 676–680. [Google Scholar] [CrossRef] [Green Version]
- Byford, A.J.; Anderson, A.; Jones, P.S.; Palin, R.; Houghton, A.K. The hypnotic, electroencephalographic, and antinociceptive properties of nonpeptide ORL1 receptor agonists after intravenous injection in rodents. Anesth. Analg. 2007, 104, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.P.; Lee, Y.; Maidment, N.T. Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res. 1999, 832, 168–170. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Panocka, I.; Polidori, C.; Regoli, D.; Massi, M. Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacology 1999, 141, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Cremeans, C.M.; Gruley, E.; Kyle, D.J.; Ko, M.C. Roles of μ-opioid receptors and nociceptin/orphanin FQ peptide receptors in buprenorphine-induced physiological responses in primates. J. Pharmacol. Exp. Ther. 2012, 343, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Devine, D.P.; Reinscheid, R.K.; Monsma, F.J., Jr.; Civelli, O.; Akil, H. The novel neuropeptide orphanin FQ fails to produce conditioned place preference or aversion. Brain Res. 1996, 727, 225–229. [Google Scholar] [CrossRef]
- Cesselin, F. Opioid and anti-opioid peptides. Fundam. Clin. Pharmacol. 1995, 9, 409–433. [Google Scholar] [CrossRef]
- Rothman, R.B. A review of the role of anti-opioid peptides in morphine tolerance and dependence. Synapse 1992, 12, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Panula, P.; Kalso, E.; Nieminen, M.; Kontinen, V.K.; Brandt, A.; Pertovaara, A. Neuropeptide FF and modulation of pain. Brain Res. 1999, 848, 191–196. [Google Scholar] [CrossRef]
- Bonini, J.A.; Jones, K.A.; Adham, N.; Forray, C.; Artymyshyn, R.; Durkin, M.M.; Smith, K.E.; Tamm, J.A.; Boteju, L.W.; Lakhlani, P.P.; et al. Identification and characterization of two G protein-coupled receptors for neuropeptide FF. J. Biol. Chem. 2000, 275, 39324–39331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, M.M.; Grisel, J.E.; Robbins, C.S.; Grandy, D.K. Antinociception mediated by the periaqueductal gray is attenuated by orphanin FQ. Neuroreport 1997, 8, 3431–3434. [Google Scholar] [CrossRef]
- Wang, J.Q.; Fibuch, E.E.; Sakurada, S.; Han, J.S. Anti-opioid peptides. In Biologically Active Peptides; Elsevier: Amsterdam, The Netherlands, 2006; Chapter 187; pp. 1345–1350. [Google Scholar]
- Zamfirova, R.; Bocheva, A.; Dobrinova, Y.; Todorov, S. Study on the antinociceptive action of Tyr-K-MIF-1, a peptide from the MIF family. Auton. Autacoid. Pharmacol. 2007, 27, 93–98. [Google Scholar] [CrossRef]
- Million, M.; Fioramonti, J.; Bueno, L. Oral administration of Tyr-MIF-1 stimulates gastric emptying and gastrointestinal motility in rodents. Peptides 1992, 13, 469–474. [Google Scholar] [CrossRef]
- Million, M.; Fioramonti, J.; Bueno, L. Central administration of Tyr-MIF-1 stimulates gastrointestinal motility in rats: Evidence for the involvement of dopamine, sigma and CCK receptors. Neuropeptides 1994, 26, 77–85. [Google Scholar] [CrossRef]
- Dufresne, M.; Seva, C.; Fourmy, D. Cholecystokinin and gastrin receptors. Physiol. Rev. 2006, 86, 805–847. [Google Scholar] [CrossRef] [Green Version]
- Ma, K.T.; Si, J.Q.; Zhang, Z.Q.; Zhao, L.; Fan, P.; Jin, J.L.; Li, X.Z.; Zhu, L. Modulatory effect of CCK-8S on GABA-induced depolarization from rat dorsal root ganglion. Brain Res. 2006, 1121, 66–75. [Google Scholar] [CrossRef]
- Rehfeld, J.F.; Friis-Hansen, L.; Goetze, J.P.; Hansen, T.V. The biology of cholecystokinin and gastrin peptides. Curr. Top. Med. Chem. 2007, 7, 1154–1165. [Google Scholar] [CrossRef]
- Dauge, V.; Sebret, A.; Beslot, F.; Matsui, T.; Roques, B.P. Behavioral profile of CCK2 receptor-deficient mice. Neuropsychopharmacology 2001, 25, 690–698. [Google Scholar] [CrossRef]
- Moran, T.H.; Schwartz, G.J. Neurobiology of cholecystokinin. Crit. Rev. Neurobiol. 1994, 9, 1–28. [Google Scholar] [PubMed]
- Pommier, B.; Beslot, F.; Simon, A.; Pophillat, M.; Matsui, T.; Dauge, V.; Roques, B.P.; Noble, F. Deletion of CCK2 receptor in mice results in an upregulation of the endogenous opioid system. J. Neurosci. 2002, 22, 2005–2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.J.; Fan, S.G.; Ren, M.F.; Han, J.S. Cholecystokinin-8 suppressed 3H-etorphine binding to rat brain opiate receptors. Life Sci. 1989, 45, 117–123. [Google Scholar] [CrossRef]
- Hill, D.R.; Campbell, N.J.; Shaw, T.M.; Woodruff, G.N. Autoradiographic localization and biochemical characterization of peripheral type CCK receptors in rat CNS using highly selective nonpeptide CCK antagonists. J. Neurosci. 1987, 7, 2967–2976. [Google Scholar] [CrossRef] [Green Version]
- Moran, T.H.; Robinson, P.H.; Goldrich, M.S.; McHugh, P.R. Two brain cholecystokinin receptors: Implications for behavioral actions. Brain Res. 1986, 362, 175–179. [Google Scholar] [CrossRef]
- Crawley, J.N. Modulation of mesolimbic dopaminergic behaviors by cholecystokinin. Ann. N. Y. Acad Sci. 1988, 537, 380–396. [Google Scholar] [CrossRef]
- Gaviraghi, G.; Feriani, A.; Marien, M.; Trist, D. Central cholecystokinin receptors: Opportunity for drug discovery. Pharmacochem. Libr. 1992, 18, 345–365. [Google Scholar]
- Faris, P.L.; Komisaruk, B.R.; Watkins, L.R.; Mayer, D.J. Evidence for the neuropeptide cholecystokinin as an antagonist of opiate analgesia. Science 1983, 219, 310–312. [Google Scholar] [CrossRef]
- Benoliel, J.J.; Mauborgne, A.; Bourgoin, S.; Legrand, J.C.; Hamon, M.; Cesselin, F. Opioid control of the in vitro release of cholecystokinin-like material from the rat substantia nigra. J. Neurochem. 1992, 58, 916–922. [Google Scholar] [CrossRef]
- You, Z.B.; Tzschentke, T.M.; Brodin, E.; Wise, R.A. Electrical stimulation of the prefrontal cortex increases cholecystokinin, glutamate, and dopamine release in the nucleus accumbens: An in vivo microdialysis study in freely moving rats. J. Neurosci. 1998, 18, 6492–6500. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, R.E.; Sacristán, M.P. In vivo release of CCK-8 from the dorsal horn of the rat: Inhibition by DAGOL. FEBS Lett. 1989, 250, 215–217. [Google Scholar] [CrossRef] [Green Version]
- Stanfa, L.C.; Dickenson, A.H. Cholecystokinin as a factor in the enhanced potency of spinal morphine following carrageenin inflammation. Br. J. Pharmacol. 1993, 108, 967–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.J.; Han, J.S. Modification by cholecystokinin octapeptide of the binding of mu-, delta-, and kappa-opioid receptors. J. Neurochem. 1990, 55, 1379–1382. [Google Scholar] [CrossRef] [PubMed]
- Verge, V.M.K.; Wiesenfeld-Hallin, Z.; Hokfelt, T. Cholecystokinin in mammalian primary sensory neurons and spinal cord: In situ hybridization studies in rat and monkey. Eur. J. Neurosci. 1993, 5, 240–250. [Google Scholar] [CrossRef]
- Itoh, S.; Katsuura, G.; Maeda, Y. Caerulein and cholecystokinin suppress beta-endorphin- induced analgesia in the rat. Eur. J. Pharmacol. 1982, 80, 421–425. [Google Scholar] [CrossRef]
- Sebret, A.; Léna, I.; Crété, D.; Matsui, T.; Roques, B.P.; Daugé, V. Rat hippocampal neurons are critically involved in physiological improvement of memory processes induced by cholecystokinin-B receptor stimulation. J. Neurosci. 1999, 19, 7230–7237. [Google Scholar] [CrossRef]
- Mitchell, J.M.; Bergren, L.J.; Chen, K.S.; Fields, H.L. Cholecystokinin is necessary for the expression of morphine conditioned place preference. Pharmacol. Biochem. Behav. 2006, 85, 787–795. [Google Scholar] [CrossRef]
- Alttoa, A.; Harro, J. Effect of CCK1 and CCK2 receptor blockade on amphetamine-stimulated exploratory behavior and sensitization to amphetamine. Eur. Neuropsychopharmacol. 2004, 14, 324–331. [Google Scholar] [CrossRef]
- Lu, L.; Huang, M.; Ma, L.; Li, J. Different role of cholecystokinin (CCK)-A and CCK-B receptors in relapse to morphine dependence in rats. Behav. Brain Res. 2001, 120, 105–110. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, B.; Liu, Z.Y.; Zhang, Z.Y. Reactivation of cocaine conditioned place preference induced by stress is reversed by cholecystokinin-B receptors antagonist in rats. Brain. Res. 2002, 954, 132–140. [Google Scholar] [CrossRef]
- Wen, D.; Ma, C.L.; Cong, B.; Zhang, Y.J.; Yang, S.C.; Meng, Y.X.; Yu, F.; Ni, Z.Y.; Li, S.J. Effects of CCK-8 and its receptor antagonists given intracerebroventricularly on withdrawal symptom of morphine dependent rats. Chin. Pharmacol. Bull. 2011, 27, 1368–1373. [Google Scholar]
- Crespi, F. The role of cholecystokinin (CCK), CCK-A or CCK-B receptor antagonists in the spontaneous preference for drugs of abuse (alcohol or cocaine) in naive rats. Methods Find. Exp. Clin. Pharmacol. 1998, 20, 679–697. [Google Scholar] [CrossRef]
- Crespi, F.; Corsi, M.; England, T.; Ratti, E.; Trist, D.G.; Gaviraghi, G. Spontaneous preference for ethanol in naive rats is influenced by cholecystokinin A receptor antagonism. Alcohol 1997, 14, 327–332. [Google Scholar] [CrossRef]
- Vasar, E.; Harro, J.; Pold, A.; Lang, A. CCK receptors and anxiety in rats. In Multiple Cholecystokinin Receptors in the CNS; Dourish, C.T., Cooper, S.J., Iversen, S.D., Iversen., L.L., Eds.; Oxford University Press: Oxford, UK, 1992; pp. 143–148. [Google Scholar]
- Kulkosky, P.J.; Clayborne, Y.J.; Sandoval, S.L. Cholecystokinin and bombesin inhibit ethanol and food intake in rats selectively bred for ethanol sensitivity. Alcohol Clin. Exp. Res. 1993, 17, 545–551. [Google Scholar] [CrossRef]
- Noble, F.; Blommaert, A.; Fournie-Zaluski, M.C.; Roques, B.P. A selective CCK-B receptor antagonist potentiates mu-, but not delta-opioid receptor-mediated antinociception in the formalin test. Eur. J. Pharmacol. 1995, 273, 145–151. [Google Scholar] [CrossRef]
- Wiesenfeld-Hallin, Z.; Xu, X.-J.; Hughes, J.; Horwell, D.C.; Ho¨kfelt, T. PD134308, a selective antagonist of cholecystokinin type-B receptor, enhances the analgesic effect of morphine and synergistically interacts with intrathecal galanin to depress spinal nociceptive reflexes. Proc. Natl. Acad. Sci. USA 1990, 87, 7105–7109. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Sun, Y.H.; Zhang, Z.W.; Han, J.S. Increased release of immunoreactive cholecystokinin octapeptide by morphine and potentiation of m-opioid analgesia by CCKB receptor antagonist L365,260 in rat spinal cord. Eur. J. Pharmacol. 1993, 234, 147–154. [Google Scholar] [CrossRef]
- Lambert, D.G. The nociceptin/orphanin FQ receptor: A target with broad therapeutic potential. Nat. Rev. Drug Discov. 2008, 7, 694–710. [Google Scholar] [CrossRef]
- Meunier, J.C.; Mollereau, C.; Toll, L.; Suaudeau, C.; Moisand, C.; Alvinerie, P.; Butour, J.L.; Guillemot, J.C.; Ferrara, P.; Monsarrat, B. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 1995, 377, 532–535. [Google Scholar] [CrossRef]
- Reinscheid, R.K.; Nothacker, H.P.; Bourson, A.; Ardati, A.; Henningsen, R.A.; Bunzow, J.R.; Grandy, D.K.; Langen, H.; Monsma, F.J., Jr.; Civelli, O. Orphanin FQ: A neuropeptide that activates an opioidlike G protein-coupled receptor. Science 1995, 270, 792–794. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.M.; Christie, M.J.; Devi, L.; Toll, L.; Traynor, J.R. Challenges for opioid receptor nomenclature: IUPHAR Review 9. Br. J. Pharmacol. 2015, 172, 317–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gintzler, A.R.; Adapa, I.D.; Toll, L.; Medina, V.M.; Wang, L. Modulation of enkephalin release by nociceptin (orphanin FQ). Eur. J. Pharmacol. 1997, 325, 29–34. [Google Scholar] [CrossRef]
- Mogil, J.S.; Pasternak, G.W. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol. Rev. 2001, 53, 381–415. [Google Scholar] [PubMed]
- Mogil, J.S.; Grisel, J.E.; Zhangs, G.; Belknap, J.K.; Grandy, D.K. Functional antagonism of mu-, delta- and kappa-opioid antinociception by orphanin FQ. Neurosci. Lett. 1996, 214, 131–134. [Google Scholar] [CrossRef]
- Rizzi, A.; Cerlesi, M.C.; Ruzza, C.; Malfacini, D.; Ferrari, F.; Bianco, S.; Tommaso Costa, T.; Guerrini, R.; Claudio Trapella, C.; Calo, G. Pharmacological characterization of cebranopadol a novel analgesic acting as mixed nociceptin/orphanin FQ and opioid receptor agonist. Pharmacol. Res. Perspect. 2016, 4, e00247. [Google Scholar] [CrossRef]
- Rizzi, A.; Ruzza, C.; Bianco, S.; Trapella, C.; Calo, C. Antinociceptive action of NOP and opioid receptor agonists in the mouse orofacial formalin test. Peptides 2017, 94, 71–77. [Google Scholar] [CrossRef]
- Ko, M.C.; Woods, J.H.; Fantegrossi, W.E.; Galuska, C.M.; Wichmann, J.; Prinssen, E.P. Behavioral effects of a synthetic agonist selective for nociceptin/orphanin FQ peptide receptors in monkeys. Neuropsychopharmacology 2009, 34, 2088–2096. [Google Scholar] [CrossRef]
- Hu, E.; Calò, G.; Guerrini, R.; Ko, M.C. Long-lasting antinociceptive spinal effects in primates of the novel nociceptin/orphanin FQ receptor agonist UFP-112. Pain 2010, 148, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Grass, S.; Hao, J.; Xu, I.S.; Wiesenfeld-Hallin, Z. Nociceptin/orphanin FQ in spinal nociceptive mechanisms under normal and pathological conditions. Peptides 2000, 21, 1031–1036. [Google Scholar] [CrossRef]
- Courteix, C.; Coudoré-Civiale, M.A.; Privat, A.M.; Pélissier, T.; Eschalier, A.; Fialip, J. Evidence for an exclusive antinociceptive effect of nociceptin/orphanin FQ, an endogenous ligand for the ORL1 receptor, in two animal models of neuropathic pain. Pain 2004, 110, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Linz, K.; Christoph, T.; Tzschentke, T.M.; Koch, T.; Schiene, K.; Gautrois, M.; Schröder, W.; Kögel, B.Y.; Beier, H.; Englberger, W.; et al. Cebranopadol: A novel potent analgesic nociceptin/orphanin FQ peptide and opioid receptor agonist. J. Pharmacol. Exp. Ther. 2014, 349, 535–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salat, K.; Furgala, A.; Salat, R. Evaluation of cebranopadol, a dually acting nociceptin/orphanin FQ and opioid receptor agonist in mouse models of acute, tonic, and chemotherapy-induced neuropathic pain. Inflammopharmacology 2018, 26, 361–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzschentke, T.M.; Linz, L.; Frosch, S.; Christoph, T. Antihyperalgesic, Antiallodynic, and Antinociceptive Effects of Cebranopadol, a Novel Potent Nociceptin/Orphanin FQ and Opioid Receptor Agonist, after Peripheral and Central Administration in Rodent Models of Neuropathic Pain. Pain Pract. 2017, 17, 1032–1041. [Google Scholar] [CrossRef]
- Calo, G.; Lambert, D.G. Nociceptin/orphanin FQ receptor ligands and translational challenges: Focus on cebranopadol as an innovative analgesic. Br. J. Anaesth. 2018, 121, 1105–1114. [Google Scholar] [CrossRef] [Green Version]
- Tzschentke, T.M.; Kögel, B.Y.; Frosch, S.; Linz, K. Limited potential of cebranopadol to produce opioid-type physical dependence in rodents. Addict. Biol. 2018, 23, 1010–1019. [Google Scholar] [CrossRef]
- Lutfy, K.; Hossain, S.M.; Khaliq, I.; Maidment, N.T. Orphanin FQ/nociceptin attenuates the development of morphine tolerance in rats. Br. J. Pharmacol. 2001, 134, 529–534. [Google Scholar] [CrossRef] [Green Version]
- Lutfy, K.; Do, T.; Maidment, N.T. Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacology 2001, 154, 1–7. [Google Scholar] [CrossRef]
- Murphy, N.P.; Ly, H.T.; Maidment, N.T. Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience 1996, 75, 1–4. [Google Scholar] [CrossRef]
- Murphy, N.P.; Maidment, N.T. Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J. Neurochem. 1999, 73, 179–186. [Google Scholar] [CrossRef]
- Rutten, K.; De Vry, J.; Bruckmann, W.; Tzschentke, T.M. Pharmacological blockade or genetic knockout of the NOP receptor potentiates the rewarding effect of morphine in rats. Drug Alcohol Depend. 2011, 114, 253–256. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Angeletti, S.; Sanna, P.P.; Weiss, F.; Massi, M. Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur. J. Pharmacol. 2000, 404, 153–159. [Google Scholar] [CrossRef]
- Kotlinska, J.; Wichmann, J.; Legowska, A.; Rolka, K.; Silberring, J. Orphanin FQ/nociceptin but not Ro 65-6570 inhibits the expression of cocaine-induced conditioned place preference. Behav. Pharmacol. 2002, 13, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Kotlinska, J.; Rafalski, P.; Biala, G.; Dylag, T.; Rolka, K.; Silberring, J. Nociceptin inhibits acquisition of amphetamine-induced place preference and sensitization to stereotypy in rats. Eur. J. Pharmacol. 2003, 474, 233–239. [Google Scholar] [CrossRef]
- Zhao, R.J.; Woo, R.S.; Jeong, M.S.; Shin, B.S.; Kim, D.G.; Kim, K.W. Orphanin FQ/nociceptin blocks methamphetamine place preference in rats. Neuroreport 2003, 14, 2383–2385. [Google Scholar] [CrossRef]
- Walker, J.R.; Spina, M.; Terenius, L.; Koob, G.F. Nociceptin fails to affect heroin self-administration in the rat. Neuroreport 1998, 9, 2243–2247. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Economidou, D.; Fedeli, A.; Angeletti, S.; Weiss, F.; Heilig, M.; Massi, M. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the anti-opioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology 2004, 172, 170–178. [Google Scholar] [CrossRef] [Green Version]
- Kuzmin, A.; Kreek, M.J.; Bakalkin, G.; Liljequist, S. The nociceptin/orphanin FQ receptor agonist Ro 646198 reduces alcohol self-administration and prevents relapse-like alcohol drinking. Neuropsychopharmacology 2007, 32, 902–910. [Google Scholar] [CrossRef] [Green Version]
- Ciccocioppo, R.; de Guglielmo, G.; Hansson, A.C.; Ubaldi, M.; Kallupi, M.; Cruz, M.T.; Oleata, C.S.; Heilig, M.; Roberto, M. Restraint stress alters nociceptin/orphanin FQ and CRF systems in the rat central amygdala: Significance for anxiety-like behaviors. J. Neurosci. 2014, 34, 363–372. [Google Scholar] [CrossRef]
- de Guglielmo, G.; Martin-Fardon, R.; Teshima, K.; Ciccocioppo, R.; Weiss, F. MT-7716, a potent NOP receptor agonist, preferentially reduces ethanol seeking and reinforcement in post-dependent rats. Addict. Biol. 2015, 20, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Cippitelli, A.; Schoch, J.; Debevec, G.; Zaveri, N.T.; Toll, L. A key role for the N/OFQ-NOP receptor system in modulating nicotine taking in a model of nicotine and alcohol co-administration. Sci. Rep. 2016, 6, 26594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotlinska, J.; Suder, P.; Legowska, A.; Rolka, K.; Silberring, J. Orphanin FQ/nociceptin inhibits morphine withdrawal. Life Sci. 2000, 66, PL119–PL123. [Google Scholar] [CrossRef]
- Devine, D.P.; Taylor, L.; Reinscheid, R.K.; Monsma, F.J., Jr.; Civelli, O.; Akil, H. Rats rapidly develop tolerance to the locomotor-inhibiting effects of the novel neuropeptide orphanin FQ. Neurochem. Res. 1996, 21, 1387–1396. [Google Scholar] [CrossRef]
- Florin, S.; Suaudeau, C.; Meunier, J.C.; Costentin, J. Nociceptin stimulates locomotion and exploratory behaviour in mice. Eur. J. Pharmacol. 1996, 317, 9–13. [Google Scholar] [CrossRef]
- Narayanan, S.; Maidment, N.T. Orphanin FQ and behavioral sensitization to cocaine. Pharmacol. Biochem. Behav. 1999, 63, 271–277. [Google Scholar] [CrossRef]
- Yang, H.Y.T.; Fratta, W.; Majane, E.A.; Costa, E. Isolation, sequencing, synthesis and pharmacological characterization of two brain neuropeptides that modulate the action of morphine. Proc. Natl. Acud. Sci. USA 1985, 82, 7757–7761. [Google Scholar] [CrossRef] [Green Version]
- Gouardères, C.; Sutak, M.; Zajac, J.M.; Jhamandas, K. Antinociceptive effects of intrathecally administered FBFamide and FMRFamide in the rat. Eur. J. Pharmucol. 1993, 237, 73–81. [Google Scholar] [CrossRef]
- Malin, D.H.; Lake, J.R.; Hammond, M.V.; Fowler, D.E.; Rogillio, R.B.; Brown, S.L.; Sims, J.L.; Leecraft, B.M.; Yang, H.Y. FMRF-NH2-like mammalian octapeptide: Possible role in opiate dependence and abstinence. Peptides 1990, 11, 969–972. [Google Scholar] [CrossRef]
- Tang, J.; Yang, H.Y.; Costa, E. Inhibition of spontaneous and opi- ate modifiate nociception by an endogenous neuropeptide with Phe-Met-Arg-Phe-NH2-like immunoreactivity. Proc. Natl. Acad. Sci. USA 1984, 81, 5002–5005. [Google Scholar] [CrossRef] [Green Version]
- Panula, P.; Aarnisalo, A.A.; Wasowicz, K. Neuropeptide FF, a mammalian neuropeptide with multiple functions. Prog. Neurobiol. 1996, 48, 461–487. [Google Scholar] [CrossRef]
- Majane, E.A.; Zhu, J.; Aarnisalo, A.A.; Panula, P.; Yang, H.Y. Origin of neurohypophyseal neuropeptide-FF (FLFQPQRF-NH2). Endocrinology 1993, 133, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Kotani, M.; Detheux, M.; Vandenbogaerde, A.; Communi, D.; Vanderwinden, J.M.; Le Poul, E.; Brézillon, S.; Tyldesley, R.; Suarez-Huerta, N.; Vandeput, F.; et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J. Biol. Chem. 2001, 276, 34631–34636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cador, M.; Marco, N.; Stinus, L.; Simonnet, G. Interaction between neuropeptide FF and opioids in the ventral tegmental area in the behavioral response to novelty. Neuroscience 2002, 110, 309–318. [Google Scholar] [CrossRef]
- Liu, Q.; Guan, X.M.; Martin, W.J.; McDonald, T.P.; Clements, M.K.; Jiang, Q.; Zeng, Z.; Jacobson, M.; Williams, D.L., Jr.; Yu, H.; et al. Identification and characterization of novel mammalian neuropeptide FF-like peptides that attenuate morphine-induced antinociception. J. Biol. Chem. 2001, 276, 36961–36969. [Google Scholar] [CrossRef] [Green Version]
- Allard, M.; Geoffre, S.; Legendre, P.; Vincent, J.D.; Simonnet, G. Characterization of rat spinal cord receptors to FLFQPQRFa-mide, a mammalian morphine modulating peptide: A binding study. Brain Res. 1989, 500, 169–176. [Google Scholar] [CrossRef]
- Raffa, R.B.; Kim, A.; Rice, K.C.; De Costa, B.R.; Codd, E.E.; Rothman, R.B. Low Affinity of FMRFamide and Four FaRPs (FMRFamide-related Peptides), Including the Mammalian-Derived FaRPs F-8-Famide (NPFF) and A-18-Famide, for Opioid Mu, Delta, Kappa 1, Kappa 2a, or Kappa 2b Receptors. Peptides 1994, 15, 401–404. [Google Scholar] [CrossRef]
- Roumy, M.; Zajac, J.M. Neuropeptide FF, Pain and Analgesia. Eur. J. Pharmacol. 1998, 345, 1–11. [Google Scholar] [CrossRef]
- Kavaliers, M. Inhibitory influences of mammalian FMRF-amide (Phe-Met-Arg-Phe-amide)-related peptides on nociception and morphine- and stress-induced analgesia in mice. Neurosci. Lett. 1990, 115, 307–312. [Google Scholar] [CrossRef]
- Lake, J.R.; Hammond, M.V.; Shaddox, R.C.; Hunsicker, L.M.; Yang, H.Y.; Malin, D.H. IgG from neuropeptide FF antiserum reverses morphine tolerance in the rat. Neurosci. Lett. 1991, 132, 29–32. [Google Scholar] [CrossRef]
- Kavaliers, M.; Yang, H.Y. Effects of mammalian FMRF-NH2-related peptides and IgG from antiserum against them on aggression and defeat-induced analgesia in mice. Peptides 1991, 12, 235–239. [Google Scholar] [CrossRef]
- Kavaliers, M.; Yang, H.Y. IgG from antiserum against endogenous mammalian FMRF-NH, -related peptides augments morphine- and stress-induced analgesia in mice. Peptides 1989, 10, 741–745. [Google Scholar] [CrossRef]
- Malin, D.H.; Lake, J.R.; Leyva, J.E.; Hammond, M.V.; Rogillio, R.B.; Arcangeli, K.R.; Ludgate, K.; Moore, G.M.; Payza, K. Analog of neuropeptide FF attenuates morphine abstinence syndrome. Peptides 1991, 12, 1011–1014. [Google Scholar] [CrossRef]
- Devillers, J.P.; Labrouche, S.A.; Castes, E.; Simonnet, G. Release of neuropeptide FF, an anti-opioid peptide, in rat spinal cord slices is voltage- and Ca*+-sensitive. Possible involvement of P-type Ca2+ channels. J. Neurochem. 1995, 64, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Stinus, L.; Allard, M.; Gold, L.; Simonnet, G. Changes in CNS neuropeptide FF-like material, pain sensitivity, and opiate dependence following chronic morphine treatment. Peptides 1995, 16, 1235–1241. [Google Scholar] [CrossRef]
- Ballet, S.; Mauborgne, A.; Gouardères, C.; Bourgoin, A.S.; Zajac, J.M.; Hamon, M.; Cesselin, F. The neuropeptide FF analogue, 1DME, enhances in vivo met-enkephalin release from the rat spinal cord. Neuropharmacology 1999, 38, 1317–1324. [Google Scholar] [CrossRef]
- Marco, N.; Stinus, L.; Allard, M.; Le Moal, M.; Simonnet, G. Neuro- peptide FF receptors within the ventral mesencephalon and dopaminergic terminal areas: Localization and functional anti-opioid involvement. Neuroscience 1995, 64, 1035–1044. [Google Scholar] [CrossRef]
- Kersanté, F.; Wang, J.Y.; Chen, J.C.; Mollereau, C.; Zajac, J.M. Anti-opioid effects of neuropeptide FF receptors in the ventral tegmental area. Neurosci. Lett. 2011, 488, 305–309. [Google Scholar] [CrossRef]
- Kotlinska, J.; Pachuta, A.; Dylag, T.; Silberring, J. Neuropeptide FF (NPFF) reduces the expression of morphine- but not of ethanol-induced conditioned place preference in rats. Peptides 2007, 28, 2235–2242. [Google Scholar] [CrossRef]
- Kotlinska, J.H.; Gibula-Bruzda, E.; Koltunowska, D.; Raoof, H.; Suder, P.; Silberring, J. Modulation of neuropeptide FF (NPFF) receptors influences the expression of amphetamine-induced conditioned place preference and amphetamine withdrawal anxiety-like behavior in rats. Peptides 2012, 33, 156–163. [Google Scholar] [CrossRef]
- Simonnet, G.; Devillers, J.P.; Boisserie, F. Pain facilitory systems activated by opiate receptor stimulation: Possible role of NPFF, an anti-opioid peptide Regulatory. Peptides 1994, 54, 277–278. [Google Scholar] [CrossRef]
- Kotlinska, J.; Pachuta, A.; Silberring, J. Neuropeptide FF (NPFF) reduces the expression of cocaine-induced conditioned place preference and cocaine-induced sensitization in animals. Peptides 2008, 29, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Takino, T.; Koshikawa, N.; Miyamori, H.; Tanaka, M.; Sasaki, T.; Okada, Y.; Seiki, M.; Sato, H. Cleavage of metastasis suppressor gene product KiSS-1 protein/metastin by matrix metalloproteinases. Oncogene 2003, 22, 4617–4626. [Google Scholar] [CrossRef] [Green Version]
- Simonin, F.; Schmitt, M.; Laulin, J.P.; Laboureyras, E.; Jhamandas, J.H.; MacTavish, D.; Matifas, A.; Mollereau, C.; Laurent, P.; Parmentier, M.; et al. RF9, a potent and selective neuropeptide FF receptor antagonist, prevents opioid-induced tolerance associated with hyperalgesia. Proc. Natl. Acad. Sci. USA 2006, 103, 466–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milton, N.G. In Vitro Activities of Kissorphin, a Novel Hexapeptide KiSS-1 Derivative, in Neuronal Cells. J. Amino Acids 2012, 2012, 691463. [Google Scholar] [CrossRef] [PubMed]
- Gibula-Bruzda, E.; Marszalek-Grabska, M.; Gawel, K.; Trzcinska, R.; Silberring, J.; Kotlinska, J.H. The new kisspeptin derivative—Kissorphin (KSO)—Attenuates acute hyperlocomotion and sensitization induced by ethanol and morphine in mice. Alcohol 2017, 64, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Gibula-Tarlowska, E.; Grochecki, P.; Silberring, J.; Kotlinska, J.H. The kisspeptin derivative kissorphin reduces the acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in rats. Alcohol 2019, 81, 11–19. [Google Scholar] [CrossRef]
- Gibula-Tarlowska, E.; Kedzierska, E.; Piechura, K.; Silberring, J.; Kotlinska, J.H. The influence of a new derivate of kisspeptin-10—Kissorphin (KSO) on the rewarding effects of morphine in the conditioned place preference (CPP) test in male rats. Behav. Brain Res. 2019, 372, 112043. [Google Scholar] [CrossRef]
- Economidou, D.; Cippitelli, A.; Stopponi, S.; Braconi, S.; Clementi, S.; Ubaldi, M.; Martin-Fardon, R.; Weiss, F.; Massi, M.; Ciccocioppo, R. Activation of brain NOP receptors attenuates acute and protracted alcohol withdrawal symptoms in the rat. Alcohol Clin. Exp. Res. 2011, 35, 747–755. [Google Scholar] [CrossRef] [Green Version]
- Shoblock, J.R.; Wichmann, J.; Maidment, N.T. The effect of a systemically active ORL-1 agonist, Ro 64-6198, on the acquisition, expression, extinction, and reinstatement of morphine conditioned place preference. Neuropharmacology 2005, 49, 439–446. [Google Scholar] [CrossRef]
- Toll, L.; Bruchas, M.R.; Calo, G.; Cox, B.M.; Zaveri, N.T. Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems. Pharmacol. Rev. 2016, 68, 419–457. [Google Scholar] [CrossRef]
- Toledo, M.A.; Pedregal, C.; Lafuente, C.; Diaz, N.; Martinez-Grau, M.A.; Jimenez, A.; Benito, A.; Torrado, A.; Mateos, C.; Joshi, E.M.; et al. Discovery of a novel series of orally active nociceptin/orphanin FQ (NOP) receptor antagonists based on a dihydrospiro(piperidine-4,7’-thieno[2,3-c]pyran) scaffold. J. Med. Chem. 2014, 57, 3418–3429. [Google Scholar] [CrossRef] [PubMed]
- Rorick-Kehn, L.M.; Ciccocioppo, R.; Wong, C.J.; Witkin, J.M.; Martinez-Grau, M.A.; Stopponi, S.; Adams, B.L.; Katner, J.S.; Perry, K.W.; Toledo, M.A.; et al. A Novel, Orally Bioavailable Nociceptin Receptor Antagonist, LY2940094, Reduces Ethanol Self-Administration and Ethanol Seeking in Animal Models. Alcohol Clin. Exp. Res. 2016, 40, 945–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Post, A.; Smart, T.S.; Jackson, K.; Mann, J.; Mohs, R.; Rorick-Kehn, L.; Statnick, M.; Anton, R.; O’Malley, S.S.; Wong, C.J. Proof-of-Concept Study to Assess the Nociceptin Receptor Antagonist LY2940094 as a New Treatment for Alcohol Dependence. Alcohol Clin. Exp. Res. 2016, 40, 1935–1944. [Google Scholar] [CrossRef]
- Kallupi, M.; Scuppa, G.; de Guglielmo, G.; Calo, G.; Weiss, F.; Statnick, M.A.; Rorick-Kehn, L.M.; Ciccocioppo, R. Genetic Deletion of the Nociceptin/Orphanin FQ Receptor in the Rat Confers Resilience to the Development of Drug Addiction. Neuropsychopharmacology 2017, 42, 695–706. [Google Scholar] [CrossRef] [Green Version]
- Ciccocioppo, R.; Borruto, A.M.; Domi, A.; Teshima, K.; Cannella, N.; Weiss, F. NOP-related mechanisms in substance use disorders. Handb. Exp. Pharmacol. 2019, 254, 187–212. [Google Scholar]
- Ciccocioppo, R.; Economidou, D.; Rimondini, R.; Sommer, W.; Massi, M.; Heilig, M. Buprenorphine reduces alcohol drinking through activation of the nociceptin/orphanin FQ-NOP receptor system. Biol. Psychiatry 2007, 61, 4–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.; Kehner, G.B.; Cowan, A.; Liu-Chen, L.Y. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: Norbuprenorphine is a potent opioid agonist. J. Pharmacol. Exp. Ther. 2001, 297, 688–695. [Google Scholar]
- Wnendt, S.; Kruger, T.; Janocha, E.; Hildebrandt, D.; Englberger, W. Agonistic effect of buprenorphine in a nociceptin/OFQ receptor-triggered reporter gene assay. Mol. Pharmacol. 1999, 56, 334–338. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Czoty, P.W.; Kiguchi, N.; Cami-Kobeci, G.; Sukhtankar, D.D.; Nader, M.A.; Husbands, S.M.; Ko, M.C. A novel orvinol analog, BU08028, as a safe opioid analgesic without abuse liability in primates. Proc. Natl. Acad. Sci. USA 2016, 113, E5511–E5518. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Kiguchi, N.; Yasuda, D.; Daga, P.R.; Polgar, W.E.; Lu, J.J.; Czoty, P.W.; Kishioka, S.; Zaveri, N.T.; Ko, M.C. A bifunctional nociceptin and mu opioid receptor agonist is analgesic without opioid side effects in nonhuman primates. Sci. Transl. Med. 2018, 10, eaar3483. [Google Scholar] [CrossRef] [Green Version]
- Kiguchi, N.; Ding, H.; Cami-Kobeci, G.; Sukhtankar, D.D.; Czoty, P.W.; DeLoid, H.B.; Hsu, F.C.; Toll, L.; Husbands, S.M.; Ko, M.C. BU10038 as a safe opioid analgesic with fewer side-effects after systemic and intrathecal administration in primates. Br. J. Anaesth. 2019, 122, e146–e156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flynn, S.M.; Epperly, P.M.; Davenport, A.T.; Cami-Kobeci, G.; Husbands, S.M.; Ko, M.C.; Czoty, P.W. Effects of stimulation of mu opioid and nociceptin/orphanin FQ peptide (NOP) receptors on alcohol drinking in rhesus monkeys. Neuropsychopharmacology 2019, 44, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- de Guglielmo, G.; Matzeu, A.; Kononoff, J.; Mattioni, J.; Martin-Fardon, R.; George, O. Cebranopadol Blocks the Escalation of Cocaine Intake and Conditioned Reinstatement of Cocaine Seeking in Rats. J. Pharmacol. Exp. Ther. 2017, 362, 378–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Q.; Deng, Y.; Ciccocioppo, R.; Cannella, N. Cebranopadol, a Mixed Opioid Agonist, Reduces Cocaine Self-administration through Nociceptin Opioid and Mu Opioid Receptors. Front. Psychiatry. 2017, 8, 234. [Google Scholar] [CrossRef] [Green Version]
- Zaveri, N.T. The nociceptin/orphanin FQ receptor (NOP) as a target for drug abuse medications. Curr. Top. Med. Chem. 2011, 11, 1151–1156. [Google Scholar] [CrossRef]
- Kantola, I.; Scheinin, M.; Gulbrandsen, T.; Meland, N.; Smerud, K.T. Safety, tolerability, and antihypertensive effect of SER100, an opiate receptor-like 1 (ORL-1) partial agonist, in patients with isolated systolic hypertension. Clin. Pharmacol. Drug Dev. 2017, 6, 584–591. [Google Scholar] [CrossRef]
- Angelico, P.; Barchielli, M.; Lazzeri, M.; Guerrini, R.; Caló, G. Nociceptin/ orphanin FQ and urinary bladder. Handb. Exp. Pharmacol. 2019, 254, 347–365. [Google Scholar]
- Mercatelli, D.; Pisanò, C.A.; Novello, S.; Morari, M. NOP receptor ligands and Parkinson’s disease. Handb. Exp. Pharmacol. 2019, 254, 213–232. [Google Scholar]
- Ferrari, F.; Rizzo, S.; Ruzza, C.; Calo, G. Detailed In Vitro Pharmacological Characterization of the Clinically Viable Nociceptin/Orphanin FQ Peptide Receptor Antagonist BTRX-246040. J. Pharmacol. Exp. Ther. 2020, 373, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Gavioli, E.C. Nociceptin/orphanin FQ receptor antagonists as innovative antidepressant drugs. Pharmacol. Ther. 2013, 140, 10–25. [Google Scholar] [CrossRef]
- Awwad, H.O.; Durand, C.D.; Gonzalez, L.P.; Tompkins, P.; Zhang, Y.; Lerner, M.R.; Brackett, D.J.; Sherry, D.M.; Awasthi, V.; Standifer, K.M. Post-blast treatment with Nociceptin/Orphanin FQ peptide (NOP) receptor antagonist reduces brain injury- induced hypoxia and signaling proteins in vestibulomotor-related brain regions. Behav. Brain Res. 2018, 340, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Witkin, J.M.; Wallace, T.L.; Martin, W.J. Therapeutic Approaches for NOP Receptor Antagonists in Neurobehavioral Disorders: Clinical Studies in Major Depressive Disorder and Alcohol Use Disorder with BTRX-246040 (LY2940094). Handb. Exp. Pharmacol. 2019, 254, 399–415. [Google Scholar]
- Carvalho, D.; Petronilho, F.; Vuolo, F.; Machado, R.A.; Constantino, L.; Guerrini, R.; Calo, G.; Gavioli, E.C.; Streck, E.L.; Dal-Pizzol, F. The nociceptin/orphanin FQ-NOP receptor antagonist effects on an animal model of sepsis. Intensive Care Med. 2008, 34, 2284–2290. [Google Scholar] [CrossRef]
- Genovese, R.F.; Dobre, S. Mitigation of adverse behavioral impact from predator exposure by the nociceptin/orphanin FQ peptide antagonist J-113397 in rats. Behav. Pharmacol. 2017, 28, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Simpson-Durand, C.D.; Standifer, K.M. Nociceptin/orphanin FQ peptide receptor antagonist JTC-801 reverses pain and anxiety symptoms in a rat model of post-traumatic stress disorder. Br. J. Pharmacol. 2015, 172, 571–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khroyan, T.V.; Polgar, W.E.; Jiang, F.; Zaveri, N.T.; Toll, L. Nociceptin/orphanin FQ receptor activation attenuates antinociception induced by mixed nociceptin/orphanin FQ/mu-opioid receptor agonists. J. Pharmacol. Exp. Ther. 2009, 331, 946–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillemyn, K.; Starnowska, J.; Lagard, C.; Dyniewicz, J.; Rojewska, E.; Mika, J.; Chung, N.N.; Utard, V.; Kosson, P.; Lipkowski, A.W.; et al. Bifunctional Peptide-Based Opioid Agonist-Nociceptin Antagonist Ligands for Dual Treatment of Acute and Neuropathic Pain. J. Med. Chem. 2016, 59, 3777–3792. [Google Scholar] [CrossRef] [Green Version]
- Christoph, A.; Eerdekens, M.H.; Kok, M.; Volkers, G.; Freynhagen, R. Cebranopadol, a novel first-in-class analgesic drug candidate: First experience in patients with chronic low back pain in a randomized clinical trial. Pain 2017, 158, 1813–1824. [Google Scholar] [CrossRef] [Green Version]
- Eerdekens, M.H.; Kapanadze, S.; Koch, E.D.; Kralidis, G.; Volkers, G.; Ahmedzai, S.H.; Meissner, W. Cancer-related chronic pain: Investigation of the novel analgesic drug candidate cebranopadol in a randomized, double-blind, noninferiority trial. Eur. J. Pain. 2019, 23, 577–588. [Google Scholar] [CrossRef]
- Tzschentke, T.M.; Linz, K.; Koch, T.; Christoph, T. Cebranopadol: A novel first- in-class potent analgesic acting via NOP and opioid receptors. Handb. Exp. Pharmacol. 2019, 254, 367–398. [Google Scholar]
- Lu, L.; Huang, M.; Liu, Z.; Ma, L. Cholecystokinin-B receptor antagonists attenuate morphine dependence and withdrawal in rats. Neuroreport 2000, 11, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Baber, N.S.; Dourish, C.T.; Hill, D.R. The role of CCK, caerulein, and CCK antagonists in nociception. Pain 1989, 39, 307–328. [Google Scholar] [CrossRef]
- Wiesenfeld-Hallin, Z.; Xu, X.-J. The role of cholecystokinin in nociception, neuropathic pain and opiate tolerance. Reg. Peptides 1996, 65, 23–28. [Google Scholar] [CrossRef]
- Maldonado, R.; Valverde, O.; Ducos, B.; Blommaert, A.G.; Fournie-Zaluski, M.C.; Roques, B.P. Inhibition of morphine withdrawal by the association of RB 101, an inhibitor of enkephalin catabolism, and the CCKB antagonist PD-134,308. Br. J. Pharmacol. 1995, 114, 1031–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastin, A.J.; Banks, W.A.; Hahn, K.; Zadina, J.E. Extreme stability of Tyr-MIF-1 in CSF. Neurosci. Lett. 1994, 174, 26–28. [Google Scholar] [CrossRef]
- Dourish, C.T.; Hawley, D.; Iversen, S.D. Enhancement of morphine analgesia and prevention of morphine tolerance in the rat by the cholecystokinin antagonist L-364,718. Eur. J. Pharmacol. 1988, 147, 469–472. [Google Scholar] [CrossRef]
- Bocheva, A.; Dzambazova-Maximova, E. Antiopioid properties of the TYR-MIF-1 family. Methods Find. Exp. Clin. Pharmacol. 2004, 26, 673–677. [Google Scholar] [CrossRef]
| Opioid Receptor | Subtypes | Previous and Unofficial Names | Effects of Activation |
|---|---|---|---|
| µ | µ1, µ2, µ3 | Mu receptor/MOP/OP3/MOPr/opioid receptor, mu 1 |
|
| δ | δ1, δ2 | DOP/DOR/OP1/Delta receptor/DOR-1/DOPr |
|
| κ | κ1, κ2, κ3 | KOR-1/Kappa receptor/OP2/KOP/KOPr |
|
| Nociceptin receptor | ORL1 | N/OFQ receptor/OP4/KOR-3/NOCIR/kappa3-related opioid receptor/MOR-C/nociceptin receptor ORL1/XOR1/NOP-r/nociceptin/orphanin FQ receptor/NOPr |
|
| Precursor | Endogenous Opioid Peptide | Relative Opioid Receptor Affinity |
|---|---|---|
| Proenkephalin (PENK) | [Met]-enkephalin [Leu]-enkephalin | µ, δ (δ >> µ) |
| Proopiomelanocortin (POMC) | β-endorphin | µ and δ (δ = µ) |
| Prodynorphin (PDYN) | Dynorphin A Dynorphin A (1–8) Dynorphin B α-neoendorphin β-neoendorphin | κ, µ, δ (κ >> µ, δ) |
| Prepronociceptin (PNOC) | Nociceptin/Orphanin FQ | ORL-1 |
| Unknown | Endomorphin-1 Endomorphin-2 | µ |
| Peptide | Effect | References |
|---|---|---|
MIF-1 PubChem Identifier: CID 92910 URL: https://pubchem.ncbi.nlm.nih.gov/compound/92910 | Attenuates morphine antinociception | [87,96,97,101,102,103,104] |
| Attenuates stress-induced antinociception | [87,99,104,105,106] | |
| Attenuates enkephalinergic analgesia | [105] | |
| Attenuates morphine-induced hypothermia and inhibit guinea pig ileum contractions | [106] | |
| Precipitate morphine withdrawal symptoms | [93] | |
| Inhibition of hypothermia and hypomotility produced by morphine | [106] | |
CCK-8 PubChem Identifier: CID 9833444 URL: https://pubchem.ncbi.nlm.nih.gov/compound/9833444 | Attenuates morphine antinociception | [107,108,109,110] |
| Attenuates foot shock antinociception | [107,110] | |
| CCK-8 antagonist attenuates morphine tolerance | [111,112] | |
| CCK-8 antagonist potentiates analgesia morphine | [111] | |
| CCK-8 antagonist does not block morphine dependence | [113] | |
| Attenuates β-endorphin (1-31) catalepsy | [113] | |
Nociceptin/Orphanin FQ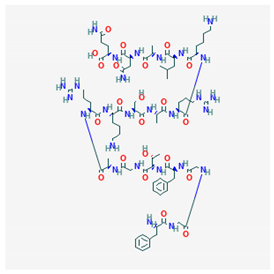 PubChem Identifier: CID 16131448 URL: https://pubchem.ncbi.nlm.nih.gov/compound/16131448 | Attenuates morphine antinociception | [114] |
| Inhibits morphine-induced CPP | [115,116,117,118] | |
| Inhibits ethanol-induced CPP | [105,115] | |
| Blockade or deprivation of NOP receptors potentiate rewarding effects of morphine | [119] | |
NPFF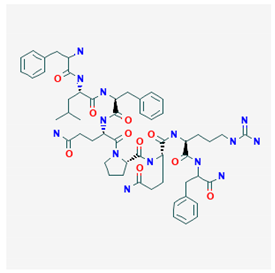 PubChem Identifier: CID 123797 URL: https://pubchem.ncbi.nlm.nih.gov/compound/123797 | Precipitates opioid withdrawal syndrome | [118] |
| Attenuates morphine antinociception | [120] | |
| Chronic morphine increases NPFF levels in cerebrospinal fluid | [118,121,122] | |
| Anti-NPFF IgG attenuates naloxone-induced | [118] | |
| Anti-NPFF IgG reverses morphine tolerance in the rat | [123] | |
| Putative NPFF antagonist attenuates morphine | [124] | |
| Suppresses DAMGO-induced inhibition of withdrawal abstinence syndrome | [125] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibula-Tarlowska, E.; Kotlinska, J.H. Crosstalk between Opioid and Anti-Opioid Systems: An Overview and Its Possible Therapeutic Significance. Biomolecules 2020, 10, 1376. https://doi.org/10.3390/biom10101376
Gibula-Tarlowska E, Kotlinska JH. Crosstalk between Opioid and Anti-Opioid Systems: An Overview and Its Possible Therapeutic Significance. Biomolecules. 2020; 10(10):1376. https://doi.org/10.3390/biom10101376
Chicago/Turabian StyleGibula-Tarlowska, Ewa, and Jolanta H. Kotlinska. 2020. "Crosstalk between Opioid and Anti-Opioid Systems: An Overview and Its Possible Therapeutic Significance" Biomolecules 10, no. 10: 1376. https://doi.org/10.3390/biom10101376
APA StyleGibula-Tarlowska, E., & Kotlinska, J. H. (2020). Crosstalk between Opioid and Anti-Opioid Systems: An Overview and Its Possible Therapeutic Significance. Biomolecules, 10(10), 1376. https://doi.org/10.3390/biom10101376




