The Role of Urocortins in Intracerebral Hemorrhage
Abstract
1. Introduction
2. Intracerebral Hemorrhage
Epidemiology and Risk Factors
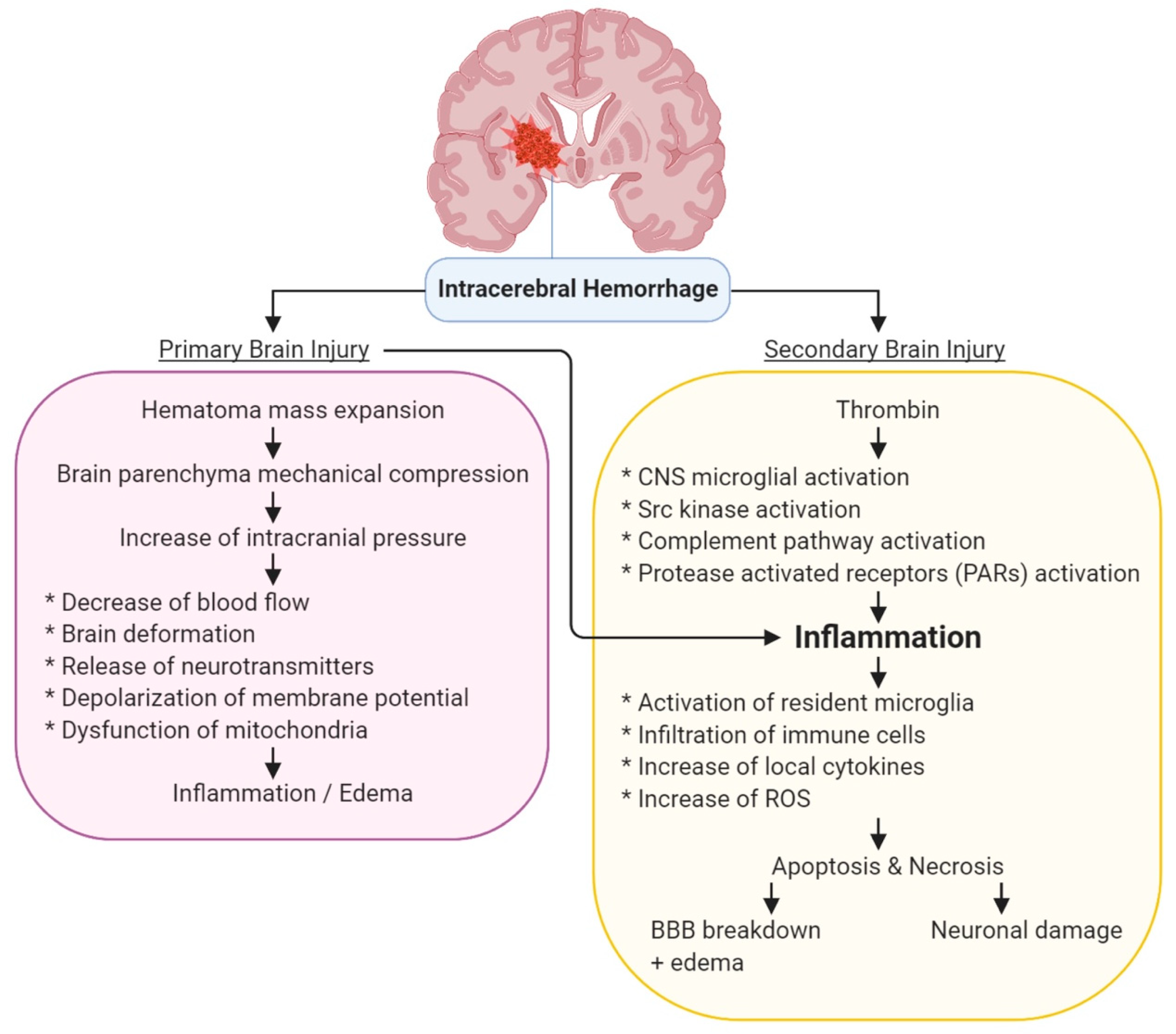
3. Molecular Pathology of ICH
3.1. Primary Injury by ICH
3.2. Secondary Injury by ICH
4. General Management for ICH
5. Urocortin
5.1. Urocortin Structure, Expression, Signaling, and Receptors
5.2. Clinical Application of Urocortin in the Treatment of Heart Failure
6. Urocortin and Intracerebral Hemorrhage
6.1. Urocortin Reduced Neurological Deficits
6.2. Urocortin Has a Hypotensive Effect, but without Changing the Other Physiological Parameter
6.3. Urocortin Reduced Brain Edema
6.4. Urocortin Reduced Pro-Inflammatory Cytokine Level in Striatal Tissue
6.5. Urocortin Reduced Blood-Brain Barrier (BBB) Leakage
7. Conclusions & Perspective
Funding
Conflicts of Interest
References
- Caceres, J.A.; Goldstein, J.N. Intracranial hemorrhage. Emerg. Med. Clin. N. Am. 2012, 30, 771–794. [Google Scholar] [CrossRef] [PubMed]
- An, S.J.; Kim, T.J.; Yoon, B.-W. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J. Stroke 2017, 19, 3–10. [Google Scholar] [CrossRef] [PubMed]
- van Asch, C.J.; Luitse, M.J.; Rinkel, G.J.; van der Tweel, I.; Algra, A.; Klijn, C.J. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet. Neurol. 2010, 9, 167–176. [Google Scholar] [CrossRef]
- Tsai, C.F.; Jeng, J.S.; Anderson, N.; Sudlow, C.L.M. Comparisons of risk factors for intracerebral hemorrhage versus ischemic stroke in chinese patients. Neuroepidemiology 2017, 48, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Fekete, E.M.; Zorrilla, E.P. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: Ancient CRF paralogs. Front. Neuroendocrinol. 2007, 28, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, S.C.; Koob, G.F. Corticotropin-releasing factor in brain: A role in activation, arousal, and affect regulation. J. Pharmacol. Exp. Ther. 2004, 311, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Tache, Y. Corticotropin-releasing factor signaling and visceral response to stress. Exp. Biol. Med. 2010, 235, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Neufeld-Cohen, A.; Tsoory, M.M.; Evans, A.K.; Getselter, D.; Gil, S.; Lowry, C.A.; Vale, W.W.; Chen, A. A triple urocortin knockout mouse model reveals an essential role for urocortins in stress recovery. Proc. Natl. Acad. Sci. USA 2010, 107, 19020–19025. [Google Scholar] [CrossRef]
- Larauche, M.; Kiank, C.; Tache, Y. Corticotropin releasing factor signaling in colon and ileum: Regulation by stress and pathophysiological implications. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2009, 60 (Suppl. 7), 33–46. [Google Scholar]
- De Bonis, M.; Torricelli, M.; Severi, F.M.; Luisi, S.; De Leo, V.; Petraglia, F. Neuroendocrine aspects of placenta and pregnancy. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2012, 28 (Suppl. 1), 22–26. [Google Scholar] [CrossRef]
- Aguilar, M.I.; Brott, T.G. Update in intracerebral hemorrhage. Neurohospitalist 2011, 1, 148–159. [Google Scholar] [CrossRef]
- Flaherty, M.L.; Woo, D.; Haverbusch, M.; Sekar, P.; Khoury, J.; Sauerbeck, L.; Moomaw, C.J.; Schneider, A.; Kissela, B.; Kleindorfer, D.; et al. Racial variations in location and risk of intracerebral hemorrhage. Stroke 2005, 36, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Grysiewicz, R.A.; Thomas, K.; Pandey, D.K. Epidemiology of ischemic and hemorrhagic stroke: Incidence, prevalence, mortality, and risk factors. Neurol. Clin. 2008, 26, 871–895. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthi, R.V.; Moran, A.E.; Forouzanfar, M.H.; Bennett, D.A.; Mensah, G.A.; Lawes, C.M.; Barker-Collo, S.; Connor, M.; Roth, G.A.; Sacco, R.; et al. The global burden of hemorrhagic stroke: A summary of findings from the gbd 2010 study. Glob. Heart 2014, 9, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, J.C., 3rd; Bonovich, D.C.; Besmertis, L.; Manley, G.T.; Johnston, S.C. The ich score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001, 32, 891–897. [Google Scholar] [CrossRef]
- Fisher, C.M. Pathological observations in hypertensive cerebral hemorrhage. J. Neuropathol. Exp. Neurol. 1971, 30, 536–550. [Google Scholar] [CrossRef]
- Ariesen, M.J.; Claus, S.P.; Rinkel, G.J.; Algra, A. Risk factors for intracerebral hemorrhage in the general population: A systematic review. Stroke 2003, 34, 2060–2065. [Google Scholar] [CrossRef]
- O’Donnell, M.J.; Xavier, D.; Liu, L.; Zhang, H.; Chin, S.L.; Rao-Melacini, P.; Rangarajan, S.; Islam, S.; Pais, P.; McQueen, M.J.; et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the interstroke study): A case-control study. Lancet 2010, 376, 112–123. [Google Scholar]
- Sturgeon, J.D.; Folsom, A.R.; Longstreth, W.T., Jr.; Shahar, E.; Rosamond, W.D.; Cushman, M. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke 2007, 38, 2718–2725. [Google Scholar] [CrossRef]
- Liew, H.K.; Pang, C.Y.; Hsu, C.W.; Wang, M.J.; Li, T.Y.; Peng, H.F.; Kuo, J.S.; Wang, J.Y. Systemic administration of urocortin after intracerebral hemorrhage reduces neurological deficits and neuroinflammation in rats. J. Neuroinflammation 2012, 9, 13. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Tuhrim, S.; Broderick, J.P.; Batjer, H.H.; Hondo, H.; Hanley, D.F. Spontaneous intracerebral hemorrhage. N. Engl. J. Med. 2001, 344, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Kazui, S.; Naritomi, H.; Yamamoto, H.; Sawada, T.; Yamaguchi, T. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke 1996, 27, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Suri, M.F.; Ostrow, P.T.; Kim, S.H.; Ali, Z.; Shatla, A.A.; Guterman, L.R.; Hopkins, L.N. Apoptosis as a form of cell death in intracerebral hemorrhage. Neurosurgery 2003, 52, 1041–1047; discussion 1047–1048. [Google Scholar]
- Lusardi, T.A.; Wolf, J.A.; Putt, M.E.; Smith, D.H.; Meaney, D.F. Effect of acute calcium influx after mechanical stretch injury in vitro on the viability of hippocampal neurons. J. Neurotrauma 2004, 21, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.I.; McIntosh, T.K.; Maxwell, W.L.; Nicoll, J.A. Recent advances in neurotrauma. J. Neuropathol. Exp. Neurol. 2000, 59, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Brott, T.; Broderick, J.; Kothari, R.; Barsan, W.; Tomsick, T.; Sauerbeck, L.; Spilker, J.; Duldner, J.; Khoury, J. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke 1997, 28, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mendelow, A.D.; Gregson, B.A.; Fernandes, H.M.; Murray, G.D.; Teasdale, G.M.; Hope, D.T.; Karimi, A.; Shaw, M.D.; Barer, D.H.; STICH investigators. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the international surgical trial in intracerebral haemorrhage (stich): A randomised trial. Lancet 2005, 365, 387–397. [Google Scholar] [CrossRef]
- Wang, J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog. Neurobiol. 2010, 92, 463–477. [Google Scholar] [CrossRef]
- Liew, H.K.; Cheng, H.Y.; Huang, L.C.; Li, K.W.; Peng, H.F.; Yang, H.I.; Lin, P.B.; Kuo, J.S.; Pang, C.Y. Acute alcohol intoxication aggravates brain injury caused by intracerebral hemorrhage in rats. J. Stroke Cereb. Dis. 2016, 25, 15–25. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Z.; Yu, J.; Yang, X.; He, F.; Liu, Z.; Che, F.; Chen, X.; Ren, H.; Hong, M.; et al. Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Prog. Neurobiol. 2019. [Google Scholar] [CrossRef]
- Keep, R.F.; Xiang, J.; Ennis, S.R.; Andjelkovic, A.; Hua, Y.; Xi, G.; Hoff, J.T. Blood-brain barrier function in intracerebral hemorrhage. Cerebral Hemorrhage 2008, 105, 73–77. [Google Scholar]
- Xi, G.; Reiser, G.; Keep, R.F. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: Deleterious or protective? J. Neurochem. 2003, 84, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Kim-Han, J.S.; Kopp, S.J.; Dugan, L.L.; Diringer, M.N. Perihematomal mitochondrial dysfunction after intracerebral hemorrhage. Stroke 2006, 37, 2457–2462. [Google Scholar] [CrossRef]
- Liu, D.Z.; Sharp, F.R. The dual role of src kinases in intracerebral hemorrhage. Intracerebral Hemorrhage Res. 2011, 111, 77–81. [Google Scholar]
- Babu, R.; Bagley, J.H.; Di, C.; Friedman, A.H.; Adamson, C. Thrombin and hemin as central factors in the mechanisms of intracerebral hemorrhage-induced secondary brain injury and as potential targets for intervention. Neurosurg. Focus 2012, 32, E8. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Mendelow, A.D.; Hanley, D.F. Intracerebral haemorrhage. Lancet 2009, 373, 1632–1644. [Google Scholar] [CrossRef]
- Keep, R.F.; Hua, Y.; Xi, G. Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet. Neurol. 2012, 11, 720–731. [Google Scholar] [CrossRef]
- Lattanzi, S.; Brigo, F.; Trinka, E.; Cagnetti, C.; Di Napoli, M.; Silvestrini, M. Neutrophil-to-lymphocyte ratio in acute cerebral hemorrhage: A system review. Transl. Stroke Res. 2019, 10, 137–145. [Google Scholar] [CrossRef]
- Lattanzi, S.; Cagnetti, C.; Rinaldi, C.; Angelocola, S.; Provinciali, L.; Silvestrini, M. Neutrophil-to-lymphocyte ratio improves outcome prediction of acute intracerebral hemorrhage. J. Neurol. Sci. 2018, 387, 98–102. [Google Scholar] [CrossRef]
- Di Napoli, M.; Slevin, M.; Popa-Wagner, A.; Singh, P.; Lattanzi, S.; Divani, A.A. Monomeric c-reactive protein and cerebral hemorrhage: From bench to bedside. Front. Immunol. 2018, 9, 1921. [Google Scholar] [CrossRef]
- Rodriguez-Luna, D.; Pineiro, S.; Rubiera, M.; Ribo, M.; Coscojuela, P.; Pagola, J.; Flores, A.; Muchada, M.; Ibarra, B.; Meler, P.; et al. Impact of blood pressure changes and course on hematoma growth in acute intracerebral hemorrhage. Eur. J. Neurol. 2013, 20, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Dastur, C.K.; Yu, W. Current management of spontaneous intracerebral haemorrhage. Stroke Vasc. Neurol. 2017, 2, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.A.; Lewin, J.J., 3rd; Rabinstein, A.A.; Aisiku, I.P.; Alexandrov, A.W.; Cook, A.M.; del Zoppo, G.J.; Kumar, M.A.; Peerschke, E.I.; Stiefel, M.F.; et al. Guideline for reversal of antithrombotics in intracranial hemorrhage: A statement for healthcare professionals from the neurocritical care society and society of critical care medicine. Neurocrit. Care 2016, 24, 6–46. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.S.; Heeley, E.; Huang, Y.; Wang, J.; Stapf, C.; Delcourt, C.; Lindley, R.; Robinson, T.; Lavados, P.; Neal, B.; et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N. Engl. J. Med. 2013, 368, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Ziai, W.; Lattanzi, S.; Divani, A.A. Response by ziai et al to letter regarding article, “blood pressure variability predicts poor in-hospital outcome in spontaneous intracerebral hemorrhage”. Stroke 2019, 50, e276. [Google Scholar] [CrossRef]
- Arima, H.; Heeley, E.; Delcourt, C.; Hirakawa, Y.; Wang, X.; Woodward, M.; Robinson, T.; Stapf, C.; Parsons, M.; Lavados, P.M.; et al. Optimal achieved blood pressure in acute intracerebral hemorrhage: Interact2. Neurology 2015, 84, 464–471. [Google Scholar] [CrossRef]
- Lattanzi, S.; Silvestrini, M. Optimal achieved blood pressure in acute intracerebral hemorrhage: Interact2. Neurology 2015, 85, 557–558. [Google Scholar] [CrossRef]
- Chan, S.; Hemphill, J.C., 3rd. Critical care management of intracerebral hemorrhage. Crit. Care Clin. 2014, 30, 699–717. [Google Scholar] [CrossRef]
- Menon, G. Surgery for spontaneous intracerebral hemorrhage: Emerging trends. Arch. Med. Health Sci. 2017, 5, 65–70. [Google Scholar]
- Jafari, M.; Di Napoli, M.; Lattanzi, S.; Mayer, S.A.; Bachour, S.; Bershad, E.M.; Damani, R.; Datta, Y.H.; Divani, A.A. Serum magnesium level and hematoma expansion in patients with intracerebral hemorrhage. J. Neurol. Sci. 2019, 398, 39–44. [Google Scholar] [CrossRef]
- Lattanzi, S.; Cagnetti, C.; Provinciali, L.; Silvestrini, M. Neutrophil-to-lymphocyte ratio and neurological deterioration following acute cerebral hemorrhage. Oncotarget 2017, 8, 57489–57494. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, J.; Majdi, A.; Lattanzi, S.; Di Napoli, M.; Bershad, E.M.; Rodrigues, C.M.P.; Divani, A.A. Imidazoline receptor agonists for managing hypertension may hold promise for treatment of intracerebral hemorrhage. Curr. Mol. Med. 2018, 18, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Tamm, A.S.; McCourt, R.; Gould, B.; Kate, M.; Kosior, J.C.; Jeerakathil, T.; Gioia, L.C.; Dowlatshahi, D.; Hill, M.D.; Coutts, S.B.; et al. Cerebral perfusion pressure is maintained in acute intracerebral hemorrhage: A CT perfusion study. AJNR Am. J. Neuroradiol. 2016, 37, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Hanggi, D.; Steiger, H.J. Spontaneous intracerebral haemorrhage in adults: A literature overview. Acta Neurochir. 2008, 150, 371–379; discussion 379. [Google Scholar] [CrossRef] [PubMed]
- Pouratian, N.; Kassell, N.F.; Dumont, A.S. Update on management of intracerebral hemorrhage. Neurosurg. Focus 2003, 15, E2. [Google Scholar] [CrossRef] [PubMed]
- Hankey, G.J.; Hon, C. Surgery for primary intracerebral hemorrhage: Is it safe and effective? A systematic review of case series and randomized trials. Stroke 1997, 28, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, J.; Donaldson, C.; Bittencourt, J.; Perrin, M.H.; Lewis, K.; Sutton, S.; Chan, R.; Turnbull, A.V.; Lovejoy, D.; Rivier, C.; et al. Urocortin, a mammalian neuropeptide related to fish urotensin i and to corticotropin-releasing factor. Nature 1995, 378, 287–292. [Google Scholar] [CrossRef]
- Reyes, T.M.; Lewis, K.; Perrin, M.H.; Kunitake, K.S.; Vaughan, J.; Arias, C.A.; Hogenesch, J.B.; Gulyas, J.; Rivier, J.; Vale, W.W.; et al. Urocortin ii: A member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 2843–2848. [Google Scholar] [CrossRef]
- Lewis, K.; Li, C.; Perrin, M.H.; Blount, A.; Kunitake, K.; Donaldson, C.; Vaughan, J.; Reyes, T.M.; Gulyas, J.; Fischer, W.; et al. Identification of urocortin iii, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 7570–7575. [Google Scholar] [CrossRef]
- Kimura, Y.; Takahashi, K.; Totsune, K.; Muramatsu, Y.; Kaneko, C.; Darnel, A.D.; Suzuki, T.; Ebina, M.; Nukiwa, T.; Sasano, H. Expression of urocortin and corticotropin-releasing factor receptor subtypes in the human heart. J. Clin. Endocrinol. Metab. 2002, 87, 340–346. [Google Scholar] [CrossRef]
- Iino, K.; Sasano, H.; Oki, Y.; Andoh, N.; Shin, R.W.; Kitamoto, T.; Totsune, K.; Takahashi, K.; Suzuki, H.; Nagura, H.; et al. Urocortin expression in human pituitary gland and pituitary adenoma. J. Clin. Endocrinol. Metab. 1997, 82, 3842–3850. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seres, J.; Bornstein, S.R.; Seres, P.; Willenberg, H.S.; Schulte, K.M.; Scherbaum, W.A.; Ehrhart-Bornstein, M. Corticotropin-releasing hormone system in human adipose tissue. J. Clin. Endocrinol. Metab. 2004, 89, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Uzuki, M.; Sasano, H.; Muramatsu, Y.; Totsune, K.; Takahashi, K.; Oki, Y.; Iino, K.; Sawai, T. Urocortin in the synovial tissue of patients with rheumatoid arthritis. Clin. Sci. 2001, 100, 577–589. [Google Scholar] [CrossRef]
- Bamberger, C.M.; Wald, M.; Bamberger, A.M.; Ergun, S.; Beil, F.U.; Schulte, H.M. Human lymphocytes produce urocortin, but not corticotropin-releasing hormone. J. Clin. Endocrinol. Metab. 1998, 83, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Papadopoulou, N.G.; Lytinas, M.; Huang, M.; Kandere-Grzybowska, K.; Madhappan, B.; Boucher, W.; Christodoulou, S.; Athanassiou, A.; Theoharides, T.C. Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinology 2004, 145, 43–48. [Google Scholar] [CrossRef]
- Yamauchi, N.; Otagiri, A.; Nemoto, T.; Sekino, A.; Oono, H.; Kato, I.; Yanaihara, C.; Shibasaki, T. Distribution of urocortin 2 in various tissues of the rat. J. Neuroendocrinol. 2005, 17, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.Y.; Hsueh, A.J. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat. Med. 2001, 7, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Totsune, K.; Murakami, O.; Shibahara, S. Urocortins as cardiovascular peptides. Peptides 2004, 25, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, P.; Vaughan, J.; Blount, A.; Chen, A.; Jamieson, P.M.; Rivier, J.; Smith, M.S.; Vale, W. Urocortin iii is expressed in pancreatic beta-cells and stimulates insulin and glucagon secretion. Endocrinology 2003, 144, 3216–3224. [Google Scholar] [CrossRef]
- Imperatore, A.; Florio, P.; Torres, P.B.; Torricelli, M.; Galleri, L.; Toti, P.; Occhini, R.; Picciolini, E.; Vale, W.; Petraglia, F. Urocortin 2 and urocortin 3 are expressed by the human placenta, deciduas, and fetal membranes. Am. J. Obstet. Gynecol. 2006, 195, 288–295. [Google Scholar] [CrossRef]
- Saruta, M.; Takahashi, K.; Suzuki, T.; Fukuda, T.; Torii, A.; Sasano, H. Urocortin 3/stresscopin in human colon: Possible modulators of gastrointestinal function during stressful conditions. Peptides 2005, 26, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Dautzenberg, F.M.; Hauger, R.L. The CRF peptide family and their receptors: Yet more partners discovered. Trends Pharm. Sci. 2002, 23, 71–77. [Google Scholar] [CrossRef]
- Deussing, J.M.; Breu, J.; Kuhne, C.; Kallnik, M.; Bunck, M.; Glasl, L.; Yen, Y.C.; Schmidt, M.V.; Zurmuhlen, R.; Vogl, A.M.; et al. Urocortin 3 modulates social discrimination abilities via corticotropin-releasing hormone receptor type 2. J. Neurosci. 2010, 30, 9103–9116. [Google Scholar] [CrossRef] [PubMed]
- Grace, C.R.; Perrin, M.H.; Cantle, J.P.; Vale, W.W.; Rivier, J.E.; Riek, R. Common and divergent structural features of a series of corticotropin releasing factor-related peptides. J. Am. Chem. Soc. 2007, 129, 16102–16114. [Google Scholar] [CrossRef] [PubMed]
- Liew, H.K.; Huang, L.C.; Yang, H.I.; Peng, H.F.; Li, K.W.; Tsai, A.P.; Chen, S.Y.; Kuo, J.S.; Pang, C.Y. Therapeutic effects of human urocortin-1, -2 and -3 in intracerebral hemorrhage of rats. Neuropeptides 2015, 52, 89–96. [Google Scholar] [CrossRef]
- Haass-Koffler, C.L. The corticotropin releasing factor binding protein: A strange case of dr. Jekyll and mr. Hyde in the stress system? Alcohol 2018, 72, 3–8. [Google Scholar] [CrossRef]
- Ketchesin, K.D.; Huang, N.S.; Seasholtz, A.F. Cell type-specific expression of corticotropin-releasing hormone-binding protein in gabaergic interneurons in the prefrontal cortex. Front. Neuroanat. 2017, 11, 90. [Google Scholar] [CrossRef]
- Huising, M.O.; Vaughan, J.M.; Shah, S.H.; Grillot, K.L.; Donaldson, C.J.; Rivier, J.; Flik, G.; Vale, W.W. Residues of corticotropin releasing factor-binding protein (CRF-BP) that selectively abrogate binding to CRF but not to urocortin 1. J. Biol. Chem. 2008, 283, 8902–8912. [Google Scholar] [CrossRef]
- Manuel, R.; Metz, J.R.; Flik, G.; Vale, W.W.; Huising, M.O. Corticotropin-releasing factor-binding protein (CRF-BP) inhibits CRF- and urotensin-i-mediated activation of CRF receptor-1 and -2 in common carp. Gen. Comp. Endocrinol. 2014, 202, 69–75. [Google Scholar] [CrossRef]
- Barra de la Tremblaye, P.; Plamondon, H. Alterations in the corticotropin-releasing hormone (crh) neurocircuitry: Insights into post stroke functional impairments. Front. Neuroendocrinol. 2016, 42, 53–75. [Google Scholar] [CrossRef]
- Pedersen, W.A.; Wan, R.; Zhang, P.; Mattson, M.P. Urocortin, but not urocortin ii, protects cultured hippocampal neurons from oxidative and excitotoxic cell death via corticotropin-releasing hormone receptor type i. J. Neurosci. 2002, 22, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Stern, C.M.; Meitzen, J.; Mermelstein, P.G. Corticotropin-releasing factor and urocortin i activate creb through functionally selective gbetagamma signaling in hippocampal pyramidal neurons. Eur. J. Neurosci. 2011, 34, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.C.; Iba, M.; Bangasser, D.A.; Valentino, R.J.; James, M.J.; Brunden, K.R.; Lee, V.M.; Trojanowski, J.Q. Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in a transgenic mouse model of tauopathy. J. Neurosci. 2011, 31, 14436–14449. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.M.; Rybka, A.E.; Townsend, P.A. The powerful cardioprotective effects of urocortin and the corticotropin releasing hormone (crh) family. Biochem. Pharmacol. 2009, 77, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramanian, S.; Griffiths, M.E.; McLean, S.G.; Miller, M.R.; Luo, R.; Lang, N.N.; Newby, D.E. Vascular effects of urocortins 2 and 3 in healthy volunteers. J. Am. Heart Assoc. 2013, 2, e004267. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramanian, S.; Philp, D.; White, A.; Lang, N. Effect of urocortin 2 and 3 on forearm arterial blood flow in patients with heart failure. J. Am. Coll. Cardiol. 2013, 61, E647. [Google Scholar] [CrossRef][Green Version]
- Davis, M.E.; Pemberton, C.J.; Yandle, T.G.; Fisher, S.F.; Lainchbury, J.G.; Frampton, C.M.; Rademaker, M.T.; Richards, M. Urocortin 2 infusion in human heart failure. Eur. Heart J. 2007, 28, 2589–2597. [Google Scholar] [CrossRef]
- Chan, W.Y.; Frampton, C.M.; Crozier, I.G.; Troughton, R.W.; Richards, A.M. Urocortin-2 infusion in acute decompensated heart failure: Findings from the unicorn study (urocortin-2 in the treatment of acute heart failure as an adjunct over conventional therapy). JACC Heart Fail. 2013, 1, 433–441. [Google Scholar] [CrossRef]
- Davis, M.E.; Pemberton, C.J.; Yandle, T.G.; Lainchbury, J.G.; Rademaker, M.T.; Nicholls, M.G.; Frampton, C.M.; Richards, A.M. Effect of urocortin 1 infusion in humans with stable congestive cardiac failure. Clin. Sci. 2005, 109, 381–388. [Google Scholar] [CrossRef]
- Cordonnier, C.; Demchuk, A.; Ziai, W.; Anderson, C.S. Intracerebral haemorrhage: Current approaches to acute management. Lancet 2018, 392, 1257–1268. [Google Scholar] [CrossRef]
- Schaar, K.L.; Brenneman, M.M.; Savitz, S.I. Functional assessments in the rodent stroke model. Exp. Transl Stroke Med. 2010, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Hsu, C.W.; Huang, W.H.; Wang, J.Y. Post-injury baicalein improves histological and functional outcomes and reduces inflammatory cytokines after experimental traumatic brain injury. Br. J. Pharm. 2008, 155, 1279–1296. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.; Oh, H. Comparisons of acute physiological parameters influencing outcome in patients with traumatic brain injury and hemorrhagic stroke. Worldviews Evid. Based Nurs. 2009, 6, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Doong, M.L.; Rivier, J.E.; Tache, Y. Intravenous urocortin ii decreases blood pressure through CRF2 receptor in rats. Regul. Pept. 2003, 113, 125–130. [Google Scholar] [CrossRef]
- Huang, Y.; Chan, F.L.; Lau, C.W.; Tsang, S.Y.; He, G.W.; Chen, Z.Y.; Yao, X. Urocortin-induced endothelium-dependent relaxation of rat coronary artery: Role of nitric oxide and k+ channels. Br. J. Pharm. 2002, 135, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, K.; Furukawa, K.; Miki, I.; Terui, K.; Motomura, S.; Suda, T. Vasodilative effects of urocortin ii via protein kinase a and a mitogen-activated protein kinase in rat thoracic aorta. J. Cardiovasc Pharm. 2003, 42, 561–565. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sanz, E.; Monge, L.; Fernandez, N.; Climent, B.; Dieguez, G.; Garcia-Villalon, A.L. Mechanisms of relaxation by urocortin in renal arteries from male and female rats. Br. J. Pharm. 2003, 140, 1003–1007. [Google Scholar] [CrossRef][Green Version]
- Schilling, L.; Kanzler, C.; Schmiedek, P.; Ehrenreich, H. Characterization of the relaxant action of urocortin, a new peptide related to corticotropin-releasing factor in the rat isolated basilar artery. Br. J. Pharm. 1998, 125, 1164–1171. [Google Scholar] [CrossRef]
- Butcher, K.S.; Jeerakathil, T.; Hill, M.; Demchuk, A.M.; Dowlatshahi, D.; Coutts, S.B.; Gould, B.; McCourt, R.; Asdaghi, N.; Findlay, J.M.; et al. The intracerebral hemorrhage acutely decreasing arterial pressure trial. Stroke 2013, 44, 620–626. [Google Scholar] [CrossRef]
- Liew, H.K.; Hsu, C.W.; Wang, M.J.; Kuo, J.S.; Li, T.Y.; Peng, H.F.; Wang, J.Y.; Pang, C.Y. Therapeutic benefit of urocortin in rats with intracerebral hemorrhage. J. Neurosurg. 2012, 116, 193–200. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, C.; Zhang, J.; Hu, Z. Mechanism and therapy of brain edema after intracerebral hemorrhage. Cereb. Dis. 2016, 42, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Tjuvajev, J.; Kolesnikov, Y.; Joshi, R.; Sherinski, J.; Koutcher, L.; Zhou, Y.; Matei, C.; Koutcher, J.; Kreek, M.J.; Blasberg, R. Anti-neoplastic properties of human corticotropin releasing factor: Involvement of the nitric oxide pathway. In Vivo (AthensGreece) 1998, 12, 1–10. [Google Scholar]
- Reubi, J.C.; Waser, B.; Vale, W.; Rivier, J. Expression of CRF1 and CRF2 receptors in human cancers. J. Clin. Endocrinol. Metab. 2003, 88, 3312–3320. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moroz, M.A.; Huang, R.; Kochetkov, T.; Shi, W.; Thaler, H.; de Stanchina, E.; Gamez, I.; Ryan, R.P.; Blasberg, R.G. Comparison of corticotropin-releasing factor, dexamethasone, and temozolomide: Treatment efficacy and toxicity in u87 and c6 intracranial gliomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 3282–3292. [Google Scholar] [CrossRef] [PubMed]
- Recht, L.D.; Mechtler, L.; Phuphanich, S.; Hormigo, A.; Hines, V.; Milsted, R.; O’Connor, P.C.; Ryan, R.P.; Wong, E.T. A placebo-controlled study investigating the dexamethasone-sparing effects of corticorelin acetate in patients with primary or metastatic brain tumors and peritumoral edema. J. Clin. Oncol. 2009, 27, 2078. [Google Scholar]
- Shapiro, W.R.; Mechtler, L.; Cher, L.; Wheeler, H.; Hines, V.; Milsted, R.; O’Connor, P.C.; Ryan, R.P.; Recht, L. A randomized, double-blind study comparing corticorelin acetate with dexamethasone in patients with primary malignant glioma who require increased dexamethasone doses to control symptoms of peritumoral brain edema. J. Clin. Oncol. 2009, 27, 2080. [Google Scholar]
- Mechtler, L.; Wong, E.T.; Hormigo, A.; Pannullo, S.; Hines, V.; Milsted, R.; O’Connor, P.C.; Ryan, R.P.; Recht, L. A long-term open-label extension study examining the steroid-sparing effects of corticorelin acetate in patients with cerebral tumors. J. Clin. Oncol. 2009, 27, 2079. [Google Scholar]
- Moliterno, J.A.; Henry, E.; Pannullo, S.C. Corticorelin acetate injections for the treatment of peritumoral brain edema. Expert Opin. Investig. Drugs 2009, 18, 1413–1419. [Google Scholar] [CrossRef]
- Liew, H.K.; Hu, W.F.; Lin, P.B.; Wang, P.K.; Tsai, A.P.; Pang, C.Y.; Chen, T.Y. Over-activated proteasome mediates neuroinflammation on acute intracerebral hemorrhage in rats. Cells 2019, 8, 1326. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Ma, C.; Geng, J.; Li, Y.; Li, S.; Yu, F.; Zhang, X.; Cong, B. Endoplasmic reticulum stress contributes to crh-induced hippocampal neuron apoptosis. Exp. Cell Res. 2012, 318, 732–740. [Google Scholar] [CrossRef]
- Mracsko, E.; Veltkamp, R. Neuroinflammation after intracerebral hemorrhage. Front. Cell Neurosci. 2014, 8, 388. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Lin, S.Z.; Kuo, J.S.; Huang, H.Y.; Tzeng, S.F.; Liao, C.H.; Chen, D.C.; Chen, W.F. Urocortin modulates inflammatory response and neurotoxicity induced by microglial activation. J. Immunol. 2007, 179, 6204–6214. [Google Scholar] [CrossRef] [PubMed]
- Keep, R.F.; Zhou, N.; Xiang, J.; Andjelkovic, A.V.; Hua, Y.; Xi, G. Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage. Fluids Barriers CNS 2014, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wu, Q.; Guo, G.; Ding, X. The protective effects of urocortin1 against intracerebral hemorrhage by activating JNK1/2 and p38 phosphorylation and further increasing VEGF via corticotropin-releasing factor receptor 2. Neurosci. Lett. 2015, 589, 31–36. [Google Scholar] [CrossRef]
- Cao, C.; Zhou, J.; Wu, X.; Qian, Y.; Hong, Y.; Mu, J.; Jin, L.; Zhu, C.; Li, S. Activation of cRHR1 contributes to cerebral endothelial barrier impairment via cPLA2 phosphorylation in experimental ischemic stroke. Cell Signal. 2020, 66, 109467. [Google Scholar] [CrossRef]
| No | Intervention | Study Design | Results | Reference |
|---|---|---|---|---|
| 1 | UCN2, UCN3 | Non-randomized clinical trial | Does not cause forearm vasodilatation in patients with stable heart failure. Highest dose of UCN3 triggers a transient tachycardia | [86] |
| 2 | UCN2 | Single-blind, placebo-controlled, dose-escalation design | Increases cardiac output, elevates left ventricular ejection fraction, decreases systemic vascular resistance and cardiac work | [87] |
| 3 | UCN | Randomized time-matched cross-over design | No effect on cardiovascular system | [89] |
| 4 | UCN2 | Randomized control trial | Reduces systolic blood pressure, decreases total peripheral resistance, elevates increased in cardiac output with nonsignificant elevation in heart rate | [88] |
| No | Study Design | Results | Reference |
|---|---|---|---|
| 1 | A placebo-controlled study | hCRF improved symptoms of peritumoral edema associated with primary or metastatic cerebral tumors. hCRF enabled them to reduce or stop dexamethasone treatment and therefore minimize the rate of steroid-related short- and long-term adverse events of myopathy, cushingoid symptoms, and skin disorders | [105] |
| 2 | Randomized control trial | hCRF treatment had similar efficacy to increased dexamethasone. There was a lower incidence of cushingoid symptoms in the hCRF groups compared to dexamethasone groups | [106] |
| 3 | Randomized double-blind control trial | hCRF has long-term safety, tolerability, and reduced steroid-sparing potential in patients with primary or secondary brain tumors and peritumoral edema | [107] |
| 4 | Randomized control trial (Phase 1) | hCRF is well tolerated and effective in lowering peritumoral brain edema and its associated systemic side effects | [108] |
| i.p. | i.c.v. | |||||||
|---|---|---|---|---|---|---|---|---|
| L-UCN (2.5 μg/kg) | H-UCN (25 μg/kg) | hUCN1 (2.5 g/kg) | hUCN2 (2.5 g/kg) | hUCN3 (2.5 g/kg) | UCN (0.05 g) | UCN (0.5 μg) | UCN (5 μg) | |
| Neurological deficits | ||||||||
| NSS | ↓↓ | ↓ | ↓ | - | - | ↓ | ↓ | ↓↓ |
| Lesion volume | ↓ | N/A | ↓ | - | - | N/A | N/A | ↓ |
| Physiological parameters | ||||||||
| Mean arterial blood pressure (MABP) | ↓ | ↓↓ | N/A | N/A | N/A | N/A | N.A. | - |
| Heart rate | ↑ | ↑ | N/A | N/A | N/A | N/A | N/A | N/A |
| pO2 | - | N/A | N/A | N/A | N/A | N/A | N/A | - |
| pCO2 | - | N/A | N/A | N/A | N/A | N/A | N/A | - |
| pH | - | N/A | N/A | N/A | N/A | N/A | N/A | - |
| Body Temperature | - | N/A | N/A | N/A | N/A | N/A | N/A | - |
| Brain edema | ||||||||
| Water content | ↓ | ↓ | ↓ | N/A | N/A | N/A | N/A | ↓ |
| Neuroinflammation | ||||||||
| IL-1b | ↓ | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| IL-6 | ↓ | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| TNF-a | ↓ | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Microglial activation | ↓ | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| BBB leakage | ||||||||
| Evan’s blue | ↓ | N/A | ↓ | N/A | N/A | N/A | N/A | ↓ |
| Apparent diffusion coefficient (ADC) | N/A | N/A | ↓ | N/A | N/A | N/A | N/A | N/A |
| References | [20] | [75] | [100] | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choy, K.W.; Tsai, A.P.-Y.; Lin, P.B.-C.; Wu, M.-Y.; Lee, C.; Alias, A.; Pang, C.-Y.; Liew, H.-K. The Role of Urocortins in Intracerebral Hemorrhage. Biomolecules 2020, 10, 96. https://doi.org/10.3390/biom10010096
Choy KW, Tsai AP-Y, Lin PB-C, Wu M-Y, Lee C, Alias A, Pang C-Y, Liew H-K. The Role of Urocortins in Intracerebral Hemorrhage. Biomolecules. 2020; 10(1):96. https://doi.org/10.3390/biom10010096
Chicago/Turabian StyleChoy, Ker Woon, Andy Po-Yi Tsai, Peter Bor-Chian Lin, Meng-Yu Wu, Chihyi Lee, Aspalilah Alias, Cheng-Yoong Pang, and Hock-Kean Liew. 2020. "The Role of Urocortins in Intracerebral Hemorrhage" Biomolecules 10, no. 1: 96. https://doi.org/10.3390/biom10010096
APA StyleChoy, K. W., Tsai, A. P.-Y., Lin, P. B.-C., Wu, M.-Y., Lee, C., Alias, A., Pang, C.-Y., & Liew, H.-K. (2020). The Role of Urocortins in Intracerebral Hemorrhage. Biomolecules, 10(1), 96. https://doi.org/10.3390/biom10010096







