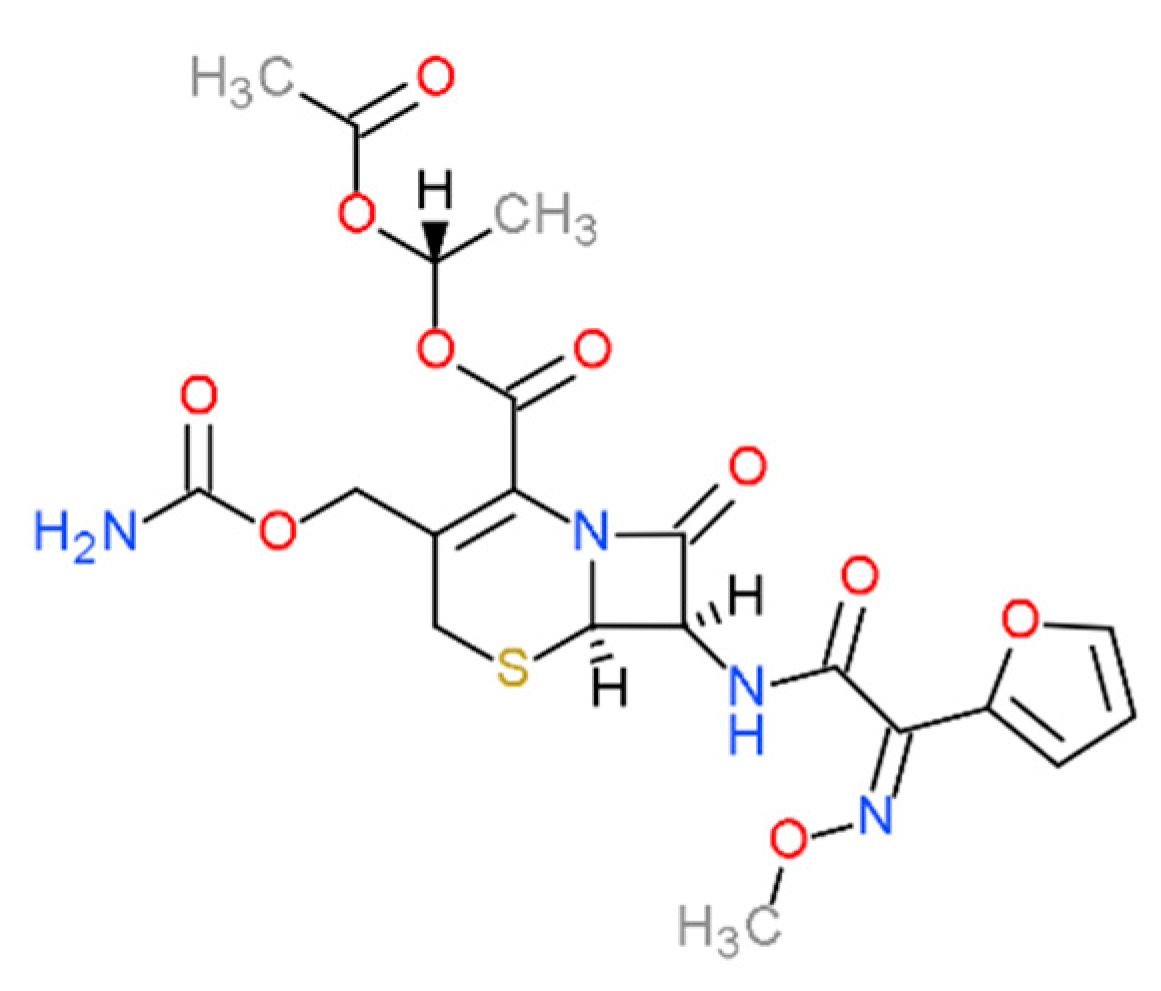
Computer-Aided Design of Cefuroxime Axetil/Cyclodextrin System with Enhanced Solubility and Antimicrobial Activity
Abstract
1. Introduction
2. Materials and Methods
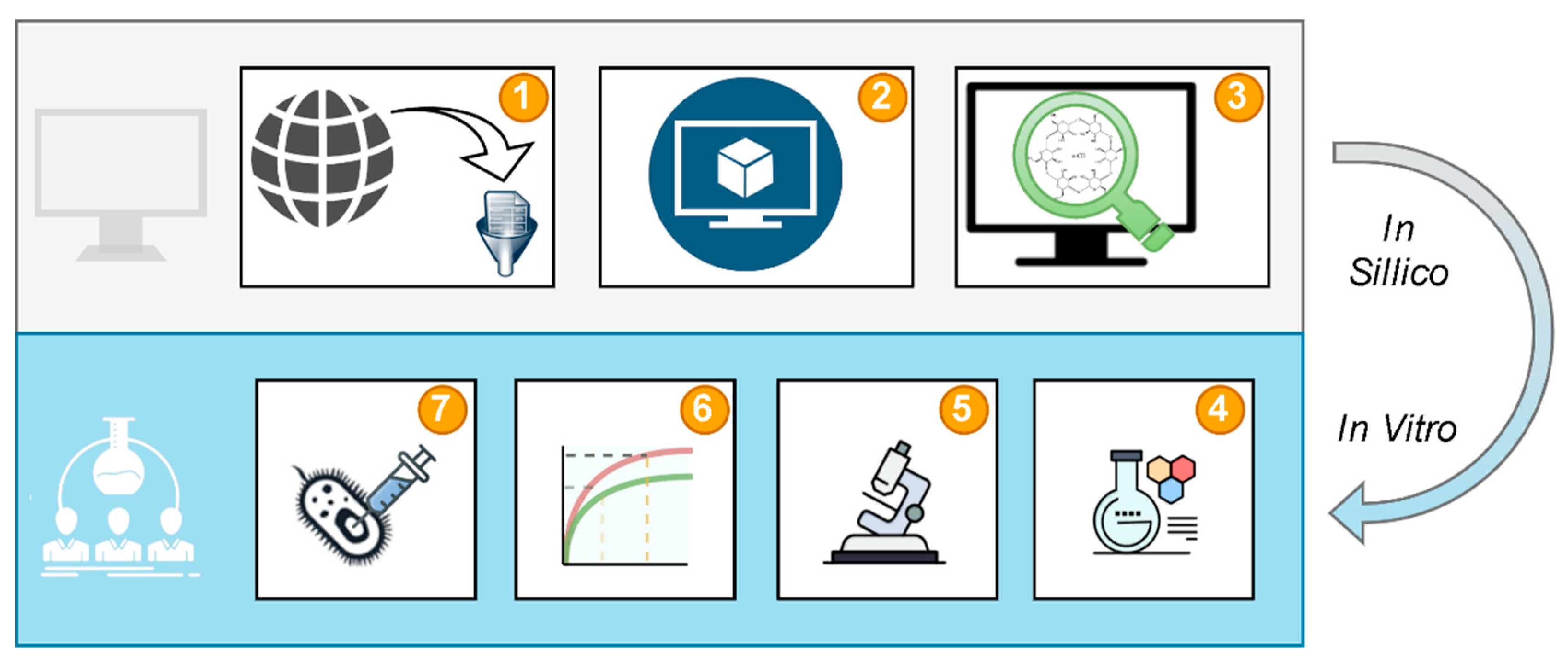
2.1. Study Design
2.2. Data Curation
2.3. Model Validation
2.4. Virtual Screening
2.5. System Preparation
2.6. System Determination
2.7. Dissolution Studies
2.8. Microbiological Study
3. Results
3.1. In Silico Study
3.2. Experimental Study
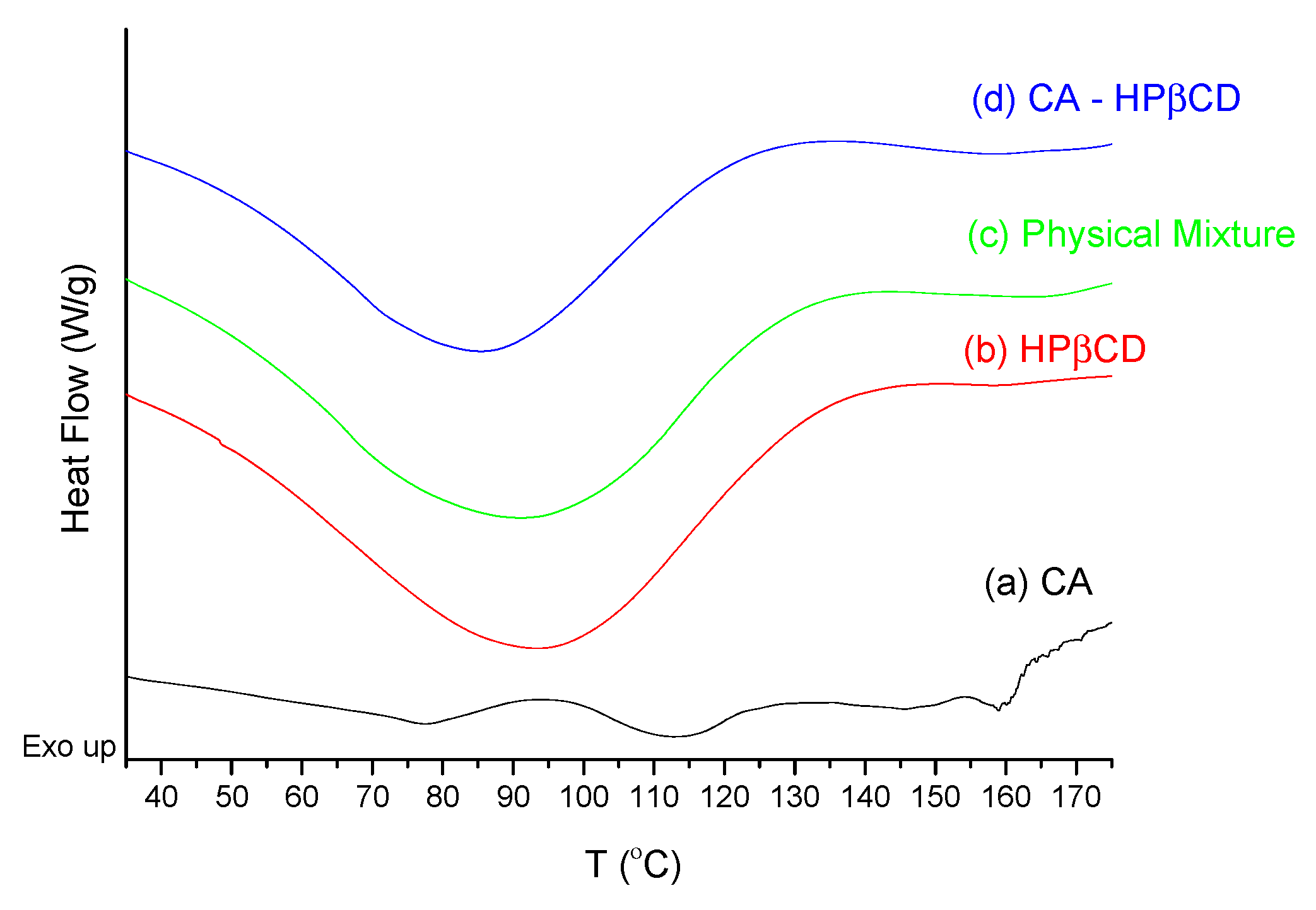
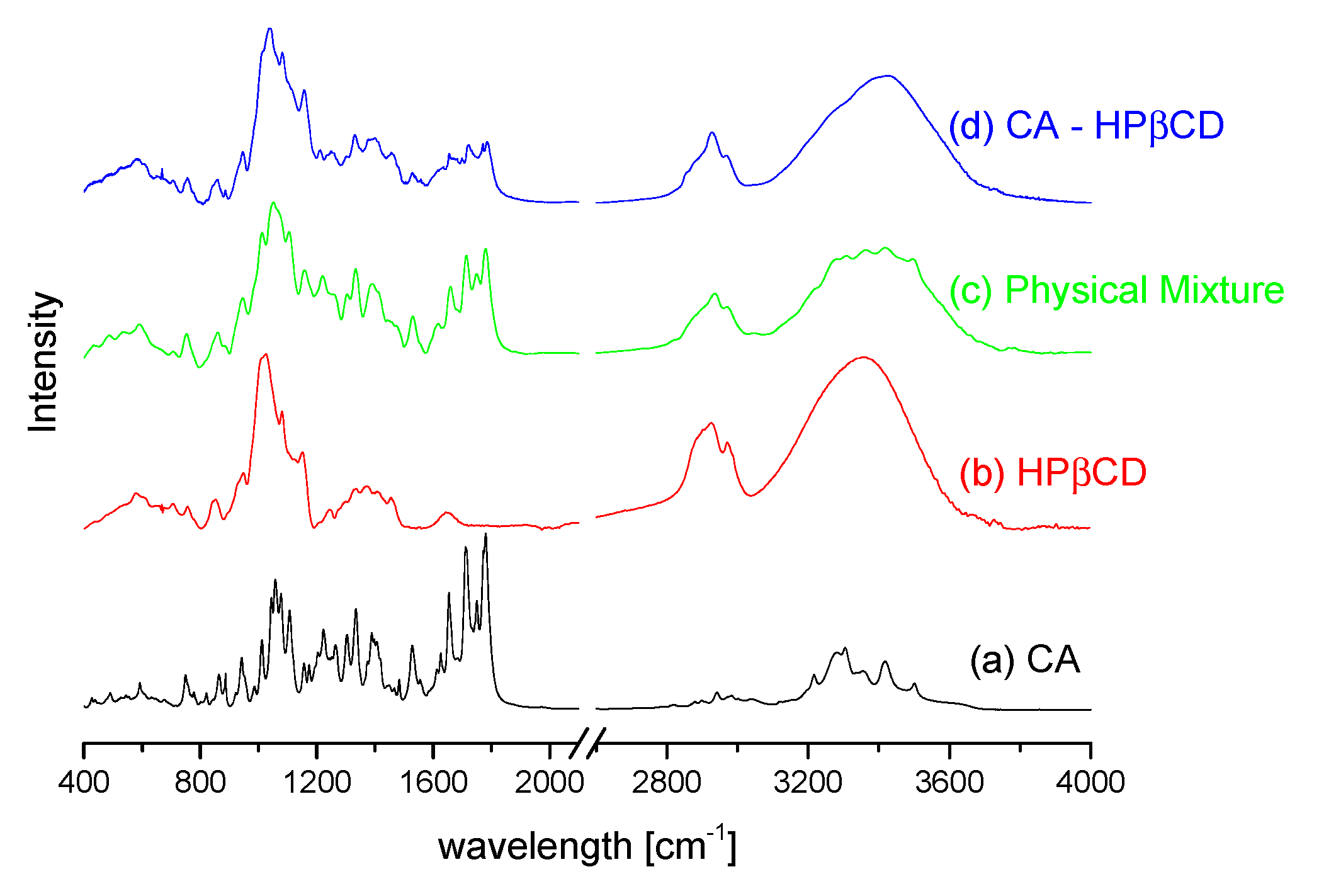
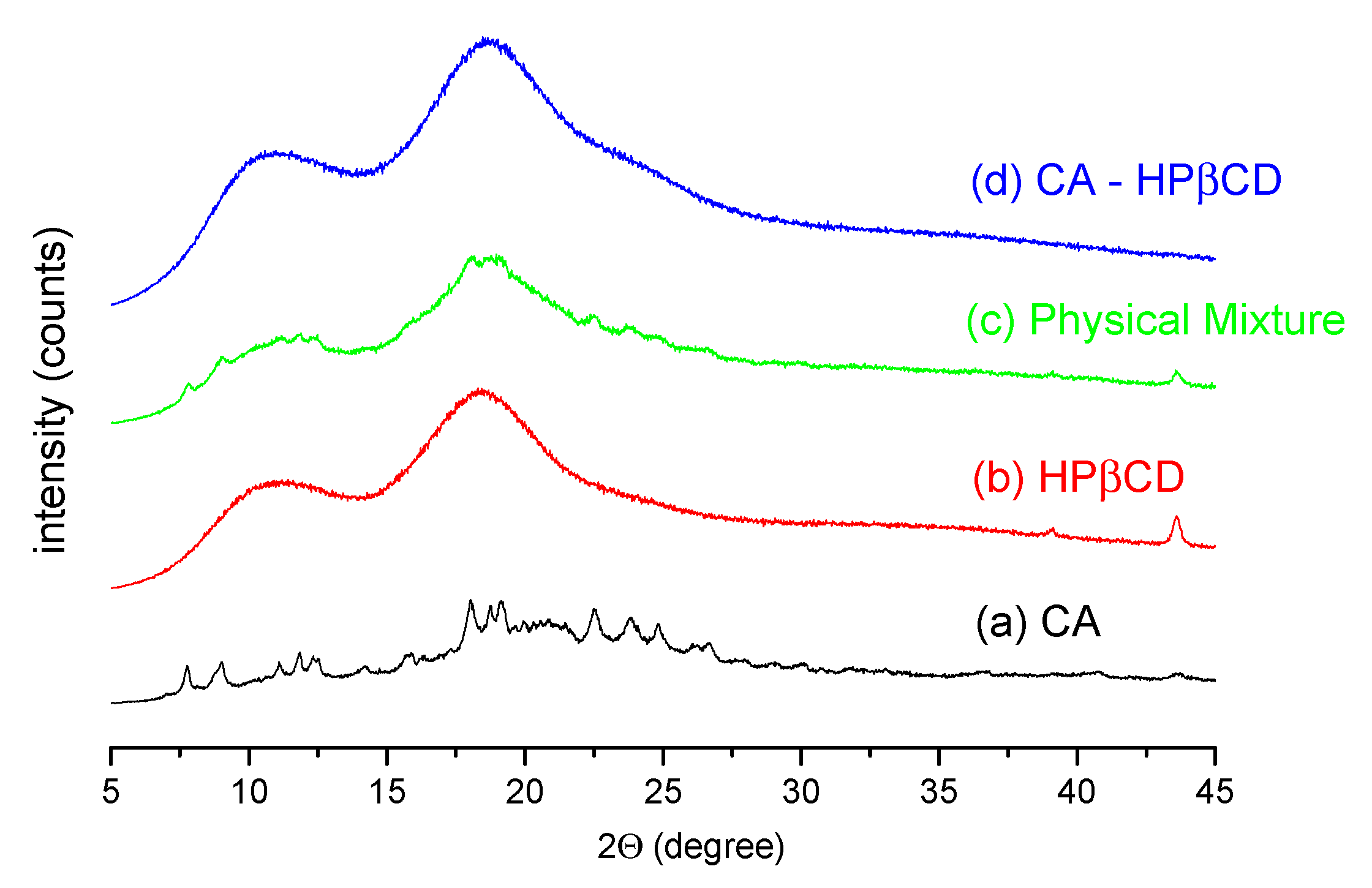
3.2.1. System Characterization
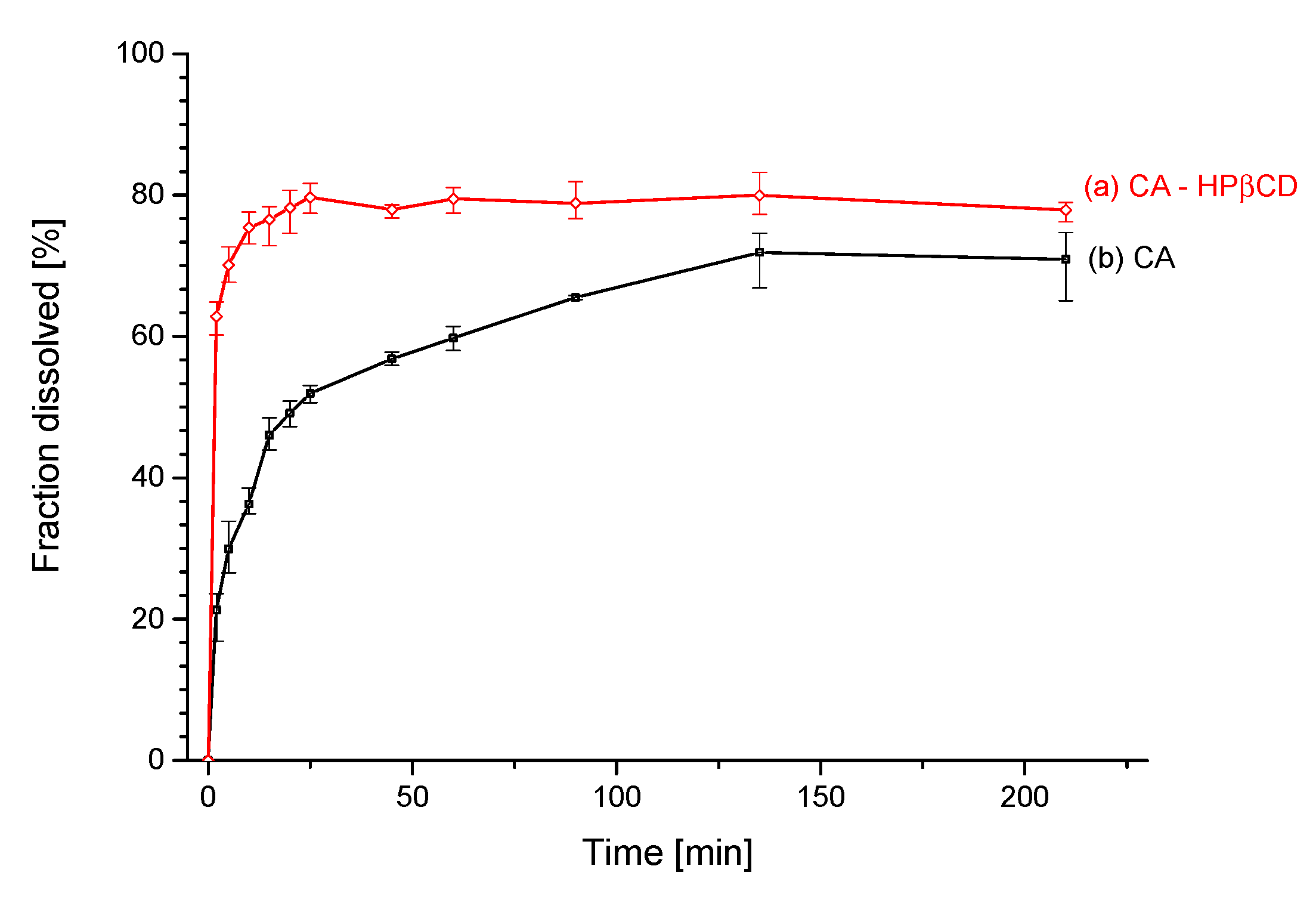
3.2.2. Solubility Studies
3.2.3. Microbiological Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rao, G.K.; Mandapalli, P.K.; Manthri, R.; Reddy, V.P. Development and in vivo evaluation of gastroretentive delivery systems for cefuroxime axetil. Saudi Pharm. J. 2013, 21, 53–59. [Google Scholar] [CrossRef]
- Rudnicka, L.; Szymańska, E.; Walecka, I.; Słowińska, M. Long-term cefuroxime axetil in subacute cutaneous lupus erythematosus. Dermatology 2000, 200, 129–131. [Google Scholar] [CrossRef]
- Lashkar, M.O.; Nahata, M.C. Antimicrobial Pharmacotherapy Management of Urinary Tract Infections in Pediatric Patients. J. Pharm. Technol. 2018, 34, 62–81. [Google Scholar] [CrossRef]
- Lang, C.C.; Moreland, T.A.; Davey, P.G. Bioavailability of cefuroxime axetil: Comparison of standard and abbreviated methods. J. Antimicrob. Chemother. 1990, 25, 645–650. [Google Scholar] [CrossRef]
- Sasinowska-Motyl, M.; Wiśniewska, I.; Gumułka, W.; Oszczapowicz, I.; Szelachowska, M.; Interewicz, B. Esters of cephalosporins. Part, I. Permeability of cefuroxime liberated from its 1-acetoxyethyl ester through biological membranes; influence of the form and size of the ester particles. Acta Pol. Pharm. 1995, 52, 391–395. [Google Scholar]
- Oszczapowicz, I.; Małafiej, E.; Szelachowska, M.; Horoszewicz-Małafiej, A.; Kuklewicz, C.; Sierańska, E.; Denys, A.; Niedworok, J. Esters of cephalosporins. Part II. Differences in the properties of various forms of the 1-acetoxyethyl ester of cefuroxime. Acta Pol. Pharm. 1995, 52, 397–401. [Google Scholar]
- Shah, M.; Pore, Y.; Dhawale, S.; Burade, K.; Kuchekar, B. Physicochemical characterization of spray dried ternary micro-complexes of cefuroxime axetil with hydroxypropyl-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2013, 76, 391–401. [Google Scholar] [CrossRef]
- Sapte, S.; Pore, Y. Inclusion complexes of cefuroxime axetil with β-cyclodextrin: Physicochemical characterization, molecular modeling and effect of L-arginine on complexation. J. Pharm. Anal. 2016, 6, 300–306. [Google Scholar] [CrossRef]
- Prabhakaran, R.; Janakiraman, K.; Harindran, J. Improvement of bioavailability of cefuroxime axetil oral suspension by inclusion complexation method. Int. J. Pharm. Pharm. Sci. 2016, 8, 361–364. [Google Scholar]
- Athanassiou, G.; Michaleas, S.; Lada-Chitiroglou, E.; Tsitsa, T.; Antoniadou-Vyza, E. Antimicrobial activity of β-lactam antibiotics against clinical pathogens after molecular inclusion in several cyclodextrins. A novel approach to bacterial resistance. J. Pharm. Pharmacol. 2003, 55, 291–300. [Google Scholar] [CrossRef]
- Paczkowska, M.; Mizera, M.; Szymanowska-Powałowska, D.; Lewandowska, K.; Błaszczak, W.; Gościańska, J.; Pietrzak, R.; Cielecka-Piontek, J. β-Cyclodextrin complexation as an effective drug delivery system for meropenem. Eur. J. Pharm. Biopharm. 2016, 99, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska, M.; Szymanowska-Powałowska, D.; Mizera, M.; Siąkowska, D.; Błaszczak, W.; Piotrowska-Kempisty, H.; Cielecka-Piontek, J. Cyclodextrins as multifunctional excipients: Influence of inclusion into β-cyclodextrin on physicochemical and biological properties of tebipenem pivoxil. PLoS ONE 2019, 14, e0210694. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Jarho, P.; Masson, M.; Järvinen, T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef]
- Paczkowska, M.; Mizera, M.; Piotrowska, H.; Szymanowska-Powałowska, D.; Lewandowska, K.; Goscianska, J.; Pietrzak, R.; Bednarski, W.; Majka, Z.; Cielecka-Piontek, J. Complex of rutin with β-cyclodextrin as potential delivery system. PLoS ONE 2015, 10, e0120858. [Google Scholar] [CrossRef]
- Paczkowska, M.; Mizera, M.; Sałat, K.; Furgała, A.; Popik, P.; Knapik-Kowalczuk, J.; Krause, A.; Szymanowska-Powałowska, D.; Fojud, Z.; Kozak, M.; et al. Enhanced pharmacological efficacy of sumatriptan due to modification of its physicochemical properties by inclusion in selected cyclodextrins. Sci. Rep. 2018, 8, 16184. [Google Scholar] [CrossRef]
- Liu, B.; Zeng, J.; Chen, C.; Liu, Y.; Ma, H.; Mo, H.; Liang, G. Interaction of cinnamic acid derivatives with β-cyclodextrin in water: Experimental and molecular modeling studies. Food Chem. 2016, 194, 1156–1163. [Google Scholar] [CrossRef]
- Shityakov, S.; Salmas, R.E.; Durdagi, S.; Salvador, E.; Pápai, K.; Yáñez-Gascón, M.J.; Pérez-Sánchez, H.; Puskás, I.; Roewer, N.; Förster, C.; et al. Characterization, in vivo evaluation, and molecular modeling of different propofol–cyclodextrin complexes to assess their drug delivery potential at the blood–brain barrier level. J. Chem. Inf. Model. 2016, 56, 1914–1922. [Google Scholar] [CrossRef]
- Khuntawee, W.; Karttunen, M.; Wong-ekkabut, J. A molecular dynamics study of conformations of beta-cyclodextrin and its eight derivatives in four different solvents. Phys. Chem. Chem. Phys. 2017, 19, 24219–24229. [Google Scholar] [CrossRef]
- Suzuki, T. A nonlinear group contribution method for predicting the free energies of inclusion complexation of organic molecules with α-and β-cyclodextrins. J. Chem. Inf. Comput. Sci. 2001, 41, 1266–1273. [Google Scholar] [CrossRef]
- Tropsha, A. Best practices for QSAR model development, validation, and exploitation. Mol. Inform. 2010, 29, 476–488. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Jacobson, M.P.; Pincus, D.L.; Rapp, C.S.; Day, T.J.; Honig, B.; Shaw, D.E.; Friesner, R.A. A hierarchical approach to all-atom protein loop prediction. Proteins Struct. Funct. Bioinform. 2004, 55, 351–367. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. Gaussian 09, Revision, A.02; Fox, Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- OriginLab Corporation. OriginPro; Version 9.0; OriginLab Corporation: Northampton, MA, USA, 1991. [Google Scholar]
- Hassan, M.A.; Suleiman, M.S.; Najib, N.M. Improvement of the in vitro dissolution characteristics of famotidine by inclusion in β-cyclodextrin. Int. J. Pharm. 1990, 58, 19–24. [Google Scholar] [CrossRef]
- Bruker AXS GmbH Karlsruhe. DIFFRAC.EVA; Version 4.2.2; Bruker AXS GmbH Karlsruhe: Karlsruhe, Germany, 2016. [Google Scholar]
- Zenoni, M.; Leone, M.; Cattaneo, A.; Marsili, L. Bioavailable crystalline form of cefuroxime axetil. U.S. Patent No. 5,677,443, 14 October 1997. [Google Scholar]
- ICDD. PDF-4/Organics 2020 (Database); Kabekkodu, S., Ed.; International Centre for Diffraction Data: Newtown Square, PA, USA, 2018. [Google Scholar]
- Wang, R.; Zhou, H.; Siu, S.W.; Gan, Y.; Wang, Y.; Ouyang, D. Comparison of three molecular simulation approaches for cyclodextrin-ibuprofen complexation. J. Nanomater. 2015, 16, 267. [Google Scholar] [CrossRef]
- Núñez-Agüero, C.J.; Escobar-Llanos, C.M.; Díaz, D.; Jaime, C.; Garduño-Juárez, R. Chiral discrimination of ibuprofen isomers in β-cyclodextrin inclusion complexes: Experimental (NMR) and theoretical (MD, MM/GBSA) studies. Tetrahedron 2006, 62, 4162–4172. [Google Scholar] [CrossRef]
- Mizera, M.; Lewandowska, K.; Miklaszewski, A.; Cielecka-Piontek, J. Machine Learning Approach for Determining the Formation of β-Lactam Antibiotic Complexes with Cyclodextrins Using Multispectral Analysis. Molecules 2019, 24, 743. [Google Scholar] [CrossRef]
- Maffeo, D.; Leondiadis, L.; Mavridis, I.M.; Yannakopoulou, K. Positive effect of natural and negatively charged cyclodextrins on the stabilization of penicillins towards β-lactamase degradation due to inclusion and external guest–host association. An NMR and MS study. Organ. Biomol. Chem. 2006, 4, 1297–1304. [Google Scholar] [CrossRef]
- Aleem, O.; Kuchekar, B.; Pore, Y.; Late, S. Effect of β-cyclodextrin and hydroxypropyl β-cyclodextrin complexation on physicochemical properties and antimicrobial activity of cefdinir. J. Pharm. Biomed. Anal. 2008, 47, 535–540. [Google Scholar] [CrossRef]
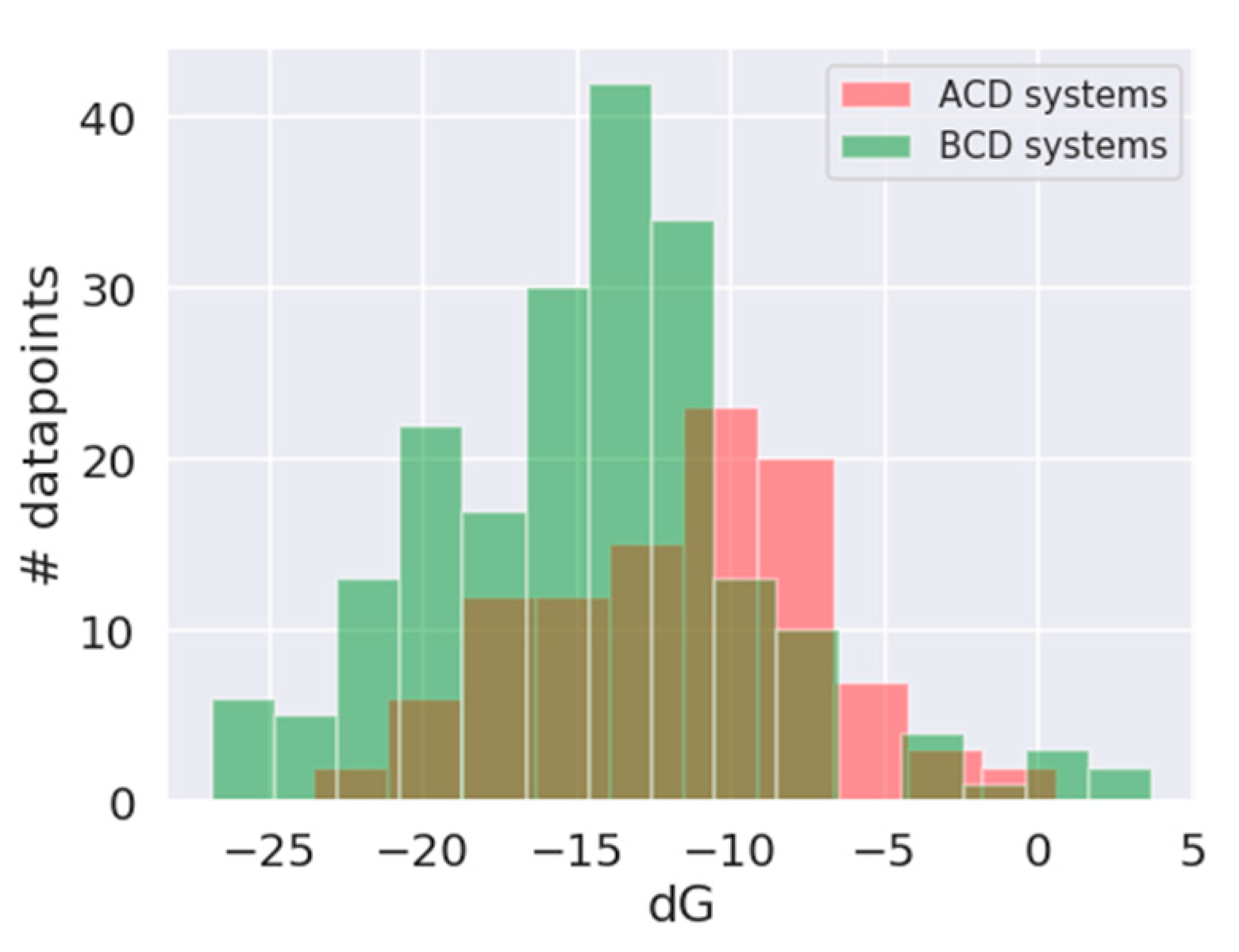
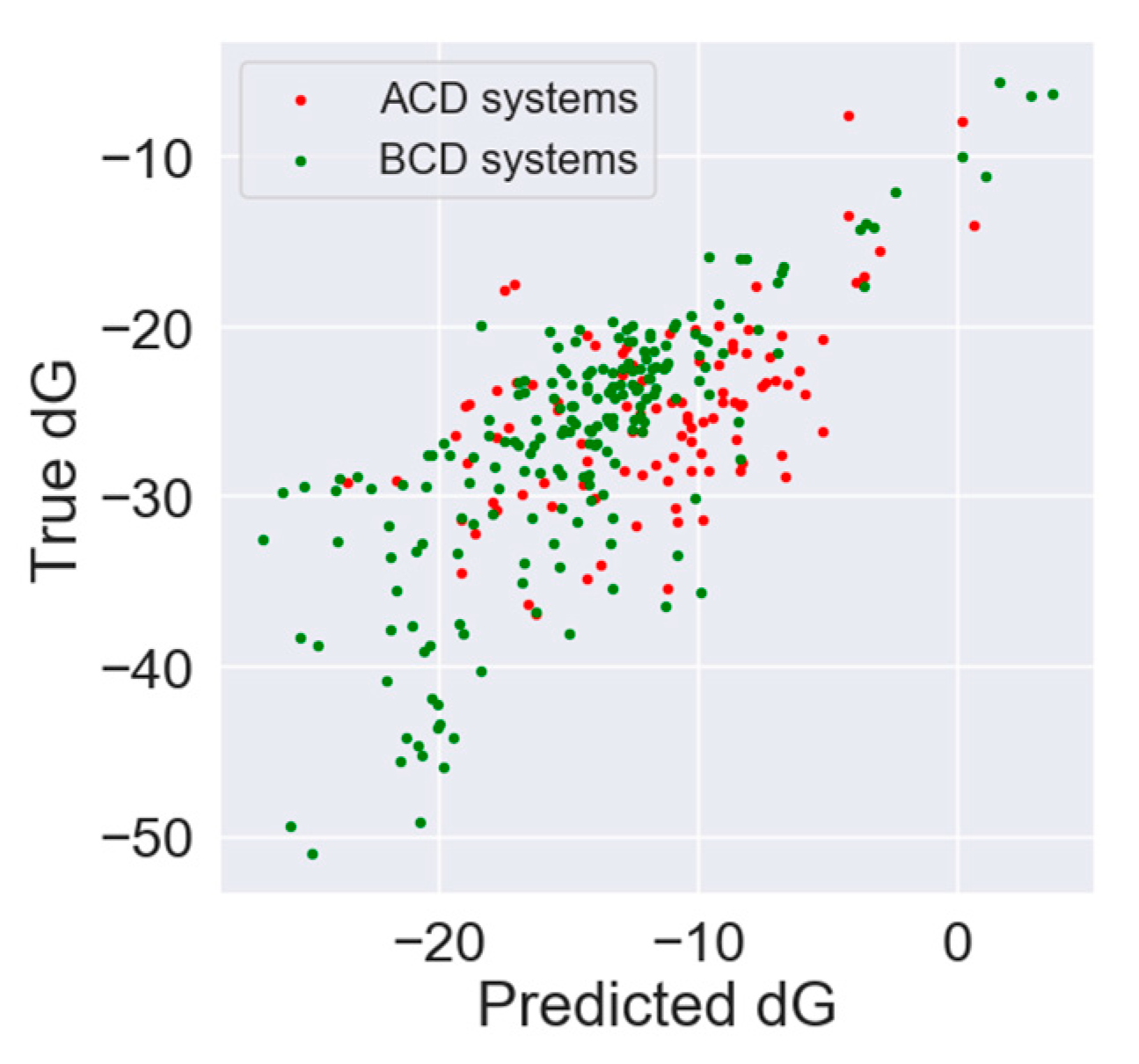
| Absolute Value [kJ/mol] | Relative Value [%] | Rank Prediction | |
|---|---|---|---|
| HPβCD | −57.84 | 100.00 | 1 |
| RMβCD | −56.64 | 97.91 | 2 |
| βCD | −51.21 | 88.53 | 3 |
| αCD | −50.55 | 87.38 | 4 |
| HPαCD | −49.24 | 85.13 | 5 |
| Microorganism | MIC (mg/L) | |
|---|---|---|
| CA | CA–HPβCD | |
| Proteus mirabilis ATCC 12453 | 16 | 8 |
| Proteus mirabilis clinical isolates | 32 | 16 |
| Klebsiella pneumoniae ATCC 31488 | 2 | 1 |
| Klebsiella pneumoniae clinical isolates | 8 | 2 |
| Enterobacter aerogenes ATCC 13048 | 64 | 32 |
| Enterobacter aerogenes clinical isolates | 128 | 64 |
| Enterococcus faecalis ATTC 29212 | 64 | 64 |
| Enterococcus faecalis clinical isolates | 128 | 128 |
| Escherichia coli ATCC 25922 | 64 | 32 |
| Escherichia coli clinical isolates | 64 | 32 |
| Staphylococcus aureus ATCC 25923 | 32 | 32 |
| Staphylococcus aureus clinical isolates | 32 | 32 |
| Acinetobacter baumanii ATCC 19606 | 32 | 16 |
| Acinetobacter baumanii clinical isolates | 64 | 32 |
| Pseudomonas aeruginosa ATCC 27853 | 16 | 8 |
| Pseudomonas aeruginosa clinical isolates | 32 | 8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizera, M.; Szymanowska, D.; Stasiłowicz, A.; Siąkowska, D.; Lewandowska, K.; Miklaszewski, A.; Plech, T.; Tykarska, E.; Cielecka-Piontek, J. Computer-Aided Design of Cefuroxime Axetil/Cyclodextrin System with Enhanced Solubility and Antimicrobial Activity. Biomolecules 2020, 10, 24. https://doi.org/10.3390/biom10010024
Mizera M, Szymanowska D, Stasiłowicz A, Siąkowska D, Lewandowska K, Miklaszewski A, Plech T, Tykarska E, Cielecka-Piontek J. Computer-Aided Design of Cefuroxime Axetil/Cyclodextrin System with Enhanced Solubility and Antimicrobial Activity. Biomolecules. 2020; 10(1):24. https://doi.org/10.3390/biom10010024
Chicago/Turabian StyleMizera, Mikołaj, Daria Szymanowska, Anna Stasiłowicz, Dominika Siąkowska, Kornelia Lewandowska, Andrzej Miklaszewski, Tomasz Plech, Ewa Tykarska, and Judyta Cielecka-Piontek. 2020. "Computer-Aided Design of Cefuroxime Axetil/Cyclodextrin System with Enhanced Solubility and Antimicrobial Activity" Biomolecules 10, no. 1: 24. https://doi.org/10.3390/biom10010024
APA StyleMizera, M., Szymanowska, D., Stasiłowicz, A., Siąkowska, D., Lewandowska, K., Miklaszewski, A., Plech, T., Tykarska, E., & Cielecka-Piontek, J. (2020). Computer-Aided Design of Cefuroxime Axetil/Cyclodextrin System with Enhanced Solubility and Antimicrobial Activity. Biomolecules, 10(1), 24. https://doi.org/10.3390/biom10010024







