Preparation, Characterization, and Immuno-Enhancing Activity of Polysaccharides from Glycyrrhiza uralensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
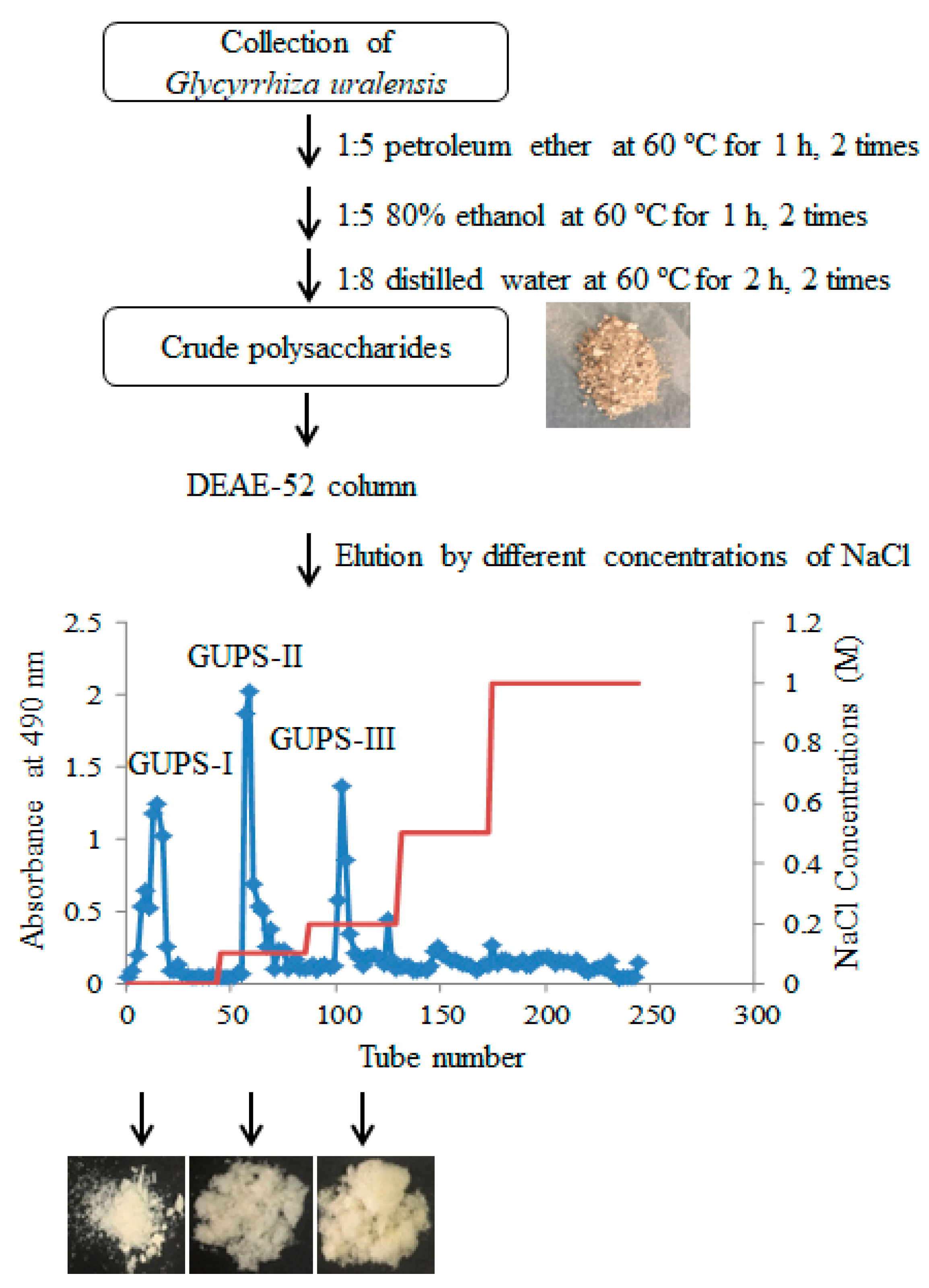
2.2. Preparation of G. uralensis Crude Polysaccharides (GUPS-C)
2.3. Purification of GUPS
2.4. Analysis of Carbohydrate, Protein, and Sulfate Contents
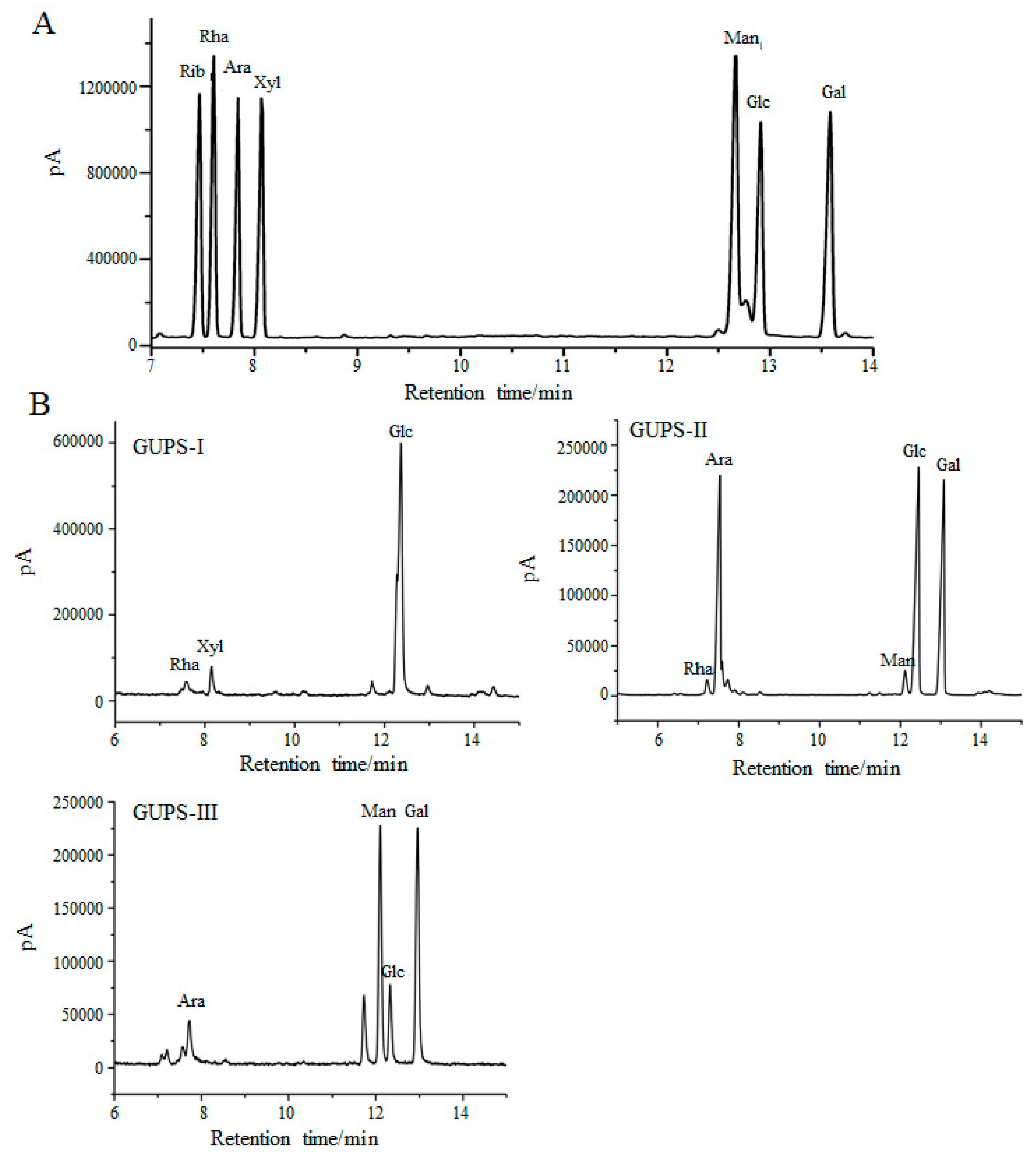
2.5. Monosaccharide Composition Analysis
2.6. Molecular Weight Determination
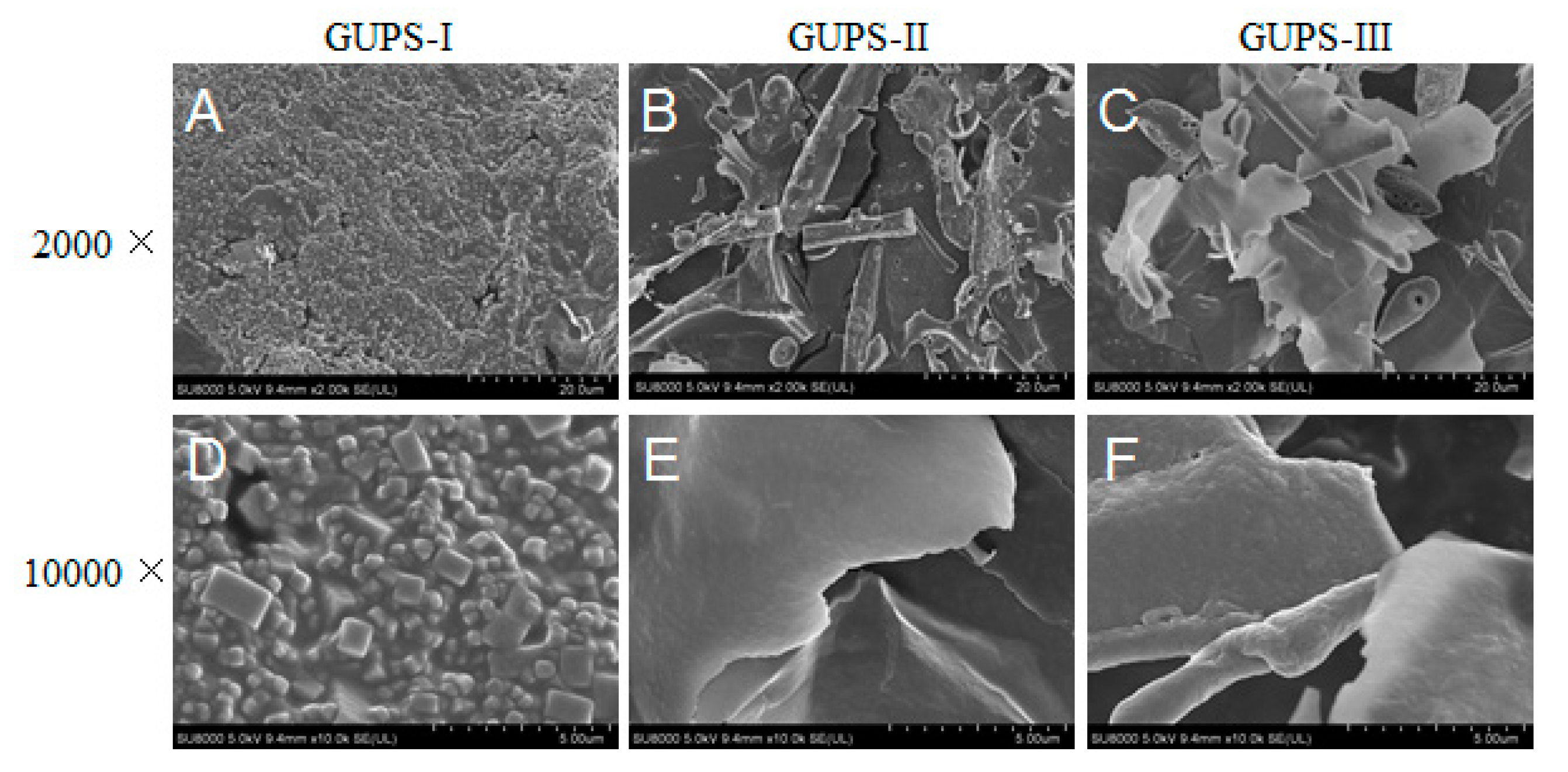
2.7. SEM Analysis
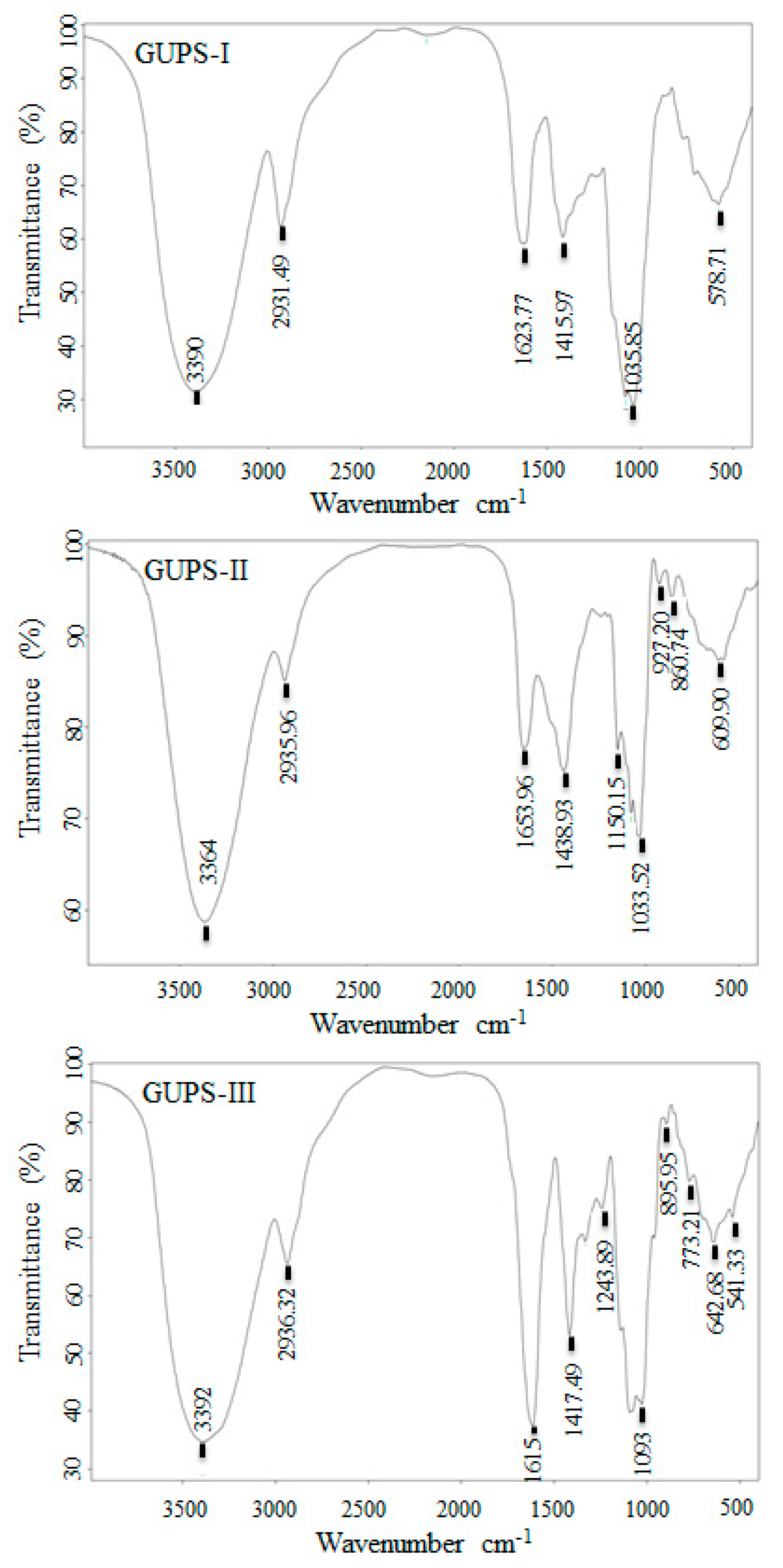
2.8. Fourier Transform Infrared (FT-IR) Spectroscopy and Nuclear Magnetic Resonance (NMR) Spectroscopy
2.9. DPPH Radical Scavenging Assay
2.10. Generation and Treatment of Bone Marrow-Derived DCs
2.11. Animal Experiment
2.12. Ethics Statements
2.13. Flow Cytometry
2.14. ELISA Assay of Cytokines
2.15. Data Analysis
3. Results
3.1. Purification and Physicochemical Characterization of GUPS
3.2. Monosaccharide Composition of GUPS
3.3. Scanning Electron Microscopy (SEM) Analysis of GUPS
3.4. The Analysis of GUPS by FT-IR Spectroscopy
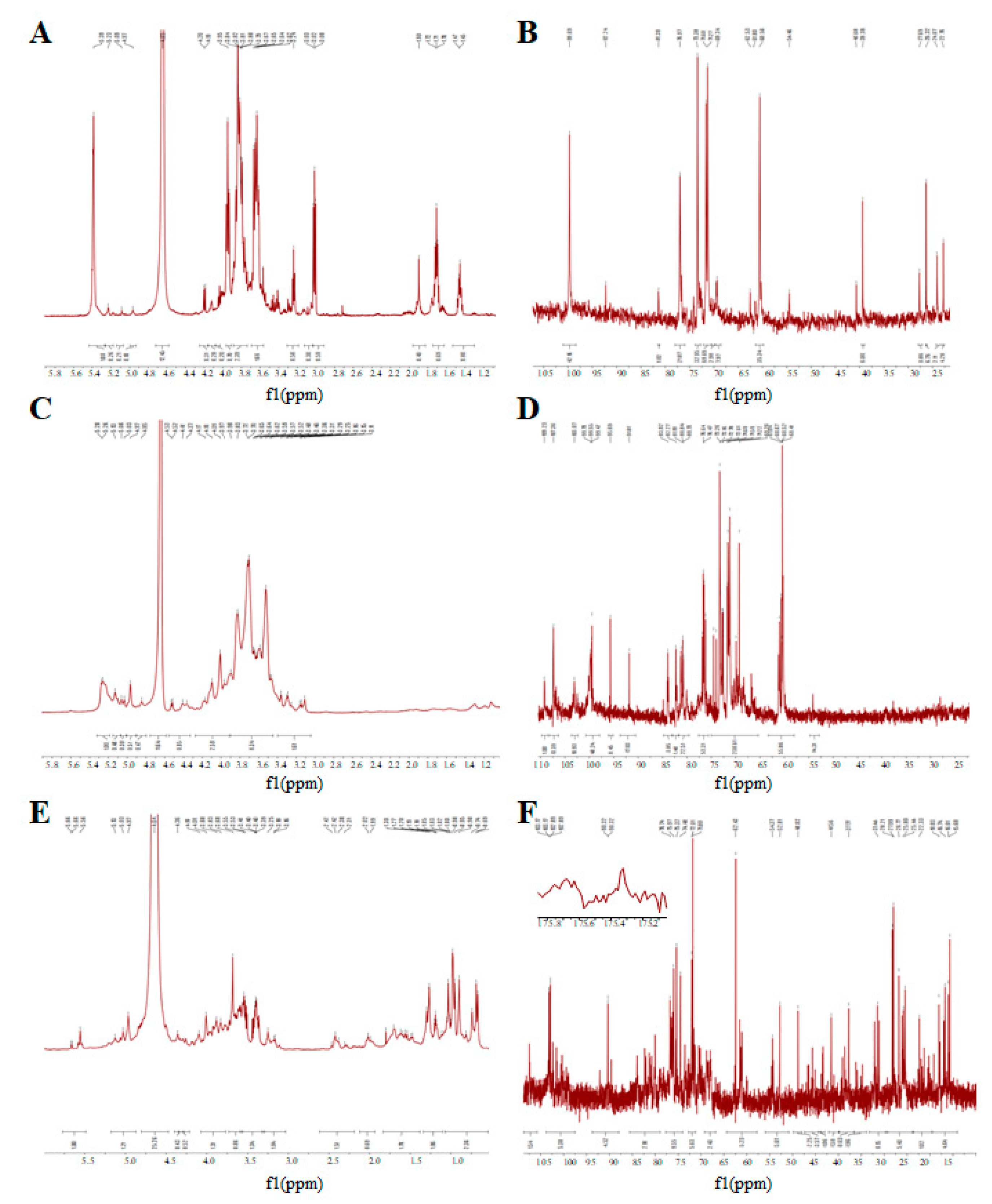
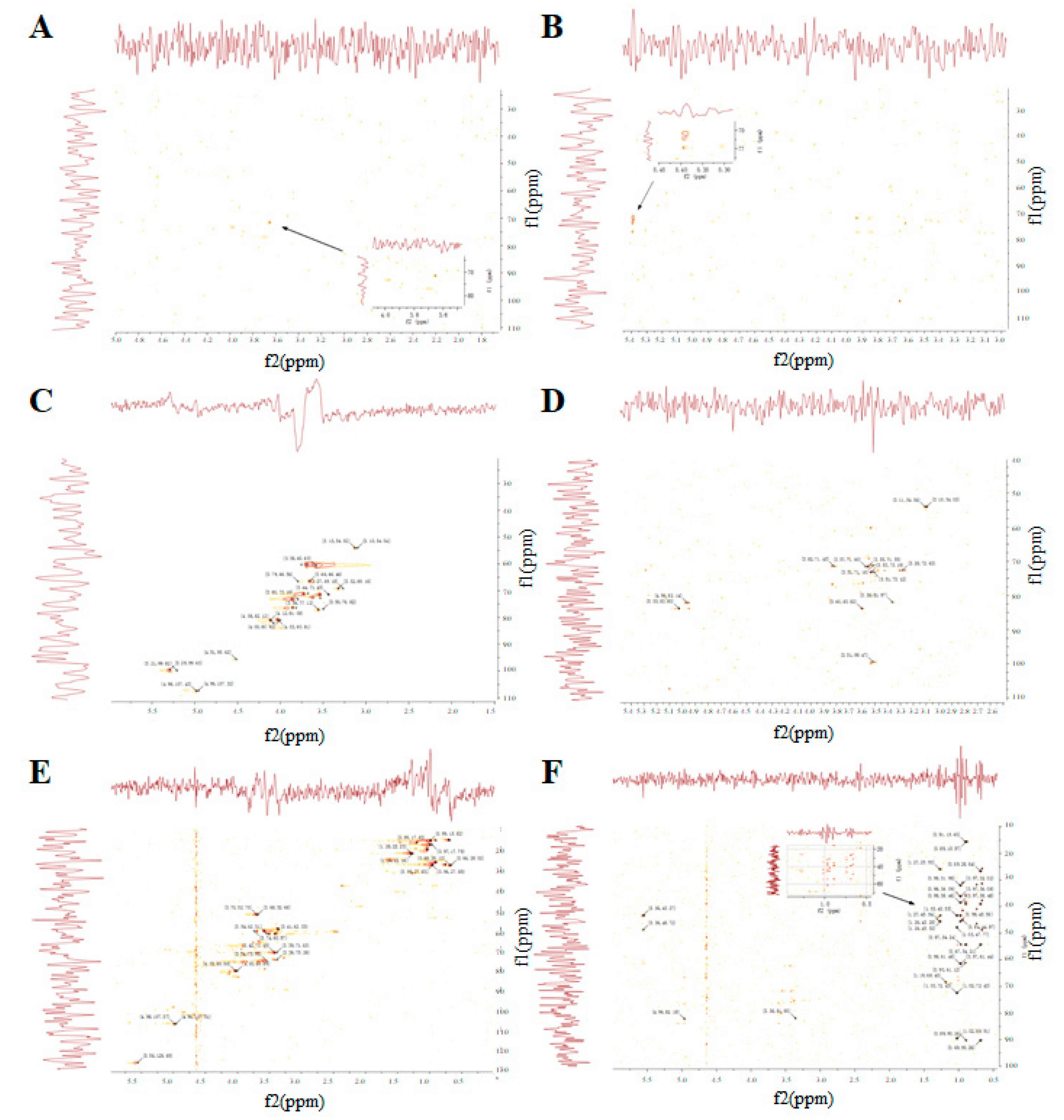
3.5. The NMR Spectrum of GUPS
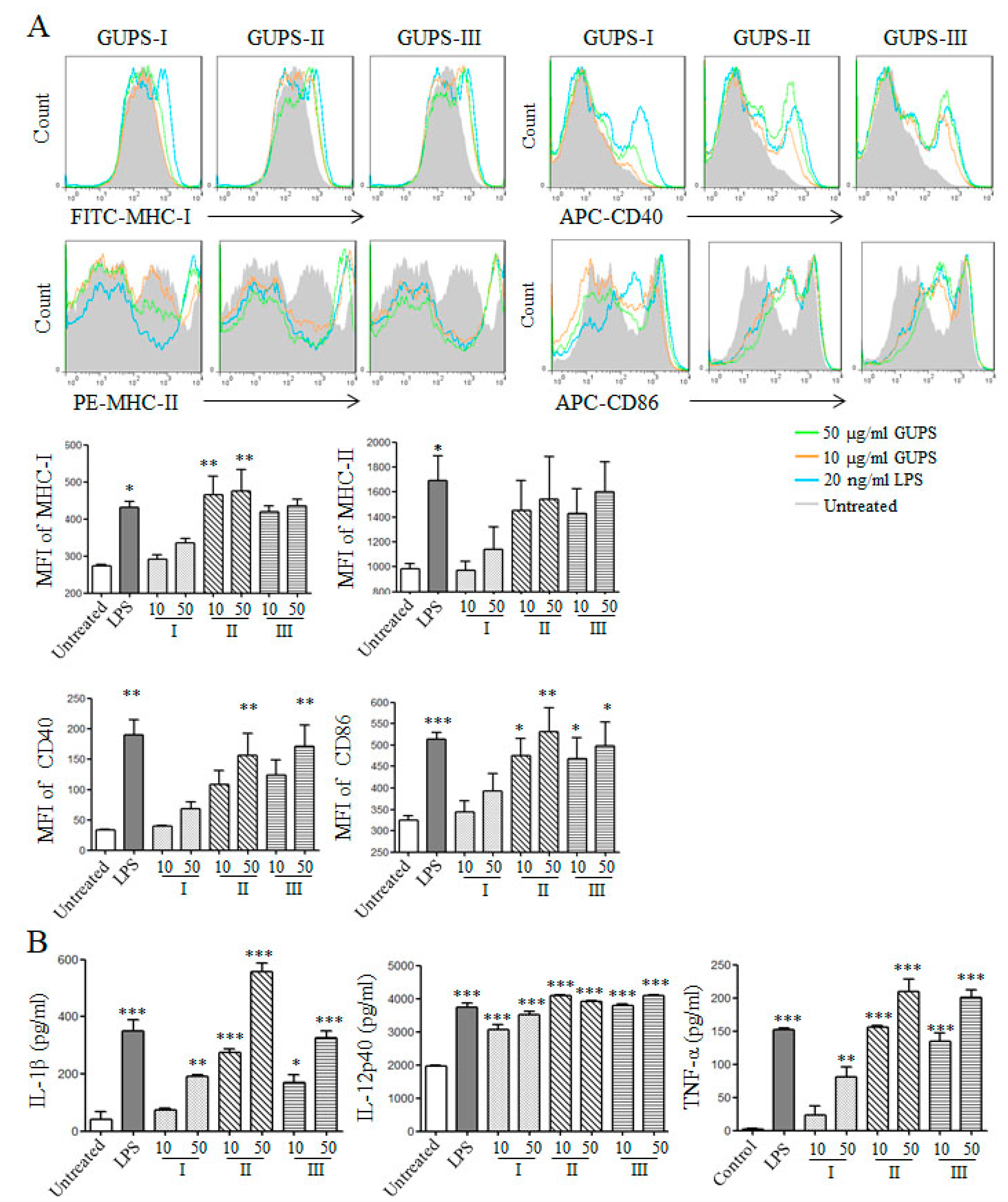
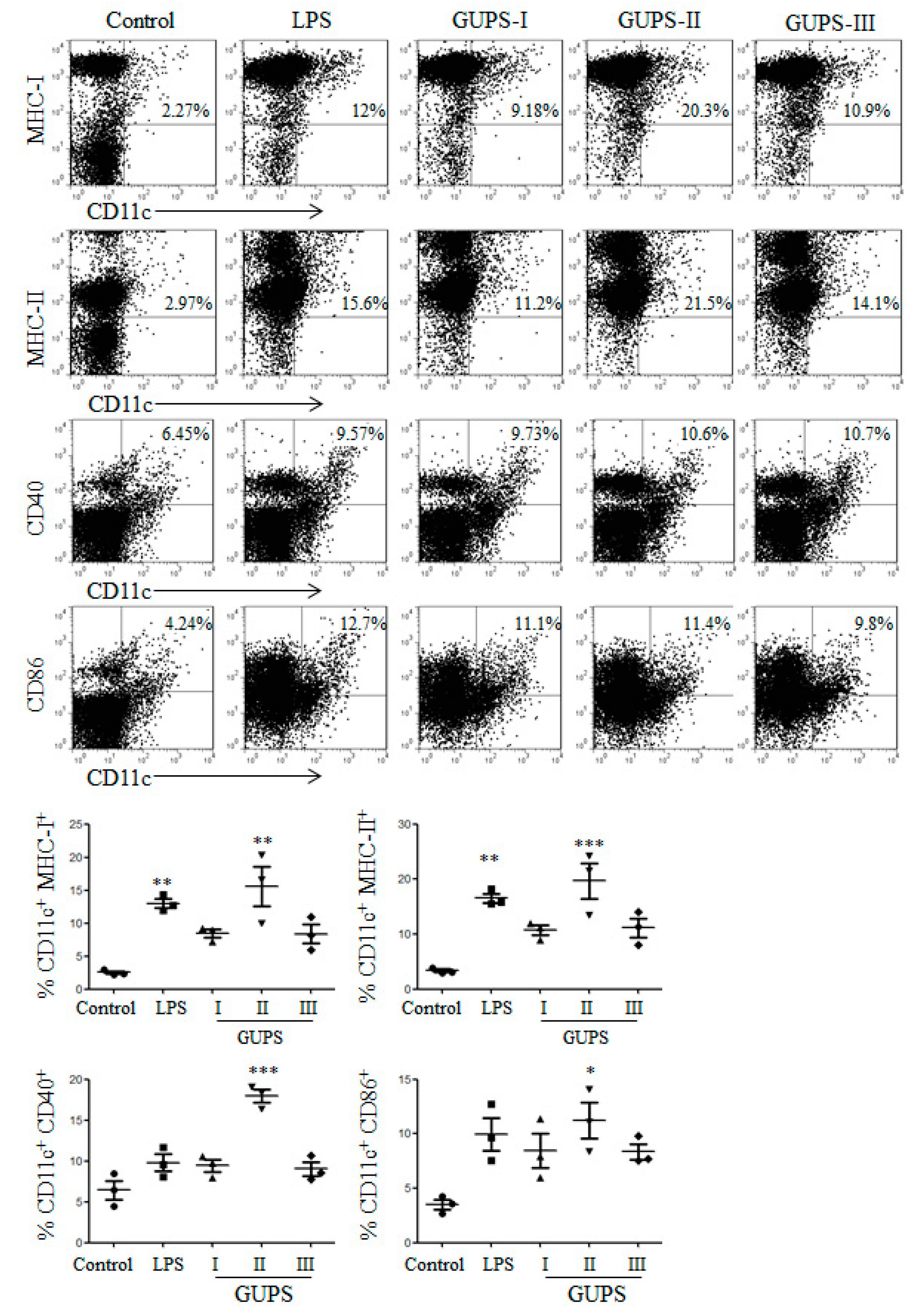
3.6. GUPS Promotes the Maturation of DCs
3.7. Diphenylpicrylhydrazyl (DPPH) Radical Scavenging Activity of GUPS
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhen, D.; Su, L.; Miao, Y.; Zhao, F.; Ren, G.; Mahfuz, S.; Song, H. Purification, partial characterization and inducing tumor cell apoptosis activity of a polysaccharide from Ganoderma applanatum. Int. J. Biol. Macromol. 2018, 115, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, X.; Xie, H.; Li, X.; Shi, L. Structural characterization and antitumor activity of a polysaccharide from Ramulus mori. Carbohydr. Polym. 2018, 190, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Meng, Z. Purification, characterization and anti-tumor activities of polysaccharides extracted from wild Russula griseocarnosa. Int. J. Biol. Macromol. 2018, 109, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, X.; Wang, F.; Xu, J.; Tang, X.; Li, N. Structural characterization and antioxidant activity of polysaccharide from ginger. Int. J. Biol. Macromol. 2018, 111, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, G.; Hu, J. Extraction, characterisation and antioxidant activity of polysaccharides from Chinese watermelon. Int. J. Biol. Macromol. 2018, 111, 1304–1307. [Google Scholar] [CrossRef]
- Deng, Y.; Li, M.; Chen, L.X.; Chen, X.Q.; Lu, J.H.; Zhao, J.; Li, S.P. Chemical characterization and immunomodulatory activity of acetylated polysaccharides from Dendrobium devonianum. Carbohydr. Polym. 2018, 180, 238–245. [Google Scholar] [CrossRef]
- Dong, Z.; Li, C.; Huang, Q.; Zhang, B.; Fu, X. Characterization of a novel polysaccharide from the leaves of Moringa oleifera and its immunostimulatory activity. J. Funct. Foods 2018, 49, 391–400. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, Y.K.; Choi, Y.R.; Park, J.; Jung, S.K.; Chang, Y.H. The characterization, selenylation and anti-inflammatory activity of pectic polysaccharides extracted from Ulmus pumila L. Int. J. Biol. Macromol. 2018, 111, 311–318. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, P.; Zhao, S.; Nie, C.; Wang, N.; Du, X.; Zhou, Y. Characterization, antioxidant activity and immunomodulatory activity of polysaccharides from the swollen culms of Zizania latifolia. Int. J. Biol. Macromol. 2017, 95, 809–817. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Li, J.; Yuan, P.; Wang, X.; Aipire, A.; Li, M.; Yang, J.; Tao, H.; Ying, T.; Fu, C.; Wei, X.; et al. Purification, characterization and bioactivities of polysaccharides from Pleurotus ferulae. Food Funct. 2017, 8, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.A.; Nunes, C.A.; Pereira, J. Relationship between the chemical components of taro rhizome mucilage and its emulsifying property. Food Chem. 2015, 178, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, H.; Li, M.; Niu, S.; Wang, J.; Shao, H.; Li, T.; Wang, H. Salvia miltiorrhiza polysaccharide activates T Lymphocytes of cancer patients through activation of TLRs mediated -MAPK and -NF-κB signaling pathways. J. Ethnopharmacol. 2017, 200, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Gan, F.; Liu, Q.; Liu, Y.; Huang, D.; Pan, C.; Song, S.; Huang, K. Lycium barbarum polysaccharides improve CCl4-induced liver fibrosis, inflammatory response and TLRs/NF-kB signaling pathway expression in wistar rats. Life Sci. 2018, 192, 205–212. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; You, L.; Fu, X.; Liu, R.H. Fractionation, preliminary structural characterization and bioactivities of polysaccharides from Sargassum pallidum. Carbohydr. Polym. 2017, 155, 261–270. [Google Scholar] [CrossRef]
- Cheng, A.; Wan, F.; Wang, J.; Jin, Z.; Xu, X. Macrophage immunomodulatory activity of polysaccharides isolated from Glycyrrhiza uralensis Fish. Int. Immunopharmacol. 2008, 8, 43–50. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, S.; Song, X.; Jia, J.; Zhang, Z.; Zhou, H.; Fu, H.; Cui, H.; Hu, S.; Fang, M.; et al. Inhibition effect of glycyrrhiza polysaccharide (GCP) on tumor growth through regulation of the gut microbiota composition. J. Pharmacol. Sci. 2018, 137, 324–332. [Google Scholar] [CrossRef]
- Wu, Y.; Yi, L.; Li, E.; Li, Y.; Lu, Y.; Wang, P.; Zhou, H.; Liu, J.; Hu, Y.; Wang, D. Optimization of Glycyrrhiza polysaccharide liposome by response surface methodology and its immune activities. Int. J. Biol. Macromol. 2017, 102, 68–75. [Google Scholar] [CrossRef]
- Tao, W.; Duan, J.; Zhao, R.; Li, X.; Yan, H.; Li, J.; Guo, S.; Yang, N.; Tang, Y. Comparison of three officinal Chinese pharmacopoeia species of Glycyrrhiza based on separation and quantification of triterpene saponins and chemometrics analysis. Food Chem. 2013, 141, 1681–1689. [Google Scholar] [CrossRef]
- Song, W.; Si, L.; Ji, S.; Wang, H.; Fang, X.M.; Yu, L.Y.; Li, R.Y.; Liang, L.N.; Zhou, D.; Ye, M. Uralsaponins M-Y, antiviral triterpenoid saponins from the roots of Glycyrrhiza uralensis. J. Nat. Prod. 2014, 77, 1632–1643. [Google Scholar] [CrossRef]
- Tang, Z.H.; Li, T.; Tong, Y.G.; Chen, X.J.; Chen, X.P.; Wang, Y.T.; Lu, J.J. A Systematic Review of the Anticancer Properties of Compounds Isolated from Licorice (Gancao). Planta Med. 2015, 81, 1670–1687. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, J.; Li, Y.J.; Zheng, Y.F.; Li, P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013, 141, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Aipire, A.; Li, J.; Yuan, P.; He, J.; Hu, Y.; Liu, L.; Feng, X.; Li, Y.; Zhang, F.; Yang, J.; et al. Glycyrrhiza uralensis water extract enhances dendritic cell maturation and antitumor efficacy of HPV dendritic cell-based vaccine. Sci. Rep. 2017, 7, 43796. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zheng, G.; You, L.; Wen, L.; Li, C.; Fu, X.; Zhou, L. Structural characterization and macrophage immunomodulatory activity of a polysaccharide isolated from Gracilaria lemaneiformis. J. Funct. Foods 2017, 33, 286–296. [Google Scholar] [CrossRef]
- Tu, W.; Zhu, J.; Bi, S.; Chen, D.; Song, L.; Wang, L.; Zi, J.; Yu, R. Isolation, characterization and bioactivities of a new polysaccharide from Annona squamosa and its sulfated derivative. Carbohydr. Polym. 2016, 152, 287–296. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Wang, W.; Luo, J.; Aipire, A.; Li, J.; Zhang, F. Pleurotus ferulae water extract enhances the maturation and function of murine bone marrow-derived dendritic cells through TLR4 signaling pathway. Vaccine 2015, 33, 1923–1933. [Google Scholar] [CrossRef]
- Liu, D.; Sun, Q.; Xu, J.; Li, N.; Lin, J.; Chen, S.; Li, F. Purification, characterization, and bioactivities of a polysaccharide from mycelial fermentation of Bjerkandera fumosa. Carbohydr. Polym. 2017, 167, 115–122. [Google Scholar] [CrossRef]
- Zhang, C.H.; Yu, Y.; Liang, Y.Z.; Chen, X.Q. Purification, partial characterization and antioxidant activity of polysaccharides from Glycyrrhiza uralensis. Int. J. Biol. Macromol. 2015, 79, 681–686. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Ma, X.; Ren, H.; Fan, W.; Leng, F.; Yang, M.; Wang, X. Extraction, purification, and bioactivities analyses of polysaccharides from Glycyrrhiza uralensis. Ind. Crops Prod. 2018, 122, 596–608. [Google Scholar] [CrossRef]
- Chylińska, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman characterization of non-cellulosic polysaccharides fractions isolated from plant cell wall. Carbohydr. Polym. 2016, 154, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, Y.; Duan, X.; Tang, T.; Shen, Y.; Hu, B.; Liu, A.; Chen, H.; Li, C.; Liu, Y. Characterization and antioxidant activities of polysaccharides from thirteen boletus mushrooms. Int. J. Biol. Macromol. 2018, 113, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zi, W.; Pei, Z.; Liu, S. Characterization of polysaccharides and their antioxidant properties from Plumula nelumbinis. Saudi. Pharm. J. 2018, 26, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, Z.; Liu, X.; Chen, H.; Lai, F.; Zhang, M. Structure characterization of two novel polysaccharides from Colocasia esculenta (taro) and a comparative study of their immunomodulatory activities. J. Funct. Foods 2018, 42, 47–57. [Google Scholar] [CrossRef]
- Sun, Y.; Gong, G.; Guo, Y.; Wang, Z.; Song, S.; Zhu, B.; Zhao, L.; Jiang, J. Purification, structural features and immunostimulatory activity of novel polysaccharides from Caulerpa lentillifera. Int. J. Biol. Macromol. 2018, 108, 314–323. [Google Scholar] [CrossRef]
- Liu, N.; Dong, Z.; Zhu, X.; Xu, H.; Zhao, Z. Characterization and protective effect of Polygonatum sibiricum polysaccharide against cyclophosphamide-induced immunosuppression in Balb/c mice. Int. J. Biol. Macromol. 2018, 107 Pt A, 796–802. [Google Scholar] [CrossRef]
- Liu, J.; Shang, F.; Yang, Z.; Wu, M.; Zhao, J. Structural analysis of a homogeneous polysaccharide from Achatina fulica. Int. J. Biol. Macromol. 2017, 98, 786–792. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, Y.; Liu, F.; Niu, X.; Wang, L.; Li, X.; Chen, H.; Yang, Y. Sulfated modification of the polysaccharides from blackcurrant and their antioxidant and α-amylase inhibitory activities. Int. J. Biol. Macromol. 2018, 109, 1344–1354. [Google Scholar] [CrossRef]
- Anand, J.; Sathuvan, M.; Babu, G.V.; Sakthivel, M.; Palani, P.; Nagaraj, S. Bioactive potential and composition analysis of sulfated polysaccharide from Acanthophora spicifera (Vahl) Borgeson. Int. J. Biol. Macromol. 2018, 111, 1238–1244. [Google Scholar] [CrossRef]
- Liang, L.; Ao, L.; Ma, T.; Ni, Y.; Liao, X.; Hu, X.; Song, Y. Sulfated modification and anticoagulant activity of pumpkin (Cucurbita pepo, Lady Godiva) polysaccharide. Int. J. Biol. Macromol. 2018, 106, 447–455. [Google Scholar] [CrossRef]
- Li, S.; Li, M.; Yue, H.; Zhou, L.; Huang, L.; Du, Z.; Ding, K. Structural elucidation of a pectic polysaccharide from Fructus Mori and its bioactivity on intestinal bacteria strains. Carbohydr. Polym. 2018, 186, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Zou, S.; Liang, D.; Luan, L. Structural characterization, antioxidant and hepatoprotective activities of polysaccharides from Sophorae tonkinensis Radix. Carbohydr. Polym. 2018, 184, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, H.; Bai, W. Characterization of Momordica charantia L. polysaccharide and its protective effect on pancreatic cells injury in STZ-induced diabetic mice. Int. J. Biol. Macromol. 2018, 115, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, F.; Chen, G.; Chen, Y.; Zhang, W.; Mao, G.; Zhao, T.; Zhang, M.; Yang, L.; Wu, X. Purification, characterization and immunomodulatory activity of a novel polysaccharide from Grifola frondosa. Int. J. Biol. Macromol. 2018, 111, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, W.C.; Gu, X.L. Optimized Extraction, Preliminary Characterization, and In Vitro Antioxidant Activity of Polysaccharides from Glycyrrhiza uralensis Fisch. Med. Sci. Monit. 2017, 23, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
| Samples | GUPS-C | GUPS-I | GUPS-II | GUPS-III |
|---|---|---|---|---|
| Color | Brown | White | Faint yellow | Yellow |
| Texture | Granulate | Granulate | Loose | Loose |
| Water solubility | Better | Good | Good | Good |
| Molecular Weight (Da) | nm | 1060 | 29100 | 14900 |
| Carbohydrate content (%) | 37.7 ± 0.34 | 29.1 ± 0.14 | 93.5 ± 0.68 | 75.2 ± 0.54 |
| Protein content (%) | 4.1 ± 0.04 | 0 | 0 | 21.5 ± 0.02 |
| Sulfate content (%) | nm | 6.5 ± 0.45 | 8.7 ± 0.23 | 20.5 ± 0.76 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aipire, A.; Yuan, P.; Aimaier, A.; Cai, S.; Mahabati, M.; Lu, J.; Ying, T.; Zhang, B.; Li, J. Preparation, Characterization, and Immuno-Enhancing Activity of Polysaccharides from Glycyrrhiza uralensis. Biomolecules 2020, 10, 159. https://doi.org/10.3390/biom10010159
Aipire A, Yuan P, Aimaier A, Cai S, Mahabati M, Lu J, Ying T, Zhang B, Li J. Preparation, Characterization, and Immuno-Enhancing Activity of Polysaccharides from Glycyrrhiza uralensis. Biomolecules. 2020; 10(1):159. https://doi.org/10.3390/biom10010159
Chicago/Turabian StyleAipire, Adila, Pengfei Yuan, Alimu Aimaier, Shanshan Cai, Mahepali Mahabati, Jun Lu, Tianlei Ying, Baohong Zhang, and Jinyao Li. 2020. "Preparation, Characterization, and Immuno-Enhancing Activity of Polysaccharides from Glycyrrhiza uralensis" Biomolecules 10, no. 1: 159. https://doi.org/10.3390/biom10010159
APA StyleAipire, A., Yuan, P., Aimaier, A., Cai, S., Mahabati, M., Lu, J., Ying, T., Zhang, B., & Li, J. (2020). Preparation, Characterization, and Immuno-Enhancing Activity of Polysaccharides from Glycyrrhiza uralensis. Biomolecules, 10(1), 159. https://doi.org/10.3390/biom10010159







