A Missing Puzzle in Dissociative Electron Attachment to Biomolecules: The Detection of Radicals
Abstract
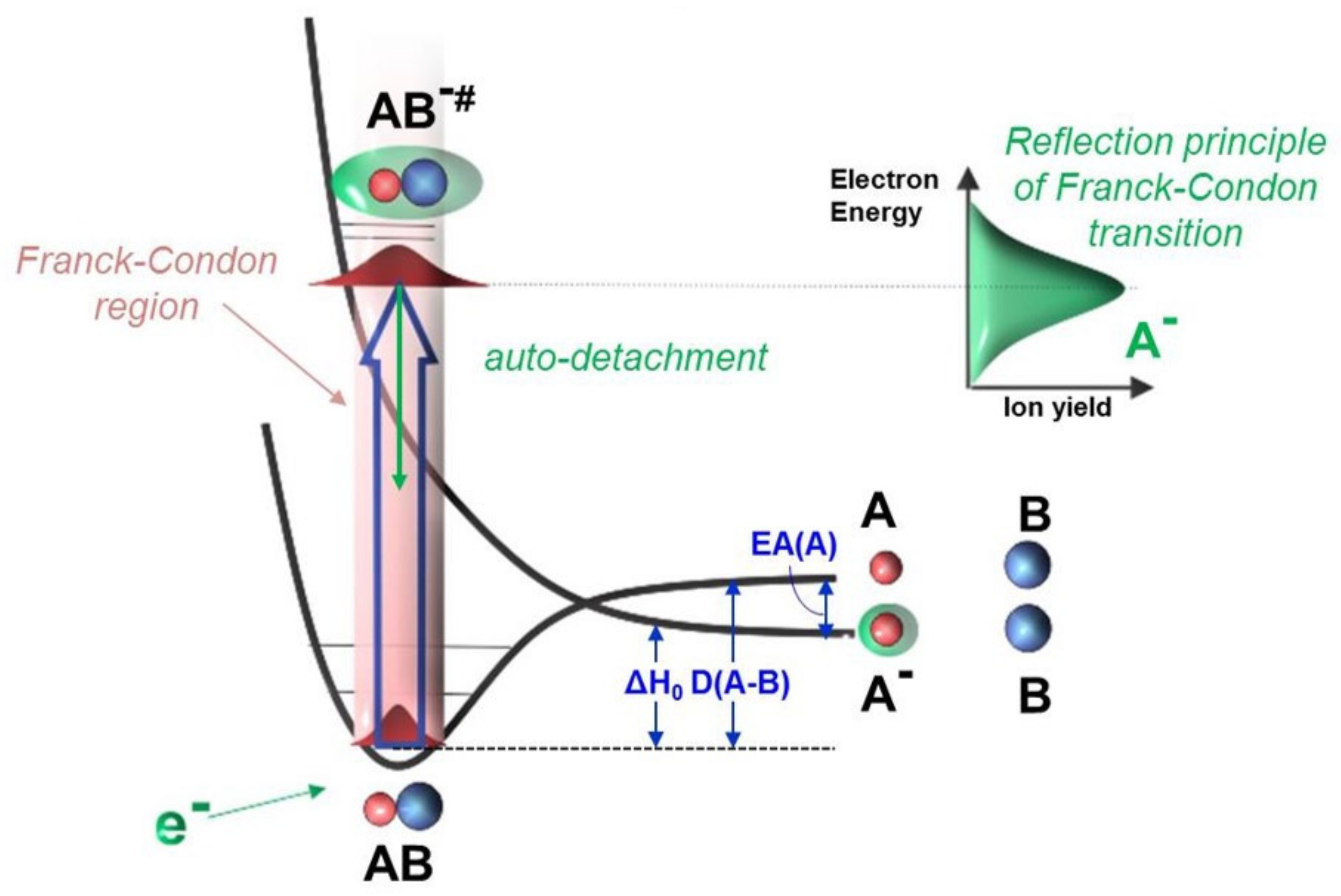
:1. Background and Knowledge Gap
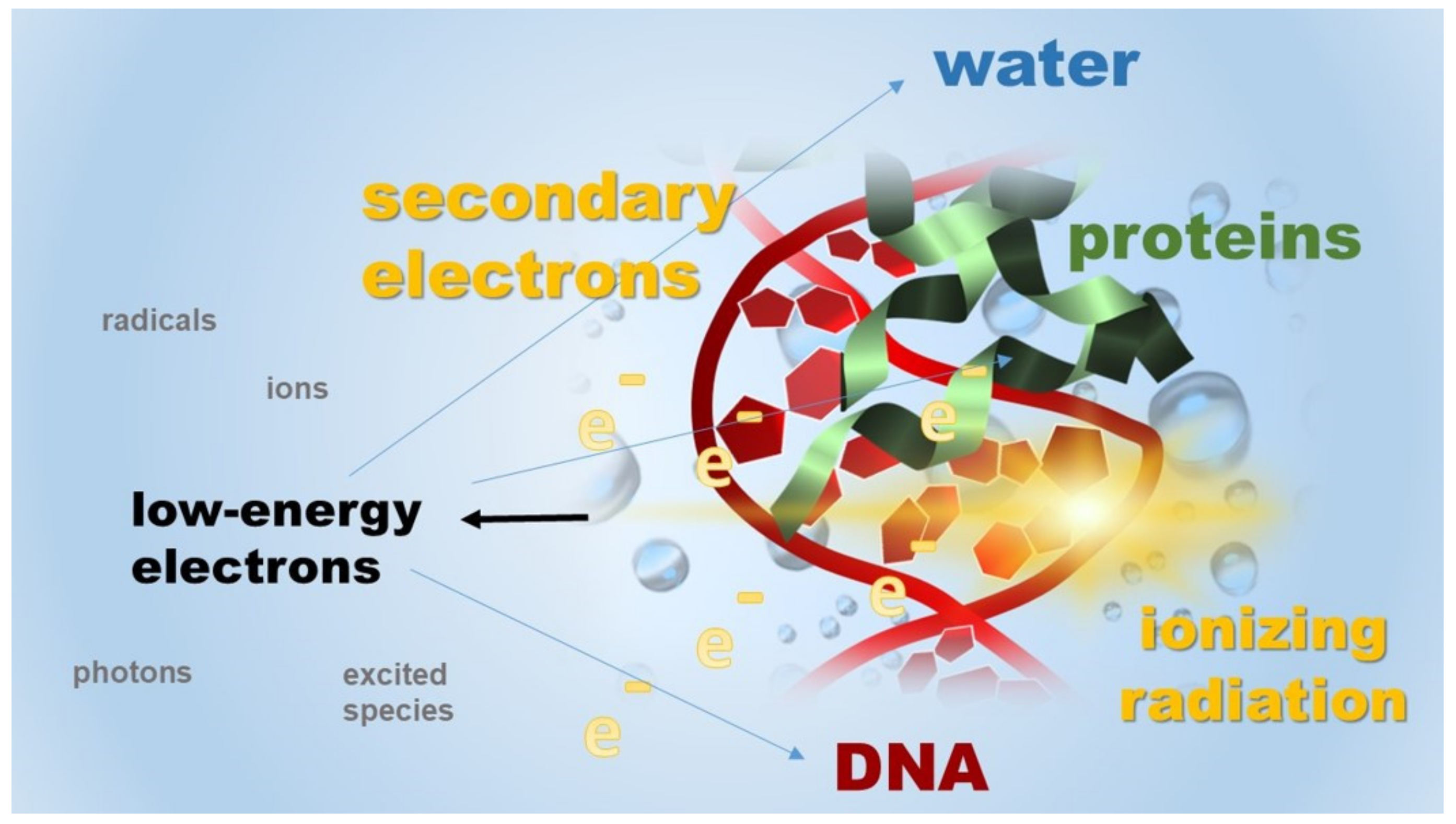
2. Radiation Damage to DNA
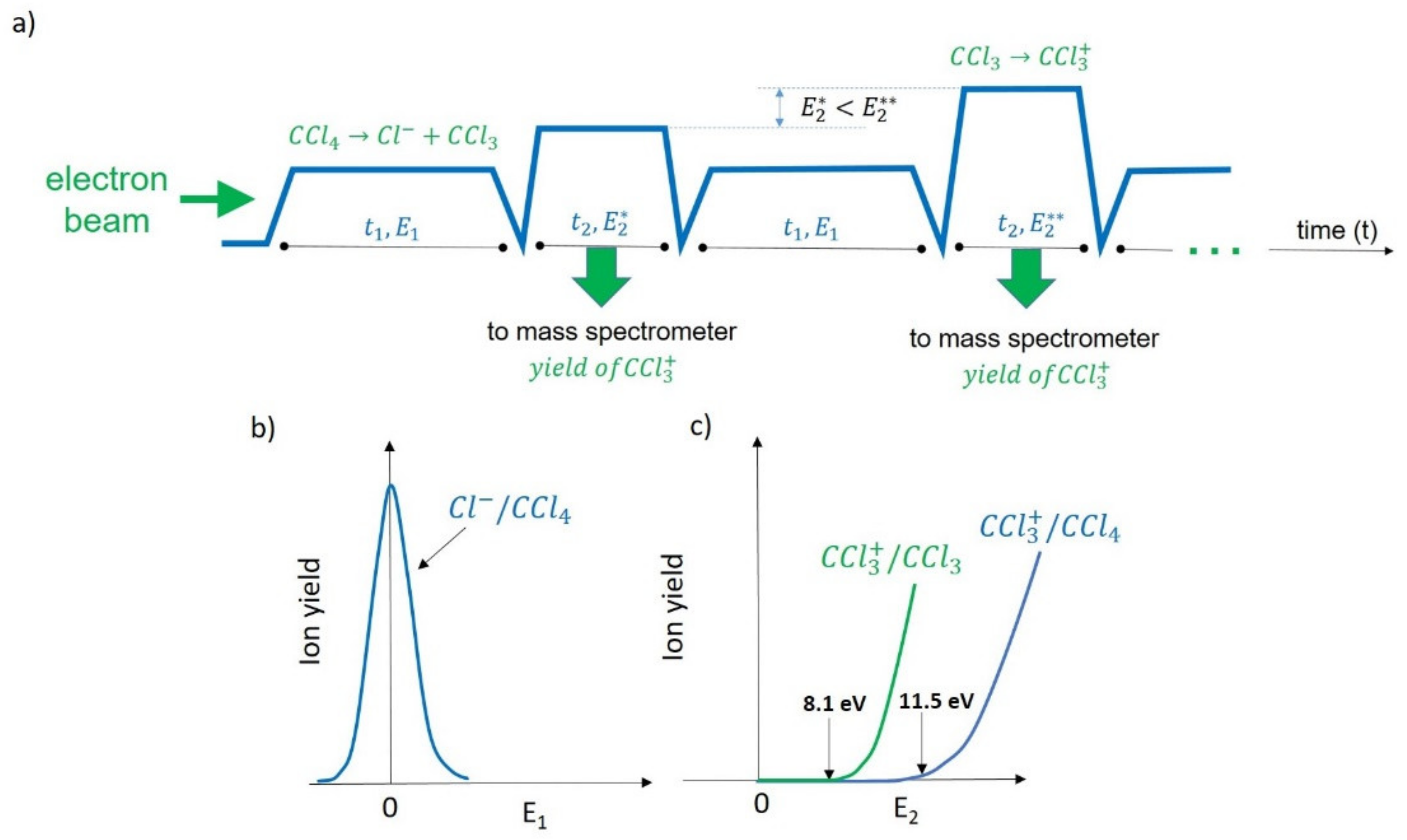
3. The State of the Art of Detection Techniques for Neutrals Formed due to LEE Impacts
4. Molecular Targets of Opportunity
4.1. Nucleobabses
4.2. Water
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Plante, I. A review of simulation codes and approaches for radiation chemistry. Phys. Med. Biol. 2021, 66, 03TR02. [Google Scholar] [CrossRef]
- Boudaïffa, B.; Cloutier, P.; Hunting, D.; Huels, M.A.; Sanche, L. Resonant Formation of DNA Strand Breaks by Low-Energy (3 to 20 eV) Electrons. Science 2000, 287, 1658–1660. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, D.S.; Rescigno, T.N. Breaking up is hard to do. Nat. Phys. 2018, 14, 109–110. [Google Scholar] [CrossRef]
- Fabrikant, I.I.; Eden, S.; Mason, N.J.; Fedor, J. Chapter Nine—Recent Progress in Dissociative Electron Attachment: From Diatomics to Biomolecules. In Advances in Atomic, Molecular, and Optical Physics; Arimondo, E., Lin, C.C., Yelin, S.F., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 66, pp. 545–657. [Google Scholar]
- Gorfinkiel, J.D.; Ptasinska, S. Electron scattering from molecules and molecular aggregates of biological relevance. J. Phys. B At. Mol. Opt. Phys. 2017, 50, 182001. [Google Scholar] [CrossRef] [Green Version]
- Sonntage, C.V. Free-Radical-Induced DNA Damage and Its Repair, 1st ed.; Springer: Berlin, Germany, 2006; p. 523. [Google Scholar]
- Ward, J.F. Radiolytic damage to genetic material. J. Chem. Educ. 1981, 58, 135–139. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Jaruga, P. Mechanisms of free radical-induced damage to DNA. Free Radic. Res. 2012, 46, 382–419. [Google Scholar] [CrossRef] [PubMed]
- Spotheim-Maurizot, M.; Davídková, M. Radiation damage to DNA in DNA–protein complexes. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2011, 711, 41–48. [Google Scholar] [CrossRef]
- Sagstuen, E.; Sanderud, A.; Hole, E.O. The Solid-State Radiation Chemistry of Simple Amino Acids, Revisited. Radiat. Res. 2004, 162, 112–119. [Google Scholar] [CrossRef]
- Ptasińska, S.; Li, Z.; Mason, N.J.; Sanche, L. Damage to amino acid–nucleotide pairs induced by 1 eV electrons. Phys. Chem. Chem. Phys. 2010, 12, 9367–9372. [Google Scholar] [CrossRef]
- Alizadeh, E.; Sanche, L. Precursors of solvated electrons in radiobiological physics and chemistry. Chem. Rev. 2012, 112, 5578–5602. [Google Scholar] [CrossRef]
- Moretto-Capelle, P.; Le Padellec, A. Electron spectroscopy in proton collisions with dry gas-phase uracil base. Phys. Rev. A 2006, 74, 062705. [Google Scholar] [CrossRef]
- Padellec, A.L.; Moretto-Capelle, P.; Richard-Viard, M.; Champeaux, J.; Cafarelli, P. Ionization and fragmentation of DNA, RNA bases induced by proton impact. J. Phys. Conf. Ser. 2008, 101, 012007. [Google Scholar] [CrossRef]
- Jahnke, T.; Hergenhahn, U.; Winter, B.; Dörner, R.; Frühling, U.; Demekhin, P.V.; Gokhberg, K.; Cederbaum, L.S.; Ehresmann, A.; Knie, A.; et al. Interatomic and Intermolecular Coulombic Decay. Chem. Rev. 2020, 120, 11295–11369. [Google Scholar] [CrossRef] [PubMed]
- Harbach, P.H.P.; Schneider, M.; Faraji, S.; Dreuw, A. Intermolecular Coulombic Decay in Biology: The Initial Electron Detachment from FADH− in DNA Photolyases. J. Phys. Chem. Lett. 2013, 4, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Schwestka, J.; Niggas, A.; Creutzburg, S.; Kozubek, R.; Heller, R.; Schleberger, M.; Wilhelm, R.A.; Aumayr, F. Charge-Exchange-Driven Low-Energy Electron Splash Induced by Heavy Ion Impact on Condensed Matter. J. Phys. Chem. Lett. 2019, 10, 4805–4811. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Guo, D.; Ma, X.; Zhu, X.; Feng, W.; Yan, S.; Zhao, D.; Gao, Y.; Zhang, S.; Ren, X.; et al. Damaging Intermolecular Energy and Proton Transfer Processes in Alpha-Particle-Irradiated Hydrogen-Bonded Systems. Angew. Chem. Int. Ed. 2018, 57, 17023–17027. [Google Scholar] [CrossRef] [PubMed]
- Grieves, G.A.; Orlando, T.M. Intermolecular Coulomb Decay at Weakly Coupled Heterogeneous Interfaces. Phys. Rev. Lett. 2011, 107, 016104. [Google Scholar] [CrossRef] [Green Version]
- Gokhberg, K.; Kolorenč, P.; Kuleff, A.I.; Cederbaum, L.S. Site- and energy-selective slow-electron production through intermolecular Coulombic decay. Nature 2014, 505, 661–663. [Google Scholar] [CrossRef] [Green Version]
- Kohanoff, J.; McAllister, M.; Tribello, G.A.; Gu, B. Interactions between low energy electrons and DNA: A perspective from first-principles simulations. J. Phys. Condens. Matter 2017, 29, 383001. [Google Scholar] [CrossRef]
- Ma, J.; Kumar, A.; Muroya, Y.; Yamashita, S.; Sakurai, T.; Denisov, S.A.; Sevilla, M.D.; Adhikary, A.; Seki, S.; Mostafavi, M. Observation of dissociative quasi-free electron attachment to nucleoside via excited anion radical in solution. Nat. Commun. 2019, 10, 102. [Google Scholar] [CrossRef]
- Dong, Y.; Liao, H.; Gao, Y.; Cloutier, P.; Zheng, Y.; Sanche, L. Early Events in Radiobiology: Isolated and Cluster DNA Damage Induced by Initial Cations and Nonionizing Secondary Electrons. J. Phys. Chem. Lett. 2021, 12, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Desouky, O.; Ding, N.; Zhou, G. Targeted and non-targeted effects of ionizing radiation. J. Radiat. Res. Appl. Sci. 2015, 8, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, N.W.; Parrish, J.M.; Sheha, E.D.; Singh, K. Intraoperative risks of radiation exposure for the surgeon and patient. Ann. Transl. Med. 2021, 9, 84. [Google Scholar] [CrossRef]
- Gauduel, Y.; Glinec, Y.; Malka, V. Femtoradical events in aqueous molecular environments: The tenuous borderline between direct and indirect radiation damages. J. Phys. Conf. Ser. 2008, 101, 012004. [Google Scholar] [CrossRef]
- Gao, Y.; Zheng, Y.; Sanche, L. Low-Energy Electron Damage to Condensed-Phase DNA and Its Constituents. Int. J. Mol. Sci. 2021, 22, 7879. [Google Scholar] [CrossRef]
- Bazin, M.; Ptasinska, S.; Bass, A.D.; Sanche, L.; Burean, E.; Swiderek, P. Electron induced dissociation in the condensed-phase nitromethane: II. Desorption of neutral fragments. J. Phys. Condens. Matter 2010, 22, 084003. [Google Scholar] [CrossRef]
- Abdoul-Carime, H.; Dugal, P.C.; Sanche, L. Damage induced by 1-30 eV electrons on thymine- and bromouracil-substituted oligonucleotides. Radiat. Res. 2000, 153, 23–28. [Google Scholar] [CrossRef]
- Houplin, J.; Amiaud, L.; Humblot, V.; Martin, I.; Matar, E.; Azria, R.; Pradier, C.-M.; Lafosse, A. Selective terminal function modification of SAMs driven by low-energy electrons (0–15 eV). Phys. Chem. Chem. Phys. 2013, 15, 7220–7227. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Aleksandrov, A.; Orlando, T.M. Probing low-energy electron induced DNA damage using single photon ionization mass spectrometry. Int. J. Mass Spectrom. 2008, 277, 314–320. [Google Scholar] [CrossRef]
- Li, Z.; Milosavljević, A.R.; Carmichael, I.; Ptasinska, S. Characterization of Neutral Radicals from a Dissociative Electron Attachment Process. Phys. Rev. Lett. 2017, 119, 053402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, T.; Toyoda, H.; Sugai, H. Electron-Impact Dissociation of Methane into CH3 and CH2 Radicals I. Relative Cross Sections. Jpn. J. Appl. Phys. 1991, 30, 2908. [Google Scholar] [CrossRef]
- Nakano, T.; Toyoda, H.; Sugai, H. Electron-Impact Dissociation of Methane into CH3 and CH2 Radicals II. Absolute Cross Sections. Jpn. J. Appl. Phys. 1991, 30, 2912. [Google Scholar] [CrossRef]
- Nakano, T.; Sugai, H. Partial Cross Sections for Electron Impact Dissociation of CF4 into Neutral Radicals. Jpn. J. Appl. Phys. 1992, 31, 2919. [Google Scholar] [CrossRef]
- Nakano, T.; Sugai, H. Cross section measurements for electron-impact dissociation of SiF4 into neutral radicals. J. Phys. D Appl. Phys. 1993, 26, 1909. [Google Scholar] [CrossRef]
- Fisher, E.R.; Kickel, B.L.; Armentrout, P.B. Collision-induced dissociation and charge transfer reactions of SF+x (x = 1 − 5): Thermochemistry of sulfur fluoride ions and neutrals. J. Chem. Phys. 1992, 97, 4859. [Google Scholar] [CrossRef]
- Iio, M.; Goto, M.; Sugai, H. Relative Cross Sections for Electron—Impact Dissociation of SF6 into SFx (x = 1 − 3) Neutral Radicals. Contrib. Plasma Phys. 1995, 35, 405–413. [Google Scholar] [CrossRef]
- Christophorou, L.G.; Olthoff, J.K. Electron Interactions With SF6. J. Phys. Chem. Ref. Data 2000, 29, 267. [Google Scholar] [CrossRef] [Green Version]
- Sugai, H.; Toyoda, H. Appearance mass spectrometry of neutral radicals in radio frequency plasmas. J. Vac. Sci. Technol. A 1992, 10, 1193. [Google Scholar] [CrossRef]
- Benedikt, J.; Kersten, H.; Piel, A. Foundations of measurement of electrons, ions and species fluxes toward surfaces in low-temperature plasmas. Plasma Sources Sci. Technol. 2021, 30, 033001. [Google Scholar] [CrossRef]
- Berdys, J.; Anusiewicz, I.; Skurski, P.; Simons, J. Damage to Model DNA Fragments from Very Low-Energy (<1 eV) Electrons. J. Am. Chem. Soc. 2004, 126, 6441–6447. [Google Scholar] [CrossRef]
- Hahn, M.B.; Meyer, S.; Schröter, M.-A.; Seitz, H.; Kunte, H.-J.; Solomun, T.; Sturm, H. Direct electron irradiation of DNA in a fully aqueous environment. Damage determination in combination with Monte Carlo simulations. Phys. Chem. Chem. Phys. 2017, 19, 1798–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alizadeh, E.; Orlando, T.M.; Sanche, L. Biomolecular Damage Induced by Ionizing Radiation: The Direct and Indirect Effects of Low-Energy Electrons on DNA. Annu. Rev. Phys. Chem. 2015, 66, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Orlando, T.M.; Oh, D.; Chen, Y.; Aleksandrov, A.B. Low-energy electron diffraction and induced damage in hydrated DNA. J. Chem. Phys. 2008, 128, 195102. [Google Scholar] [CrossRef] [PubMed]
- Denifl, S.; Sulzer, P.; Zappa, F.; Moser, S.; Kräutler, B.; Echt, O.; Bohme, D.K.; Märk, T.D.; Scheier, P. Isotope effects in dissociative electron attachment to the DNA base thymine. Int. J. Mass Spectrom. 2008, 277, 296–299. [Google Scholar] [CrossRef]
- Kopyra, J.; Koenig-Lehmann, C.; Illenberger, E. On the absolute value for the cross-section of dissociative electron attachment (DEA) to the DNA base thymine. Int. J. Mass Spectrom. 2009, 281, 89–91. [Google Scholar] [CrossRef]
- Ptasinska, S.; Denifl, S.; Scheier, P.; Illenberger, E.; Märk, T.D. Bond- and Site-Selective Loss of H Atoms from Nucleobases by Very-Low-Energy Electrons (<3 eV). Angew. Chem. Int. Ed. 2005, 44, 6941–6943. [Google Scholar] [CrossRef]
- Ptasińska, S.; Denifl, S.; Grill, V.; Märk, T.D.; Scheier, P.; Gohlke, S.; Huels, M.A.; Illenberger, E. Bond-Selective H− Ion Abstraction from Thymine. Angew. Chem. Int. Ed. 2005, 44, 1647–1650. [Google Scholar] [CrossRef]
- Chernyshova, I.V.; Kontrosh, E.E.; Shpenik, O.B. Collisions of Slow Electrons with Thymine Molecules. Opt. Spectrosc. 2018, 125, 845–852. [Google Scholar] [CrossRef]
- Ptasińska, S.; Denifl, S.; Grill, V.; Märk, T.D.; Illenberger, E.; Scheier, P. Bond- and Site-Selective Loss of H− from Pyrimidine Bases. Phys. Rev. Lett. 2005, 95, 093201. [Google Scholar] [CrossRef] [Green Version]
- Ptasińska, S.; Denifl, S.; Mróz, B.; Probst, M.; Grill, V.; Illenberger, E.; Scheier, P.; Märk, T.D. Bond selective dissociative electron attachment to thymine. J. Chem. Phys. 2005, 123, 124302. [Google Scholar] [CrossRef]
- Burrow, P.D.; Gallup, G.A.; Scheer, A.M.; Denifl, S.; Ptasinska, S.; Märk, T.; Scheier, P. Vibrational Feshbach resonances in uracil and thymine. J. Chem. Phys. 2006, 124, 124310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.; Leszczynski, J.; Schaefer, H.F. Interactions of Electrons with Bare and Hydrated Biomolecules: From Nucleic Acid Bases to DNA Segments. Chem. Rev. 2012, 112, 5603–5640. [Google Scholar] [CrossRef] [PubMed]
- Melton, C.E. Radiolysis of water vapor in a wide range radiolysis source of a mass spectrometer. I. Individual and total cross sections for the production of positive ions, negative ions, and free radicals by electrons. J. Phys. Chem. 1970, 74, 582–587. [Google Scholar] [CrossRef]
- Song, M.-Y.; Cho, H.; Karwasz, G.P.; Kokoouline, V.; Nakamura, Y.; Tennyson, J.; Faure, A.; Mason, N.J.; Itikawa, Y. Cross Sections for Electron Collisions with H2O. J. Phys. Chem. Ref. Data 2021, 50, 023103. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. Available online: http://webbook.nist.gov (accessed on 30 August 2021).
- Arumainayagam, C.R.; Lee, H.L.; Nelson, R.B.; Haines, D.R.; Gunawardane, R.P. Low-energy electron-induced reactions in condensed matter. Surf. Sci. Rep. 2010, 65, 1–44. [Google Scholar] [CrossRef]
- Tsuchida, H.; Kai, T.; Kitajima, K.; Matsuya, Y.; Majima, T.; Saito, M. Relation between biomolecular dissociation and energy of secondary electrons generated in liquid water by fast heavy ions. Eur. Phys. J. D 2020, 74, 212. [Google Scholar] [CrossRef]
- Mason, N.J. Electron Induced Processing; Applications and Data Needs. AIP Conf. Proc. 2007, 901, 74–84. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ptasinska, S. A Missing Puzzle in Dissociative Electron Attachment to Biomolecules: The Detection of Radicals. Atoms 2021, 9, 77. https://doi.org/10.3390/atoms9040077
Ptasinska S. A Missing Puzzle in Dissociative Electron Attachment to Biomolecules: The Detection of Radicals. Atoms. 2021; 9(4):77. https://doi.org/10.3390/atoms9040077
Chicago/Turabian StylePtasinska, Sylwia. 2021. "A Missing Puzzle in Dissociative Electron Attachment to Biomolecules: The Detection of Radicals" Atoms 9, no. 4: 77. https://doi.org/10.3390/atoms9040077
APA StylePtasinska, S. (2021). A Missing Puzzle in Dissociative Electron Attachment to Biomolecules: The Detection of Radicals. Atoms, 9(4), 77. https://doi.org/10.3390/atoms9040077





