Rosetta Mission: Electron Scattering Cross Sections—Data Needs and Coverage in BEAMDB Database
Abstract
1. Introduction
2. Rosetta Instruments and Their Observation of Electron Scattering Processes in the Cometary Coma
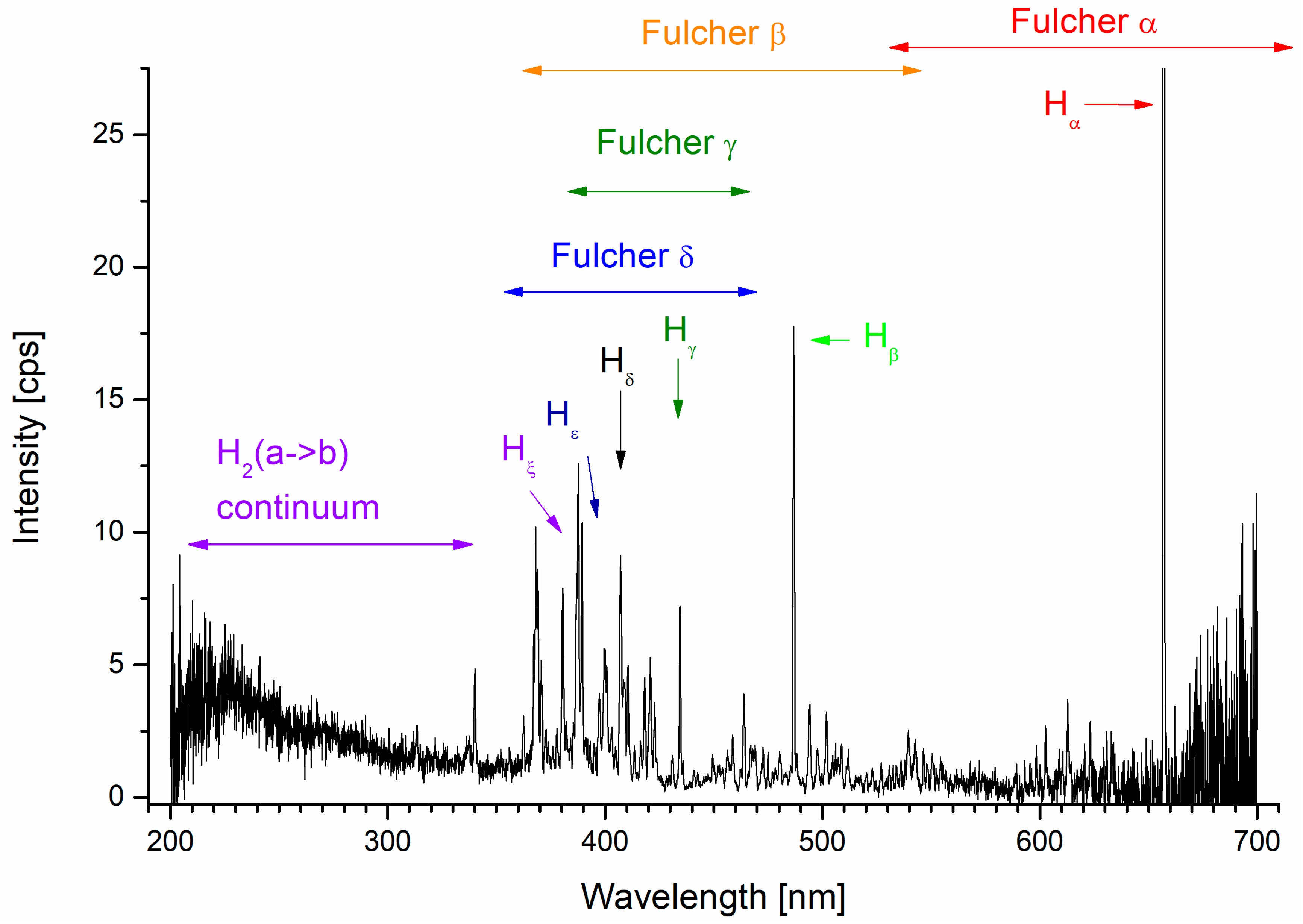
2.1. FUV Emissions Measured by the ALICE Instrument
2.2. Observations from the OSIRIS Instrument
2.3. Detection of Organic Molecules on the Comet Surface—COSAC Mass Spectrometry
2.4. Electrons in the Cometary Coma
3. Atomic and Molecular Data Needed for Analysing Electron Scattering Processes Relevant to Comet 67P
3.1. Databases
3.2. VAMDC and BEAMDB Databases
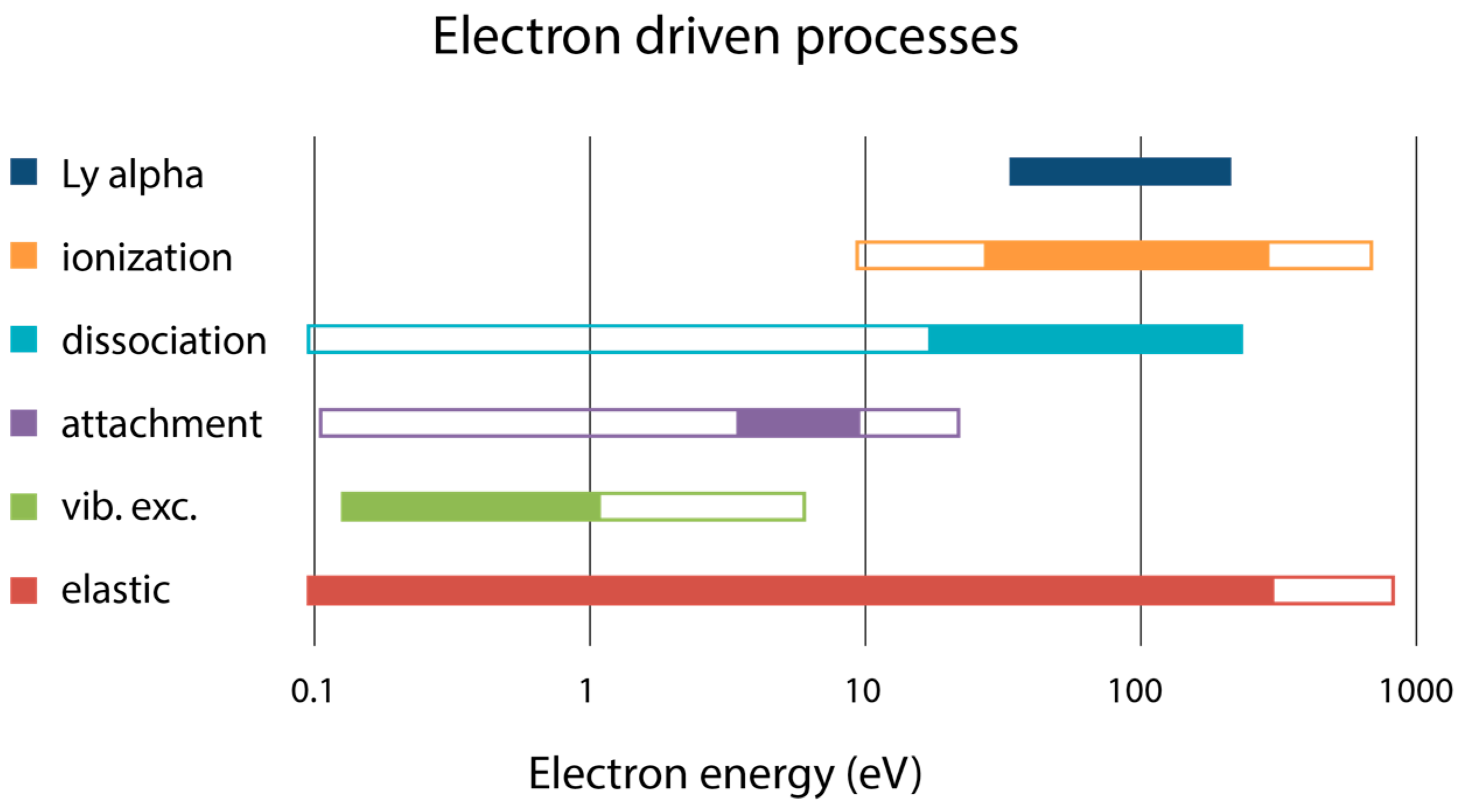
4. Electron Scattering Processes and Cross Sections—Data Needs
4.1. Elastic Electron Scattering and Cross Sections
4.2. Electron Impact Ionisation Cross Sections
4.3. Anion Production
4.4. Electron Impact Excitation and Dissociation
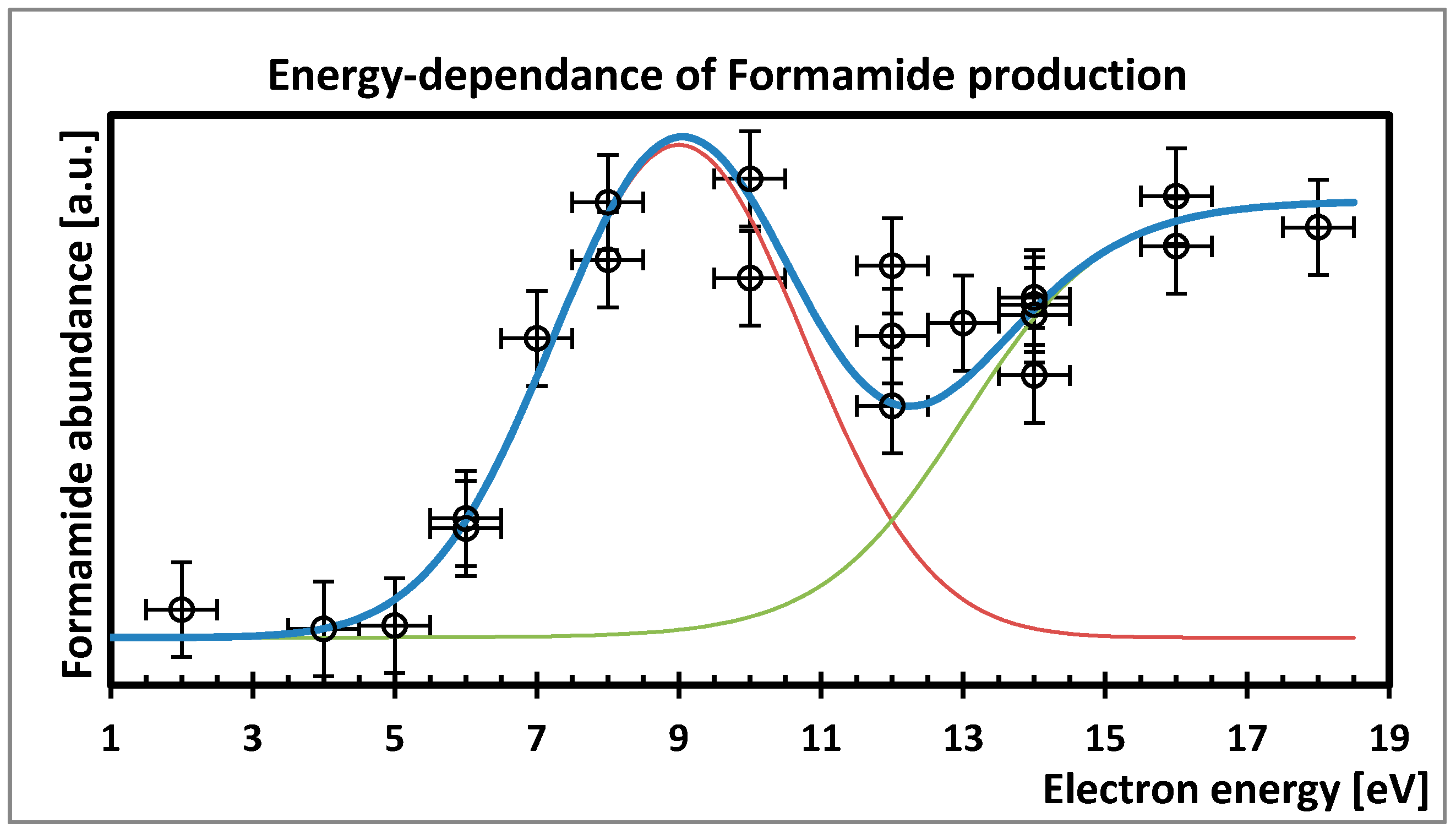
5. Possibility of Electron Induced Surface Chemistry
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Altwegg, K.; Balsiger, H.; Bar-Nun, A.; Berthelier, J.J.; Bieler, A.; Bochsler, P.; Briois, C.; Calmonte, U.; Combi, M.; De Keyser, J.; et al. 67P/Churyumov-Gerasimenko, a Jupiter family comet with a high D/H ratio. Science 2015, 347. [Google Scholar] [CrossRef] [PubMed]
- Feldman, P.D.; A’Hearn, M.F.; Bertaux, J.-L.; Feaga, L.M.; Parker, J.W.; Schindhelm, E.; Steffl, A.J.; Stern, S.A.; Weaver, H.A.; Sierks, H.; et al. Measurements of the near-nucleus coma of comet 67P/Churyumov-Gerasimenko with the Alice far-ultraviolet spectrograph on Rosetta. Astron. Astrophys. 2015, 583, A8. [Google Scholar] [CrossRef]
- Itikawa, Y.; Mason, N.J. Cross Sections for Electron Collisions with Water Molecules. Phys. Chem. Ref. Data 2005, 34, 1–22. [Google Scholar] [CrossRef]
- Galand, M.; Héritier, K.L.; Odelstad, E.; Henri, P.; Broiles, T.W.; Allen, A.J.; Altwegg, K.; Beth, A.; Burch, J.L.; Carr, C.M.; et al. Ionospheric plasma of comet 67P probed by Rosetta at 3 au from the Sun. Mon. Not. R. Astron. Soc. 2016, S331–S351. [Google Scholar] [CrossRef]
- Héritier, K.L.; Henri, P.; Vallières, X.; Galand, M.; Odelstad, E.; Eriksson, A.I.; Johansson, F.L.; Altwegg, K.; Behar, E.; Beth, A.; et al. Vertical structure of the near-surface expanding ionosphere of comet 67P probed by Rosetta. Mon. Not. R. Astron. Soc. 2017, S130–S141. [Google Scholar] [CrossRef]
- Bodewits, D.; Lara, L.M.; A’Hearn, M.F.; La Forgia, F.; Gicquel, A.; Kovacs, G.; Knollenberg, J.; Lazzarin, M.; Lin, Z.-Y.; Shi, X.; et al. Changes in the physical environment of the inner coma of 67P/Churyumov–Gerasimenko with decreasing heliocentric distance. Astron. J. 2016, 152. [Google Scholar] [CrossRef]
- Krüger, H.; Goesmann, F.; Giri, C.; Wright, I.; Morse, A.; Bredehöft, J.H.; Ulamec, S.; Cozzoni, B.; Ehrenfreund, P.; Gautier, T.; et al. Decay of COSAC and Ptolemy mass spectra at comet 67P/Churyumov-Gerasimenko. Astron. Astrophys. 2017, 600, A56. [Google Scholar] [CrossRef]
- Goesmann, F.; Rosenbauer, H.; Bredehöft, J.H.; Cabane, M.; Ehrenfreund, P.; Gautier, T.; Giri, C.; Krüger, H.; Le Roy, L.; MacDermott, A.J.; et al. Organic compounds on comet 67P/Churyumov-Gerasimenko revealed by COSAC mass spectrometry. Science 2015, 349. [Google Scholar] [CrossRef] [PubMed]
- Altwegg, K.; Balsiger, H.; Berthelier, J.J.; Bieler, A.; Calmonte, U.; Fuselier, S.A.; Goesmann, F.; Gasc, S.; Gombosi, T.I.; Le Roy, L.; et al. Organics in comet 67P—A first comparative analysis of mass spectra from ROSINA-DFMS, COSAC and Ptolemy. Mon. Not. R. Astron. Soc. 2017, S130–S141. [Google Scholar] [CrossRef]
- Wright, I.P.; Sheridan, S.; Barber, S.J.; Morgan, G.H.; Andrews, D.J.; Morse, A.D. CHO-bearing organic compounds at the surface of 67P/Churyumov-Gerasimenko revealed by Ptolemy. Science 2015, 349. [Google Scholar] [CrossRef] [PubMed]
- Bieler, A.; Altwegg, K.; Balsiger, H.; Bar-Nun, A.; Berthelier, J.-J.; Bochsler, P.; Briois, C.; Calmonte, U.; Combi, M.; De Keyser, J.; et al. Abundant molecular oxygen in the coma of comet 67P/Churyumov–Gerasimenko. Nature 2015, 526, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Carr, C.; Cupido, E.; Lee, C.G.Y.; Balogh, A.; Beek, T.; Burch, J.L.; Dunford, C.N.; Eriksson, A.I.; Gill, R.; Glassmeier, K.H.; et al. RPC: The Rosetta Plasma Consortium. Space Sci. Rev. 2007, 128, 629–647. [Google Scholar] [CrossRef]
- Burch, J.L.; Goldstein, R.; Cravens, T.E.; Gibson, W.C.; Lundin, R.N.; Pollock, C.J.; Winningham, J.D.; Young, D.T. RPC-IES: The Ion and Electron Sensor of the Rosetta Plasma Consortium. Space Sci. Rev. 2007, 128, 697–712. [Google Scholar] [CrossRef]
- Eriksson, A.I.; Boström, R.; Gill, R.; Åhlén, L.; Jansson, S.-E.; Wahlund, J.-E.; André, M.; Mälkki, A.; Holtet, J.A.; Lybekk, B.; et al. RPC-LAP: The Rosetta Langmuir Probe Instrument. Space Sci. Rev. 2007, 128, 723–744. [Google Scholar] [CrossRef]
- Trotignon, J.G.; Michau, J.L.; Lagoutte, D.; Chabassière, M.; Chalumeau, G.; Colin, F.; Décréau, P.M.E.; Geiswiller, J.; Gille, P.; Grard, R.; et al. RPC-MIP: The Mutual Impedance Probe of the Rosetta Plasma Consortium. Space Sci. Rev. 2007, 128, 713–728. [Google Scholar] [CrossRef]
- Cravens, T.E.; Kozyra, J.U.; Nagy, A.F.; Gombosi, T.I.; Kurtz, M. Electron impact ionization in the vicinity of comets. J. Geophys. Res. Space 1987, 92, 7341–7353. [Google Scholar] [CrossRef]
- Edberg, N.J.T.; Eriksson, A.I.; Odelstad, E.; Henri, P.; Lebreton, J.P.; Gasc, S.; Rubin, M.; André, M.; Gill, R.; Johansson, E.P.G.; et al. Spatial distribution of low-energy plasma around comet 67P/CG from Rosetta measurements. Geophys. Res. Lett. 2015, 42, 4263–4269. [Google Scholar] [CrossRef]
- Clark, G.; Broiles, T.W.; Burch, J.L.; Collinson, G.A.; Cravens, T.; Frahm, R.A.; Goldstein, J.; Goldstein, R.; Mandt, K.; Mokashi, P.; et al. Suprathermal electron environment of comet 67P/Churyumov-Gerasimenko: Observations from the Rosetta Ion and Electron Sensor. Astron. Astrophys. 2015, 583, A24. [Google Scholar] [CrossRef]
- Broiles, T.W.; Burch, J.L.; Chae, K.; Clark, G.; Cravens, T.E.; Eriksson, A.; Fuselier, S.A.; Frahm, R.A.; Gasc, S.; Goldstein, R.; et al. Statistical analysis of suprathermal electron drivers at 67P/Churyumov–Gerasimenko. Mon. Not. R. Astron. Soc. 2016, 462, S312–S322. [Google Scholar] [CrossRef]
- Deca, J.; Divin, A.; Henri, P.; Eriksson, A.; Markidis, S.; Olshevsky, V.; Horányi, M. Electron and Ion Dynamics of the Solar Wind Interaction with a Weakly Outgassing Comet. Phys. Rev. Lett. 2017, 118. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, L.; Wegg, K.; Balsiger, H.; Berthelier, J.-J.; Bieler, A.; Briois, C.; Calmonte, U.; Combi, M.R.; De Keyser, J.; Dhooghe, F.; et al. Rosetta mission results pre-perihelion Special feature Inventory of the volatiles on comet 67P/Churyumov-Gerasimenko from Rosetta/ROSINA. Astron. Astrophys. 2015, 583, A1. [Google Scholar] [CrossRef]
- Calmonte, U.; Altwegg, K.; Balsiger, H.; Berthelier, J.J.; Bieler, A.; Cessateur, G.; Dhooghe, F.; van Dishoeck, E.F.; Fiethe, B.; Fuselier, S.A.; et al. Sulphur-bearing species in the coma of comet 67P/Churyumov–Gerasimenko. Mon. Not. Roy. Astron. Soc. 2016, 462, S253–S273. [Google Scholar] [CrossRef]
- Pitchford, L.C.; Alves, L.L.; Bartschat, K.; Biagi, S.F.; Bordage, M.-C.; Bray, I.; Brion, C.E.; Brunger, M.J.; Campbell, L.; Chachereau, A.; et al. LXCat: An Open-Access, Web-Based Platformfor Data Needed for Modeling LowTemperature Plasmas. Plasma Process. Polym. 2016, 14. [Google Scholar] [CrossRef]
- Wakelam, V.; Herbst, E.; Loison, J.-C.; Smith, I.W.M.; Chandrasekaran, V.; Pavone, B.; Adams, N.G.; Bacchus-Montabonel, M.-C.; Bergeat, A.; Béroff, K.; et al. A KInetic Database for Astrochemistry (KIDA). Astrophys. J. Suppl. Ser. 2012, 199. [Google Scholar] [CrossRef]
- Hibbert, A. Calculation of Rates of 4p–4d Transitions in Ar II. Atoms 2017, 5, 8. [Google Scholar] [CrossRef]
- Dubernet, M.L.; Antony, B.K.; Ba, Y.A.; Babikov, Y.L.; Bartschat, K.; Boudon, V.; Braams, B.J.; Chung, H.-K.; Daniel, F.; Delahaye, F.; et al. The virtual atomic and molecular data centre (VAMDC) consortium. J. Phys. B 2016. [Google Scholar] [CrossRef]
- VAMDC Dictionary. Available online: http://dictionary.vamdc.eu/ (accessed on 30 August 2017).
- Marinković, B.P.; Jevremović, D.; Srećković, V.A.; Vujčić, V.; Ignjatović, L.M.; Dimitrijević, M.S.; Mason, N.J. BEAMDB and MolD—Databases for atomic and molecular collisional and radiative processes: Belgrade nodes of VAMDC. Eur. Phys. J. D 2017, 71. [Google Scholar] [CrossRef]
- Denifl, S.; Garcia, G.; Huber, B.A.; Marinković, B.P.; Mason, N.; Postler, J.; Rabus, H.; Rixon, G.; Solov’yov, A.V.; Suraud, E.; et al. Radiation damage of biomolecules (RADAM) database development: Current status. J. Phys. Conf. Ser. 2013, 438. [Google Scholar] [CrossRef]
- Register, D.F.; Trajmar, S.; Srivastava, S.K. Absolute elastic differential electron scattering cross sections for He: A proposed calibration standard from 5 to 200 eV. Phys. Rev. A 1980, 21, 1134–1151. [Google Scholar] [CrossRef]
- Maljković, J.B.; Blanco, F.; García, G.; Marinković, B.P.; Milosavljević, A.R. Elastic electron scattering from formamide molecule. Nucl. Instrum. Methods Phys. Res. B 2012, 279, 124–127. [Google Scholar] [CrossRef]
- Belić, D.S.; Urbain, X.; Cherkani-Hassani, H.; Defrance, P. Electron-impact dissociation and ionization of CN+ ions. Phys. Rev. A 2017, 95. [Google Scholar] [CrossRef]
- Huebner, W.F. Composition of comets: Observations and models. Earth Moon Planets 2002, 89, 179–195. [Google Scholar] [CrossRef]
- Campbell, L.; Brunger, M.J. Modelling of plasma processes in cometary and planetary atmospheres. Plasma Sources Sci. Technol. 2013, 22. [Google Scholar] [CrossRef]
- Simmons, J.D.; Bass, A.M.; Tilford, S.G. The Fourth Positive System of Carbon Monoxide Observed in Absorption at High Resolution in the Vacuum Ultraviolet Region. Astrophys. J. 1969, 155, 345–358. [Google Scholar] [CrossRef]
- Campbell, L.; Brunger, M.J. Electron collisions in atmospheres. Int. Rev. Phys. Chem. 2016, 35, 297–351. [Google Scholar] [CrossRef]
- Zecca, A.; Karwasz, G.P.; Brusa, R.S. One century of experiments on electron-atom and molecule scattering. A critical review of integral cross-sections, I. Atoms and diatomic molecules. Riv. Nuovo Cimento 1996, 19, 1–146. [Google Scholar] [CrossRef]
- Bransden, B.H.; McDowell, M.R.C. Electron scattering by atoms at intermediate energies II. Theoretical and experimental data for light atoms. Phys. Rep. 1978, 46, 249–394. [Google Scholar] [CrossRef]
- Brunger, M.J.; Buckman, S.J. Electron–molecule scattering cross-sections. I. Experimental techniques and data for diatomic molecules. Phys. Rep. 2002, 357, 215–458. [Google Scholar] [CrossRef]
- Flower, D.R. Molecular collision processes in interstellar clouds. Phys. Rep. 1989, 174, 1–66. [Google Scholar] [CrossRef]
- Anzai, K.; Kato, H.; Hoshino, M.; Tanaka, H.; Itikawa, Y.; Campbell, L.; Brunger, M.J.; Buckman, S.J.; Cho, H.; Blanco, F.; et al. Cross section data sets for electron collisions with H2, O2, CO, CO2, N2O and H2O. Eur. Phys. J. D 2012, 66. [Google Scholar] [CrossRef]
- King, G.C. The Use of the Magnetic Angle Changer in Electron Spectroscopy. In Changer in Electron Scattering. Physics of Atoms and Molecules; Whelan, C.T., Mason, N.J., Eds.; Springer: Boston, MA, USA, 2005; pp. 111–120. [Google Scholar]
- Khakoo, M.A.; Silva, H.; Muse, J.; Lopes, M.C.A.; Winstead, C.; McKoy, V. Electron scattering from H2O: Elastic scattering. Phys. Rev. A 2008, 78. [Google Scholar] [CrossRef]
- Muñoz, A.; Oller, J.C.; Blanco, F.; Gorfinkiel, J.D.; Limão-Vieira, P.; Maira-Vidal, A.; Borge, M.J.G.; Tengblad, O.; Huerga, C.; Téllez, M.; et al. Energy deposition model based on electron scattering cross section data from water molecules. J. Phys. Conf. Ser. 2008, 133. [Google Scholar] [CrossRef]
- de Urquijo, J.; Basurto, E.; Juárez, A.M.; Ness, K.F.; Robson, R.E.; Brunger, M.J.; White, R.D. Electron drift velocities in He and water mixtures: Measurements and an assessment of the water vapour cross-section sets. J. Chem. Phys. 2014, 141. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-Y.; Yoon, J.-S.; Cho, H.; Itikawa, Y.; Karwasz, G.P.; Kokoouline, V.; Nakamura, Y.; Tennyson, J. Cross Sections for Electron Collisions with Methane. J. Phys. Chem. Ref. Data 2015, 44. [Google Scholar] [CrossRef]
- Song, M.-Y.; Yoon, J.-S.; Cho, H.; Karwasz, G.P.; Kokoouline, V.; Nakamura, Y.; Tennyson, J. Cross Sections for Electron Collisions with Acetylene. J. Phys. Chem. Ref. Data 2017, 46. [Google Scholar] [CrossRef]
- Sanz, A.G.; Fuss, M.C.; Blanco, F.; Sebastianelli, F.; Gianturco, F.A.; García, G. Electron scattering cross sections from HCN over a broad energy range (0.1–10 000 eV): Influence of the permanent dipole moment on the scattering process. J. Chem. Phys. 2012, 137. [Google Scholar] [CrossRef] [PubMed]
- Ranković, M.L.; Maljković, J.B.; Tökesi, K.; Marinković, B.P. Elastic electron differential cross sections for argon atom in the intermediate energy range from 40 eV to 300 eV. Eur. Phys. J. D 2017. submitted. [Google Scholar]
- McEachran, R.P.; Stauffer, A.D. An optical potential method for elastic electron and positron scattering from argon. J. Phys. B 2009, 42. [Google Scholar] [CrossRef]
- Paikeday, J.M.; Alexander, J. Polarization Potential for e-Argon Scattering by Differential Scattering Minimization at Intermediate Energies. Int. J. Quant. Chem. 2002, 90, 778–785. [Google Scholar] [CrossRef]
- Nahar, S.N.; Wadehra, J.M. Elastic scattering of positrons and electrons by argon. Phys. Rev. A 1987, 35, 2051–2064. [Google Scholar] [CrossRef]
- Fon, W.C.; Berrington, K.A.; Burke, P.G.; Hibbert, A. The elastic scattering of electrons from inert gases. III. Argon. J. Phys. B 1983, 16, 307–321. [Google Scholar] [CrossRef]
- McCarthy, I.E.; Noble, C.J.; Phillips, B.A.; Turnbull, A.D. Optical model for electron scattering by inert gases. Phys. Rev. A 1977, 15, 2173–2185. [Google Scholar] [CrossRef]
- Joachain, C.J.; Vanderpoorten, R.; Winters, K.H.; Byron, F.W., Jr. Optical model theory of elastic electron- and positron-argon scattering at inter mediate energies. J. Phys. B 1977, 10, 227–238. [Google Scholar] [CrossRef]
- Milosavljević, A.R.; Telega, S.; Šević, D.; Sienkiewicz, J.E.; Marinković, B.P. Elastic electron scattering by argon in the vicinity of the high-energy critical minimum. Rad. Phys. Chem. 2004, 70, 669–676. [Google Scholar] [CrossRef]
- Panajotović, R.; Filipović, D.M.; Marinković, B.P.; Pejčev, V.; Kurepa, M.; Vušković, L. Critical minima in elastic electron scattering by argon. J. Phys. B 1997, 30, 5875–5894. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Tanaka, H.; Chutjian, A.; Trajmar, S. Elastic scattering of intermediate-energy electrons by Ar and Kr. Phys. Rev. A 1981, 23, 2156–2166. [Google Scholar] [CrossRef]
- DuBois, R.D.; Rudd, M.E. Differential cross sections for elastic scattering of electrons from argon, neon, nitrogen and carbon monoxide. J. Phys. B 1976, 9, 2657–2667. [Google Scholar] [CrossRef]
- Jansen, R.H.J.; de Heer, F.J.; Luyken, H.J.; van Wingerden, B.; Blaauw, H.J. Absolute differential cross sections for elastic scattering of electrons by helium, neon, argon and molecular nitrogen. J. Phys. B 1976, 9, 185–212. [Google Scholar] [CrossRef]
- Williams, J.F.; Willis, B.A. The scattering of electrons from inert gases, I. Absolute differential elastic cross sections for argon atoms. J. Phys. B 1975, 8, 1670–1682. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. Available online: http://webbook.nist.gov/chemistry/ (accessed on 30 August 2017).
- Tanaka, H.; Brunger, M.J.; Campbell, L.; Kato, H.; Hoshino, M.; Rau, A.R.P. Scaled plane-wave Born cross sections for atoms and molecules. Rev. Mod. Phys. 2016, 88, 025004. [Google Scholar] [CrossRef]
- IDEADB. Available online: http://ideadb.uibk.ac.at/ (accessed on 18 October 2017).
- Cordiner, M.A.; Charnley, S.B. Negative ion chemistry in the coma of comet 1P/Halley. Meteorit. Planet. Sci. 2014, 49, 21–27. [Google Scholar] [CrossRef]
- Barnett, S.M.; Mason, N.J.; Newell, W.R. Production of the N2(A1Σu+) Metastable State by Electron Dissociation of N2O. Chem. Phys. 1991, 153, 283–295. [Google Scholar] [CrossRef]
- Barnett, S.M.; Mason, N.J.; Newell, W.R. Dissociative Excitation of Metastable Fragments by Electron Impact on Carbonyl Sulphide, Carbon Dioxide and Carbon Monoxide. J. Phys B 1992, 25, 1307–1320. [Google Scholar] [CrossRef]
- Danko, M.; Ribar, A.; Ďurian, M.; Országh, J.; Matejčík, Š. Electron induced fluorescence of the H2 molecule—Balmer lines and Fulcher α system. Plasma Sources Sci. Technol. 2016, 25. [Google Scholar] [CrossRef]
- Harb, T.; Kedzierski, W.; McConkey, J.W. Production of ground state OH following electron impact on H2O. J. Chem. Phys. 2001, 115, 5507–5512. [Google Scholar] [CrossRef]
- Cosby, P.C. Electron-impact dissociation of carbon monoxide. J. Chem. Phys. 1993, 98, 7804–7818. [Google Scholar] [CrossRef]
- Bredehöft, J.H.; Böhler, E.; Schmidt, F.; Borrmann, T.; Swiderek, P. Electron-Induced Synthesis of Formamide in Condensed Mixtures of Carbon Monoxide and Ammonia. ACS Earth Space Chem. 2017, 1, 50–59. [Google Scholar] [CrossRef]
- Boyer, M.C.; Rivas, N.; Tran, A.A.; Verish, C.A.; Arumainayagam, C.R. The role of low-energy (≤20 eV) electrons in astrochemistry. Surf. Sci. 2016, 652, 26–32. [Google Scholar] [CrossRef]
- Mason, N.J.; Nair, B.; Jheeta, S.; Szymańska, E. Electron induced chemistry: A new frontier in astrochemistry. Faraday Discuss. 2014, 168, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Jewitt, D.; Kaiser, R.I. Formation of Hydrogen, Oxygen and Hydrogen Peroxide in Electron-irradiated Crystalline Water Ice. Astrophys. J. 2006, 639, 534–548. [Google Scholar] [CrossRef]
- Zheng, W.; Jewitt, D.; Kaiser, R.I. Temperature Dependence of the Formation of Hydrogen, Oxygen and Hydrogen Peroxide in Electron-Irradiated Crystalline Water Ice. Ice. Astrophys. J. 2006, 648, 753–761. [Google Scholar] [CrossRef]



| Name of Compound | Sum Formula | Abundance wrt Water |
|---|---|---|
| Methane | CH4 | 0.5% |
| Water | H2O | 100% |
| Hydrogen cyanide | HCN | 0.9% |
| Carbon monoxide | CO | 1.2% |
| Methylamine | CH3NH2 | 0.6% |
| Acetonitrile | CH3CN | 0.3% |
| Isocyanic acid | HNCO | 0.3% |
| Acetaldehyde | CH3CHO | 0.5% |
| Formamide | HCONH2 | 1.8% |
| Ethylamine | C2H5NH2 | 0.3% |
| Methyl isocyanate | CH3NCO | 1.3% |
| Acetone | CH3COCH3 | 0.3% |
| Propionaldehyde | C2H5CHO | 0.1% |
| Acetamide | CH3CONH2 | 0.7% |
| Glycolaldehyde | CH2OHCHO | 0.4% |
| Ethylene glycol | HOC2H4OH | 0.2% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinković, B.P.; Bredehöft, J.H.; Vujčić, V.; Jevremović, D.; Mason, N.J. Rosetta Mission: Electron Scattering Cross Sections—Data Needs and Coverage in BEAMDB Database. Atoms 2017, 5, 46. https://doi.org/10.3390/atoms5040046
Marinković BP, Bredehöft JH, Vujčić V, Jevremović D, Mason NJ. Rosetta Mission: Electron Scattering Cross Sections—Data Needs and Coverage in BEAMDB Database. Atoms. 2017; 5(4):46. https://doi.org/10.3390/atoms5040046
Chicago/Turabian StyleMarinković, Bratislav P., Jan Hendrik Bredehöft, Veljko Vujčić, Darko Jevremović, and Nigel J. Mason. 2017. "Rosetta Mission: Electron Scattering Cross Sections—Data Needs and Coverage in BEAMDB Database" Atoms 5, no. 4: 46. https://doi.org/10.3390/atoms5040046
APA StyleMarinković, B. P., Bredehöft, J. H., Vujčić, V., Jevremović, D., & Mason, N. J. (2017). Rosetta Mission: Electron Scattering Cross Sections—Data Needs and Coverage in BEAMDB Database. Atoms, 5(4), 46. https://doi.org/10.3390/atoms5040046





