Abstract
To better understand the hydrogen corrosion mechanism on uranium surfaces and assess the hydrogen penetration resistance of Al-U alloys, the adsorption of hydrogen atoms on U(110), Al(111), and nAl/U(110) alloy surfaces was systematically studied through density functional theory (DFT) calculations. The results reveal that when U is alloyed with Al, the adsorption behavior of H atom on its surface thereon changes greatly. Specifically, the adsorption energy of H decreases with increasing Al content, indicating a weakening of the interaction between the H atom and the surface. A correlation between binding strength and alloy composition was established using d-band center theory. The incorporation of Al atoms alters the electronic structure of the U(110) surface, shifting the d-band center of uranium atoms downward. This shift results in a weakened interaction between the adsorbed H atom and the alloy surface.
1. Introduction
Uranium, with its unique electronic configuration (5f36d17s2) and low standard electrode potential, is a highly reactive metal that plays a crucial role as a nuclear fuel [1,2]. Three distinct phases of uranium exist at different temperatures: the body-centered tetragonal β-phase (bct), the face-centered orthorhombic α-phase (fco) in the ground state, and the body-centered cubic γ-phase (bcc) [3]. Among these, the γ-phase is favored due to its cubic polycrystalline structure [3]. During the use or storage of uranium materials, hydrogen molecules from the surrounding environment can react with uranium to form UH₃ species, leading to uranium embrittlement [4]. Hydrogen embrittlement, as a result of hydrogen corrosion, is a well-documented issue in various steel materials [5]. Common methods to mitigate hydrogen embrittlement include electroplating, chemical plating, and magnetron sputtering, which involve applying hydrogen barrier coatings on metal substrates [6,7]. Typical materials used for such coatings include glass, ceramic, and metals [8,9]. Ion implantation [10] is another effective technique for creating hydrogen barrier coatings, as it forms a new alloy phase on the metal surface without obvious interfaces or stress concentrations, preventing peeling or cracking.
Studies have shown that Al or Al-rich coatings provide excellent resistance to hydrogen corrosion [11]. For example, Bland et al. [12] investigated the corrosion performance of ion-plated Cu, Cr, and Al coatings on uranium surfaces exposed to water vapor and found that Al coatings offered the best corrosion protection. Similarly, Yamabe et al. [13] reported that alumina/Fe–Al and alumina/Al/Fe–Al layers deposited on type 304 austenitic stainless steel demonstrated high resistance to hydrogen penetration at 10 MPa. This is likely due to the extremely low hydrogen diffusion rate in Al [14], which is in the range of 10−11 to 10−15 m2/s at room temperature [15]. Musket et al. [16] used aluminum ion implantation to create a hydrogen permeation barrier that effectively prevented tritium from permeating polycrystalline iron. Shan [17] also found that injecting aluminum, oxygen, Molybdenum, and Chromium onto uranium surfaces could reduce tritium permeability by ~2–3 orders of magnitude, with Al being particularly effective. While much of the research on hydrogen barrier coatings has focused on preventing hydrogen diffusion into the metal matrix, the hydrogen corrosion process begins with the H adsorption onto the metal surface, which is then followed by their diffusion into the subsurface. Therefore, reducing H adsorption on the U surface is a key step in reduce the infiltration of hydrogen into the U metal at the source. However, because uranium metal is a radioactive and biotoxic substance, there are special safety requirements in processing and surface treatment, so there is a lack of experimental studies to reduce or prevent hydrogen adsorption on the surface of U metal. Through the first principles calculation and simulation, the adsorption behavior of H on the surface of U and its alloy can be studied on an electronic level, which can provide a theoretical basis for the experimental preparation of hydrogen corrosion-resistant U materials.
In our previous study [18], the effect of gold (Au) substitution on the hydrogen adsorption performance of the U(110) surface was investigated using DFT calculations. The results suggest that the adsorption energy of H decreases with increasing Au content, suggesting that an Au-U alloy could serve as an effective hydrogen barrier coating on the U surface. A comparable approach was employed in this study. For comparative analysis, DFT calculations were performed to investigate the interaction between a H atom and the most stable surfaces, bcc uranium (U(110)) and fcc aluminum (Al(111)). Additionally, the interaction between H and U(110) surfaces with different aluminum concentrations, referred to as nAl/U(110) surfaces, was further explored. The adsorption energy of a H atom on these surfaces, along with their geometric and electronic properties, is analyzed. The potential of Al-U alloys to improve hydrogen corrosion protection was also assessed. The results offer valuable theoretical insights into the atomic-level interaction mechanisms between hydrogen and metal surfaces, which are crucial for enhancing the H corrosion resistance of uranium-based materials.
2. Computational Methods
All ab initio DFT calculations were conducted using the plane-wave-based Cambridge Sequential Total Energy Package (CASTEP) [19]. The exchange–correlation energy was calculated using the Perdew–Burke–Erzenhof (PBE) functional, a generalized gradient approximation (GGA) [20]. Ionic potentials were represented by ultrasoft pseudopotentials, and the Kohn–Sham wavefunctions [21] were expanded in a plane-wave basis set. The surface Brillouin zone was sampled using uniform k-point meshes: A 6 × 6 × 1 mesh for the U(110) surface and a 7 × 7 × 1 mesh for the Al(111) surface. The cutoff energies were set to 520 eV and 300 eV for the U(110) and Al(111) surfaces, respectively, in order to ensure accurate and converged results. Geometry optimization was carried out using the Broyden–Fletcher–Goldfarb–Shanno method [22]. Self-consistent convergence was achieved with maximum energy differences of 1 × 10−6 eV/atom, a force of 0.03 eV/Å, and displacements of 1 × 10−3 Å.
The U(110), Al(111), and nAl/U(110) slabs were modeled using a flat plate approach with periodic boundary conditions. Each flat plate consisted of five metal atom layers, with the top three layers and all adsorbed atoms fully relaxed, while the bottom two layers were fixed. In the p(2 × 2) supercell, which contained four atoms per layer, a vacuum spacing of 15 Å was introduced perpendicular to the surface to prevent interactions between adjacent slabs. Adsorption was restricted to one side of the plate, corresponding to a coverage of 0.25 ML, and the electrostatic potential was adjusted accordingly. A dipole moment was induced by placing adsorbed atoms on only one side of the atomically relaxed surface, necessitating a dipole correction to mitigate the asymmetry-induced dipole [23]. Given the strongly correlated nature of the 5f electrons in uranium, the DFT + U method was initially considered to accurately predict ground state properties [24]. However, Adak et al. successfully applied generalized DFT to investigate α-U metal properties without accounting for strong electronic correlations, obtaining results in good agreement with experimental data [25]. Moreover, the hybridization of uranium’s 5f electrons with 6d and 7s orbitals, which exhibit mobile behavior [26], suggests that generalized DFT is appropriate for describing metallic uranium without requiring additional modifications to the electronic states. Consequently, 5f electron correlations were neglected in the calculations.
Before investigating the adsorption of H atoms, the bulk properties of the Al and U metals were examined. The calculated lattice constants for Al and U are presented in Table 1. The crystal parameters obtained for both metals agree with experimental values [27,28] and previously reported results [29,30], confirming the validity of the chosen computational setup. Additionally, hydrogen molecules were placed in a 10 × 10 × 10 lattice, yielding an energy of −31.678 eV and an H-H bond length of 0.754 Å, which agrees excellently with other theoretical values (0.752 Å) [31].

Table 1.
Comparison of the calculated results from this study with other theoretical and experimental results.
3. Results and Discussion
3.1. H Adsorption on the Pure Al(111) and U(110) Surfaces
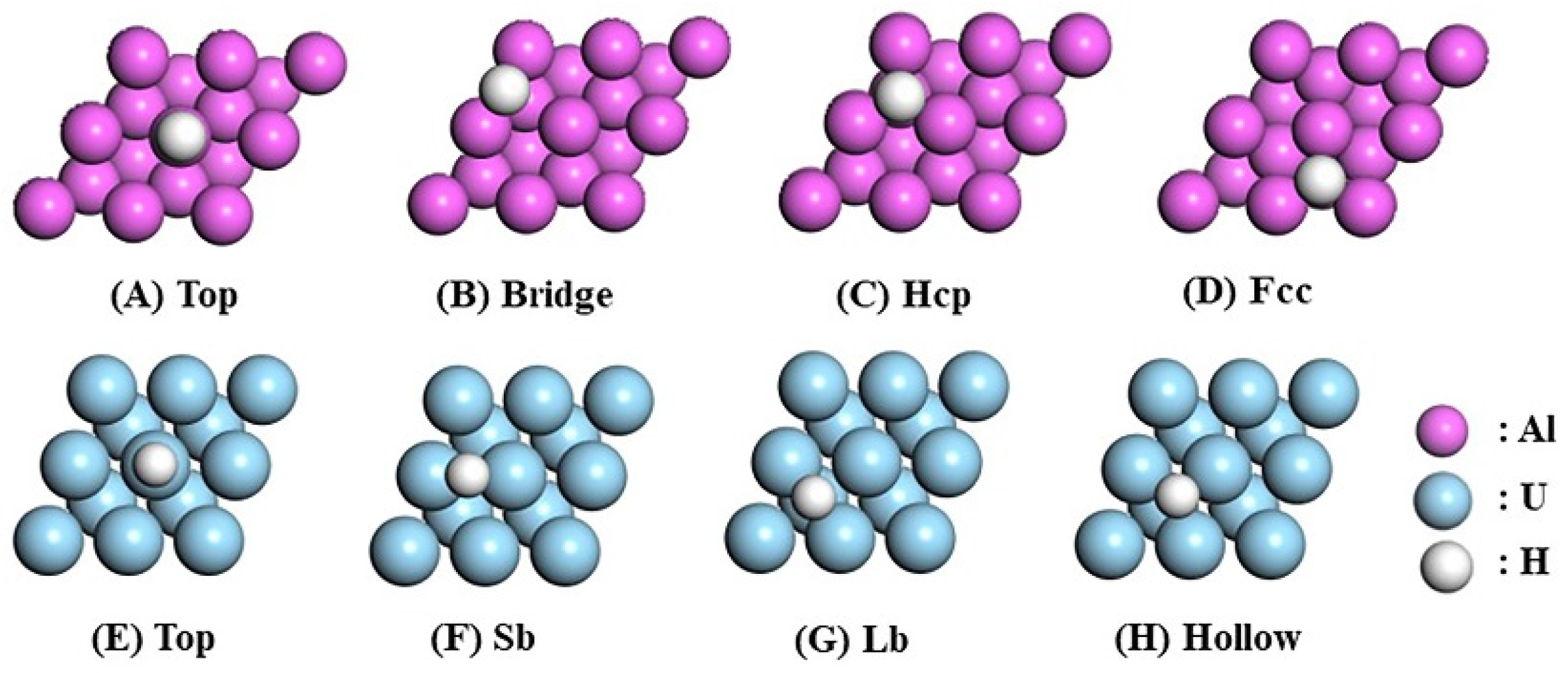
As shown in Figure 1, four distinct adsorption sites are available on the pure Al(111) surface: Top (directly above the surface atom), Bridge (above the midpoint between two surface Al atoms), Hcp (above the midpoint of three surface Al atoms and directly above an Al atom in the second layer), and Fcc (above the midpoint of three surface Al atoms and directly above an Al atom in the third layer). Similarly, the pure U(110) surface contains four distinct adsorption sites: Top (directly above the U atom on the surface), Short Bridge (Sb) (above the midpoint of two surface U atoms), Long Bridge (Lb) (above the midpoint of four surface U atoms and simultaneously above a U atom in the second layer), and Hollow (above the midpoint of three surface U atoms). The adsorption energy () is calculated using Equation (1).
where represents the energy of adsorption system, denotes the energy of a pure metal slab, and is the energy of the isolated H2 molecule. A negative indicates an exothermic adsorption process, with a more negative value corresponding to stronger metal–hydrogen interactions.
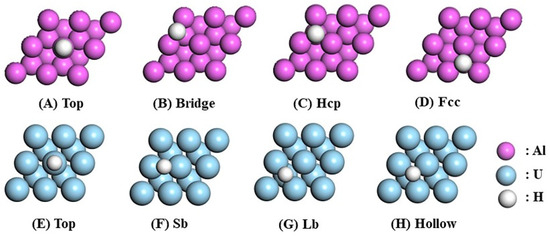
Figure 1.
Top view of the pure Al(111) and U(110) surfaces with H adsorption sites.
Table 2 presents the and key geometrical parameters for H adsorption on the pure metal surfaces. For the Al(111) surface, all values are positive, indicating that H adsorption requires heat and that the process is unstable. The adsorption of H at the Fcc site is the most stable, with an of 0.349 eV, which is consistent with the findings of Stumpf et al. [34]. The value at the Top site is 0.427 eV, which is marginally lower than that at the Hcp site. Additionally, the bond length between H and Al () at the Top site is 1.627 Å, shorter than that at the Hcp site, suggesting that there is no significant surface deformation, and that H adsorption at the Top site is more stable. During structure optimization, the H atom migrates from the Bridge site to the Fcc site, a behavior similar to that observed in previous studies [35]. Furthermore, the relatively high for H on the Al(111) surface may be attributed to the low solubility of H in the Al lattice [36].

Table 2.
Calculated energies and geometric parameters for the H-metal system.
For the pure U(110) surface, hydrogen adsorption at the Hollow site is the most thermodynamically stable, with an of −3.828 eV. The shortest H–U distance at this site is 1.192 Å, indicating the strongest H–U interaction and stable adsorption. Adsorption at the Lb and Sb sites (−3.674 and −3.683 eV, respectively) is also favorable, with values close to that at the Hollow site, suggesting a smooth potential energy surface for hydrogen diffusion across the U(110) surface. In contrast, adsorption at the Top site was found to be unstable. The bond lengths between H and U atoms () range from 2.130 to 2.269 Å, closely matching the H–U bond length (2.320 Å) in uranium hydride UH3 [37], indicating that when enough H is adsorbed, there is a tendency to form uranium hydride on the U(110) surface, and H is chemically adsorbed on the U(110) surface.
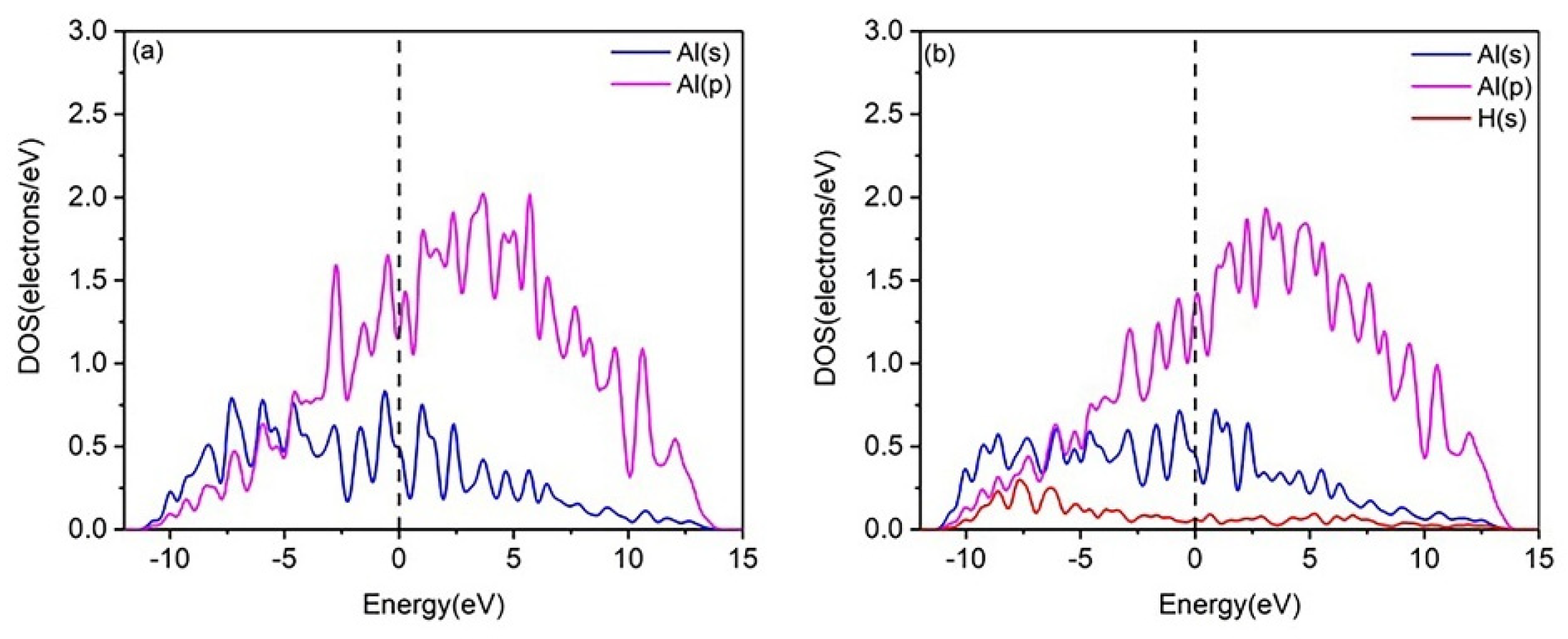
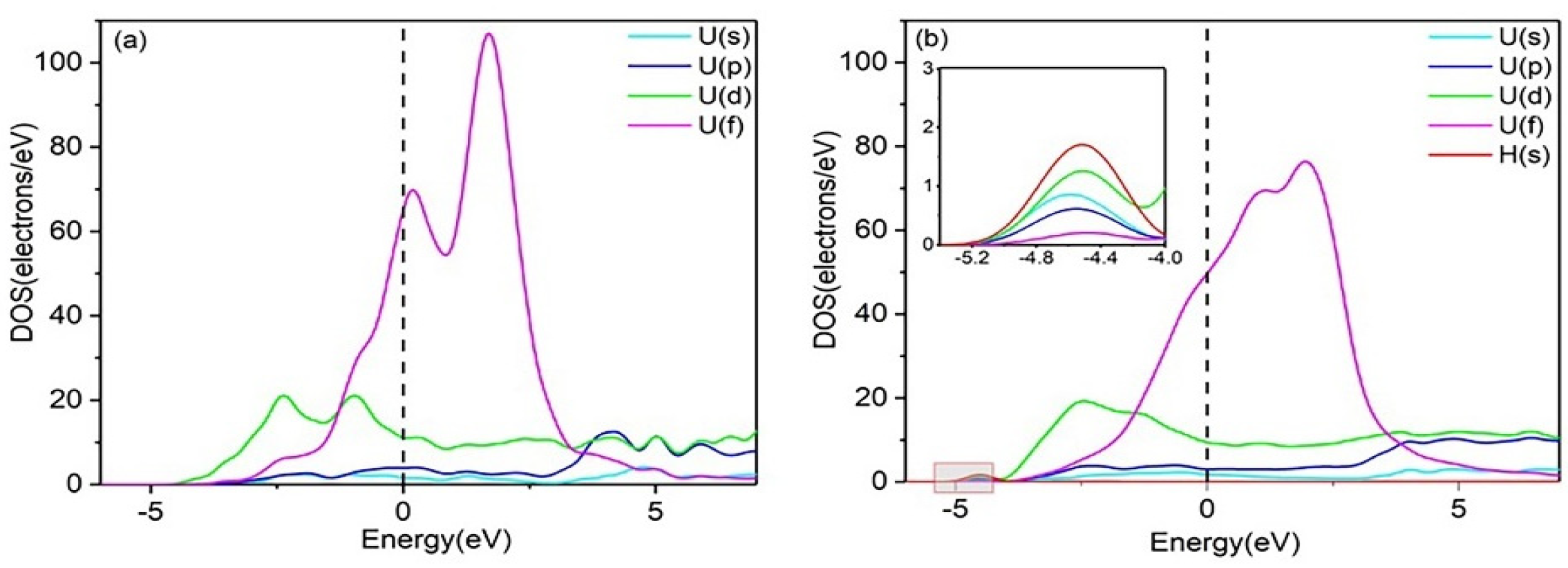
To gain deeper insight into the interaction mechanisms of H with the Al(111) and U(110) surfaces, the partial densities of states (PDOSs) were calculated. Figure 2 shows the PDOS diagram for H adsorption at the Fcc site on the pure Al(111) surface, both before and after adsorption. After hydrogen adsorption, the PDOS curve of the Al(111) surface undergoes only slight changes, with no new hybridization peaks observed. This suggests weak or negligible bonding between the H and Al atoms, leading to unstable hydrogen adsorption. In contrast, the PDOS plots of the U(110) surface, before and after hydrogen adsorption, are shown in Figure 3. Following hydrogen adsorption, the PDOS curve exhibits significant changes, with the formation of a new hybridization peak around −4.000 eV to −5.200 eV, indicating the formation of a strong H–U bond and stable hydrogen adsorption. It can thus be predicted that the adsorption of multiple hydrogen atoms on the uranium surface, followed by diffusion into the bulk phase, will result in embrittlement. Moreover, the electronic hybridization between the H-1s and U-6d states is stronger than that between the H-1s and U-5f states (Figure 3b), suggesting that charge transfer primarily occurs between the H-1s and U-6d electronic states. Consequently, the contribution of U-5f states is neglected in the subsequent analysis.
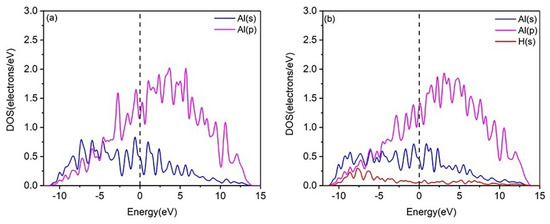
Figure 2.
PDOS plots of (a) the clean Al(111) surface and (b) the hydrogen-adsorbed Al(111) surface.
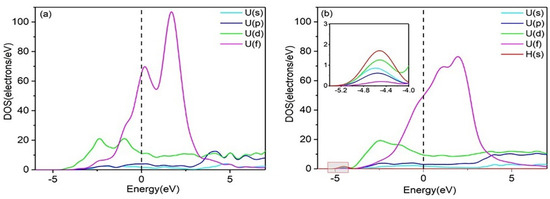
Figure 3.
PDOS plots of (a) the clean U (110) surface and (b) the hydrogen-adsorbed U (110) surface.
3.2. H Adsorption on the nAl/U(110) Alloy Surfaces
An Al-U alloy model was constructed by substituting the surface atoms of the pure U(110) surface with Al atoms, referred to as the nAl/U(110) surface, where n refers to the number of U atoms substituted by Al atoms. The value of n can be equal to 1, 2, 3, 4, or 8, with specific configurations as follows: at n = 4, the top layer of U(110) is fully replaced by Al atoms, effectively covering the surface with a monolayer of Al. At n = 8, both the top and second layers of U are replaced by Al atoms. The five alloy configurations in Table 3 were thus considered. The stability of the Al-substituted configurations was examined by calculating the substitution energy () defined by the following:
where is the energy of the Al-substituted configuration, and are the energies of individual U and Al atoms in their respective bulk phases, and is the energy of the clean slab. A negative indicates a stable Al substitution configuration. All calculated values (Table 3) were negative, confirming the stability and energetically favorable nature of Al substitution on the U(110) surface, and validating the reliability of the simulation method.

Table 3.
The substitution energy of the nAl/U(110) surfaces.
Compared to the pure U(110) surface, the substitution of U atoms with Al atoms induces local structural distortions on the nAl/U(110) alloy surface. The degree of atomic layer contraction or expansion was calculated using the following:
where is the interlayer spacing between adjacent atomic layers i and j, and is the corresponding spacing in the bulk structure. The results, shown in Table 4, reveal that the atomic layer spacing on the 1Al/U(110) surface contracted by 0.286% and 1.535% between the first and second, and second and third layers, respectively, compared to the bulk values. On the 2Al/U(110) surface, the first layer expanded outward by 3.236% relative to the second, while the second layer contracted by 2.949% towards the third. Relaxations on the 3Al/U(110) and 4Al/U(110) surfaces were similar to those on the 2Al/U(110) surface. For the 8Al/U(110) surface, the spacing between the first and second layers contracted by 1.893%, whereas the spacing between the second and third layers expanded by 2.448%. These results indicate that, although some relaxation of the atomic spacing occurred after structural optimization, no significant structural reconstruction was observed on the nAl/U(110) surfaces.

Table 4.
Interlayer relaxation data for the alloy surfaces.
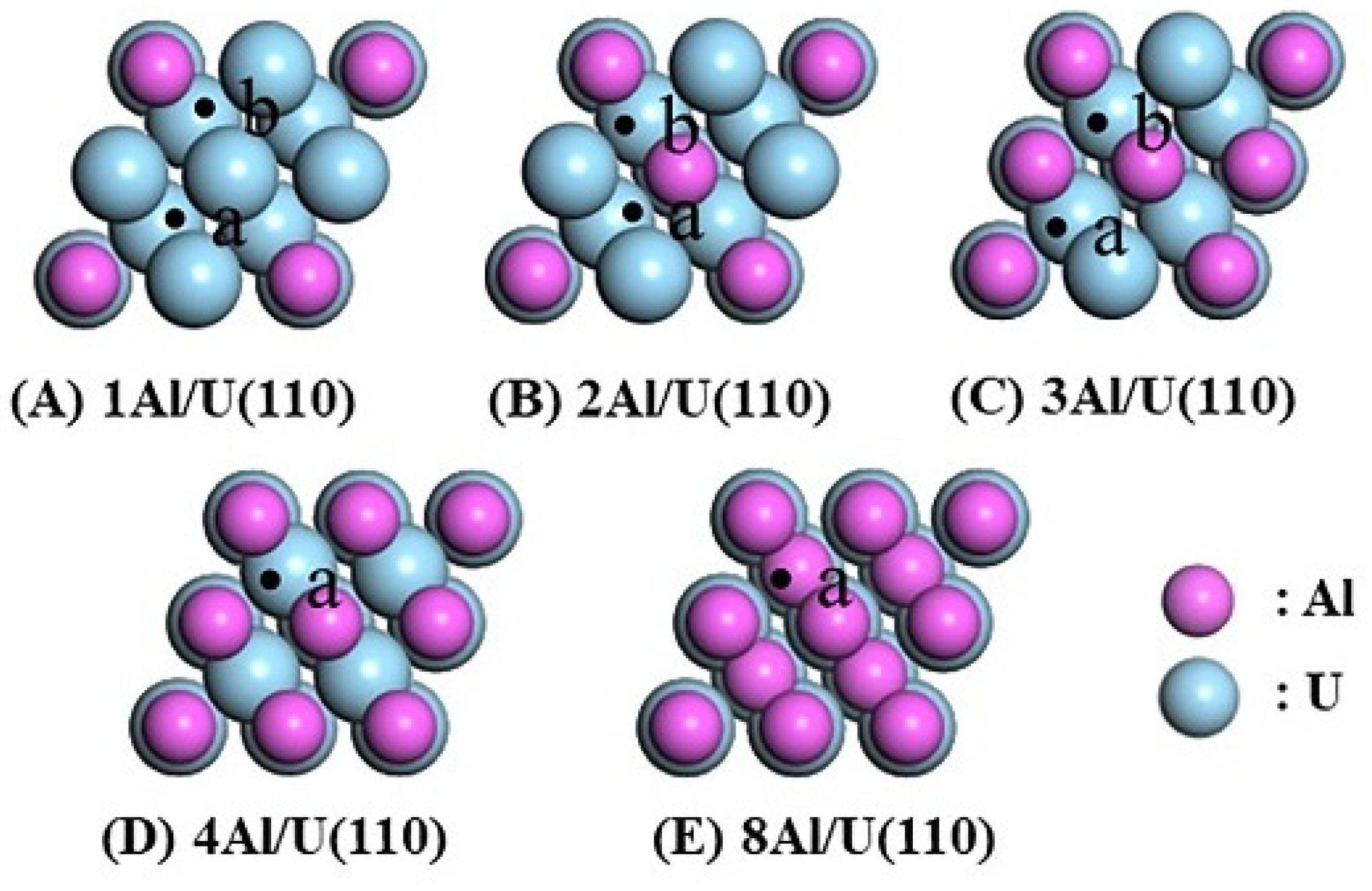
The adsorption of H at the Hollow site of the nAl/U(110) alloy surface was investigated, as this site represents the most stable H adsorption site on the pure U(110) surface. The adsorption sites on the nAl/U(110) surfaces differ based on the atomic composition around the Hollow site. As illustrated in Figure 4, on the 1Al/U(110) surface, the following configurations were considered: (a) Hollow(3U), where all three surrounding atoms are U atoms, and (b) Hollow(1Al2U), where one Al atom and two U atoms surround the Hollow site. Similarly, for the 2Al/U(110) surface, the (a) Hollow(1Al2U) and (b) Hollow(2Al1U) sites were considered, while the 3Al/U(110) surface had the (a) Hollow(2Al1U) and (b) Hollow(3Al) sites. The 4Al/U(110) surface only had the (a) Hollow(3Al) site, and for the 8Al/U(110) surface, the second layer is composed of Al atoms, creating a distinct Hollow(3Al) site. These variations arise due to the differences in atomic composition in the subsurface layers of the nAl/U(110) configurations.
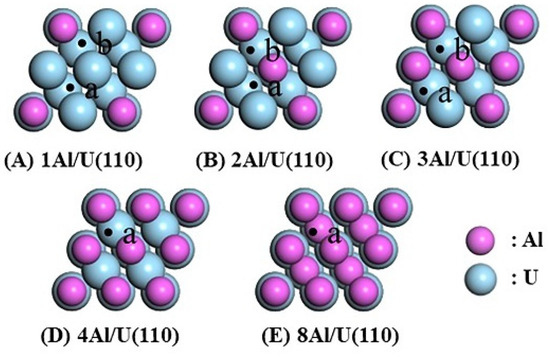
Figure 4.
Top view of the nAl/U(110) alloy surface with H adsorption sites.
Table 5 provides the calculated and geometric parameters for H adsorption at various sites on the alloy surfaces. For the 1Al/U(110) surface, the most stable adsorption site was Hollow(3U), with an of −0.950 eV, which is notably higher than the on the pure U(110) surface (−3.828 eV). This indicates that substituting U by Al on the surface weakens the hydrogen adsorption strength on uranium. Additionally, among the different Hollow sites, Hollow(1Al2U) had a slightly higher than Hollow(3U), further suggesting that Al atoms at the surface influence H adsorption. The weakening of adsorption at the Hollow(1Al2U) site can be attributed to the presence of the Al atom, which decreases the stability of hydrogen adsorption. This is consistent with Section 3.1, which shows that the hydrogen adsorption energy on the pure Al(111) surface (positive) is higher than that on the pure U(110) surface (negative), indicating that the catalytic activity of U is greater than that of Al. Consequently, H consistently prefers to adsorb at sites near the U atom on the nAl/U (110) surface.

Table 5.
Adsorption energies and geometric parameters of the H-metal system.
The Hollow(1Al2U) site is the most stable hydrogen adsorption site on the 2Al/U (110) surface, with an of −0.670 eV, which was 0.232 eV higher than the corresponding site on the 1Al/U(110) surface. The bond lengths, RH–U and RH–Al, were slightly increased (by 0.051 and 0.039 Å, respectively), indicating weaker adsorption at this site. On the Hollow(2Al1U) site, the hydrogen adsorption is unstable, and the adsorbed H atom migrates to the Bridge site upon optimization. This observation suggests that increasing the number of Al atoms on the surface weakens hydrogen adsorption, with a higher number of Al atoms near the adsorption site leading to a more unstable adsorption process. For the 3Al/U(110) surface, the most stable adsorption site is the Hollow(2Al1U) site, with an of −0.167 eV, whereas adsorption at the Hollow(3Al) site is unstable. Similarly, when three Al atoms are present on the alloy surface, the hydrogen adsorption energy increases, indicating that the substitution of three Al atoms significantly affects the stability of H adsorption.
Hydrogen remains unstable at the Hollow(3Al) site on the 4Al/U(110) surface, further confirming that Al substitution negatively impacts the hydrogen adsorption process. The of hydrogen at the Hollow(3Al) site on the 8Al/U(110) surface was −2.066 eV, significantly lower than that of the previous four configurations, but slightly higher than the on the clean U(110) surface (−3.828 eV). This difference is attributed to the strong influence of the subsurface atomic configuration on adsorption at the Hollow site. Consequently, the substitution of U atoms with Al in the nAl/U(110) alloy weakens hydrogen adsorption. This weakening is most pronounced when the surface is fully saturated with Al atoms, and it slightly diminishes when U atoms are replaced by bilayer Al atoms.
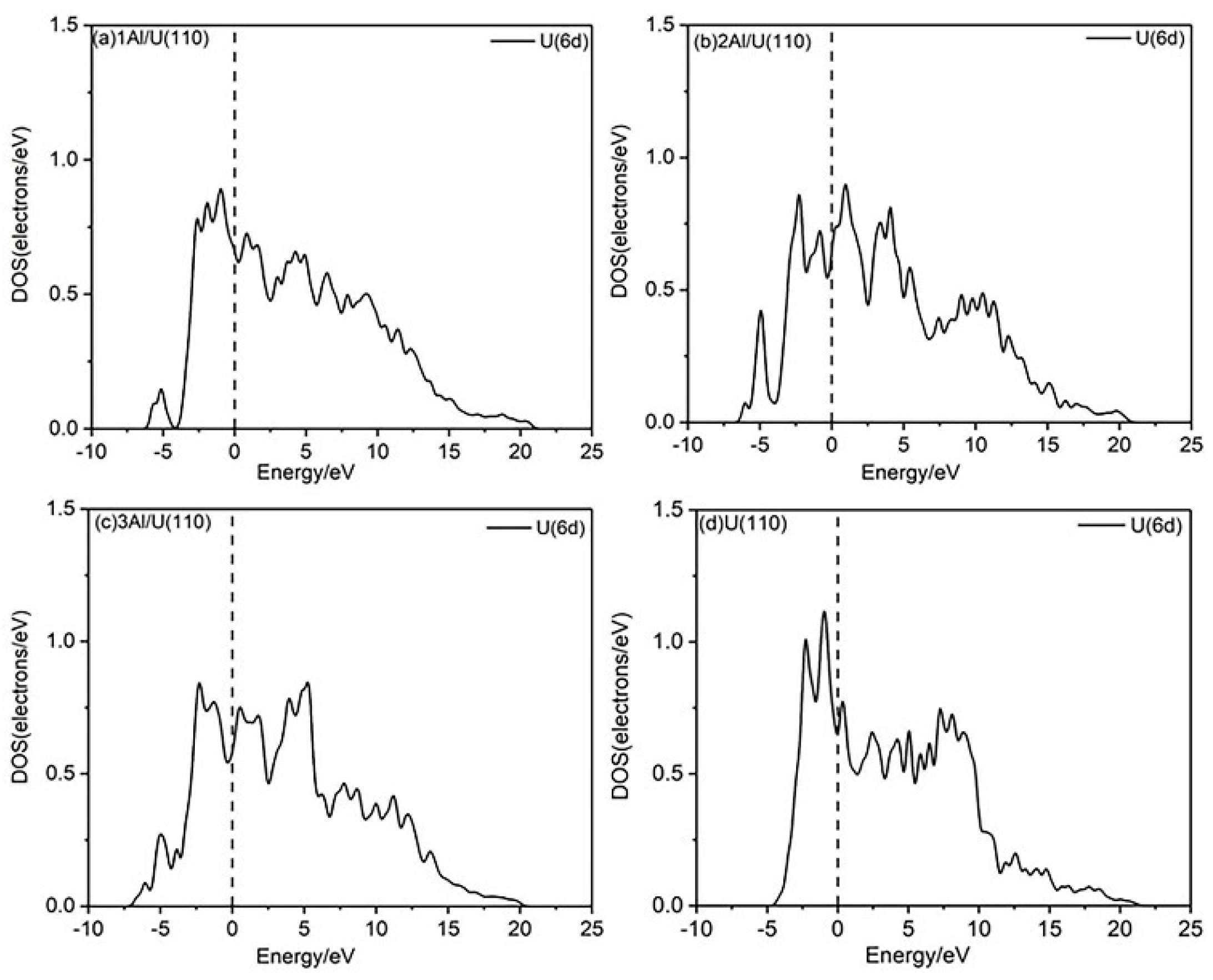
In general, the introduction of Al atoms alters the physicochemical properties of uranium surface by rearranging surface electrons, which in turn modifies the surface electronic density of states. The d-band densities of states for both U(110) and nAl/U(110) were calculated, as shown in Figure 5. The electron density distribution on the alloy surface differs from that of the pure U(110) surface. When Al atoms replace U atoms, the overall d-band density of states shifts to lower energy, resulting in the appearance of a new peak around 5.000 eV.
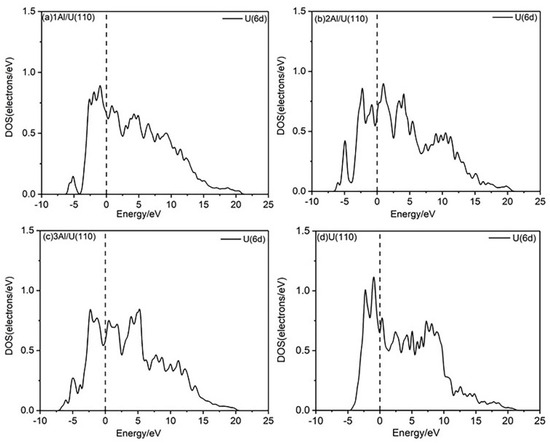
Figure 5.
PDOS plots of the nAl/U(110) and U(110) surfaces.
Multiple theoretical studies have demonstrated that the d-band centers of metal surfaces are reliable indicators of the adsorption energy for various small molecules. In particular, the adsorption energy is strongly correlated with the d-band center of the metal [38,39]. According to the d-band model proposed by Nørskov and Hammer, when the d-band center shifts upward relative to the Fermi level, the d-band becomes broader, leading to a reduction in antibonding interactions between the metal surface atoms and adsorbed atoms. This results in more stable adsorption and lower adsorption energy, as defined in this study. Conversely, a downward shift in the d-band center increases antibonding interactions, weakening adsorption. The introduction of Al atoms alters the ensemble of adsorption sites on the U surface, and the catalytic activity of the alloy surface is influenced by a combination of ligand, strain, and ensemble effects [40], all of which are reflected in the position of the d-band center of the surface atoms. The magnitude of d-band center () was calculated using Equation (4).
where ρ is the density of states of the d-orbiting electrons, and E is the electron energy. The calculated from Equation (4) is presented in Table 6.

Table 6.
The magnitude of the d-band center on each surface.
The introduction of Al atoms, with their larger atomic radius, into the relatively smaller U lattice induces tensile strain. This strain leads to an upward shift in the d-band center of surface U atoms relative to the Fermi level, enhancing the interaction between the adsorbed atoms and the metal surface. Conversely, the ligand effect of Al atoms causes a downward shift in the d-band center of surface U atoms relative to the Fermi level, weakening the interaction between the adsorbed atoms and the metal surface. As shown in Table 6, the of surface U atoms decreases progressively and shifts downward with an increasing number of surface Al atoms. These results suggest that, following the introduction of Al atoms onto the U(110) surface, the ligand effect predominates over the strain effect, leading to a downward shift in the d-band center relative to the Fermi level. This shift weakens the interaction between the U surface and H atoms, thereby increasing the adsorption energy of H atoms.
4. Conclusions
DFT calculations were employed to investigate the adsorption of H on the Al(111), U(110), and nAl/U(110) surfaces. The effect of Al atom substitution on H adsorption at the U(110) surface was assessed by calculating the adsorption energies, geometric structures, and density of states. The results demonstrate that H adsorption on the pure Al surface is less favorable compared to the pure U surface, thereby reducing the risk of hydrogen-induced corrosion. Furthermore, the overall hydrogen adsorption on the U surface is weakened when alloyed with Al; the weak H adsorption on Al-U alloys suggests their potential for hydrogen corrosion protection.
This study provides theoretical support for reducing the infiltration of hydrogen into U metal from the source, and provides a theoretical basis for the experimental preparation of hydrogen corrosion-resistant U materials. Further studies of the diffusion of hydrogen from the surface to the bulk of Al-U alloys are needed to elucidate the hydrogen barrier properties, which we are undertaking, and these results will be reported in a subsequent publication. In addition, despite the radiological and biological toxicity of uranium metal, the conclusions of this study still need to be verified by design experiments.
Author Contributions
Conceptualization, D.X.; methodology, X.W.; software, M.G. and D.Y.; validation, M.H.; formal analysis, M.G. and M.H.; resources, D.Y.; data curation, M.H.; writing—original draft preparation, X.W., M.G. and D.Y.; writing—review and editing, D.X.; visualization, D.Y. and M.H.; supervision, Y.L.; project administration, Y.L. and D.X.; funding acquisition, D.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Sichuan Province, China (2022NSFSC0292).
Data Availability Statement
The original contributions presented in this study are included in this article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Tian, X.F.; Wang, Y.; Li, L.S.; Wu, M.D.; Yu, Y. First principles studies of oxygen adsorption on the γ-U (110) surface and influences of mo doping. Comp. Mater. Sci. 2020, 179, 109633. [Google Scholar] [CrossRef]
- Sheng, J.; Liu, Y.; Shi, X.M.; Wang, Y.C.; Chen, Z.H.; Xu, K.; Wu, S.; Huang, H.B.; Sun, B.; Liu, H.F.; et al. A multiphase-field model for simulating the hydrogen-induced multi-spot corrosion on the surface of polycrystalline metals: Application to uranium metal. Comp. Mater. Sci. 2024, 235, 112779. [Google Scholar] [CrossRef]
- Qin, C.L.; Yu, Y.S.; Xu, Z.H.; Du, J.G.; Zhao, L.; Jiang, G. The interaction of oxygen with the γ-U (001) and (110) surfaces: An ab initio study. Comp. Mater. Sci. 2023, 219, 112025. [Google Scholar] [CrossRef]
- Chen, J.F.; Tang, T. First-principles thermodynamical modeling of molecular reactions on α-U (001) and α-UH 3 (001) surfaces and their influence on hydrogen activation. J. Nucl. Mater. 2025, 603, 155455. [Google Scholar] [CrossRef]
- Chen, L.C.; Ji, H.F.; Su, B.; Chen, P.H.; Wang, X.L. The role of nb in enhancing the corrosion resistance of U-Nb alloy to hydrogen. Corros. Sci. 2025, 243, 112594. [Google Scholar] [CrossRef]
- Singh, S.; Singh, H. Effect of electroplated interlayers on bonding mechanism of cold-sprayed copper on SS316L steel substrate. Vacuum 2020, 172, 109092. [Google Scholar] [CrossRef]
- Depla, D. Sputter deposition with powder targets: An overview. Vacuum 2021, 184, 109892. [Google Scholar] [CrossRef]
- Yugeswaran, S.; Kobayashi, A. Metallic glass coatings fabricated by gas tunnel type plasma spraying. Vacuum 2014, 110, 177–182. [Google Scholar] [CrossRef]
- Anna, K.; Subr, M.; Kylián, O.; Kús, P.; Hanus, J.; Procházka, M. Nanostructured metal coatings for surface-enhanced raman spectroscopy (sers) prepared by means of low-pressure plasma. Vacuum 2019, 170, 108951. [Google Scholar]
- Tan, Y.; Yip, W.S.; Zhao, T.; To, S.; Zhao, Z.J. Subsurface damage and brittle fracture suppression of monocrystalline germanium in ultra-precision machining by multiple ion implantation surface modification. J. Mater. Process. Technol. 2024, 334, 118640. [Google Scholar] [CrossRef]
- Levchuk, D.; Koch, F.; Maier, H.; Bolt, H. Gas-driven deuterium permeation through alo coated samples. Phys. Scripta 2004, T108, 119–123. [Google Scholar] [CrossRef]
- Bland, R.D. A parametric study of ion-plated aluminum coatings on uranium. Electrochem. Technol. 1968, 6, 272–278. [Google Scholar]
- Yamabe, J.; Matsuoka, S.; Murakami, Y. Surface coating with a high resistance to hydrogen entry under high-pressure hydrogen-gas environment. Int. J. Hydrogen Energy 2013, 38, 10141–10154. [Google Scholar] [CrossRef]
- Qiu, C.A.; Olson, G.B.; Opalka, S.M.; Anton, D.L. Thermodynamic evaluation of the Al-H system. J. Phase Equilib. Diff. 2004, 25, 520–527. [Google Scholar] [CrossRef]
- Kamoutsi, H.; Haidemenopoulos, G.N.; Bontozoglou, V.; Pantelakis, S. Corrosion-induced hydrogen embrittlement in aluminum alloy 2024. Corros. Sci. 2006, 48, 1209–1224. [Google Scholar] [CrossRef]
- Musket, R.G.; Steward, S.A.; Brown, D.W.; Uribe, F.S. Reduction of tritium permeation through iron using aluminum-ion implantation. In Conference: American Vacuum Society Meeting, Boston, MA, USA, 1 Nov 1983; Lawrence Livermore National Lab.: Livermore, CA, USA, 1983; 6p. [Google Scholar]
- Shan, C.; Wu, A.; Chen, Q.; Wang, Q.; Ni, R. Investigation on behavior of diffusion and permeation of tritium in uranium implanted with aluminum, oxygen, molybdenum and chromium. At. Energy Sci. Technol. 1993, 26, 75–79. [Google Scholar]
- You, D.; Xie, D.; Wang, X.T.; Wei, L.J.; Liang, C.H.; Leng, Y.X. Hydrogen adsorption on Au (111), U (110), and nAu/U (110) alloy surfaces: A first-principles study. J. Nucl. Mater. 2023, 577, 154331. [Google Scholar] [CrossRef]
- Fan, Y.M.; Zhuo, Y.Q.; Lou, Y.; Zhu, Z.W.; Li, L.L. SeO2 adsorption on cao surface: Dft study on the adsorption of a single SeO2 molecule. Appl. Surf. Sci. 2017, 413, 366–371. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Baerends, E.J. Perspective on “self-consistent equations including exchange and correlation effects”. Theor. Chem. Acc. 2000, 103, 265–269. [Google Scholar] [CrossRef]
- Fischer, T.H.; Almlof, J. General-methods for geometry and wave-function optimization. J. Phys. Chem. 1992, 96, 9768–9774. [Google Scholar] [CrossRef]
- Neugebauer, J.; Scheffler, M. Adsorbate-substrate and adsorbate-adsorbate interactions of Na and K adlayers on al(111). Phys. Rev. B 1992, 46, 16067–16080. [Google Scholar] [CrossRef]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An Lsda + U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Adak, S.; Nakotte, H.; de Châtel, P.F.; Kiefer, B. Uranium at high pressure from first principles. Phys. B Condens. Matter 2011, 406, 3342–3347. [Google Scholar] [CrossRef]
- Huda, M.N.; Ray, A.K. Density functional study of o adsorption on (100) surface of γ-uranium. Int. J. Quantum. Chem. 2005, 102, 98–105. [Google Scholar] [CrossRef]
- Shiroka, T. Introduction to solid state physics. Contemp. Phys. 2020, 61, 221–222. [Google Scholar] [CrossRef]
- Dholabhai, P.P.; Ray, A.K. A density functional study of carbon monoxide adsorption on (100) surface of γ-uranium. J. Alloys Compd. 2007, 444, 356–362. [Google Scholar] [CrossRef]
- Beeler, B.; Good, B.; Rashkeev, S.; Deo, C.; Baskes, M.; Okuniewski, M. First principles calculations for defects in U. J. Phys.-Condens Mat. 2010, 22, 505703. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, C.H.; Jin, Y. Tensile strain induced surface reactions for co-adsorption of ho and oh-on vacancy Al (111) surface. Vacuum 2021, 192, 110459. [Google Scholar] [CrossRef]
- Guo, J.X.; Guan, L.; Bian, F.; Li, Q.; Geng, B.; Wang, Y.L.; Zhao, Q.X.; Liu, B.T. First-principles calculations of hydrogen molecule adsorption on Ti (0001)-(2 × 1) surface. Appl. Surf. Sci. 2009, 255, 7512–7516. [Google Scholar] [CrossRef]
- Zhu, S.L.; Yang, Y.X.; Zhang, Z.F.; Liu, X.H.; Tian, X.F.; Yu, Y.; Li, D. Density functional theory study of adsorption of H2O on γ-U(110) surface. Indian J. Phys. 2023, 97, 2297–2306. [Google Scholar] [CrossRef]
- Mei, Z.G.; Liang, L.Y.; Yacout, A.M. First-principles study of the surface properties of U-Mo system. Comp. Mater. Sci. 2018, 142, 355–360. [Google Scholar] [CrossRef]
- Stumpf, R. H-induced reconstruction and faceting of al surfaces. Phys. Rev. Lett. 1997, 78, 4454–4457. [Google Scholar] [CrossRef]
- Ducéré, J.M.; Rouhani, M.D.; Rossi, C.; Estève, A. Role of impurities, defects and their complexes on the trapping of hydrogen in bulk aluminum and on the Al (111) surface. Comp. Mater. Sci. 2017, 126, 272–279. [Google Scholar] [CrossRef]
- Saitoh, H.; Machida, A.; Katayama, Y.; Aoki, K. Formation and decomposition of alh in the aluminum-hydrogen system. Appl. Phys. Lett. 2008, 93, 151918. [Google Scholar] [CrossRef]
- Levason, B. Recent developments in the chemistry of the actinide (5f) elements preface. Coordin. Chem. Rev. 2014, 266, 1. [Google Scholar]
- Norskov, J.K.; Bligaard, T.; Logadottir, A.; Bahn, S.; Hansen, L.B.; Bollinger, M.; Bengaard, H.; Hammer, B.; Sljivancanin, Z.; Mavrikakis, M.; et al. Universality in heterogeneous catalysis. J. Catal. 2002, 209, 275–278. [Google Scholar] [CrossRef]
- Hammer, B.; Norskov, J.K. Theoretical surface science and catalysis—Calculations and concepts. Adv. Catal. 2000, 45, 71–129. [Google Scholar]
- Chen, M.S.; Kumar, D.; Yi, C.W.; Goodman, D.W. The promotional effect of gold in catalysis by palladium-gold. Science 2005, 310, 291–293. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).