Reactions of CH2OO, CH3CHOO, and (CH3)2COO with Methane through the Formation of Intermediate Complex
Abstract
:1. Introduction
2. Calculation Method
3. Results and Discussion
3.1. CH2OO + CH4 Reaction
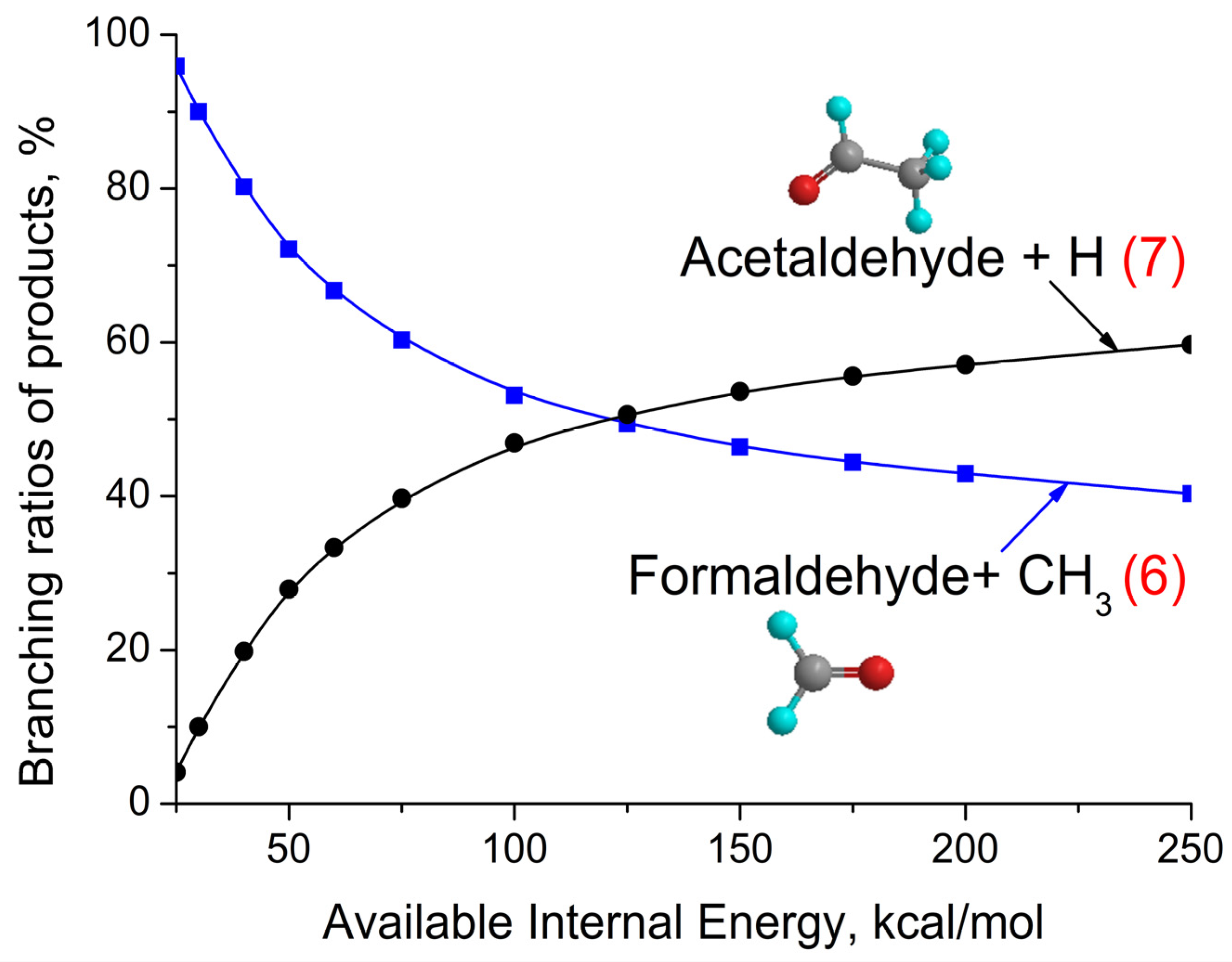
3.2. CH3CHOO + CH4 Reaction
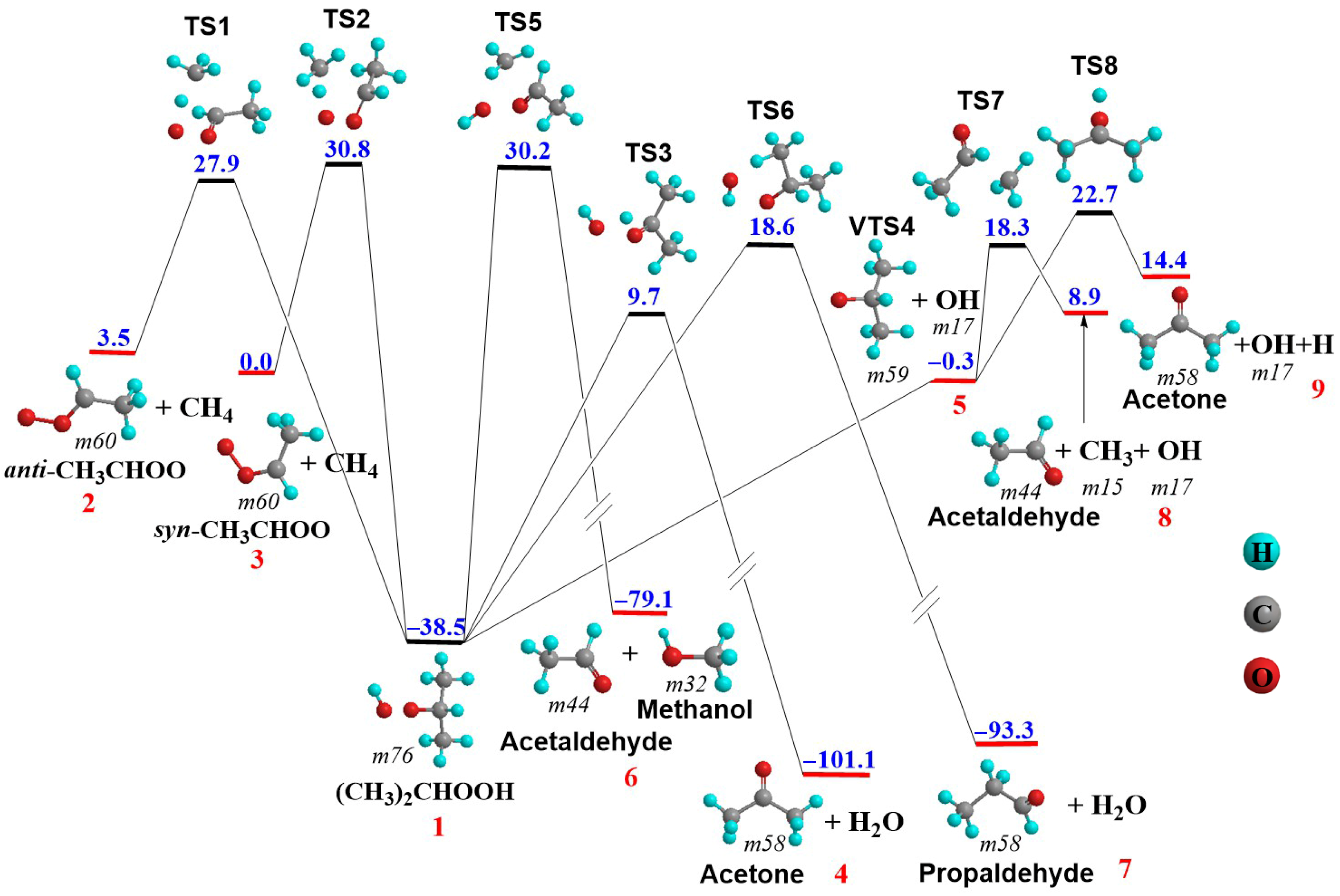
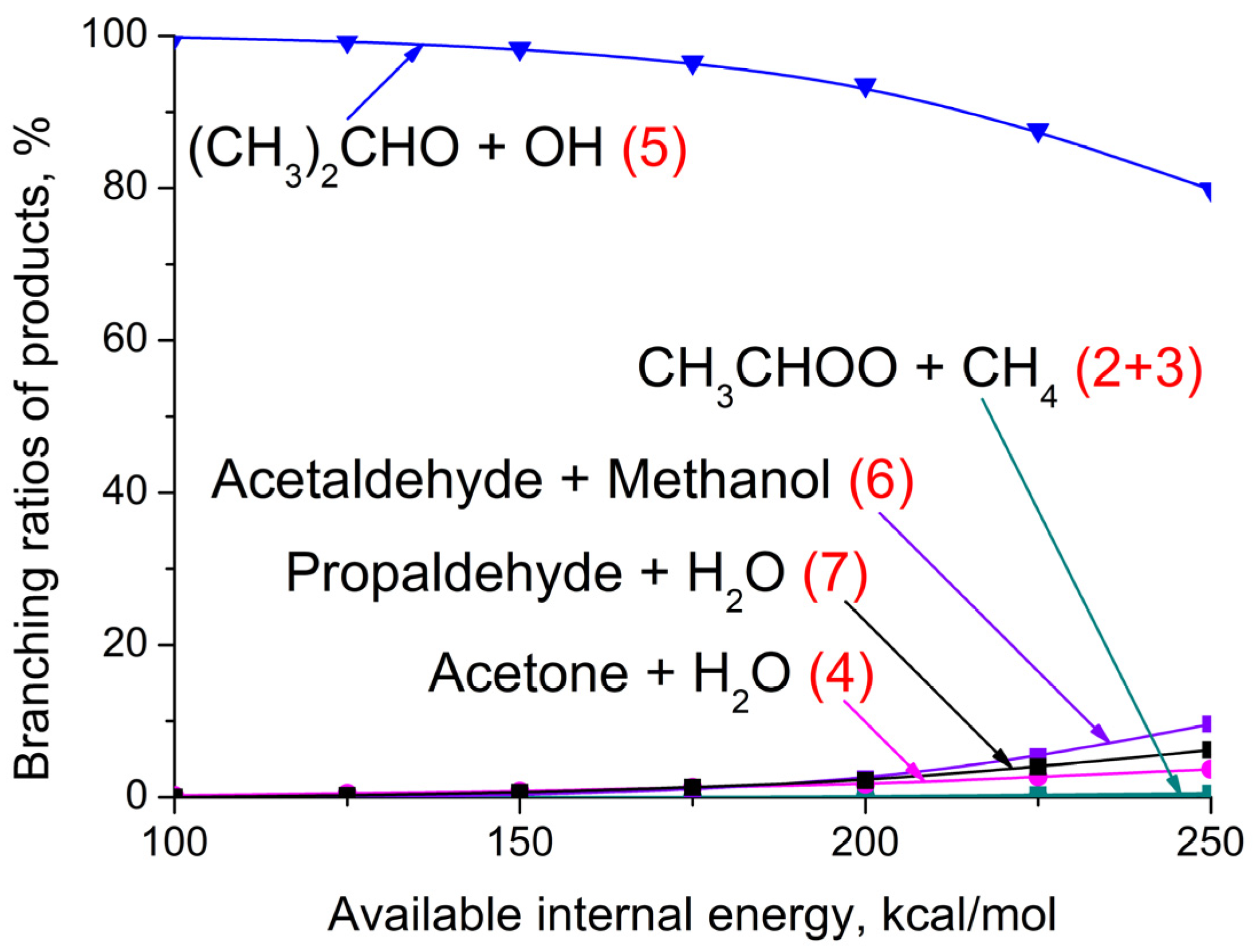
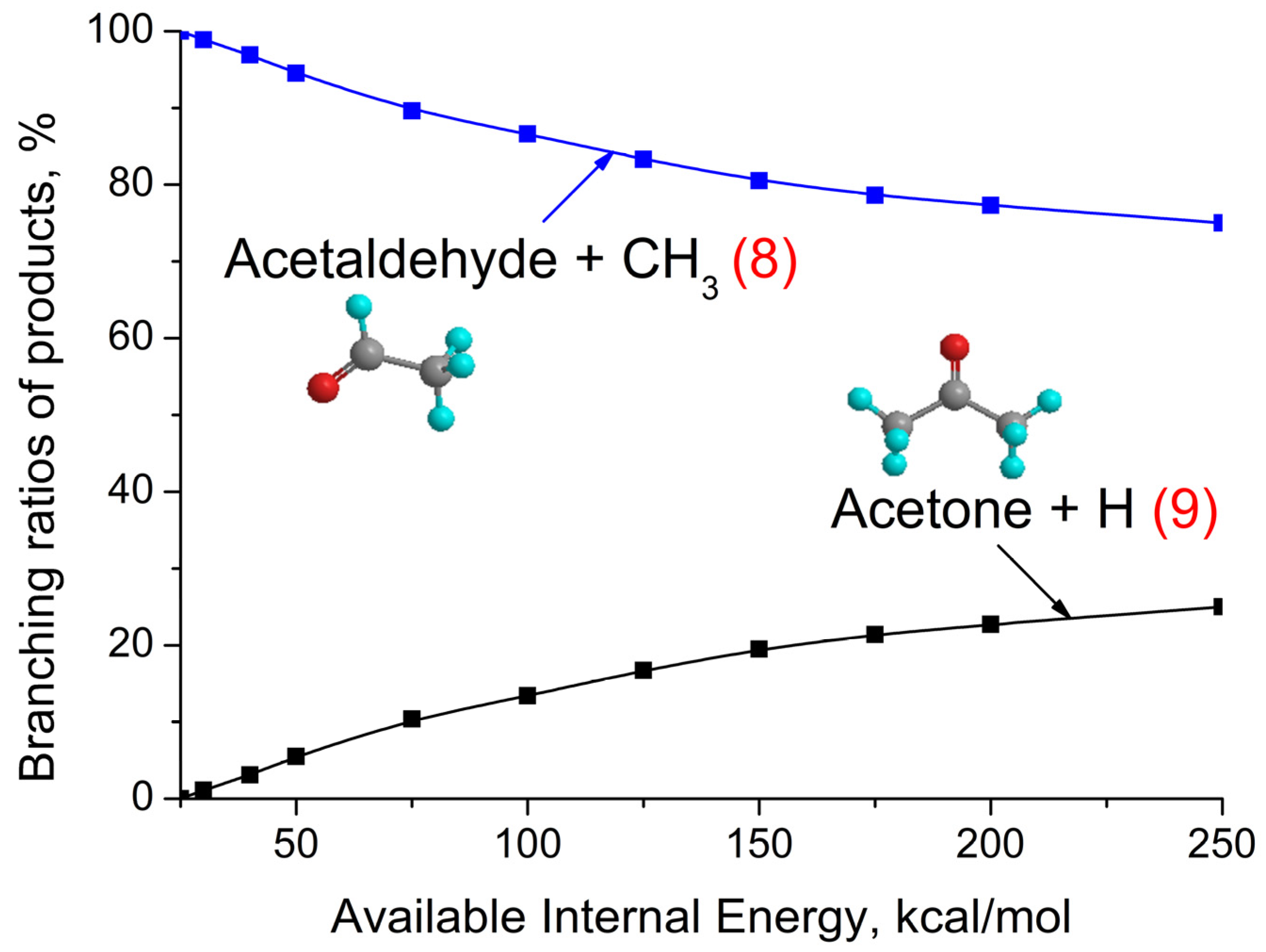
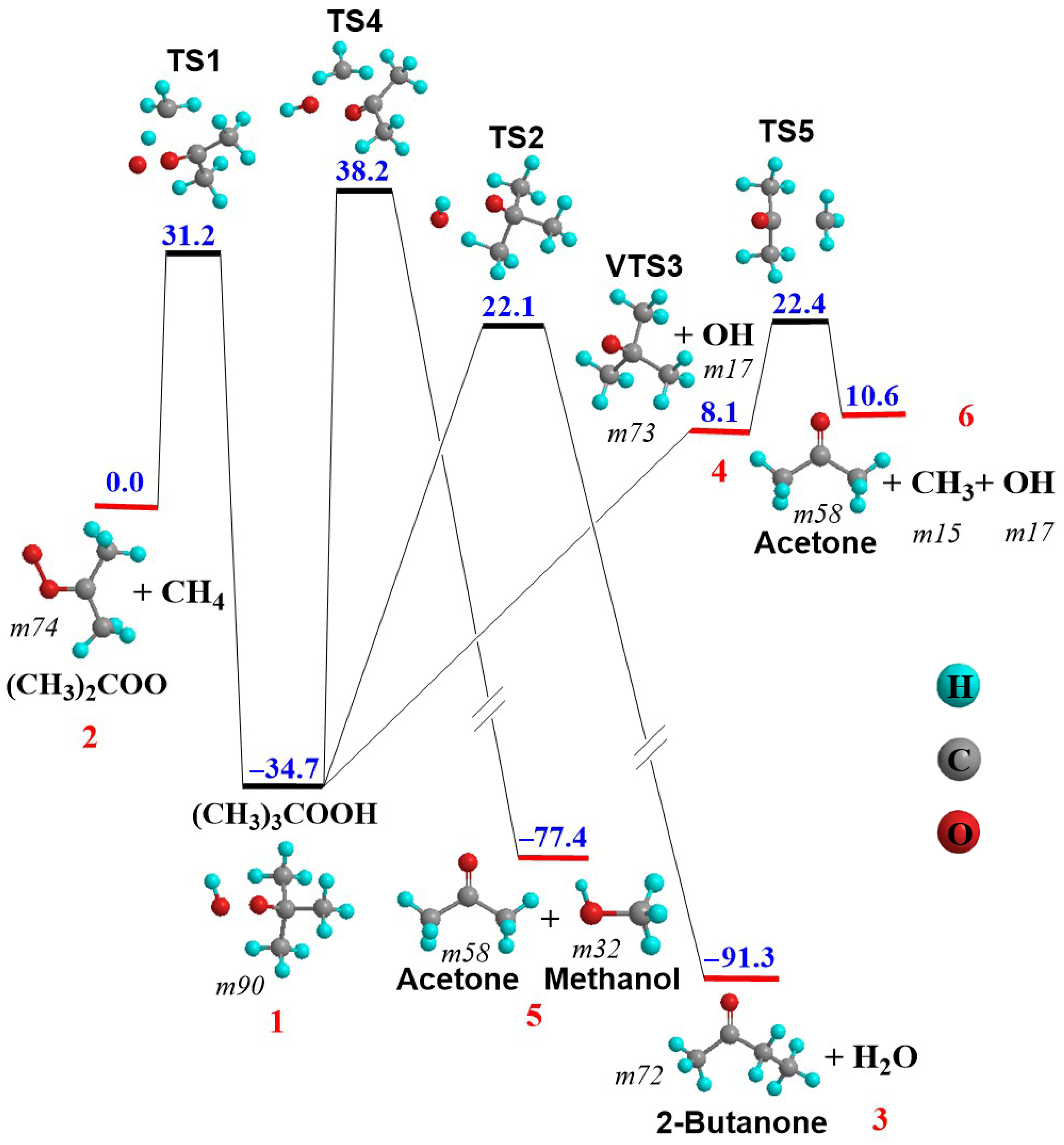
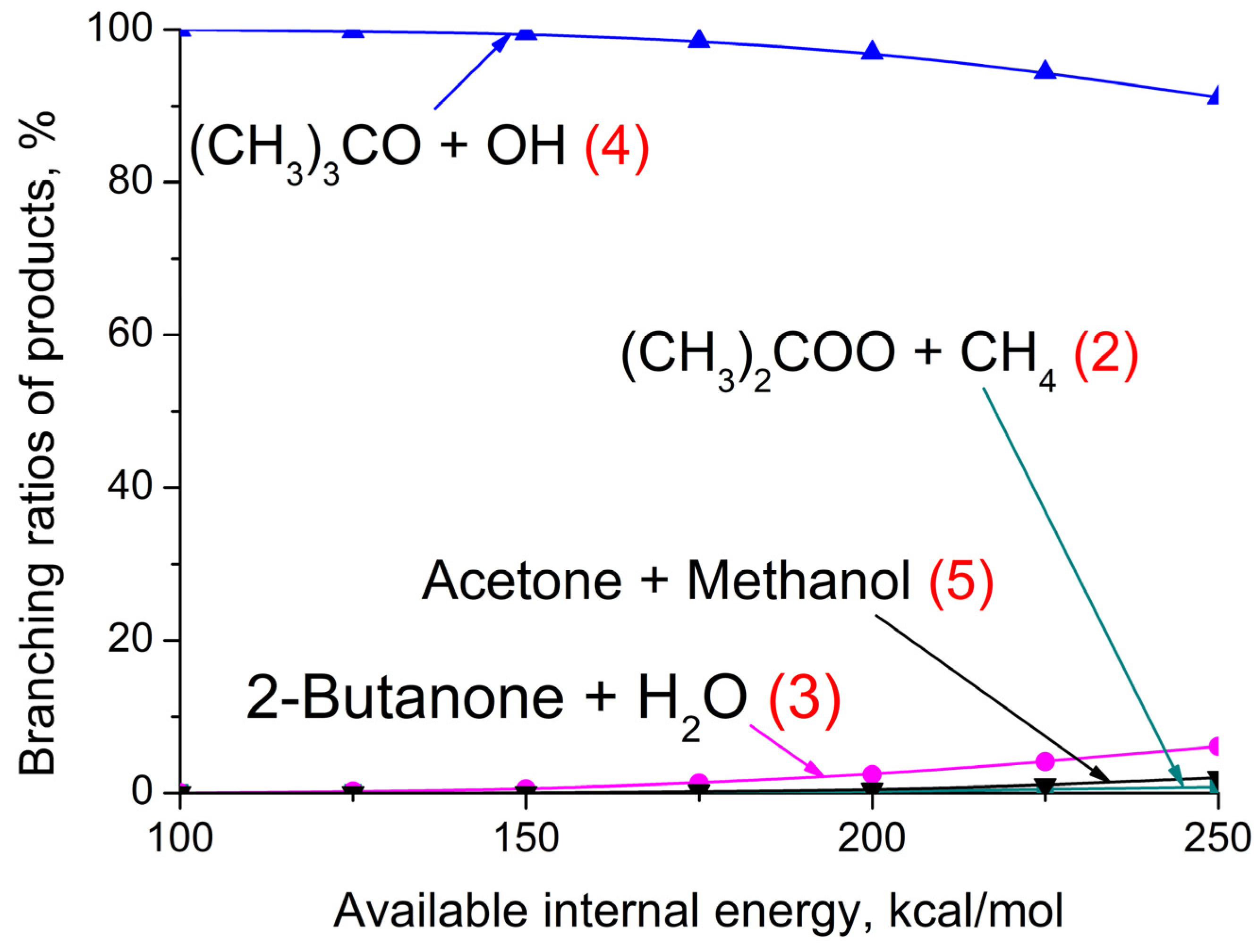
3.3. (CH3)2COO + CH4 Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pachauri, R.K.; Meyer, L.A. (Eds.) Climate Change 2014: Synthesis Report; IPCC: Geneva, Switzerland, 2014; 151p.
- Choi, W.; Kim, S.; Grant, W.B.; Shiotani, M.; Sasano, Y.; Schoeberl, M.R. Transport of methane in the stratosphere associated with the breakdown of the Antarctic polar vortex. J. Geophys. Res. Atmos. 2002, 107, D24. [Google Scholar] [CrossRef]
- Nair, P.R.; Kavitha, M. Stratospheric distribution of methane over a tropical region as observed by MIPAS on board ENVISAT. Int. J. Remote Sens. 2020, 41, 8380–8405. [Google Scholar] [CrossRef]
- Borchevkina, O.P.; Kurdyaeva, Y.A.; Dyakov, Y.A.; Karpov, I.V.; Golubkov, G.V.; Wang, P.K.; Golubkov, M.G. Disturbances of the thermosphere and the ionosphere during a meteorological storm. Atmosphere 2021, 12, 1384. [Google Scholar] [CrossRef]
- Golubkov, G.V.; Adamson, S.O.; Borchevkina, O.P.; Wang, P.K.; Dyakov, Y.A.; Efishov, I.I.; Karpov, I.V.; Kurdyaeva, Y.A.; Lukhovitskaya, E.E.; Olkhov, O.A.; et al. Coupling of ionospheric disturbances with dynamic processes in the troposphere. Russ. J. Phys. Chem. B 2022, 16, 508–530. [Google Scholar] [CrossRef]
- Mohammad, S.; Wang, P.K.; Chou, Y.L. A cloud model study of internal gravity wave breaking atop a high shear supercell in US high plains. Russ. J. Phys. Chem. B 2022, 16, 549–563. [Google Scholar] [CrossRef]
- Shinbori, A.; Otsuka, Y.; Sori, T.; Nishioka, M.; Perwitasari, S.; Tsuda, T.; Nishitani, N. Electromagnetic conjugacy of ionospheric disturbances after the 2022 Hunga Tonga-Hunga Ha’apai volcanic eruption as seen in GNSS-TEC and SuperDARN Hokkaido pair of radars observations. Earth Planets Sp. 2022, 74, 106:1–106:17. [Google Scholar] [CrossRef]
- Kesselmeier, J.; Staudt, M. Biogenic volatile organic compounds (VOC): An overview on emission, physiology and ecology. J. Atmos. Chem. 1999, 33, 23–88. [Google Scholar] [CrossRef]
- Sindelarova, K.; Granier, C.; Bouarar, I.; Guenther, A.; Tilmes, S.; Stavrakou, T.; Müller, J.F.; Kuhn, U.; Stefani, P.; Knorr, W. Global data set of biogenic VOC emissions calculated by the MEGAN model over the last 30 years. Atmos. Chem. Phys. 2014, 14, 9317–9341. [Google Scholar] [CrossRef]
- Levy, H. Normal atmosphere: Large radical and formaldehyde concentrations predicted. Science 1971, 173, 141–143. [Google Scholar] [CrossRef]
- Lelieveld, J.; Dentener, F.J.; Peters, W.; Krol, M.C. On the role of hydroxyl radicals in the self-cleansing capacity of the troposphere. Atmos. Chem. Phys. 2004, 4, 2337–2344. [Google Scholar] [CrossRef]
- Criegee, R.; Wenner, G. Die ozonisierung des 9,10-oktalins. Justus Liebigs Ann. Chem. 1949, 564, 9–15. [Google Scholar] [CrossRef]
- Taatjes, C.A.; Welz, O.; Eskola, A.J.; Savee, J.D.; Scheer, A.M.; Shallcross, D.E.; Rotavera, B.; Lee, E.P.F.; Dyke, J.M.; Mok, D.K.W.; et al. Direct measurements of conformer-dependent reactivity of the Criegee intermediate CH3CHOO. Science 2013, 340, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Chung, C.Y.; Lee, Y.P. Infrared spectral identification of the Criegee intermediate (CH3)2COO. J. Chem. Phys. 2016, 145, 154303:1–154303:6. [Google Scholar] [CrossRef]
- Chao, W.; Hsieh, J.T.; Chang, C.H.; Lin, J.J.M. Direct kinetic measurement of the reaction of the simplest Criegee intermediate with water vapor. Science 2015, 347, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Bao, J.L.; Truhlar, D.G. Atmospheric chemistry of Criegee intermediates: Unimolecular reactions and reactions with water. J. Am. Chem. Soc. 2016, 138, 14409–14422. [Google Scholar] [CrossRef]
- Smith, M.C.; Chang, C.H.; Chao, W.; Lin, L.C.; Takahashi, K.; Boering, K.A.; Lin, J.J.M. Strong negative temperature dependence of the simplest Criegee intermediate CH2OO reaction with water dimer. J. Phys. Chem. Lett. 2015, 6, 2708–2713. [Google Scholar] [CrossRef]
- Lin, L.C.; Chang, H.T.; Chang, C.H.; Chao, W.; Smith, M.C.; Chang, C.H.; Lin, J.J.M.; Takahashi, K. Competition between H2O and (H2O)2 reactions with CH2OO/CH3CHOO. Phys. Chem. Chem. Phys. 2016, 18, 4557–4568. [Google Scholar] [CrossRef]
- Sheps, L.; Scully, A.M.; Au, K. UV absorption probing of the conformer-dependent reactivity of a Criegee intermediate CH3CHOO. Phys. Chem. Chem. Phys. 2014, 16, 26701–26706. [Google Scholar] [CrossRef]
- Welz, O.; Eskola, A.J.; Sheps, L.; Rotavera, B.; Savee, J.D.; Scheer, A.M.; Osborn, D.L.; Lowe, D.; Murray Booth, A.; Xiao, P.; et al. Rate coefficients of C1 and C2 Criegee intermediate reactions with formic and acetic acid near the collision limit: Direct kinetics measurements and atmospheric implications. Angew. Chemie Int. Ed. 2014, 53, 4547–4550. [Google Scholar] [CrossRef]
- Taatjes, C.A.; Shallcross, D.E.; Percival, C.J. Research frontiers in the chemistry of Criegee intermediates and tropospheric ozonolysis. Phys. Chem. Chem. Phys. 2014, 16, 1704–1718. [Google Scholar] [CrossRef]
- Foreman, E.S.; Kapnas, K.M.; Murray, C. Reactions between Criegee intermediates and the inorganic acids HCl and HNO3: Kinetics and atmospheric implications. Angew. Chemie Int. Ed. 2016, 55, 10419–10422. [Google Scholar] [CrossRef]
- Chhantyal-Pun, R.; McGillen, M.R.; Beames, J.M.; Khan, M.A.H.; Percival, C.J.; Shallcross, D.E.; Orr-Ewing, A.J. Temperature-dependence of the rates of reaction of trifluoroacetic acid with Criegee intermediates. Angew. Chemie Int. Ed. 2017, 56, 9044–9047. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.H.; Percival, C.J.; Caravan, R.L.; Taatjes, C.A.; Shallcross, D.E. Criegee intermediates and their impacts on the troposphere. Environ. Sci. Process. Impacts 2018, 20, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.; Takahashi, K.; Lee, Y.P. Mechanism and kinetics of the reaction of the Criegee intermediate CH2OO with acetic acid studied using a step-scan Fourier-transform IR spectrometer. Phys. Chem. Chem. Phys. 2022, 24, 18568–18581. [Google Scholar] [CrossRef] [PubMed]
- Kanakidou, M.; Seinfeld, J.H.; Pandis, S.N.; Barnes, I.; Dentener, F.J.; Facchini, M.C.; Van Dingenen, R.; Ervens, B.; Nenes, A.; Nielsen, C.J.; et al. Organic aerosol and global climate modelling: A review. Atmos. Chem. Phys. 2005, 5, 1053–1123. [Google Scholar] [CrossRef]
- Hallquist, M.; Wenger, J.C.; Baltensperger, U.; Rudich, Y.; Simpson, D.; Claeys, M.; Dommen, J.; Donahue, N.M.; George, C.; Goldstein, A.H.; et al. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmos. Chem. Phys. 2009, 9, 5155–5236. [Google Scholar] [CrossRef]
- Taatjes, C.A.; Khan, M.A.H.; Eskola, A.J.; Percival, C.J.; Osborn, D.L.; Wallington, T.J.; Shallcross, D.E. Reaction of perfluorooctanoic acid with Criegee intermediates and implications for the atmospheric fate of perfluorocarboxylic acids. Environ. Sci. Technol. 2019, 53, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Herron, J.T.; Martinez, R.I.; Huie, R.E. Kinetics and energetics of the Criegee intermediate in the gas phase. II. The Criegee intermediate in the photooxidation of formaldehyde, in alkyldioxy disproportionation and O + oxoalkane addition reactions. Int. J. Chem. Kinet. 1982, 14, 225–236. [Google Scholar] [CrossRef]
- Wang, Z.; Dyakov, Y.A.; Bu, Y. Dynamics insight into isomerization and dissociation of hot Criegee intermediate CH3CHOO. J. Phys. Chem. A 2019, 123, 1085–1090. [Google Scholar] [CrossRef]
- Dyakov, Y.A.; Adamson, S.O.; Wang, P.K.; Golubkov, G.V.; Olkhov, O.A.; Peskov, V.D.; Rodionov, I.D.; Rodionova, I.P.; Rodionov, A.I.; Shapovalov, V.L.; et al. Isomerization and decay of a Criegee intermediate CH3CHOO in the Earth’s upper atmosphere. Russ. J. Phys. Chem. B 2021, 15, 559–565. [Google Scholar] [CrossRef]
- Stone, D.; Au, K.; Sime, S.; Medeiros, D.J.; Blitz, M.; Seakins, P.W.; Decker, Z.; Sheps, L. Unimolecular decomposition kinetics of the stabilised Criegee intermediates CH2OO and CD2OO. Phys. Chem. Chem. Phys. 2018, 20, 24940–24954. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, F.; Barber, V.P.; Klippenstein, S.J.; McCoy, A.B.; Lester, M.I. Communication: Real time observation of unimolecular decay of Criegee intermediates to OH radical products. J. Chem. Phys. 2016, 144, 061102:1–061102:4. [Google Scholar] [CrossRef]
- Kidwell, N.M.; Li, H.; Wang, X.; Bowman, J.M.; Lester, M.I. Unimolecular dissociation dynamics of vibrationally activated CH3CHOO Criegee intermediates to OH radical products. Nat. Chem. 2016, 8, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bowman, J.M. Two pathways for dissociation of highly energized syn-CH3CHOO to OH plus vinoxy. J. Phys. Chem. Lett. 2016, 7, 3359–3364. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; McCaslin, L.; McCarthy, M.C.; Stanton, J.F. Communication: Thermal unimolecular decomposition of syn-CH3CHOO: A kinetic study. J. Chem. Phys. 2016, 145, 131102:1–131102:5. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, Y.; Dong, W.; Yang, X. Unimolecular reaction rate measurement of syn-CH3CHOO. J. Phys. Chem. Lett. 2019, 10, 4817–4821. [Google Scholar] [CrossRef]
- Beames, J.M.; Liu, F.; Lu, L.; Lester, M.I. UV spectroscopic characterization of an alkyl substituted Criegee intermediate CH3CHOO. J. Chem. Phys. 2013, 138, 244307:1–244307:9. [Google Scholar] [CrossRef]
- Sheps, L. Absolute ultraviolet absorption spectrum of a Criegee intermediate CH2OO. J. Phys. Chem. Lett. 2013, 4, 4201–4205. [Google Scholar] [CrossRef]
- Liu, F.; Beames, J.M.; Green, A.M.; Lester, M.I. UV spectroscopic characterization of dimethyl- and ethyl-substituted carbonyl oxides. J. Phys. Chem. A 2014, 118, 2298–2306. [Google Scholar] [CrossRef]
- Ting, W.L.; Chen, Y.H.; Chao, W.; Smith, M.C.; Lin, J.J.M. The UV absorption spectrum of the simplest Criegee intermediate CH2OO. Phys. Chem. Chem. Phys. 2014, 16, 10438–10443. [Google Scholar] [CrossRef]
- Samanta, K.; Beames, J.M.; Lester, M.I.; Subotnik, J.E. Quantum dynamical investigation of the simplest Criegee intermediate CH2OO and its O–O photodissociation channels. J. Chem. Phys. 2014, 141, 134303:1–134303:12. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.P. Perspective: Spectroscopy and kinetics of small gaseous Criegee intermediates. J. Chem. Phys. 2015, 143, 020901:1–020901:16. [Google Scholar] [CrossRef] [PubMed]
- Ting, A.W.L.; Lin, J.J.M. UV Spectrum of the simplest deuterated Criegee intermediate CD2OO. J. Chinese Chem. Soc. 2017, 64, 360–368. [Google Scholar] [CrossRef]
- Esposito, V.J.; Werba, O.; Bush, S.A.; Marchetti, B.; Karsili, T.N.V. Insights into the ultrafast dynamics of CH2OO and CH3CHOO following excitation to the bright 1 ππ* state: The role of singlet and triplet states. Photochem. Photobiol. 2022, 98, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.F.; Tzeng, C.M.; Dyakov, Y.A.; Ni, C.K. Photostability of amino acids: Internal conversion versus dissociation. J. Chem. Phys. 2007, 126, 241104:1–241104:5. [Google Scholar] [CrossRef] [PubMed]
- Dyakov, Y.A.; Toliautas, S.; Trakhtenberg, L.I.; Valkunas, L. Excited state photodissociation dynamics of 2-, 3-, 4-hydroxyacetophenone: Theoretical study. Chem. Phys. 2018, 515, 672–678. [Google Scholar] [CrossRef]
- Dyakov, Y.A.; Adamson, S.O.; Wang, P.K.; Vetchinkin, A.S.; Golubkov, G.V.; Peskov, V.D.; Rodionov, I.D.; Syromyatnikov, A.G.; Umanskii, S.Y.; Shestakov, D.V.; et al. Excited state dynamics of CH3CHOO Criegee intermediates in the upper atmosphere of the Earth. Russ. J. Phys. Chem. B 2022, 16, 543–548. [Google Scholar] [CrossRef]
- Larsson, M.; Orel, A.E. Dissociative Recombination of Molecular Ions; Cambridge University Press: Cambridge, UK, 2008; 392p. [Google Scholar]
- Hsu, C.H.; Tsai, M.T.; Dyakov, Y.A.; Ni, C.K. Energy transfer of highly vibrationally excited molecules studied by crossed molecular beam/time-sliced velocity map ion imaging. Int. Rev. Phys. Chem. 2012, 31, 201–233. [Google Scholar] [CrossRef]
- Huang, H.L.; Chao, W.; Lin, J.J.M. Kinetics of a Criegee intermediate that would survive high humidity and may oxidize atmospheric SO2. Proc. Natl. Acad. Sci. USA 2015, 112, 10857–10862. [Google Scholar] [CrossRef]
- Mauldin III, R.L.; Berndt, T.; Sipilä, M.; Paasonen, P.; Petäjä, T.; Kim, S.; Kurtén, T.; Stratmann, F.; Kerminen, V.M.; Kulmala, M. A new atmospherically relevant oxidant of sulphur dioxide. Nature 2012, 488, 193–196. [Google Scholar] [CrossRef]
- Kumar, M.; Francisco, J.S. Reactions of Criegee intermediates with non-water greenhouse gases: Implications for metal free chemical fixation of carbon dioxide. J. Phys. Chem. Lett. 2017, 8, 4206–4213. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, W.; Wei, W.; Feng, W.; Sun, Q.; Li, P. Insights into the reaction mechanism of Criegee intermediate CH2OO with methane and implications for the formation of methanol. J. Phys. Chem. A 2017, 121, 7236–7245. [Google Scholar] [CrossRef] [PubMed]
- Dyakov, Y.A.; Adamson, S.O.; Wang, P.K.; Vetchinkin, A.S.; Golubkov, G.V.; Morozov, I.I.; Umanskii, S.Y.; Chaikina, Y.A.; Golubkov, M.G. Collisional dissociation of Crieege CH3CHOO and methane intermediates in the Earth’s upper atmosphere. Russ. J. Phys. Chem. B 2021, 15, 782–788. [Google Scholar] [CrossRef]
- Kalinowski, J.; Foreman, E.S.; Kapnas, K.M.; Murray, C.; Räsänen, M.; Benny Gerber, R. Dynamics and spectroscopy of CH2OO excited electronic states. Phys. Chem. Chem. Phys. 2016, 18, 10941–10946. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gong, Q.; Yue, L.; Wang, W.; Liu, F. Photochemistry of the simplest Criegee intermediate, CH2OO: Photoisomerization channel toward dioxirane revealed by CASPT2 calculations and trajectory surface-hopping dynamics. J. Phys. Chem. Lett. 2018, 9, 978–981. [Google Scholar] [CrossRef]
- Marchetti, B.; Esposito, V.J.; Bush, R.E.; Karsili, T.N.V. The states that hide in the shadows: The potential role of conical intersections in the ground state unimolecular decay of a Criegee intermediate. Phys. Chem. Chem. Phys. 2022, 24, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. I. The effect of the exchange-only gradient correction. J. Chem. Phys. 1992, 96, 2155–2160. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. II. The effect of the Perdew–Wang generalized-gradient correlation correction. J. Chem. Phys. 1992, 97, 9173–9177. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Eyring, H.; Lin, S.H.; Lin, S.M. Basic Chemical Kinetics; JohnWiley & Sons: New York, NY, USA, 1980; 493p. [Google Scholar]
- Robinson, P.J.; Holbrook, K.A. Unimolecular Reactions; JohnWiley & Sons: New York, NY, USA, 1972; 372p. [Google Scholar]
- Steinfeld, J.I.; Francisco, J.S.; Hase, W.L. Chemical Kinetics and Dynamics; Prentice Hall: Upper Saddle River, NJ, USA, 1999; 518p. [Google Scholar]
- Kislov, V.V.; Nguyen, T.L.; Mebel, A.M.; Lin, S.H.; Smith, S.C. Photodissociation of benzene under collision-free conditions: An ab initio/Rice–Ramsperger–Kassel–Marcus study. J. Chem. Phys. 2004, 120, 7008–7017. [Google Scholar] [CrossRef] [PubMed]


| Transition State | Reaction | Reaction Path Degeneracy | Internal Energies, kcal/mol | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 100 | 125 | 150 | 175 | 200 | 225 | 250 | |||
| TS1 | 1 → 2 | 1 | 1.1·106 | 6.0·107 | 6.6·108 | 3.4·109 | 1.1·1010 | 2.9·1010 | 6.1·1010 |
| TS2 | 1 → 3 | 2 | 7.6·109 | 5.2·1010 | 2.0·1011 | 4.7·1011 | 9.7·1011 | 1.6·1012 | 2.6·1012 |
| TS3 | 1 → 3 | 3 | 9.9·108 | 1.3·1010 | 7.1·1010 | 2.3·1011 | 5.6·1011 | 1.1·1012 | 2.0·1012 |
| VTS4 | 1 → 4 | 1 | 3.9·1011 | 1.2·1012 | 2.5·1012 | 4.3·1012 | 6.6·1012 | 8.8·1012 | 1.0·1013 |
| TS5 | 1 → 5 | 1 | 8.1·106 | 6.8·108 | 9.0·109 | 5.1·1010 | 1.8·1011 | 4.9·1011 | 1.1·1012 |
| Transition State | Reaction | Reaction Path Degeneracy | Internal Energies, kcal/mol | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 100 | 125 | 150 | 175 | 200 | 225 | 250 | |||
| TS1 | 1 → 2 | 2 | 3.6·105 | 2.3·107 | 3.2·108 | 2.0·109 | 7.6·109 | 2.2·1010 | 5.6·1010 |
| TS2 | 1 → 3 | 2 | 7.0·104 | 6.5·106 | 1.1·108 | 7.3·108 | 3.0·109 | 9.4·109 | 2.3·1010 |
| TS3 | 1 → 4 | 1 | 2.1·108 | 2.5·109 | 1.3·1010 | 4.2·1010 | 1.0·1011 | 2.1·1011 | 3.8·1011 |
| VTS4 | 1 → 5 | 1 | 1.0·1011 | 5.2·1011 | 1.6·1012 | 3.3·1012 | 5.5·1012 | 6.9·1012 | 8.3·1012 |
| TS5 | 1 → 6 | 2 | 2.3·106 | 2.6·108 | 4.4·109 | 3.2·1010 | 1.4·1011 | 4.2·1011 | 1.0·1012 |
| TS6 | 1 → 7 | 6 | 4.4·107 | 1.1·109 | 9.3·109 | 4.2·1010 | 1.3·1011 | 3.2·1011 | 6.5·1011 |
| Transition State | Reaction | Reaction Path Degeneracy | Internal Energies, kcal/mol | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 100 | 125 | 150 | 175 | 200 | 225 | 250 | |||
| TS1 | 1 → 2 | 3 | 3.0·104 | 3.3·106 | 6.2·107 | 5.1·108 | 2.4·109 | 8.3·109 | 2.2·1010 |
| TS2 | 1 → 3 | 6 | 2.7·106 | 1.1·108 | 1.2·109 | 6.7·109 | 2.4·1010 | 6.9·1010 | 2.6·1011 |
| VTS3 | 1 → 4 | 1 | 9.2·109 | 6.1·1010 | 2.3·1011 | 5.3·1011 | 9.7·1011 | 1.6·1012 | 2.4·1012 |
| TS4 | 1 → 5 | 3 | 3.5·103 | 1.5·106 | 5.5·107 | 6.5·108 | 4.0·109 | 1.7·1010 | 5.2·1012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyakov, Y.A.; Adamson, S.O.; Golubkov, G.V.; Morozov, I.I.; Nigmatullin, D.R.; Olkhov, O.A.; Wang, P.K.; Golubkov, M.G. Reactions of CH2OO, CH3CHOO, and (CH3)2COO with Methane through the Formation of Intermediate Complex. Atoms 2023, 11, 157. https://doi.org/10.3390/atoms11120157
Dyakov YA, Adamson SO, Golubkov GV, Morozov II, Nigmatullin DR, Olkhov OA, Wang PK, Golubkov MG. Reactions of CH2OO, CH3CHOO, and (CH3)2COO with Methane through the Formation of Intermediate Complex. Atoms. 2023; 11(12):157. https://doi.org/10.3390/atoms11120157
Chicago/Turabian StyleDyakov, Yuri A., Sergey O. Adamson, Gennady V. Golubkov, Igor I. Morozov, Danil R. Nigmatullin, Oleg A. Olkhov, Pao K. Wang, and Maxim G. Golubkov. 2023. "Reactions of CH2OO, CH3CHOO, and (CH3)2COO with Methane through the Formation of Intermediate Complex" Atoms 11, no. 12: 157. https://doi.org/10.3390/atoms11120157
APA StyleDyakov, Y. A., Adamson, S. O., Golubkov, G. V., Morozov, I. I., Nigmatullin, D. R., Olkhov, O. A., Wang, P. K., & Golubkov, M. G. (2023). Reactions of CH2OO, CH3CHOO, and (CH3)2COO with Methane through the Formation of Intermediate Complex. Atoms, 11(12), 157. https://doi.org/10.3390/atoms11120157








