Abstract
Auroral events are the prominent manifestation of solar/stellar forcing on planetary atmospheres. They are closely related to the energy deposition by and evolution of planetary atmospheres, and their observations are widely used to analyze the composition, structure, and chemistry of the atmosphere under study, as well as energy fluxes of the precipitating particles that affect the atmosphere. A numerical kinetic Monte Carlo model had been developed, allowing us to study the processes of precipitation of high-energy auroral electrons into the N2-O2 atmospheres of the rocky planets in the Solar and exosolar planetary systems. This model describes on a molecular level the collisions of auroral electrons and atmospheric gas, taking into account the stochastic nature of collisional scattering at high kinetic energies. The current status of the kinetic model is illustrated in the applications to the auroral events on the Earth such as the production of suprathermal nitrogen atoms due to the electron impact dissociation of N2. It was found that electron impact dissociation of N2 can potentially be an important source of suprathermal N atoms in the auroral regions of the N2-O2 atmosphere of terrestrial-type planets. Such research will allow us to study the odd nitrogen chemistry as an atmospheric marker of the N2-O2 atmosphere of rocky exoplanets.
1. Introduction
The main component of the Earth’s atmosphere is N2, whose presence indicates the availability of tectonic activity and is a unique property of the terrestrial planets in the Solar System. The study of the origin of atmospheric nitrogen and its stability gives an idea of the uniqueness of the habitat on Earth. The simultaneous existence of N2 and O2 in the Earth’s atmosphere has no analogues in the entire Solar System. The various sources of atmospheric nitrogen, the stability of nitrogen-dominated atmospheres and the development of the early nitrogen atmosphere of the Earth were recently discussed in certain papers [1,2]. In particular, it has been shown that the presence of an N2-O2 atmosphere is a clear biomarker for aerobic life forms. The fact is that the primary atmospheres of such planets are easily destroyed and a chemically stable mixture of N2-O2 can only be formed by strong secondary sources of these molecules. The only currently known complex capable of providing such massive secondary flows are aerobic life forms. Thus, the detection of an N2-O2 atmosphere on an exoplanet orbiting in the habitable zone around a solar-type star is a strong sign of the presence of a developed aerobic extraterrestrial biosphere [1,2].
To increase the probability of finding a real habitat similar to Earth, it is necessary to find the rocky planets orbiting a solar-type star. Detecting N2 atmospheres remains a challenging task. Due to the absence of a transient dipole moment, the N2 molecule has no absorption features in the near-IR and optical spectra, and probably will not be remotely detected [3]. So far, only a few molecules consisting of N, namely N2O and NO, are considered as possible biomarkers. The frequently discussed detection of N2O is complicated by the fact that its infrared absorption lines are very weak (see, for example, [4,5]), and the transition lines of N2O and NO2 in the visible and near UV spectrum are overlapped by strong lines of O2 and O3 [6].
The NO radical is a direct indicator of the atmosphere dominated by N2 and O2 since its formation is a consequence of the presence of molecular nitrogen and oxygen as the main components in the atmosphere of the planet [2]. The idea of using NO as a potential biomarker was known earlier (see, for example, [7]), but the question of the possibility of observing nitric oxide on exoplanets remains open. The fact is that NO is easily destroyed by radiation, and in order to achieve a concentration sufficient for its identification in the upper atmosphere, it is necessary to analyze not only the processes of formation and loss, but also, as the example of the Earth shows, the dynamics of the upper atmosphere. To do this, it is necessary to conduct a complex study of the chemistry and dynamics of nitric oxide NO in the upper atmosphere using the modifications of the previously developed kinetic Monte Carlo and the multi-component MHD models [8] to describe the evolution of the N2-O2 upper atmospheres of the sub-neptunes and exo-earths under the influence of hard radiation and stellar winds from the parent star.
Two mechanisms have been identified from observations as sources of nitric oxide in the upper atmospheres of terrestrial-type planets. At high latitudes, the precipitation of auroral electrons (1–10 keV) produces ionization that leads to the production of nitric oxide [9]. At low latitudes, the predominant source is now thought to be solar soft X-rays [10]. Space observations [11,12] confirm the presence of these two major sources of nitric oxide in the upper atmosphere of Earth. The largest density of nitric oxide which occurs at 106–110 km is produced by 1–10 keV electrons precipitating into the auroral region (60°–70° geomagnetic latitude). This source dominates in the auroral region, but its influence extends to midlatitudes as well. During geomagnetic storms, nitric oxide is present at midlatitudes equatorward of the auroral region [10,11].
Aurora is a spectacular manifestation of the link between our magnetized planet and the plasma that constantly streams out from the Sun. Earth’s polar atmosphere is exactly the area where solar-terrestrial connections are most intensely manifested. One of the most important manifestations of this forcing is the auroral UV glow induced by the precipitation of the high-energy electrons, protons and hydrogen atoms into the atmosphere. Different approaches have been used to describe the transport and energy deposition of auroral electrons which are based on the analytical or numerical solutions of the Boltzmann equation. The first group of models is based on the two-stream approximation in which the upward and downward fluxes of electrons propagating along a magnetic field line are described [13,14]. Further, this group was extended by numerical models based on the multi-stream approach when the electron fluxes for a large number of pitch angles are considered (e.g., [15,16]). Whereas the two-stream models are suitable for estimating integrated quantities, such as excitation and heating rates, the multi-stream models are usually required for comparison with observed electron fluxes or for studying anisotropy. The second group of electron transport models involves the direct solution of the Boltzmann equation for suprathermal electrons (e.g., [17,18]). The third type of model is based on the Monte Carlo approach, which is a stochastic method based on the tracking of numerous model particles representing the auroral electrons with the high kinetic energies (e.g., [19,20]). Detailed analysis of the electron transport codes and their applications to auroral events could be found in the recent reviews (e.g., [21,22]).
The kinetic Monte Carlo model [20] of high-energy electron precipitation was developed with an aim to interpret the UV emissions observed in the upper atmospheres of terrestrial planets. Such a model provides a statistical solution of the Boltzmann integro-differential equation including sources and sinks of electrons. It has been validated in comparisons with other numerical models of auroral precipitation and used to interpret the airglow and auroral emissions in the upper atmospheres of terrestrial planets [23,24,25].
In the paper, we describe the modification of the kinetic Monte Carlo model [20], which resulted in the calculations of source functions of suprathermal nitrogen atoms due to the auroral electron precipitation. The detail of our kinetic Monte Carlo model and justifications of the model inputs are presented in Section 2. Section 3 shows the results of simulation for two considered test cases—monoenergetic electron flux penetrating into the upper atmosphere of the (exo)planet with monodirectional and isotropic pitch-angle distributions at upper boundary for the Earth’s polar atmosphere. The energy spectra of down- and upward fluxes of precipitating electrons, height profiles of the energy fluxes of auroral electrons and the energy spectra of source functions for suprathermal nitrogen atoms formed due to the electron impact N2 dissociation were presented and discussed. Finally, a summary of our work and possible extension of the current study are given in Section 4.
The proposed modification of the kinetic Monte Carlo model [20] of auroral electron precipitation will allow us as a first step to calculate the production rates and energy spectra of suprathermal nitrogen atoms formed in the N2 dissociation by precipitating high-energy electrons. It was found that electron impact dissociation of N2 potentially can be an important source of suprathermal N atoms in the auroral regions of the N2-O2 atmospheres of terrestrial-type planets. The calculated source functions will be used in our next studies as an input to the kinetic Monte Carlo model [8] to investigate the kinetics and transport of hot N atoms in the polar upper atmosphere with an aim to calculated the steady-state energy distributions of suprathermal nitrogen atoms in the polar regions of the planetary atmospheres under study. Finally, all these steps will allow us to estimate the input of hot fractions into the odd nitrogen chemistry in the N2-O2 atmospheres of terrestrial-type exoplanets with the final aim being the search for habitable worlds.
2. Materials and Methods
2.1. N2-O2 Atmosphere
The study of the atmospheres of exoplanets has recently achieved impressive success [26,27]. Using spectroscopy methods for exoplanet transits implemented on space and ground-based observation facilities, a large amount of information was obtained about the composition and structure of the atmospheres of exoplanets, mainly hot jupiters and neptunes (see, e.g., [8,26,27]). Even more success should be expected in the near future—most of the current and future space missions now place the study of exoplanets as one of the main mission goals.
There are only a few investigations that have thoroughly studied the reaction of the nitrogen-dominated atmosphere to exposure to XUV radiation for early Earth and Earth-type exoplanets [28,29]. From these studies, it follows that the modern terrestrial atmosphere of N2-O2 would not have been stable in the Hadean and early Archean eons and, therefore, would have had to grow to its current mass later. In these studies, it was found that such an atmosphere begins to expand adiabatically for a certain range of the flux of hard stellar XUV radiation; for the Earth, this is usually about 5–6 times higher than the modern solar flux in the XUV radiation range (XUVʘ). Such hydrodynamic expansion of the upper atmosphere is usually accompanied by a strong increase in atmosphere volume, whereas the rate of gas heating approaches approximately the values characteristic of a stellar flux of 10 XUVʘ [28], which leads to high thermal rates of atmospheric loss. M-stars remain very active, probably for a billion years or even longer [30,31]. Any nitrogen-dominated atmosphere on exoplanets orbiting such stars will not survive its high-activity phase of the parent star, and the planet will probably lose most of its volatiles before it can theoretically maintain an Earth-like atmosphere.
The radical NO is a direct indicator of the atmosphere dominated by N2-O2 since its formation is a consequence of the presence of molecular nitrogen and oxygen as the main components in the atmosphere of the planet. It is known from modern studies of the upper and middle atmosphere of the Earth that radical NO plays an important role in the structure and energy budget of the upper atmosphere of the Earth. NO is a polar molecule and therefore effectively emits in the infrared range and is considered as an important source of radiation cooling in the upper atmosphere. When transferred to lower altitudes, NO catalytically destroys ozone. Its atmospheric chemistry is characterized by a set of chemical reactions involving both N2 and O2. The so-called odd chemistry of nitrogen—N(4S), N(2D) и NO—is described by a simple set of chemical reactions in the upper layers of the Earth’s atmosphere (see, e.g., [32,33]). Nitric oxide is formed in chemical reactions with dissociation products of N2 with O2 and is lost in collisions with atomic nitrogen N(4S, 2D), reproducing N2 molecules. The production and loss of NO clearly depend on the relative amounts of nitrogen atoms in the excited and ground states. This simple chemical set is a rigid set of thermal chemical reactions and is usually expanded by additional reactions involving suprathermal nitrogen atoms [32] formed in the dissociation and dissociative ionization by solar soft X-rays in the Earth’s upper atmosphere [34].
Observations of NO luminescence in planetary atmospheres are usually made in the ultraviolet and infrared ranges using space instruments. The most noticeable signs of NO in the ultraviolet spectrum are gamma-lines in the wavelength range of 205.3–247.9 nm [11]. Another notable feature of NO is its fundamental line at 5.3 microns, usually observed, for example, in the thermospheric infrared airglow in the upper layers of the Earth’s atmosphere at altitudes from 130 km to 190 km by various space instruments [35,36]. All these features of atmospheric NO luminescence indicate that observations of ultraviolet NO radiation in the atmospheres of exoplanets are quite real. If the possibility of NO observations becomes clear, then there will be a unique opportunity to detect signs of a developed aerobic extraterrestrial biosphere, which will bring us one step closer to answering the question of how unique aerobic forms of life on Earth are.
We studied the odd nitrogen chemistry in the Earth’s upper atmosphere [32,33,34], and when for the first time, the contribution of suprathermal nitrogen atoms to the chemistry of odd nitrogen was taken into account. Since then, the situation has improved somewhat, as several satellites have been launched, partly aimed at investigating this problem [12,36].
2.2. The Kinetic Monte Carlo Model for Electron Precipitation
2.2.1. Physical Processes
The fresh energetic electrons of magnetospheric origin are transported in the polar thermosphere and lose their kinetic energy in elastic, inelastic and ionization collisions with ambient atmospheric gas—e(E) + X → {e(E′) + X; e(E′) + X*; e(E′) + X+ + e(Es)}. Here, E and E′(<E) are the kinetic energies of primary electron before and after collision; X = O2, O, N2; X* and X+ are atmospheric species in excited and ionized states; Es is energy of secondary electron formed in the ionizing collision. Precipitating electrons cause the formation of emission features due to electron impact excitation, ionization and dissociative excitation. In the model, we take into account the physical processes given in Table A1, Table A2 and Table A3 in Appendix A. The electron impact cross sections for N2, O2, O were taken from the compilations [37,38,39,40]. For partial ionization cross sections, we use the approximative formulas from [41,42].
The auroral electron transport model operates in a spatial dimension, an angular dimension, and Vx, Vy and Vz velocity dimensions. The spatial dimension is along a magnetic field line, and the angular dimension is the pitch angle to that field line. Other than an interpolation “mesh” in energy and altitude for calculation of cross sections and densities and tabulation of results, there is no explicit gridding or binning of an electron’s location in space. In our model, we use the constant grid on energy from 0 to 100 keV with grid step 1 eV. If the collision produces ionization, a secondary electron is created and an isotropic pitch angle is randomly assigned as well as an energy, using an integral form of the approximate formula of [42] based on the laboratory results of [43].
At low energy, elastic cross sections become so much larger than inelastic cross sections that an electron makes repeated collisions without losing significant energy. Transport is generally negligible in this regime, except at the highest altitudes, so the way this is dealt with in the Monte Carlo code is that below 2 eV, local energy deposition is assumed. For inelastic collisions, we use the forward scattering approximation: it is assumed that the differential cross section for these collisions is so strongly peaked in the forward direction that angular redistribution by this process is negligible. This is a good approximation at all energies but the lowest energies. Below 100 eV can be considerable backscatter, particularly from forbidden excitation transitions, but the flux becomes so isotropic and the relative size of the elastic cross sections becomes so large that this has little effect on the final pitch angle distribution. The underlying assumptions are those characteristic of collisional electron transport models: that electric field acceleration and gravity have negligible effect; that the electrons are confined to spiral paths around a magnetic field line characterized by a pitch angle distribution, and that wave-plasma interactions are unimportant in the thermosphere. The magnetic field variation, i.e., magnetic line convergence in Earth’s polar regions, was taken into account by conservation of the adiabatic invariant in the model.
2.2.2. Kinetic Description
In the model, the fresh electrons lose their excess kinetic energy in collisions with other atmospheric particles and are distributed in the transition region between the thermosphere and the exosphere. Their kinetics and transport is described by the kinetic Boltzmann equation:
where fe(r,v), and fm(r,v) are the distribution functions by velocities v for electrons, and components of ambient gas at space point r, respectively. The left side of the kinetic equation describes the transport of electrons in the planetary gravitational and/or magnetic fields s. This force could be expressed as s = (g + e/me[v × B]), where g is a gravitational acceleration force, e and me are electron charge and mass and B—the intensity of the planetary magnetic field. In the right-hand side of the kinetic equation, the Qe,aur term describes the source of auroral electrons due to the precipitation at upper boundary, and the Qe,sec term describes the formation rate of secondary electrons. The elastic and inelastic scattering terms J for electron collisions with ambient atmospheric species are written in a standard form [44]. It is assumed that the ambient atmospheric gas is characterized by local Maxwellian velocity distribution functions.
Kinetics of aeronomic processes in rarefied atmospheric gas can be formulated as the statistical behaviour of an ensemble of atoms, molecules and their ions under influence of solar electromagnetic and corpuscular radiation. For a given set of elementary processes, the microscopic kinetic description of the atmospheric particle ensemble is characterized by energy distribution functions, populations of quantum levels and energy exchange in the collisional processes. The dynamical evolution of this ensemble is described by transport processes.
The kinetic Monte Carlo method is an efficient tool for atmospheric kinetic systems in stochastic approximation [44,45]. The details of the algorithmic realization of the kinetic Monte Carlo model could be found in [20,45]. In numerical simulations, the evolution of the system of modelling particles due to collisional processes and particle transport is calculated from the initial to the steady state. The relative importance of the collisional processes is governed by their cross sections.
2.2.3. Numerical Realization of Kinetic Monte Carlo Model
The direct methods of solving the Boltzmann kinetic equation consist of setting up and solving a system of equations for the probabilities of all possible paths of the suprathermal particles in the ambient atmospheric gas. Unfortunately, this direct procedure can be performed only for a few very simple physical and chemical systems [45] and involves enormous computational difficulties for real systems.
The Monte-Carlo method, which consists of generating a sample of paths for the state of suprathermal particles, is an efficient tool for studying such complex physical and chemical systems in the stochastic approximation. The path generation procedure is much simpler—a sequence of transitions between the states of suprathermal particles in the atmospheric gas and transition-separating times should be drawn based on the proper probability distributions. To perform this procedure, a homogeneous jump-like Markovian process is substituted with an equivalent homogeneous Markovian chain, where transitions between its states in time t are caused by collisions of suprathermal particles with the atmospheric atoms and molecules. This equivalent substitution form the basis for the algorithmic implementation because the procedure for numerically implementing the chain could be rigorously defined [44,45]. Such procedure is an analog Monte-Carlo algorithm for solving the kinetic Boltzmann equation for suprathermal particles—high-energy electrons, protons and hydrogen atoms [20,46,47]. Because the analog Monte-Carlo method for its solution is linear, the dynamical, physical and chemical parameters of the suprathermal particles in the atmospheric gas are calculated by averaging the realizations of the paths of the random process, states of which simulate the transport and collisions of suprathermal particles with surrounding atmospheric gas.
3. Results of Calculations
3.1. Energy Spectra of Precipitating Electrons
The calculations were conducted for the Earth’s polar atmosphere, and the runs were made for two test cases—monoenergetic flux of precipitating electrons with energy E0 = 1.0 keV; additionally, (b) the pitch-angle distribution for precipitating electrons was take as (a) a monodirectional one (strictly downward flux) and (b) an isotropic one [48], e.g., the following values were used E0 = 1.0 keV, Q0 = 1.0 erg cm−2 s−1 for the electron flux at an upper boundary at 700 km in the Earth’s polar atmosphere. The magnetic field variation, i.e., magnetic line convergence in Earth’s polar regions, was taken into account by conservation of the adiabatic invariant in the model.
In the numerical simulations, the evolution of the system of modelling particles due to collisional processes and particle transport is calculated from the initial to the steady state. The region of the Earth’s upper atmosphere under study (between 80 km and 700 km) was divided into N radial cells. In order to minimize boundary effects, the lower boundary was set at an altitude 80 km and the upper boundary was set at 800 km where the atmospheric gas flow is practically a collision-free one. The altitude distributions of the main neutral species—O2, N2, and O, and their temperature—were adopted with the MSISE-90 reference model [49]. For the validation runs, the model atmosphere was calculated for the day 29.12.1975 at noon local time. The value of latitude was equal to 37°, and of longitude—to 0°. The solar and geomagnetic conditions were at a low level corresponding to F10.7 = 75, and Ap = 15.
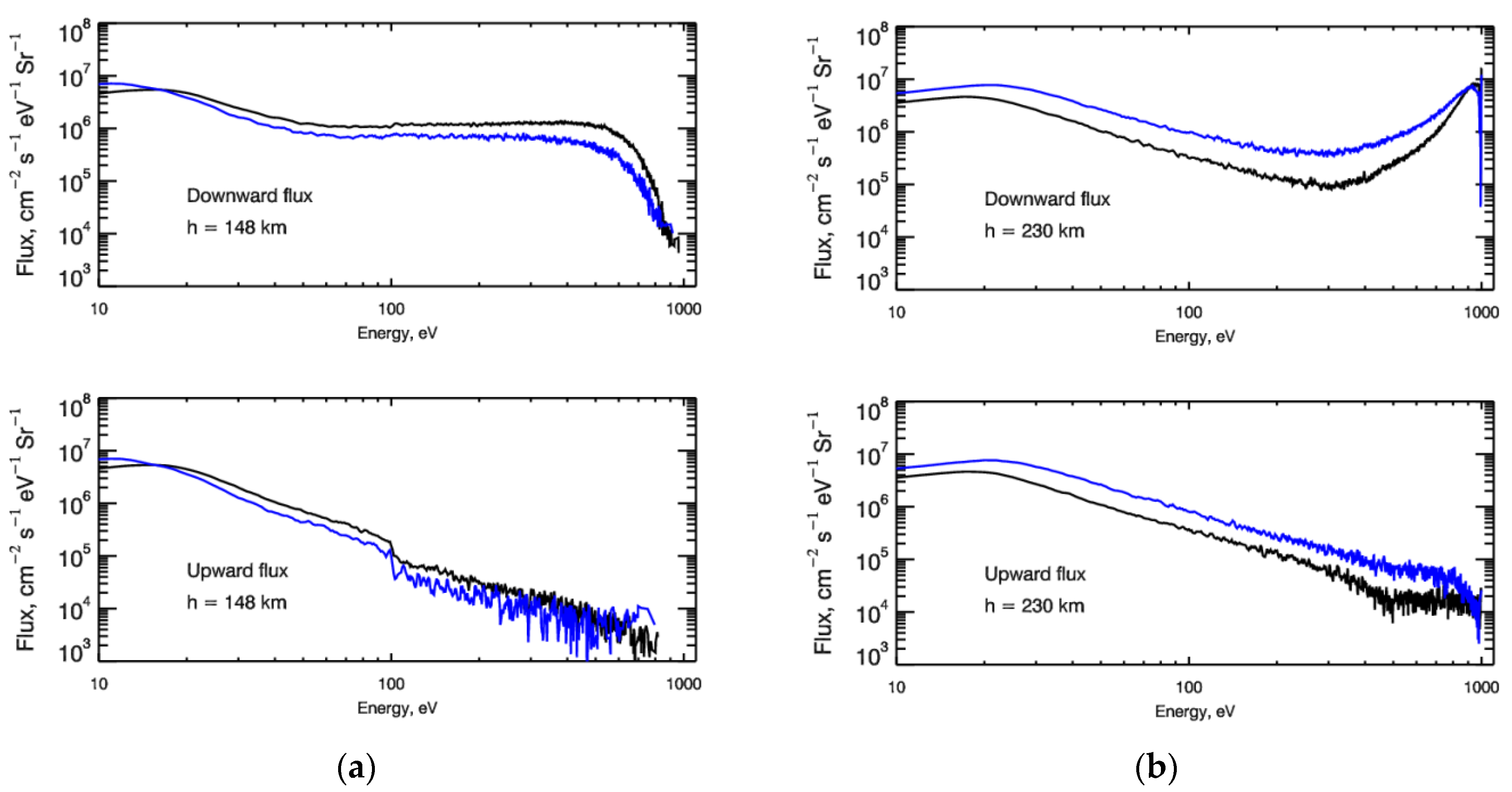
The developed kinetic Monte Carlo model of auroral electron transport allows us to calculate energy distribution functions (EDFs) in the region under study. The structure of the EDF is defined by the set of collisional processes, which were taken into account in the model, electron transport (see Table A1, Table A2 and Table A3) and input of precipitating flux. As an example in Figure 1a,b the steady-state energy spectra of down- and upward fluxes for auroral electrons at heights of 140 km (where the maximum of the electron energy deposition is observed) and 230 km are presented. From Figure 1a,b, it is clear seen that at low altitudes the energy spectra of both down- and upward fluxes are characterized by the Maxwellian cores that indicate the dominance of local electron energy deposition at these altitudes. Nevertheless, the presence of high energy precipitating electrons is well seen even at low altitudes. Upward flux at higher altitudes is defined by transport of thermalized electrons from a dense atmosphere. The presence of precipitated electrons is still seen even at 140 km (Figure 1a), which means that even in the dense thermosphere for correct consideration, it is necessary to use the non-LTE methods and kinetic equations. The presence of the high energetic tail is extremely important for calculations of the electron energy deposition and emission rates.
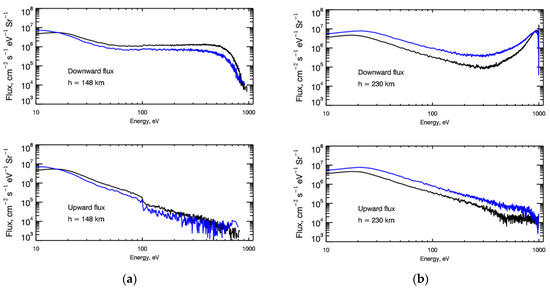
Figure 1.
(a) Energy spectra of downward (top panel) and upward (bottom panel) fluxes of auroral electrons calculated at height of 148 km. Auroral electrons are represented by monoenergetic flux with energy E0 = 1 keV and are characterized by monodirectional (black lines) and isotropic (blue lines) pitch-angle distributions at the upper boundary. (b) Same as (a) but at height 230 km.
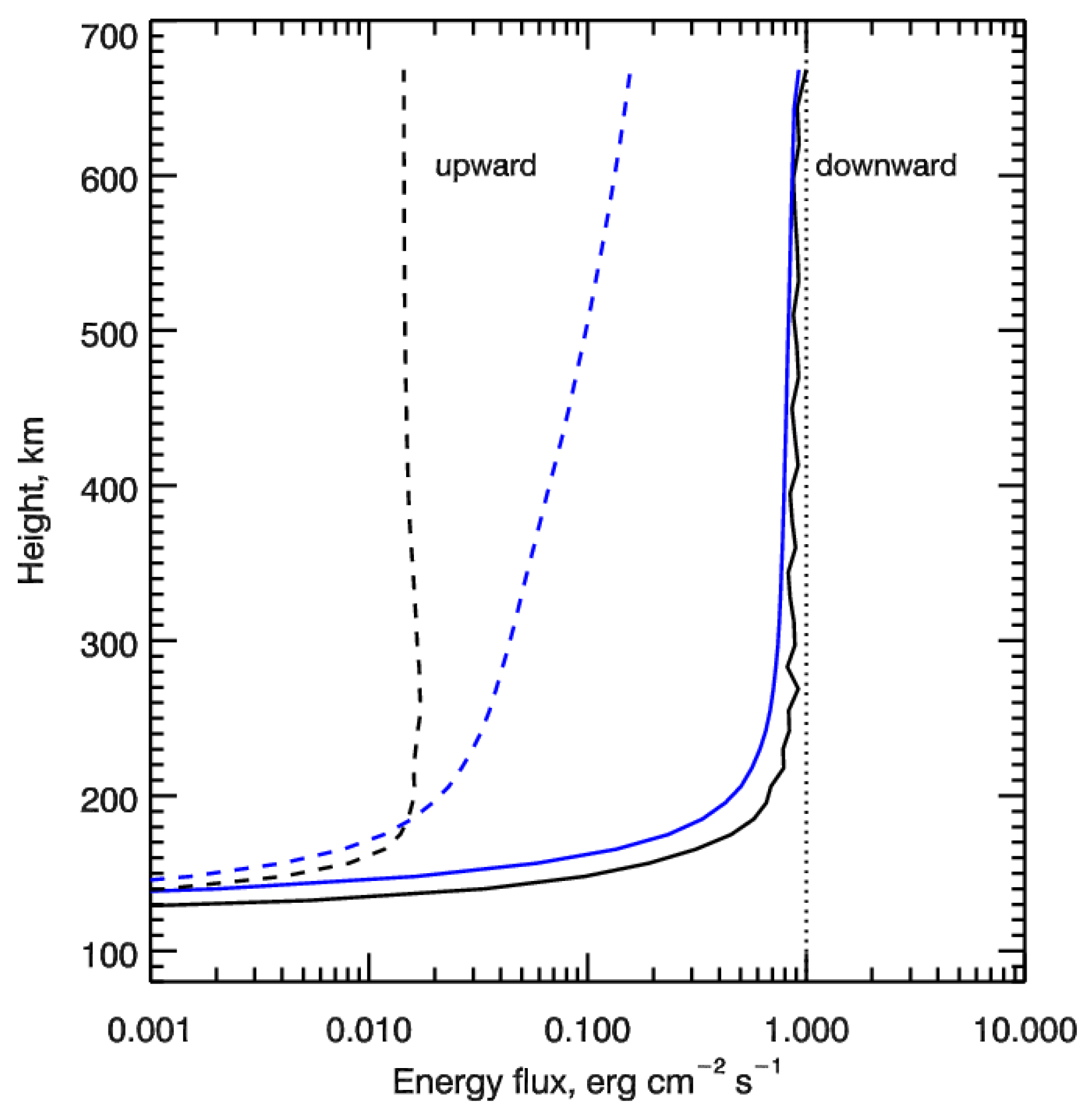
Height profiles of the calculated downward (solid lines) and upward (dashed lines) energy fluxes of auroral electrons are shown in Figure 2. The vertical dotted line shows the value of initial electron energy flux of 1 erg cm−2 s−1 transferring into the upper atmosphere at a height of 700 km—the upper boundary condition. It is seen that the electrons penetrate rather deep into the polar atmosphere with the main region of the deposition being below 200 km. The backscattered flux is low comparing with the precipitating flux—about two orders of magnitude for monodirectional pitch-angle distribution and about one order of magnitude—for isotropic pitch-angle distribution for electrons penetrating into the upper atmosphere at a height of 700 km.
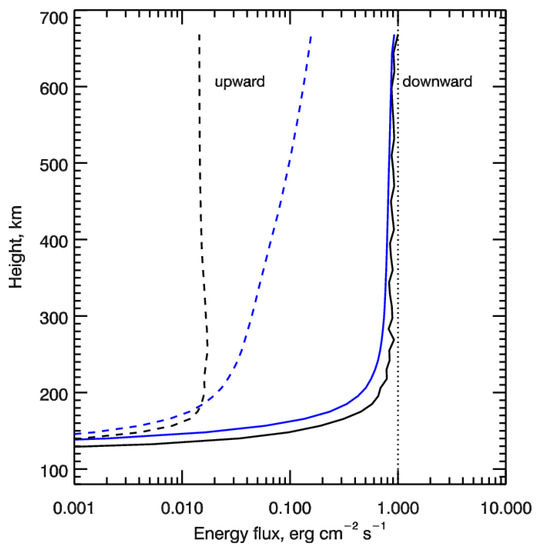
Figure 2.
Height profiles of the calculated downward (solid lines) and upward (dashed lines) energy fluxes of auroral electrons. Auroral electrons precipitate as the monoenegetic flux at characteristic energy E0 = 1 keV and are represented by monodirectional (black lines) and isotropic (blue lines) pitch-angle distributions at the upper boundary. The vertical dotted line shows the value of initial energy flux of 1 erg cm−2 s−1 of electrons penetrating into the upper atmosphere at height of 700 km—the upper boundary condition.
The calculated energy spectra of precipitating electrons could be used to estimate the parameters of auroral events such as electron energy deposition rates, emission excitation rates and formation rate of thermal and suprathermal atoms and molecules due to the electron impact. As concerns the odd nitrogen chemistry in the polar regions of N2-O2 atmospheres, the auroral electron precipitation could be considered as an important source of atomic nitrogen [9,10,11,12,50] in the different electronically excited states due to the electron impact dissociation of molecular nitrogen N2:
e(E) + N2(X1Σ+g) → e(E′) + N(4S) + N(4S,2D,2P)
Here, E and E′ < E are the kinetic energies of auroral electron before and after collision, and N(4S,2D,2P) are the nitrogen atoms in the ground and metastable electronic states. As it follows from laboratory studies (see, e.g., [51,52]), the electron impact dissociation of molecular nitrogen results in the formation of nitrogen atoms with an excess of kinetic energy and in the different states of electronic excitation. In our calculations, we used the laboratory data on the energy-dependent cross sections of reaction (1), the energy spectra of fresh nitrogen atoms formed in reaction (1) and branching ratios for the formation of N in the ground and electron excited states provided in papers [51,52]. The reaction (1) can be considered as an source term
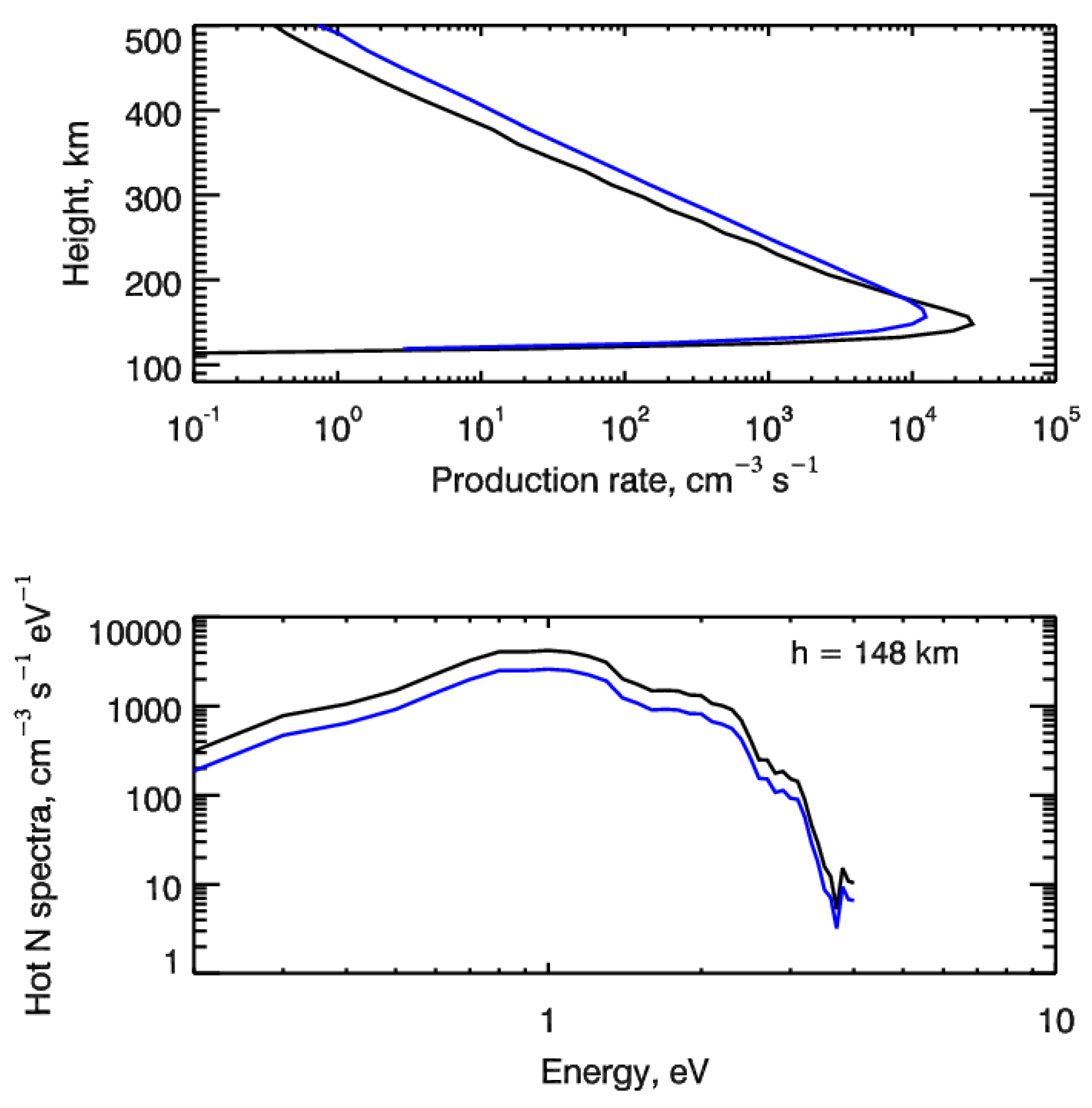
for the kinetic Boltzmann equation describing production, kinetics and transport of suprathermal nitrogen atoms in the polar upper atmosphere (see, e.g., [44,45]). Here, qNh(h) is the production rate of suprathermal N(4S) atoms in the reaction (1) at height h, and fNh(h,E) is the normalized to unity energy spectrum of the N(4S) production rate at height h. As an example, Figure 3 shows the height profiles of the calculated production rates qNh(h) of suprathermal nitrogen atoms N(4S) formed in the electron impact dissociation of atmospheric N2 molecules for two considered test cases. The calculated energy spectra of suprathermal N atoms are given in the bottom panel in Figure 3 at a height of 148 km, where the maximum of electron energy deposition is found. It is seen that fresh nitrogen atoms formed due to the electron impact N2 dissociation are populating the region of suprathermal energies (ambient atmospheric gas temperature was about 730 K at a height of 148 km in the model atmosphere used) and their energy spectra are strongly non-equilibrium. Therefore, to calculate the input of suprathermal nitrogen atoms into the odd nitrogen chemistry and, especially, in the formation of nitric oxide NO, it is necessary to solve the kinetic Boltzmann equation, taking into account both kinetics and transport of suprathermal N atoms in the polar atmosphere [32,44].
QNh(h,E) = qNh(h) × fNh(h,E)
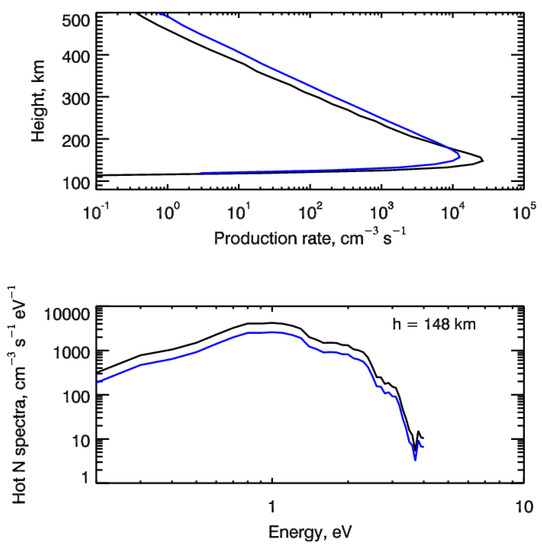
Figure 3.
(top panel) Height profiles of the production rate of suprathermal nitrogen atoms formed in the electron impact dissociation of atmospheric N2 molecules. (bottom panel) Calculated energy spectra of suprathermal N atoms formed at height of 148 km. Auroral electrons precipitate with energy E0 = 1 keV and are characterized by monodirectional (black lines) and isotropic (blue lines) pitch-angle distributions at the upper boundary.
3.2. Source Functions of Hot N Atoms from the N2 Electron Impact Dissociation
Production of nitric oxide in collisions between thermal and/or suprathermal nitrogen atoms and oxygen molecules is an important process in odd nitrogen atmospheric chemistry [53,54,55],
N(4S) + O2(X3Σ−g) → NO(X2Π) + O(3P)
This reaction together with the highly endothermic reaction,
constitute the well-known Zeldovich mechanism of nitrogen oxidation [56]. Due to an activation energy of about 0.3 eV, reaction (2) proceeds slowly at room temperatures. Thus, only energetic N atoms react with O2 at temperatures of the ambient atmosphere. The non-equilibrium energy distribution function of the nitrogen atoms has been investigated for the midlatitude Earth’s atmosphere [34,55,57] and it has been established that a significant population of hot nitrogen atoms is created. To determine their contribution to the formation of NO, the cross-section for reaction (2) as a function of the energy of relative motion is needed. However, the relatively large exothermic energy of about 1.4 eV results in production of NO with high vibrational-rotational excitation. Infrared emission from rovibrationally excited NO is considered to be an important mechanism for the night-time cooling of the thermosphere and much effort has been devoted to the determination of rovibrational level populations of NO resulting from (2) (see, e.g., [35]).
O(3P) + N2(X1Σ+g) → NO(X2Π) + N(4S)
Reaction (2) has been extensively studied in a large number of experimental (see, e.g., [58]) and theoretical (see, e.g., [36,59,60]) investigations. The experimental studies have focused on two aspects: first, to evaluate thermal rate coefficients over a wide range of temperatures from 300 to 5000 K, and second, to determine the rovibrational level populations of the NO products. There are some available experimental data about thermal rate constants and NO vibrational distributions. Two compilations of kinetic data cover a wide range of temperatures: 1.5 × 10−11 × e−3600/T cm3 molecule−1 s−1 at 280–910 K [61] or 1.5 × 10−14 T × e−3270/T cm3 molecule−1 s−1 at 298–5000 K [62]. In the paper [63], the following value 1.45 × 10−16T1.6 × e−2894/T cm3 molecule−1 s−1 at temperatures 300–5000 K of rate coefficient for reaction (2) was provided as an approximation of the experimental measurements and theoretical calculations.
Calculations [57] have shown that reaction (2) dominates NO production at altitudes above 120 km and the reaction between electronically excited N(2D) atoms and O2 dominates at lower altitudes, but in these calculations, the transport of suprathermal N(4S) was not taken into account. This effect could be important for auroral regions of terrestrial planets [50].
Basing on the calculated source functions for the electron impact N2 dissociation, it is possible to estimate the kinetic characteristics of the endothermic reaction (2). We calculated the differential rate coefficients for the source function QNh(h,E) of the suprathermal N atoms, namely
where v(E) is the relative velocity of interacting particles in (2), —is the energy-dependent cross section for reaction (2). Results of theoretical calculations of this reaction cross section [59,60] were used to estimate the differential rate coefficient for reaction (2).
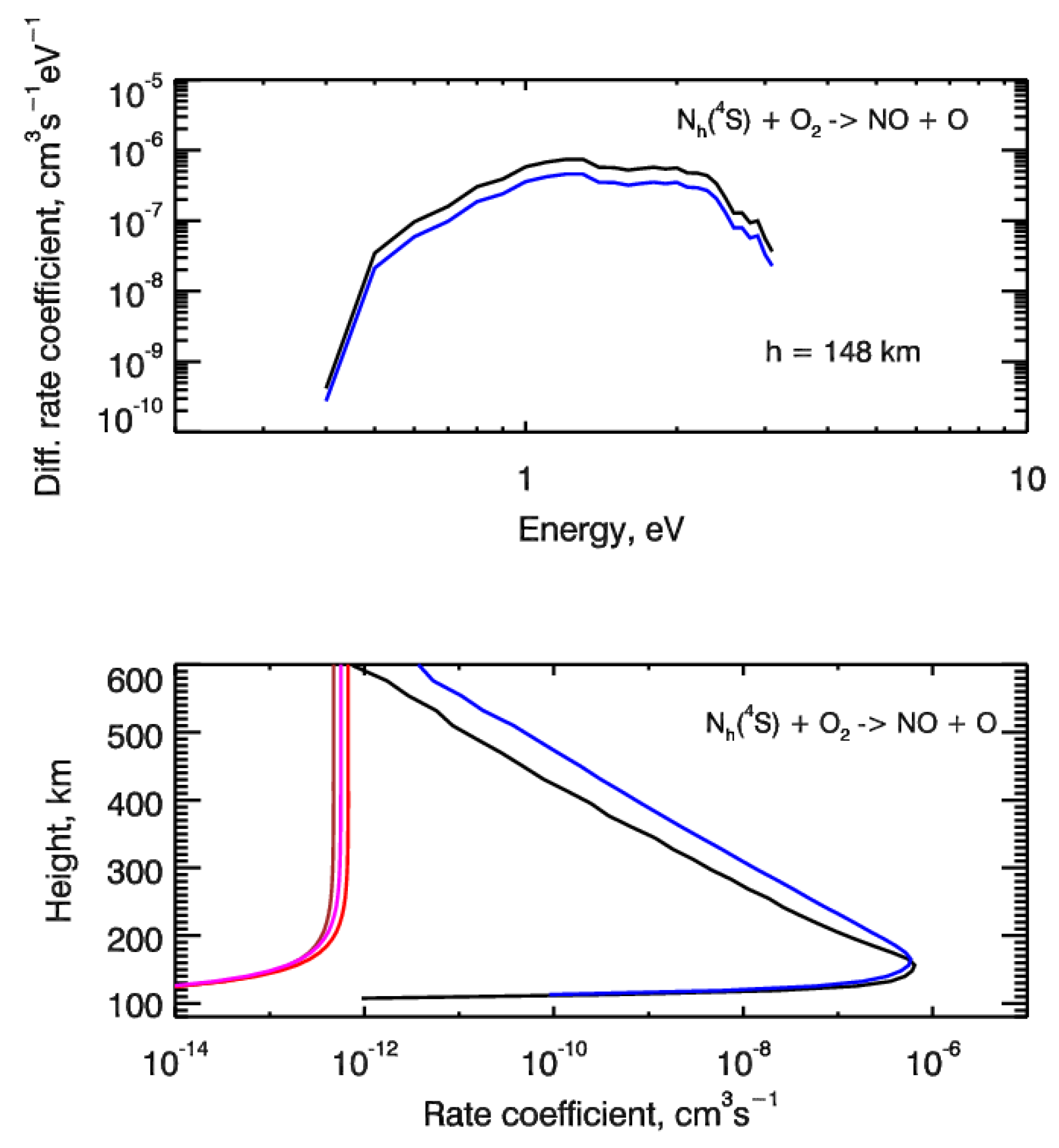
Results of our calculations are given in Figure 4. The differential rate coefficients for reaction (2) with the source functions for two test cases of auroral electron precipitation are shown in the top panel of Figure 4. The height profiles of integrated rate coefficients were presented in the bottom panel. For comparison, the thermal rate coefficients for reaction (2) were also shown. From this comparison, it is seen that electron impact dissociation of N2 can potentially be an important source of suprathermal N atoms in the auroral regions of N2-O2 atmospheres of terrestrial-type planets. Moreover, further calculations of the steady-state energy distributions for suprathermal N(4S) atoms will allow us to estimate the possible input into the nitric oxide NO formation, because as it follows from Figure 4 non-thermal rate coefficients for reaction (2) and could be much higher than thermal ones. To obtain the estimates of this input, it is necessary to calculate the input of suprathermal N atoms through the reaction (2) into the odd nitrogen chemistry. This procedure realization is in progress and the results of calculations will be presented in the near future.
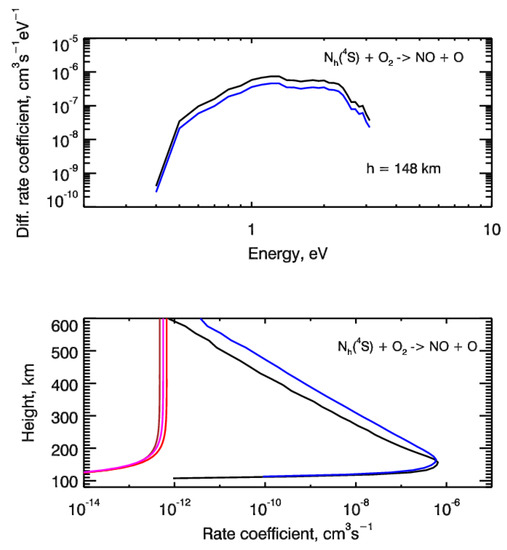
Figure 4.
(top panel) Differential rate coefficients for chemical reaction between suprathermal Nh(4S) atoms and molecular oxygen O2 resulting in formation of nitric oxide NO calculated at height of 148 km. (bottom panel) Height profiles of the non-thermal rate coefficients calculated for the source function of the chemical reaction (2). Auroral electrons precipitate with monoenergetic spectrum at characteristic energy E0 = 1 keV, and are described by monodirectional (black lines) and isotropic (blue lines) pitch-angle distributions at the upper boundary. The thermal rate coefficients for reaction (2) are also shown by the brown line [61], red line [62] and magenta line [63].
4. Discussion
The kinetic Monte Carlo model [20] for auroral electron precipitation was modified and updated with an aim to calculate the source functions of suprathermal atoms formed in the electron impact dissociation of the main atmospheric molecules—N2 and O2. Calculations were performed for two test cases of electron precipitation in the Earth’s polar atmosphere—a monoenergetic electron flux at the upper boundary with a givenmonodirectional or isotropic distribution of the pitch angle. It should be noted that the measured energy spectra and pitch-angle distributions of the precipitating electrons usually have quite arbitrary shapes but can be successfully reproduced in the kinetic model by superposition of the test distributions considered in this paper (see, for procedure details, [34,55]). The energy spectra of down- and upward fluxes of precipitating electrons, and height profiles of the energy fluxes of auroral electrons were calculated. These data allow us to estimate the energy spectra of source functions for suprathermal nitrogen atoms formed due to the electron impact N2 dissociation. It was found that electron impact dissociation of N2 potentially can be an important source of suprathermal N atoms in the auroral regions of the N2-O2 atmospheres of terrestrial-type planets.
The presented modification of the kinetic Monte Carlo model [20] of auroral electron precipitation could be considered as a first step resulting in the calculations of the production rates and energy spectra of suprathermal nitrogen atoms formed in the N2 dissociation by precipitating high-energy electrons. In our future studies, these source functions will be used as an input to the kinetic Monte Carlo model [8] to investigate the kinetics and transport of hot N atoms in the polar upper atmosphere with an aim to calculate the steady-state energy distributions of suprathermal nitrogen atoms in the polar regions of the planetary atmospheres under study. Finally, all these steps will result in understanding of the input of hot fractions to the odd nitrogen chemistry in N2-O2 atmospheres of terrestrial-type exoplanets and to reveal the relationship of the potential atmospheric biomarker of nitric oxide NO with the fraction of suprathermal particles. The chemistry and dynamics of NO in N2-O2 atmospheres of hot sub-neptunes and exo-earths will be studied using the current and earlier developed kinetic Monte Carlo models [64,65,66], designed to investigate the kinetics and transport of suprathermal particles in planetary atmospheres. In addition, in our future studies, the recently developed multi-component MHD model [67] will be used to calculate the effect of the stellar wind on the process of destruction of nitric oxide and the removal of NO from the upper atmosphere by the polar wind. Finally, this will allow us to calculate the distribution of nitric oxide NO in the upper atmosphere of the terrestrial-type exoplanet depending on the activity level of the host star and to assess the possibility of observing this potential atmospheric biomarker.
Progress in observational capabilities and the launch of space telescopes will allow in the near future to carry out transit spectroscopy of relatively small planets in a wide range of wavelengths from UV to IR range. Sub-neptunes and exo-earths located in low orbits to the parent star are the objects well-suited to study the formation of secondary N2-O2 atmospheres. Their sufficiently hot atmospheres, heated by the radiation of the host star, are in the mode of gas-dynamic outflow—photo-evaporation—as a result of which the absorption region in the resonant lines of the evaporated elements should significantly exceed the optical size of the planet. Accordingly, it becomes possible to observe such potential biomarkers as nitric oxide NO in the UV range. The most important feature of this study is a possibility to verify the developed numerical models in calculations of NO concentrations in the Earth’s polar atmosphere where the extended database on nitric oxide observations and measurements exists [11,12].
Author Contributions
D.B.—conceptualization, data analysis and writing—editing; V.S.—numerical modelling and writing—editing; B.H.—data analysis and writing—editing. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge the financial support of the Russian Science Foundation, grant # 22-12-00364.
Informed Consent Statement
Not applicable.
Data Availability Statement
All necessary data are contained in this paper.
Acknowledgments
The authors are grateful to the reviewers for useful comments.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
The following electron impact processes, which were taken into account in the kinetic Monte Carlo model for high-energy electron precipitation, are listed in Table A1, Table A2 and Table A3 for the main atmospheric species—N2, O2 and O. The energetic threshold and maximum value of cross section for each process were taken from compilations [37,38,39,40] and are also given in Table A1, Table A2 and Table A3.

Table A1.
Electron impact on N2.
Table A1.
Electron impact on N2.
| Process | Threshold, eV | σmax (×10−19), cm2 |
|---|---|---|
| Momentum transfer | 0 | 2300 |
| Rotational excitation J = 0→2 | 0.0015 | 210 |
| Total vibrational excitation | 0.29 | 2000 |
| Excitation to A3Σu+ | 6.17 | 240 |
| Excitation to B3∏g | 7.35 | 330 |
| Excitation to W3Δu | 7.36 | 320 |
| Excitation to B′3Σu− | 8.16 | 120 |
| Excitation to a′1Σu− | 8.4 | 90 |
| Excitation to a1∏g | 8.55 | 340 |
| Excitation to w1Δu | 8.89 | 110 |
| Excitation to b1∏u | 12.5 | 200 |
| Excitation to b′1Σu+ | 12.9 | 160 |
| Excitation to c1∏u | 12.1 | 250 |
| Excitation to c′41Σu+ | 12.9 | 140 |
| Excitation to C3∏u | 11.0 | 520 |
| Excitation to E3Σg+ | 11.9 | 130 |
| Excitation to a″1Σg+ | 12.3 | 70 |
| Total ionization | 15.6 | 280 |
| N + N production | 9.76 | 130 |

Table A2.
Electron impact on O2.
Table A2.
Electron impact on O2.
| Process | Threshold, eV | σmax (×10−18), cm2 |
|---|---|---|
| Momentum transfer | 0 | 1150 |
| Excitation of vibrational levels | 0.3 | 59.8 |
| Excitation to 13Πg | 7.6 | 7 |
| Excitation to B3Σu | 8.3 | 60.6 |
| Excitation to 8.9 eV peak | 8.9 | 14.5 |
| Excitation to second band 3Σ | 10.3 | 1.0 |
| Excitation to a1Δu | 1.0 | 8.7 |
| Excitation to b1Σu+ | 1.6 | 3.5 |
| Excitation to longest band 3Σ | 10.0 | 6.8 |
| Excitation to A1Σu+ + A′3Δu + c1Σu− | 4.5 | 17.3 |
| Excitation to Rydber’s states | 16.0 | 140.9 |
| Total ionization | 12.1 | 297.8 |

Table A3.
Electron impact on O.
Table A3.
Electron impact on O.
| Process | Threshold, eV | σmax (×10−18), cm2 |
|---|---|---|
| Momentum transfer | 0 | 779.1 |
| Excitation to O(1D) | 1.96 | 25.1 |
| Excitation to O(1S) | 4.17 | 2.2 |
| Excitation to O(5S0) | 9.29 | 11.5 |
| Excitation to O(3S0) | 9.53 | 17.2 |
| Excitation to O(5P0, 3P0, 5S0, 3D0, 5S0, 3′D0) | 12.1 | 9.5 |
| Ionization to O+(4S) | 13.6 | 50.9 |
| Ionization to O+(2D) | 16.9 | 55. |
| Ionization to O+(2P) | 18.6 | 29.5 |
References
- Lammer, H.; Sproß, L.; Grenfell, J.L.; Scherf, M.; Fossati, L.; Lendl, M.; Cubillos, P.E. The Role of N2 as a Geo-Biosignature for the Detection and Characterization of Earth-like Habitats. Astrobiology 2019, 19, 927–950. [Google Scholar] [CrossRef] [PubMed]
- Sproß, L.; Scherf, M.; Shematovich, V.I.; Bisikalo, D.V.; Lammer, H. Life is the Only Reason for the Existence of N2-O2-Dominated Atmospheres. Astron. Rep. 2021, 65, 275–296. [Google Scholar] [CrossRef]
- Schwieterman, E.W.; Robinson, T.D.; Meadows, V.S.; Misra, A.; Domagal-Goldman, S. Detecting and Constraining N2 Abundances in Planetary Atmospheres Using Collisional Pairs. Astrophys. J. 2015, 810, 57. [Google Scholar] [CrossRef]
- Grenfell, J.L.; Grießmeier, J.M.; von Paris, P.; Patzer, A.B.C.; Lammer, H.; Stracke, B.; Gebauer, S.; Schreier, F.; Rauer, H. Response of atmospheric biomarkers to NOx-induced photochemistry generated by stellar cosmic rays for Earth-like planets in the habitable zone of M dwarf stars. Astrobiology 2012, 12, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Hedelt, P.; von Paris, P.; Godolt, M.; Gebauer, S.; Grenfell, J.L.; Rauer, H.; Schreier, F.; Selsis, F.; Trautmann, T. Spectral features of Earth-like planets and their detectability at different orbital distances around F, G, and K-type stars. Astron. Astrophys. 2013, 553, A9. [Google Scholar] [CrossRef]
- Betremieux, Y.; Kaltenegger, L. Transmission spectrum of Earth as a transiting exoplanet from the ultraviolet to the near-infrared. Astrophys. J. 2013, 772, L31. [Google Scholar] [CrossRef]
- Seager, S.; Bains, W.; Hu, R. An Astrophysical View of Earth-Based Metabolic Biosignature Gases. Astrobiology 2012, 12, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Bisikalo, D.V.; Shematovich, V.I.; Kaygorodov, P.V.; Zhilkin, A.G. Extended envelopes of hot Jupiters. Phys. Uspiekhy 2021, 64, 747–800. [Google Scholar] [CrossRef]
- Gérard, J.C.; Barth, C.A. High-latitude nitric oxide in the lower thermosphere. J. Geophys. Res. 1977, 82, 674–680. [Google Scholar] [CrossRef]
- Barth, C.A.; Bailey, S.C.; Solomon, S.C. Solar–terrestrial coupling: Solar soft X-rays and thermospheric nitric oxide. Geophys. Res. Lett. 1999, 26, 1251–1254. [Google Scholar] [CrossRef]
- Barth, C.A.; Baker, D.N.; Mankoff, K.D.; Bailey, S.M. The northern auroral region as observed in nitric oxide. Geophys. Res. Lett. 2001, 28, 1463–1466. [Google Scholar] [CrossRef]
- Barth, C.A.; Mankoff, K.D.; Bailey, S.M.; Solomon, S.C. Global observations of nitric oxide in the thermosphere. J. Geophys. Res. 2003, 108, 1027–1038. [Google Scholar] [CrossRef]
- Nagy, A.F.; Banks, P.M. Photoelectron fluxes in the ionosphere. J. Geophys. Res. 1970, 75, 6260–6270. [Google Scholar] [CrossRef]
- Banks, P.M.; Chappell, C.R.; Nagy, A.F. A new model for the interaction of auroral electrons with the thermosphere: Spectral degradation, backscatter, optical emission, and ionization. J. Geophys. Res. 1974, 79, 1459–1470. [Google Scholar] [CrossRef]
- Stamnes, K. Analytic approach to auroral electron transport and energy degradation. Planet. Space Sci. 1980, 28, 427–441. [Google Scholar] [CrossRef]
- Porter, H.S.; Varosi, F.; Mayr, H.G. Iterative solution of the multistream electron transport equation, 1, Comparison with laboratory beam injection experiments. J. Geophys. Res. 1987, 92, 5933. [Google Scholar] [CrossRef]
- Lummerzheim, D.; Lilensten, J. Electron transport and energy degradation in the ionosphere: Evaluation of the numerical solution, comparison with laboratory experiments and auroral observations. Ann. Geophys. 1994, 12, 1039–1051. [Google Scholar] [CrossRef]
- Simon, C.; Lilensten, J.; Moen, J.; Holmes, J.M.; Ogawa, Y.; Oksavik, K.; Denig, W.F. TRANS4: A new coupled electron/proton transport code—Comparison to observations above Svalbard using ESR, DMSP and optical measurements. Ann. Geophys. 2007, 25, 661–673. [Google Scholar] [CrossRef]
- Solomon, S.C. Auroral particle transport using Monte Carlo and hybrid methods. J. Geophys. Res. 2001, 106, 107–116. [Google Scholar] [CrossRef]
- Shematovich, V.I.; Bisikalo, D.V.; Gérard, J.-C.; Cox, C.; Bougher, S.W.; Leblanc, F. Monte Carlo model of electron transport for the calculation of Mars dayglow emissions. J. Geophys. Res. 2008, 113, E02011. [Google Scholar] [CrossRef]
- Fox, J.L.; Galand, M.I.; Johnson, R.E. Energy Deposition in Planetary Atmospheres by Charged Particles and Solar Photons. Space Sci. Rev. 2008, 139, 3–62. [Google Scholar] [CrossRef]
- Solomon, S.C. Global modeling of thermospheric airglow in the far ultraviolet. J. Geophys. Res. Space Phys. 2017, 122, 7834–7848. [Google Scholar] [CrossRef]
- Gérard, J.-C.; Hubert, B.; Shematovich, V.I.; Bisikalo, D.V.; Gladstone, G.R. The Venus ultraviolet oxygen dayglow and aurora: Model comparison with observations. Planet. Space Sci. 2008, 56, 542–552. [Google Scholar] [CrossRef]
- Hubert, B.; Gérard, J.-C.; Gustin, J.; Shematovich, V.I.; Bisikalo, D.V.; Stewart, A.I.; Gladstone, R.G. Cassini-UVIS observations of OI and CO Venus dayglow. Icarus 2010, 207, 549–557. [Google Scholar] [CrossRef]
- Hubert, B.; Gérard, J.-C.; Gustin, J.; Bisikalo, D.V.; Shematovich, V.I.; Gladstone, G.R. Cassini-UVIS observation of dayglow FUV emissions of carbon in the thermosphere of Venus. Icarus 2012, 220, 635–646. [Google Scholar] [CrossRef][Green Version]
- Madhusudhan, N. Exoplanetary Atmospheres: Key Insights, Challenges, and Prospects. Annu. Rev. Astron. Astrophys. 2019, 57, 617–663. [Google Scholar] [CrossRef]
- Wordsworth, R.; Kreidberg, L. Atmospheres of Rocky Exoplanets. Annu. Rev. Astron. Astrophys. 2022, 60, 159–201. [Google Scholar] [CrossRef]
- Tian, F.; Kasting, J.F.; Liu, H.-L.; Roble, R.G. Hydrodynamic planetary thermosphere model: 1. Response of the Earth’s thermosphere to extreme solar EUV conditions and the significance of adiabatic cooling. J. Geophys. Res. 2008, 113, E05008. [Google Scholar] [CrossRef]
- Johnstone, C.P.; Lammer, H.; Kysliakova, K.G.; Scherf, M.; Güdel, M. The young Sun’s XUV-activity as a constraint for lower CO2-limits in the Earth’s Archean atmosphere. Earth Planet. Sci. Lett. 2021, 576, 117197. [Google Scholar] [CrossRef]
- Johnstone, C.P.; Khodachenko, M.L.; Lueftinger, T.; Kislyakova, K.G.; Lammer, H.; Güdel, M. Extreme hydrodynamic losses of Earth-like atmospheres in the habitable zones of very active stars. Astron. Astrophys. 2019, 624, L10. [Google Scholar] [CrossRef]
- Luger, R.; Barnes, R.; Lopez, E.; Fortney, J.; Jackson, B.; Meadows, V. Habitable evaporated cores: Transforming mini-Neptunes into super-Earths in the habitable zones of M dwarfs. Astrobiology 2015, 15, 57–88. [Google Scholar] [CrossRef] [PubMed]
- Shematovich, V.I.; Bisikalo, D.V.; Gérard, J.-C. Non-thermal nitrogen atoms in the earth’s thermosphere. I—Kinetics of hot N(4S). Geophys. Res. Lett. 1991, 18, 1691–1693. [Google Scholar] [CrossRef]
- Gérard, J.-C.; Shematovich, V.I.; Bisikalo, D.V. Non-thermal nitrogen atoms in the earth’s thermosphere. II—A source of nitric oxide. Geophys. Res. Lett. 1991, 18, 1695–1697. [Google Scholar]
- Gérard, J.C.; Shematovich, V.I.; Bisikalo, D.V.; Duff, J.W. An updated model of the hot nitrogen atom kinetics and thermospheric nitric oxide. J. Geophys. Res. 1997, 102, 285–292. [Google Scholar] [CrossRef]
- Hubert, B.; Gérard, J.-C.; Shematovich, V.I.; Bisikalo, D.V. High rotational excitation of NO infrared thermospheric airglow: A signature of superthermal nitrogen atoms? Geophys. Res. Lett. 1996, 23, 2215–2218. [Google Scholar] [CrossRef]
- Venkataramani, K.; Yonker, J.D.; Bailey, S.M. Contribution of chemical processes to infrared emissions from nitric oxide in the thermosphere. J. Geophys. Res. Space Phys. 2016, 121, 2450–2461. [Google Scholar] [CrossRef]
- Tabata, T.; Shirai, T.; Sataka, M.; Kubo, H. Analytic cross sections for electron impact collisions with nitrogen molecules. Atom. Data Nucl. Data Tables 2006, 92, 375–406. [Google Scholar] [CrossRef]
- Itikawa, Y. Cross Sections for Electron Collisions with Nitrogen Molecules. J. Phys. Chem. Ref. Data 2006, 35, 31–53. [Google Scholar] [CrossRef]
- Itikawa, Y. Cross Sections for Electron Collisions with Oxygen Molecules. J. Phys. Chem. Ref. Data 2009, 38, 1. [Google Scholar] [CrossRef]
- Anzai, K.; Kato, H.; Hoshino, M.; Tanaka, H.; Itikawa, Y.; Campbell, L.; Brunger, M.J.; Buckman, S.J.; Cho, H.; Blanco, F.; et al. Cross section data sets for electron collisions with H2, O2, CO, CO2, N2O and H2O. Eur. Phys. J. D 2012, 66, 36. [Google Scholar] [CrossRef]
- Porter, H.S.; Jackman, C.H.; Green, A.E.S. Efficiencies for production of atomic nitrogen and oxygen by relativistic proton impact in air. J. Chem. Phys. 1976, 65, 154–167. [Google Scholar] [CrossRef]
- Jackman, C.H.; Garvey, R.H.; Green, A.E.S. Electron impact on atmospheric gases. I—Updated cross sections. J. Geophys. Res. 1977, 82, 5081–5090. [Google Scholar]
- Opal, C.B.; Peterson, W.K.; Beaty, E.C. Measurements of Secondary-Electron Spectra Produced by Electron Impact Ionization of a Number of Simple Gases. J. Chem. Phys. 1971, 55, 4100–4106. [Google Scholar] [CrossRef]
- Marov, M.Y.; Shematovich, V.I.; Bisikalo, D.V. Non-equilibrium aeronomic processes. A kinetic approach to the mathematical models. Space Sci. Rev. 1996, 76, 1–202. [Google Scholar]
- Shematovich, V.I. Suprathermal particles in astrochemistry. Russ. Chem. Rev. 2019, 88, 1013–1045. [Google Scholar] [CrossRef]
- Gérard, J.-C.; Hubert, B.; Bisikalo, D.V.; Shematovich, V.I. A model of the Lyman-α line profile in the proton aurora. J. Geophys. Res. 2000, 105, 15795–15806. [Google Scholar] [CrossRef]
- Shematovich, V.I.; Bisikalo, D.V.; Diéval, C.; Barabash, S.; Stenberg, G.; Nilsson, H.; Futaana, Y.; Holmstrom, M.; Gérard, J.C. Proton and hydrogen atom transport in the Martian upper atmosphere with an induced magnetic field. J. Geophys. Res. 2011, 116, 11. [Google Scholar] [CrossRef]
- Decker, D.T.; Kozelov, B.V.; Basu, B.; Jasperse, J.R.; Ivanov, V.E. Collisional degradation of the proton-H atom fluxes in the atmosphere: A comparison of theoretical techniques. J. Geophys.Res. 1996, 101, 26947–26960. [Google Scholar] [CrossRef]
- Hedin, A.E. Extension of the MSIS thermosphere model into the middle and lower atmosphere. J. Geophys. Res. 1991, 96, 1159–1172. [Google Scholar] [CrossRef]
- Sætre, C.; Barth, C.A.; Stadsnes, J. Thermospheric nitric oxide at higher latitudes: Model calculations with auroral energy input. J. Geophys. Res. 2007, 112, 11. [Google Scholar] [CrossRef]
- Cosby, P.C. Electron-impact dissociation of nitrogen. J. Chem. Phys. 1993, 98, 9544–9553. [Google Scholar] [CrossRef]
- Walter, C.W.; Cosby, P.C.; Helm, H. N(4S0), N(2D0), and N(2P0) yields in predissociation of excited singlet states of N2. J. Chem. Phys. 1993, 99, 3553–3561. [Google Scholar] [CrossRef]
- Solomon, S. The possible effect of translationally excited nitrogen atoms on lower thermospheric odd nitrogen. Planet. Space Sci. 1983, 31, 135–144. [Google Scholar] [CrossRef]
- Gérard, J.-C. Thermospheric odd nitrogen. Planet. Space Sci. 1992, 40, 337–348. [Google Scholar] [CrossRef]
- Shematovich, V.I.; Bisikalo, D.V.; Gérard, J.-C. The thermospheric odd nitrogen photochemistry: Role of non-thermal N(4S) atoms. Ann. Geophys. 1992, 10, 792–801. [Google Scholar]
- Zeldovich, J.B.; Sadovnikov, P.J.; Frank-Kamenetski, D.A. Nitrogen Oxidation under Combustion; Academy of Sciences: Moscow, Russia, 1947; pp. 64–87. [Google Scholar]
- Balakrishnan, N.; Sergueeva, E.; Kharchenko, V.; Dalgarno, A. Kinetics and thermalization of hot N(4S) atoms in the upper atmosphere. J. Geophys. Res. 2000, 105, 18549–18555. [Google Scholar] [CrossRef]
- Caledonia, G.E.; Krech, R.H.; Oakes, D.B.; Lipson, S.J.; Blumberg, W.A. Products of the reaction of 8 km/s N (4S) and O2. J. Geophys. Res. 2000, 105, 12833–12837. [Google Scholar] [CrossRef]
- Balakrishnan, N.; Dalgarno, A. Rate coefficients for NO formation in energetic N+O2 collisions. Chem. Phys. Lett. 1999, 302, 485–488. [Google Scholar] [CrossRef]
- Sultanov, R.A.; Balakrishnan, N. Quantum mechanical investigations of the N(4S)+O2(X 3Σg−)-->NO(X 2Π)+O(3P) reaction. J. Chem. Phys. 2006, 124, 124321–124327. [Google Scholar] [CrossRef]
- De More, W.B.; Golden, D.M.; Hampson, R.F.; Sander, S.P. Chemical Kinetics and Photochemical Data for Use in Stratospheric Modelling; Evaluation 12; Jet Propulsion Laboratory: Pasadena, CA, USA, 1997. [Google Scholar]
- Baulch, D.L.; Cobos, C.J.; Cox, R.A. Evaluated Kinetic Data for Combustion Modeling. Supplement I. J. Phys. Chem. Ref. Data 1999, 23, 847–848. [Google Scholar] [CrossRef]
- Sayos, R.; Oliva, C.; Gonzalez, M. New analytical surfaces and theoretical rate constants for the N(4S)+O2 reaction. J. Chem. Phys. 2002, 117, 670–678. [Google Scholar] [CrossRef]
- Bisikalo, D.V.; Shematovich, V.I.; Gérard, J.-C.; Hubert, B. Monte Carlo simulations of the interaction of fast proton and hydrogen atoms with the Martian atmosphere and comparison with in situ measurements. J. Geophys. Res. Space Phys. 2018, 123, 5850–5861. [Google Scholar] [CrossRef]
- Shematovich, V.I.; Bisikalo, D.V.; Zhilkin, A.G. Effects of column density variations of extended hydrogen corona of Mars on the charge exchange efficiency with solar wind protons. Astron. Rep. 2021, 65, 203–208. [Google Scholar] [CrossRef]
- Zhilkin, A.G.; Bisikalo, D.V.; Shematovich, V.I. Numerical model to investigate the proton auroras at Mars. Astron. Rep. 2022, 66, 245–249. [Google Scholar] [CrossRef]
- Zhilkin, A.G.; Bisikalo, D.V. Multi-Component MHD Model of Hot Jupiter Envelopes. Universe 2021, 7, 422. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).