Metabolomic Profiling of the Synergistic Effects of Melittin in Combination with Cisplatin on Ovarian Cancer Cells
Abstract
:1. Introduction
2. Results
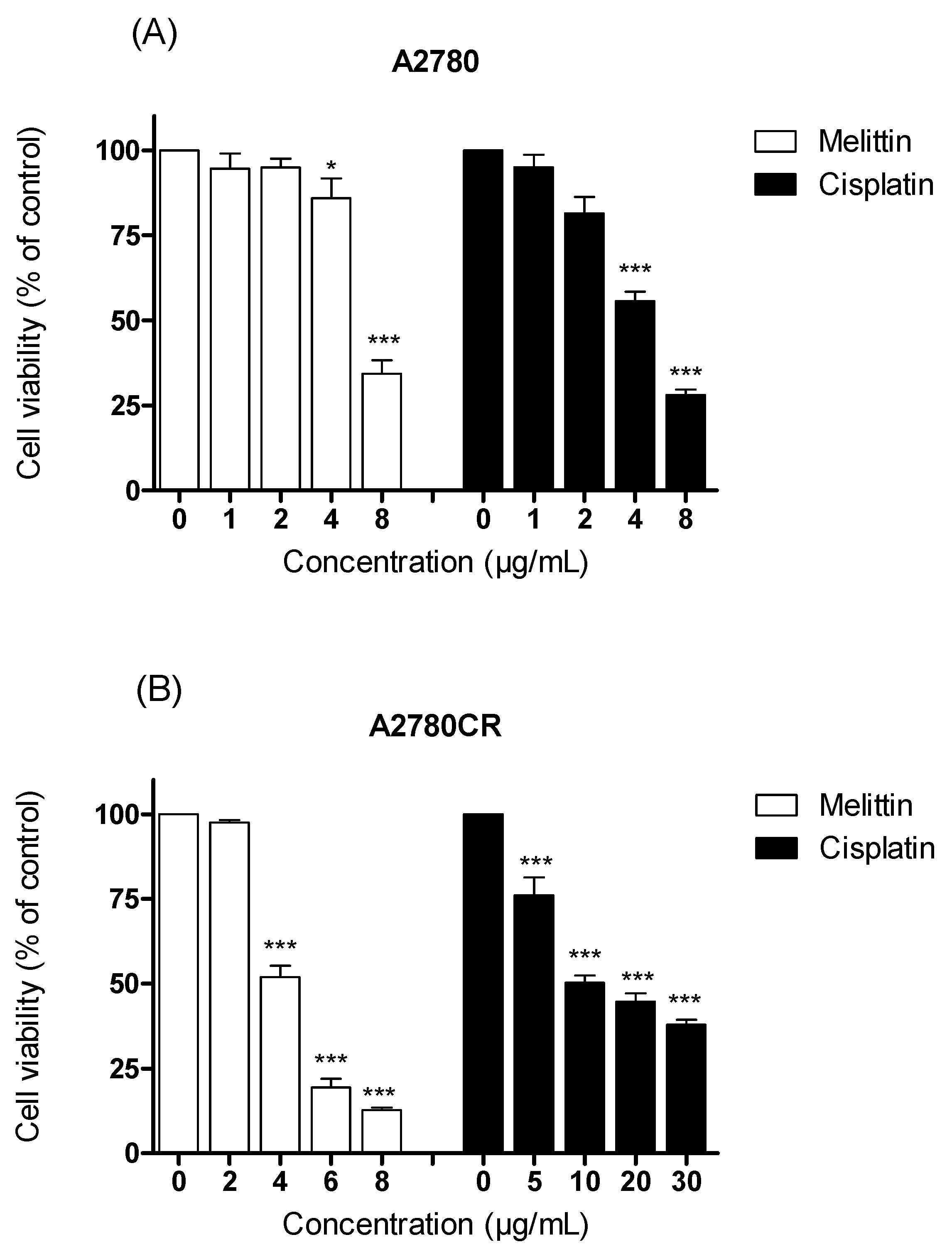
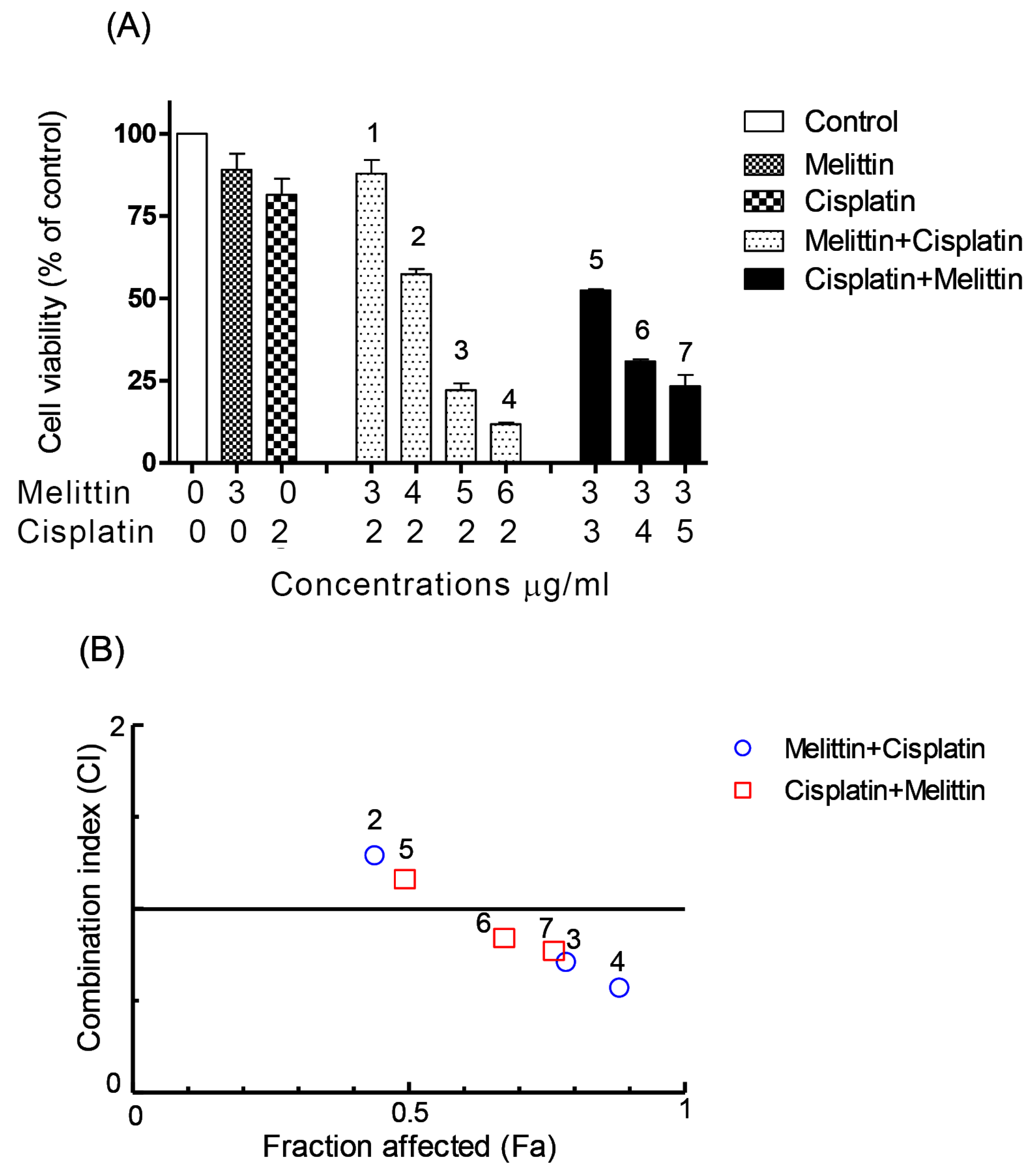
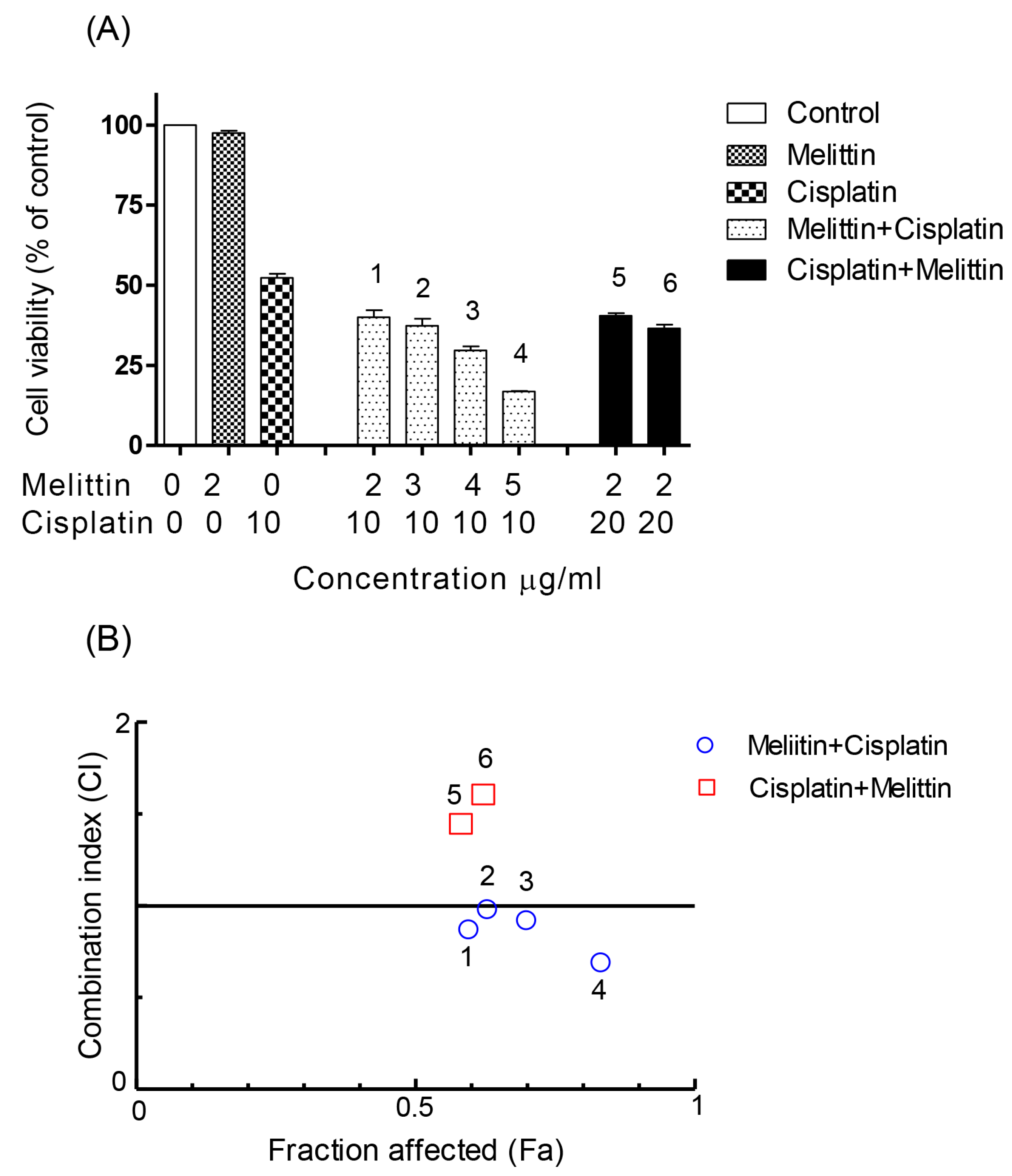
2.1. The Cytotoxicity of Melittin in Combination with Cisplatin
2.2. The Combination Index (CI)
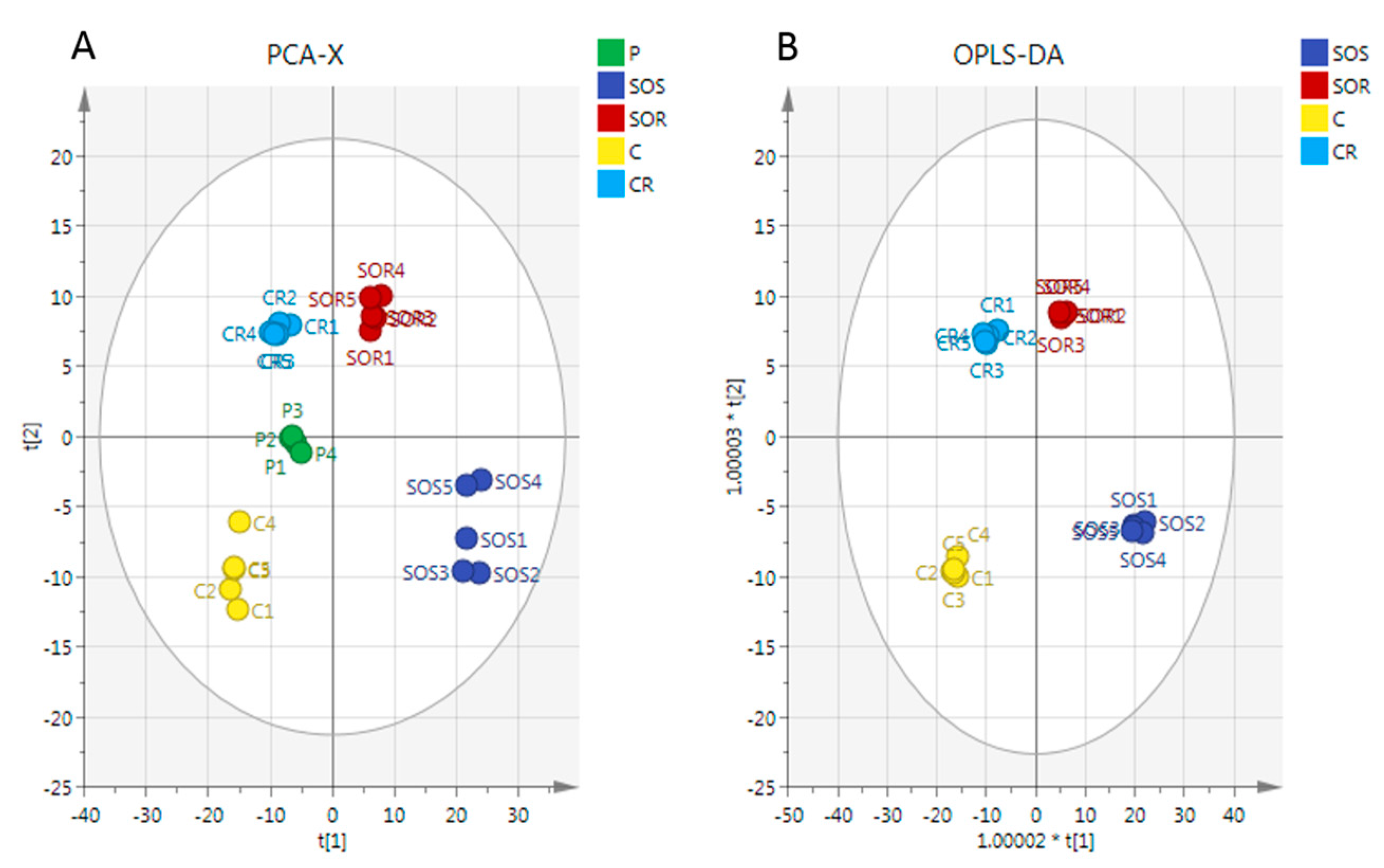
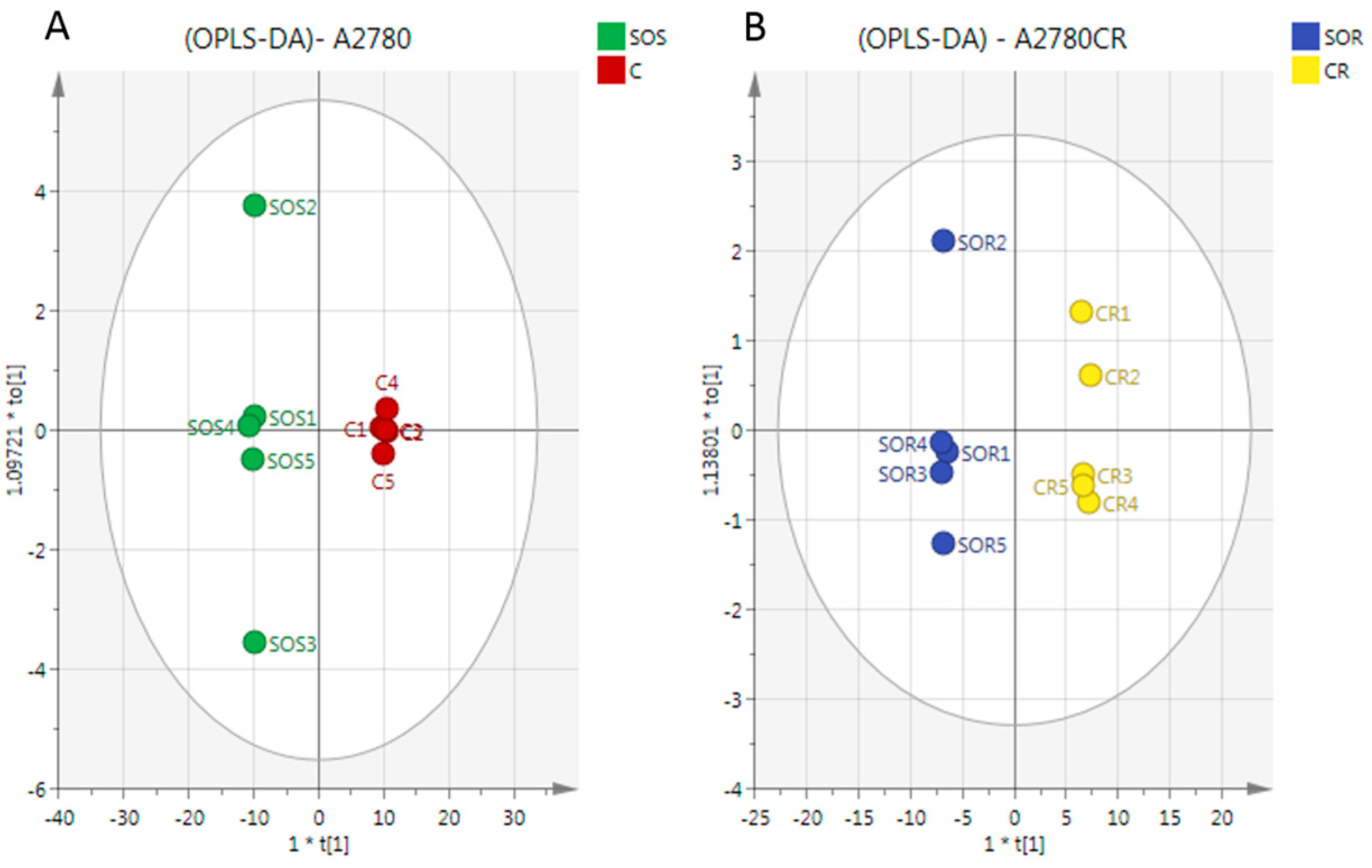
2.3. Metabolome Analysis
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cultures
4.2. Cell Viability Assay
4.3. Calculation of CI
4.4. Statistical Analysis
4.5. Determination of the Effect of Melittin in Combination with Cisplatin on Cell Metabolomes
4.6. LC-MS Conditions
4.7. Data Extraction and Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Takakura, M.; Nakamura, M.; Kyo, S.; Hashimoto, M.; Mori, N.; Ikoma, T.; Mizumoto, Y.; Fujiwara, T.; Urata, Y.; Inoue, M. Intraperitoneal administration of telomerase-specific oncolytic adenovirus sensitizes ovarian cancer cells to cisplatin and affects survival in a xenograft model with peritoneal dissemination. Cancer Gene Ther. 2010, 17, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.C.; Moreira, J.N.; Simões, S. Combination Chemotherapy in Cancer: Principles, Evaluation and Drug Delivery Strategies; INTECH Open Access Publisher: Rijeka, Croatia, 2011. [Google Scholar]
- Fontaine, F.; Overman, J.; François, M. Pharmacological manipulation of transcription factor protein-protein interactions: Opportunities and obstacles. Cell Regen. 2015, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, G.; Park, S.; Chung, H.-S.; Lee, H.; Kim, J.-Y.; Nam, S.; Kim, S.K.; Bae, H. Bee venom mitigates cisplatin-induced nephrotoxicity by regulating CD4. Evid.-Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.; Lee, G.; Jang, H.; Kim, S.-S.; Yoon, H.; Kang, G.-H.; Hwang, D.-S.; Kim, S.K.; Chung, H.-S. Phospholipase A2 inhibits cisplatin-induced acute kidney injury by modulating regulatory T cells by the CD206 mannose receptor. Kidney Int. 2015, 88, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Chvetzoff, G.; Bonnotte, B.; Chauffert, B. Anticancer chemotherapy. Prevention of toxicity. Presse Med. 1998, 27, 2106–2112. [Google Scholar] [PubMed]
- Choi, K.E.; Hwang, C.J.; Gu, S.M.; Park, M.H.; Kim, J.H.; Park, J.H.; Ahn, Y.J.; Kim, J.Y.; Song, M.J.; Song, H.S. Cancer cell growth inhibitory effect of bee venom via increase of death receptor 3 expression and inactivation of NF-kappa B in NSCLC cells. Toxins 2014, 6, 2210–2228. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.-S.; Moon, H.J.; Li, D.X.; Gil, M.; Min, J.K.; Lee, G.; Bae, H.; Kim, S.K.; Min, B.-I. Effect of bee venom acupuncture on oxaliplatin-induced cold allodynia in rats. Evid.-Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Jeon, J.-H.; Lee, Y.-W.; Cho, C.-K.; Kwon, K.-R.; Shin, J.-E.; Sagar, S.; Wong, R.; Yoo, H.-S. Sweet bee venom pharmacopuncture for chemotherapy-induced peripheral neuropathy. J. Acupunct. Meridian Stud. 2012, 5, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Gajski, G.; Čimbora-Zovko, T.; Rak, S.; Osmak, M.; Garaj-Vrhovac, V. Antitumour action on human glioblastoma A1235 cells through cooperation of bee venom and cisplatin. Cytotechnology 2016, 68, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Gajski, G.; Čimbora-Zovko, T.; Rak, S.; Rožman, M.; Osmak, M.; Garaj-Vrhovac, V. Combined antitumor effects of bee venom and cisplatin on human cervical and laryngeal carcinoma cells and their drug resistant sublines. J. Appl. Toxicol. 2014, 34, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Bae, H. Bee venom phospholipase A2: Yesterday’s enemy becomes today’s friend. Toxins 2016, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Kinsey, G.R.; Okusa, M.D. Expanding role of T cells in acute kidney injury. Curr. Opin. Nephrol. Hypertens. 2014, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Alizadehnohi, M.; Nabiuni, M.; Nazari, Z.; Safaeinejad, Z.; Irian, S. The synergistic cytotoxic effect of cisplatin and honey bee venom on human ovarian cancer cell line A2780cp. J. Venom Res. 2012, 3, 22. [Google Scholar] [PubMed]
- Alonezi, S.; Tusiimire, J.; Wallace, J.; Dufton, M.J.; Parkinson, J.A.; Young, L.C.; Clements, C.J.; Park, J.K.; Jeon, J.W.; Ferro, V.A. Metabolomic profiling of the effects of melittin on cisplatin resistant and cisplatin sensitive ovarian cancer cells using mass spectrometry and Biolog microarray technology. Metabolites 2016, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Palmnas, M.S.; Vogel, H.J. The future of NMR metabolomics in cancer therapy: Towards personalizing treatment and developing targeted drugs? Metabolites 2013, 3, 373–396. [Google Scholar] [CrossRef] [PubMed]
- Spratlin, J.L.; Serkova, N.J.; Eckhardt, S.G. Clinical applications of metabolomics in oncology: A review. Clin. Cancer Res. 2009, 15, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Odunsi, K.; Wollman, R.M.; Ambrosone, C.B.; Hutson, A.; McCann, S.E.; Tammela, J.; Geisler, J.P.; Miller, G.; Sellers, T.; Cliby, W. Detection of epithelial ovarian cancer using 1H-NMR-based metabonomics. Int. J. Cancer 2005, 113, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-F.; Lu, J.; Wang, X.-Z.; Guo, W.-H.; Zhang, J.-X. Comparative metabolomic profiling of hepatocellular carcinoma cells treated with sorafenib monotherapy vs. sorafenib-everolimus combination therapy. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 1781. [Google Scholar] [CrossRef] [PubMed]
- Patrick Reynolds, C.; Maurer, B.J. Evaluating response to antineoplastic drug combinations in tissue culture models. Chemosensitivity 2005, 110, 173–183. [Google Scholar]
- Chou, T.-C.; Talalay, P. Analysis of combined drug effects: A new look at a very old problem. Trends Pharmacol. Sci. 1983, 4, 450–454. [Google Scholar] [CrossRef]
- Chou, T.-C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2016. [Google Scholar] [CrossRef]
- Alonezi, S.; Al Washih, M.; Clements, C.J.; Young, L.; Ferro, V.A.; Watson, D.G. Current liquid chromatography mass spectrometry (LCMS) and phenotype microarray profiling of ovarian cancer cells after exposure to cisplatin. Curr. Metabol. 2017, 5. [Google Scholar] [CrossRef]
- Chou, T.-C.; Motzer, R.J.; Tong, Y.; Bosl, G.J. Computerized quantitation of synergism and antagonism of taxol, topotecan, and cisplatin against human teratocarcinoma cell growth: A rational approach to clinical protocol design. J. Natl. Cancer Inst. 1994, 86, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Gulati, S.; Chou, T.; Vega, R.; Gandola, L.; Ibrahim, S.E.; Yopp, J.; Colvin, M.; Clarkson, B. Synergistic effect of 4-hydroperoxycyclophosphamide and etoposide on a human promyelocytic leukemia cell line (HL-60) demonstrated by computer analysis. Cancer Res. 1985, 45, 2434–2439. [Google Scholar] [PubMed]
- Bible, K.C.; Kaufmann, S.H. Cytotoxic synergy between flavopiridol (NSC 649890, L86-8275) and various antineoplastic agents: The importance of sequence of administration. Cancer Res. 1997, 57, 3375–3380. [Google Scholar] [PubMed]
- Wang, J.; Jin, L.; Li, X.; Deng, H.; Chen, Y.; Lian, Q.; Ge, R.; Deng, H. Gossypol induces apoptosis in ovarian cancer cells through oxidative stress. Mol. Biosyst. 2013, 9, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Vermeersch, K.A.; Wang, L.; McDonald, J.F.; Styczynski, M.P. Distinct metabolic responses of an ovarian cancer stem cell line. BMC Syst. Biol. 2014, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Tolstikov, V.; Nikolayev, A.; Dong, S.; Zhao, G.; Kuo, M.-S. Metabolomics analysis of metabolic effects of nicotinamide phosphoribosyltransferase (NAMPT) inhibition on human cancer cells. PLoS ONE 2014, 9, e114019. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Bell, L.N.; Okamura, J.; Kim, M.S.; Mohney, R.P.; Guerrero-Preston, R.; Ratovitski, E.A. Phospho-ΔNp63α/SREBF1 protein interactions: Bridging cell metabolism and cisplatin chemoresistance. Cell Cycle 2012, 11, 3810–3827. [Google Scholar] [CrossRef] [PubMed]
- Chaney, S.G.; Campbell, S.L.; Bassett, E.; Wu, Y. Recognition and processing of cisplatin- and oxaliplatin-DNA adducts. Crit. Rev. Oncol./Hematol. 2005, 53, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Luo, R. Metabolomic response to sorafenib treatment in human hepatocellular carcinoma cells. FASEB J. 2013, 27, 663–667. [Google Scholar]
- Gong, H.; Zölzer, F.; Von Recklinghausen, G.; Havers, W.; Schweigerer, L. Arginine deiminase inhibits proliferation of human leukemia cells more potently than asparaginase by inducing cell cycle arrest and apoptosis. Leukemia 2000, 14, 826. [Google Scholar] [CrossRef] [PubMed]
- Szlosarek, P.W.; Klabatsa, A.; Pallaska, A.; Sheaff, M.; Smith, P.; Crook, T.; Grimshaw, M.J.; Steele, J.P.; Rudd, R.M.; Balkwill, F.R. In vivo loss of expression of argininosuccinate synthetase in malignant pleural mesothelioma is a biomarker for susceptibility to arginine depletion. Clin. Cancer Res. 2006, 12, 7126–7131. [Google Scholar] [CrossRef] [PubMed]
- Feun, L.; You, M.; Wu, C.; Kuo, M.; Wangpaichitr, M.; Spector, S.; Savaraj, N. Arginine deprivation as a targeted therapy for cancer. Curr. Pharm. Des. 2008, 14, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Ensor, C.M.; Holtsberg, F.W.; Bomalaski, J.S.; Clark, M.A. Pegylated arginine deiminase (ADI-SS PEG20, 000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res. 2002, 62, 5443–5450. [Google Scholar] [PubMed]
- Lind, D.S. Arginine and cancer. J. Nutr. 2004, 134 (Suppl. S10), 2837S–2841S. [Google Scholar] [PubMed]
- Shuvayeva, G.; Bobak, Y.; Igumentseva, N.; Titone, R.; Morani, F.; Stasyk, O.; Isidoro, C. Single amino acid arginine deprivation triggers prosurvival autophagic response in ovarian carcinoma SKOV3. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, L.J.; Smith, P.R.; Hiller, L.; Szlosarek, P.W.; Kimberley, C.; Sehouli, J.; Koensgen, D.; Mustea, A.; Schmid, P.; Crook, T. Epigenetic silencing of argininosuccinate synthetase confers resistance to platinum-induced cell death but collateral sensitivity to arginine auxotrophy in ovarian cancer. Int. J. Cancer 2009, 125, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Poisson, L.M.; Munkarah, A.; Madi, H.; Datta, I.; Hensley-Alford, S.; Tebbe, C.; Buekers, T.; Giri, S.; Rattan, R. A metabolomic approach to identifying platinum resistance in ovarian cancer. J. Ovarian Res. 2015, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Mohell, N.; Alfredsson, J.; Fransson, Å.; Uustalu, M.; Byström, S.; Gullbo, J.; Hallberg, A.; Bykov, V.; Björklund, U.; Wiman, K. Apr-246 overcomes resistance to cisplatin and doxorubicin in ovarian cancer cells. Cell Death Dis. 2015, 6, e1794. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.L.; Fallon, H.J.; Morris, H.P. Two enzymes of serine metabolism in rat liver and hepatomas. Cancer Res. 1970, 30, 2917–2920. [Google Scholar] [PubMed]
- Snell, K. Enzymes of serine metabolism in normal, developing and neoplastic rat tissues. Adv. Enzyme Regul. 1984, 22, 325–400. [Google Scholar] [CrossRef]
- Locasale, J.W. Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nat. Rev. Cancer 2013, 13, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Mattaini, K.R.; Sullivan, M.R.; Vander Heiden, M.G. The importance of serine metabolism in cancer. J. Cell Biol. 2016, 214, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, O.D.; Berkers, C.R.; Mason, S.M.; Zheng, L.; Blyth, K.; Gottlieb, E.; Vousden, K.H. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 2013, 493, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Vousden, K.H.; Prives, C. Blinded by the light: The growing complexity of p53. Cell 2009, 137, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Amelio, I.; Cutruzzolá, F.; Antonov, A.; Agostini, M.; Melino, G. Serine and glycine metabolism in cancer. Trends Biochem. Sci. 2014, 39, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Lemasters, J.J.; Qian, T.; He, L.; Kim, J.-S.; Elmore, S.P.; Cascio, W.E.; Brenner, D.A. Role of mitochondrial inner membrane permeabilization in necrotic cell death, apoptosis, and autophagy. Antioxid. Redox Signal. 2002, 4, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Skulachev, V. Bioenergetic aspects of apoptosis, necrosis and mitoptosis. Apoptosis 2006, 11, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Vanlangenakker, N.; Berghe, T.V.; Krysko, D.V.; Festjens, N.; Vandenabeele, P. Molecular mechanisms and pathophysiology of necrotic cell death. Curr. Mol. Med. 2008, 8, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Dong, S.; Shepard, R.L.; Kays, L.; Roth, K.D.; Geeganage, S.; Kuo, M.-S.; Zhao, G. Inhibition of nicotinamide phosphoribosyltransferase (NAMPT), an enzyme essential for NAD+ biosynthesis, leads to altered carbohydrate metabolism in cancer cells. J. Biol. Chem. 2015, 290, 15812–15824. [Google Scholar] [CrossRef] [PubMed]
- Giannetti, A.M.; Zheng, X.; Skelton, N.J.; Wang, W.; Bravo, B.J.; Bair, K.W.; Baumeister, T.; Cheng, E.; Crocker, L.; Feng, Y. Fragment-based identification of amides derived from trans-2-(pyridin-3-yl) cyclopropanecarboxylic acid as potent inhibitors of human nicotinamide phosphoribosyltransferase (NAMPT). J. Biol. Chem. 2014, 57, 770–792. [Google Scholar]
- Zheng, X.; Bauer, P.; Baumeister, T.; Buckmelter, A.J.; Caligiuri, M.; Clodfelter, K.H.; Han, B.; Ho, Y.-C.; Kley, N.; Lin, J. Structure-based discovery of novel amide-containing nicotinamide phosphoribosyltransferase (NAMPT) inhibitors. J. Biol. Chem. 2013, 56, 6413–6433. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-W.; Wang, H.; Poitras, M.F.; Coombs, C.; Bowers, W.J.; Federoff, H.J.; Poirier, G.G.; Dawson, T.M.; Dawson, V.L. Mediation of poly (ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 2002, 297, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhang, X.; Han, Y.Y.; Burke, N.A.; Kochanek, P.M.; Watkins, S.C.; Graham, S.H.; Carcillo, J.A.; Szabó, C.; Clark, R.S. Intra-mitochondrial poly (ADP-ribosylation) contributes to NAD+ depletion and cell death induced by oxidative stress. J. Biol. Chem. 2003, 278, 18426–18433. [Google Scholar] [CrossRef] [PubMed]
- Tusiimire, J.; Wallace, J.; Dufton, M.; Parkinson, J.; Clements, C.J.; Young, L.; Park, J.K.; Jeon, J.W.; Watson, D.G. An LCMS method for the assay of melittin in cosmetic formulations containing bee venom. Anal. Bioanal. Chem. 2015, 407, 3627–3635. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.; Martin, N. Compusyn for Drug Combinations: Pc Software and User’s Guide: A Computer Program for Quantitation of Synergism and Antagonism in Drug Combinations, and the Determination of IC50 and ED50 and LD50 Values; ComboSyn Inc.: Paramus, NJ, USA, 2005. [Google Scholar]
- Zhang, R.; Watson, D.G.; Wang, L.; Westrop, G.D.; Coombs, G.H.; Zhang, T. Evaluation of mobile phase characteristics on three zwitterionic columns in hydrophilic interaction liquid chromatography mode for liquid chromatography-high resolution mass spectrometry based untargeted metabolite profiling of leishmania parasites. J. Chromatogr. A 2014, 1362, 168–179. [Google Scholar] [CrossRef] [PubMed]
| m/z | RT (min) | Metabolites | SR/CR | SS/C | ||
|---|---|---|---|---|---|---|
| p-Value | Ratio | p-Value | Ratio | |||
| Citrate cycle (TCA cycle)/glycolysis | ||||||
| 338.989 | 18.1 | * D-Fructose 1,6-bisphosphate | <0.001 | 0.278 | <0.001 | 0.246 |
| 115.004 | 16.0 | * Fumarate | <0.001 | 0.227 | <0.01 | 0.402 |
| 133.014 | 16.1 | * (S)-Malate | <0.001 | 0.218 | <0.001 | 0.062 |
| 145.014 | 15.7 | * 2-Oxoglutarate | <0.001 | 0.324 | <0.001 | 0.048 |
| 191.02 | 18.1 | * Citrate | <0.001 | 0.588 | <0.001 | 0.172 |
| 173.009 | 17.9 | * cis-Aconitate | <0.001 | 0.490 | <0.001 | 0.072 |
| 166.975 | 17.5 | * Phosphoenolpyruvate | <0.001 | 0.344 | <0.05 | 0.082 |
| Oxidative phosphorylation | ||||||
| 664.116 | 14.2 | * NAD+ | <0.001 | 0.294 | <0.001 | 0.067 |
| 508.003 | 16.2 | * ATP | <0.001 | 0.362 | <0.001 | 0.073 |
| Glycine/Serine/Cysteine and Glutathione | ||||||
| 241.031 | 16.3 | * L-Cystine | ns | 1.739 | ns | 1.832 |
| 427.095 | 17.1 | S-Glutathionyl-L-cysteine | ns | 0.750 | <0.001 | 0.218 |
| 152.002 | 14.6 | 3-Sulfino-L-alanine | ns | 0.706 | <0.001 | 0.139 |
| 308.091 | 14.5 | * Glutathione (GSH) | <0.001 | 0.562 | <0.001 | 0.092 |
| 179.048 | 14.4 | L- Cysteinylglycine (Cys-Gly) | <0.001 | 0.521 | <0.001 | 0.067 |
| 223.074 | 17.1 | * L-Cystathionine | <0.001 | 0.438 | <0.001 | 0.004 |
| 76.0394 | 15.7 | * Glycine | ns | 1.050 | <0.001 | 0.142 |
| 116.035 | 10.8 | L-2-Amino-3-oxobutanoic acid | <0.001 | 0.319 | <0.001 | 0.317 |
| 106.050 | 15.8 | * L-Serine | <0.001 | 7.112 | <0.001 | 0.420 |
| Pentose phosphate pathway | ||||||
| 195.051 | 13.7 | * D-Gluconic acid | <0.001 | 0.147 | <0.001 | 0.093 |
| 308.978 | 16.5 | D-Ribose 1,5-bisphosphate | <0.001 | 0.259 | <0.001 | 0.124 |
| 149.046 | 11.9 | * D-Ribose | <0.001 | 0.222 | <0.001 | 0.285 |
| 229.012 | 15.8 | * D-Ribose 5-phosphate | <0.001 | 0.545 | <0.001 | 0.131 |
| Lysine biosynthesis | ||||||
| 170.046 | 14.3 | 2,3,4,5-Tetrahydrodipicolinate | <0.001 | 0.245 | <0.001 | 0.112 |
| 147.113 | 24.3 | * L-Lysine | <0.001 | 1.966 | ns | 0.724 |
| 162.112 | 13.3 | * L-Carnitine | <0.001 | 0.508 | <0.001 | 0.135 |
| 243.074 | 17.2 | 5-Phosphonooxy-L-lysine | <0.001 | 0.303 | <0.001 | 0.006 |
| 128.071 | 15.5 | 2,3,4,5-Tetrahydropyridine-2-carboxylate | <0.001 | 0.433 | <0.001 | 0.271 |
| Purine metabolism | ||||||
| 137.046 | 10.2 | * Hypoxanthine | <0.001 | 16.99 | <0.01 | 7.87 |
| 152.056 | 12.4 | * Guanine | <0.001 | 13.19 | <0.01 | 102.21 |
| 348.07 | 13.8 | * AMP | <0.001 | 0.372 | <0.001 | 0.190 |
| 428.036 | 15.0 | * ADP | <0.001 | 0.376 | <0.001 | 0.163 |
| 442.018 | 17.8 | GDP | <0.001 | 0.509 | ns | 0.104 |
| 521.984 | 18.9 | * GTP | <0.001 | 0.442 | <0.001 | 0.079 |
| 426.013 | 16.9 | Adenylyl sulfate | <0.001 | 0.271 | <0.01 | 0.014 |
| Pyrimidine metabolism | ||||||
| 155.01 | 10.3 | * Orotate | <0.001 | 0.218 | <0.001 | 0.050 |
| 129.066 | 14.8 | 5,6-Dihydrothymine | <0.001 | 0.326 | <0.001 | 0.120 |
| 480.982 | 15.8 | dTTP | <0.001 | 0.337 | <0.001 | 0.347 |
| 175.036 | 16.8 | N-Carbamoyl-L-aspartate | <0.001 | 0.036 | <0.001 | 0.017 |
| 115.05 | 14.7 | 5,6-Dihydrouracil | <0.001 | 0.383 | <0.001 | 0.079 |
| 402.995 | 16.4 | * UDP | <0.001 | 0.485 | <0.001 | 0.035 |
| 484.975 | 17.6 | * UTP | <0.001 | 0.465 | <0.001 | 0.059 |
| 323.029 | 15.2 | * UMP | ns | 1.195 | <0.001 | 0.282 |
| Arginine/Proline/Glutamate/Methionine | ||||||
| 188.057 | 14.3 | N-Acetyl-L-glutamate | <0.001 | 0.241 | <0.001 | 0.029 |
| 176.103 | 15.8 | * L-Citrulline | <0.001 | 0.521 | <0.001 | 0.189 |
| 173.104 | 25.8 | * L-Arginine | ns | 1.161 | <0.001 | 0.379 |
| 130.051 | 14.5 | L-Glutamate-5-semialdehyde | <0.001 | 0.538 | <0.001 | 0.235 |
| 116.071 | 12.8 | * L-Proline | <0.001 | 0.568 | <0.001 | 0.228 |
| 399.144 | 16.3 | * S-Adenosyl-L-methionine | <0.001 | 2.005 | <0.001 | 0.115 |
| 298.096 | 6.4 | * 5′-Methylthioadenosine | <0.05 | 2.016 | <0.001 | 0.158 |
| 146.093 | 15.1 | * 4-Guanidinobutanoate | <0.001 | 0.325 | <0.001 | 0.055 |
| 291.129 | 16.8 | N-(L-Arginino) succinate | <0.001 | 0.260 | <0.001 | 0.010 |
| 247.14 | 14.2 | N2-(D-1-Carboxyethyl)-L-arginine | <0.001 | 0.170 | <0.001 | 0.010 |
| 174.087 | 15.3 | 5-Guanidino-2 oxopentanoate | <0.001 | 0.680 | <0.001 | 0.058 |
| 132.077 | 14.7 | * Creatine | <0.001 | 0.403 | <0.001 | 0.075 |
| 210.029 | 15.2 | * Phosphocreatine | <0.001 | 0.422 | <0.001 | 0.051 |
| Miscellaneous | ||||||
| 110.027 | 14.9 | Hypotaurine | <0.001 | 0.139 | <0.001 | 0.009 |
| 115.04 | 8.2 | 3-Methyl-2-oxobutanoic acid | <0.001 | 0.191 | <0.05 | 5.604 |
| 166.053 | 13.4 | L-Methionine S-oxide | <0.01 | 0.470 | <0.001 | 0.250 |
| 218.067 | 13.9 | O-Succinyl-L-homoserine | <0.001 | 0.082 | <0.001 | 0.011 |
| 181.051 | 9.0 | 3-(4-Hydroxyphenyl)lactate | <0.001 | 0.656 | <0.001 | 0.087 |
| 204.123 | 11.1 | * O-Acetylcarnitine | <0.001 | 0.137 | <0.001 | 0.021 |
| 176.056 | 10.3 | 4-Hydroxy-4-methylglutamate | ns | 1.911 | <0.001 | 0.022 |
| 159.076 | 15.8 | 4-Methylene-L-glutamine | <0.001 | 0.607 | ns | 0.258 |
| 175.025 | 14.4 | * Ascorbate | <0.001 | 0.067 | <0.001 | 0.024 |
| 165.041 | 13.0 | L-Arabinonate | <0.001 | 0.372 | <0.001 | 0.166 |
| 179.056 | 17.1 | Hexose | <0.05 | 0.670 | <0.05 | 0.334 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonezi, S.; Tusiimire, J.; Wallace, J.; Dufton, M.J.; Parkinson, J.A.; Young, L.C.; Clements, C.J.; Park, J.-K.; Jeon, J.-W.; Ferro, V.A.; et al. Metabolomic Profiling of the Synergistic Effects of Melittin in Combination with Cisplatin on Ovarian Cancer Cells. Metabolites 2017, 7, 14. https://doi.org/10.3390/metabo7020014
Alonezi S, Tusiimire J, Wallace J, Dufton MJ, Parkinson JA, Young LC, Clements CJ, Park J-K, Jeon J-W, Ferro VA, et al. Metabolomic Profiling of the Synergistic Effects of Melittin in Combination with Cisplatin on Ovarian Cancer Cells. Metabolites. 2017; 7(2):14. https://doi.org/10.3390/metabo7020014
Chicago/Turabian StyleAlonezi, Sanad, Jonans Tusiimire, Jennifer Wallace, Mark J. Dufton, John A. Parkinson, Louise C. Young, Carol J. Clements, Jin-Kyu Park, Jong-Woon Jeon, Valerie A. Ferro, and et al. 2017. "Metabolomic Profiling of the Synergistic Effects of Melittin in Combination with Cisplatin on Ovarian Cancer Cells" Metabolites 7, no. 2: 14. https://doi.org/10.3390/metabo7020014
APA StyleAlonezi, S., Tusiimire, J., Wallace, J., Dufton, M. J., Parkinson, J. A., Young, L. C., Clements, C. J., Park, J.-K., Jeon, J.-W., Ferro, V. A., & Watson, D. G. (2017). Metabolomic Profiling of the Synergistic Effects of Melittin in Combination with Cisplatin on Ovarian Cancer Cells. Metabolites, 7(2), 14. https://doi.org/10.3390/metabo7020014







