Metabolomic Screening of Tumor Tissue and Serum in Glioma Patients Reveals Diagnostic and Prognostic Information
Abstract
:1. Introduction
2. Results
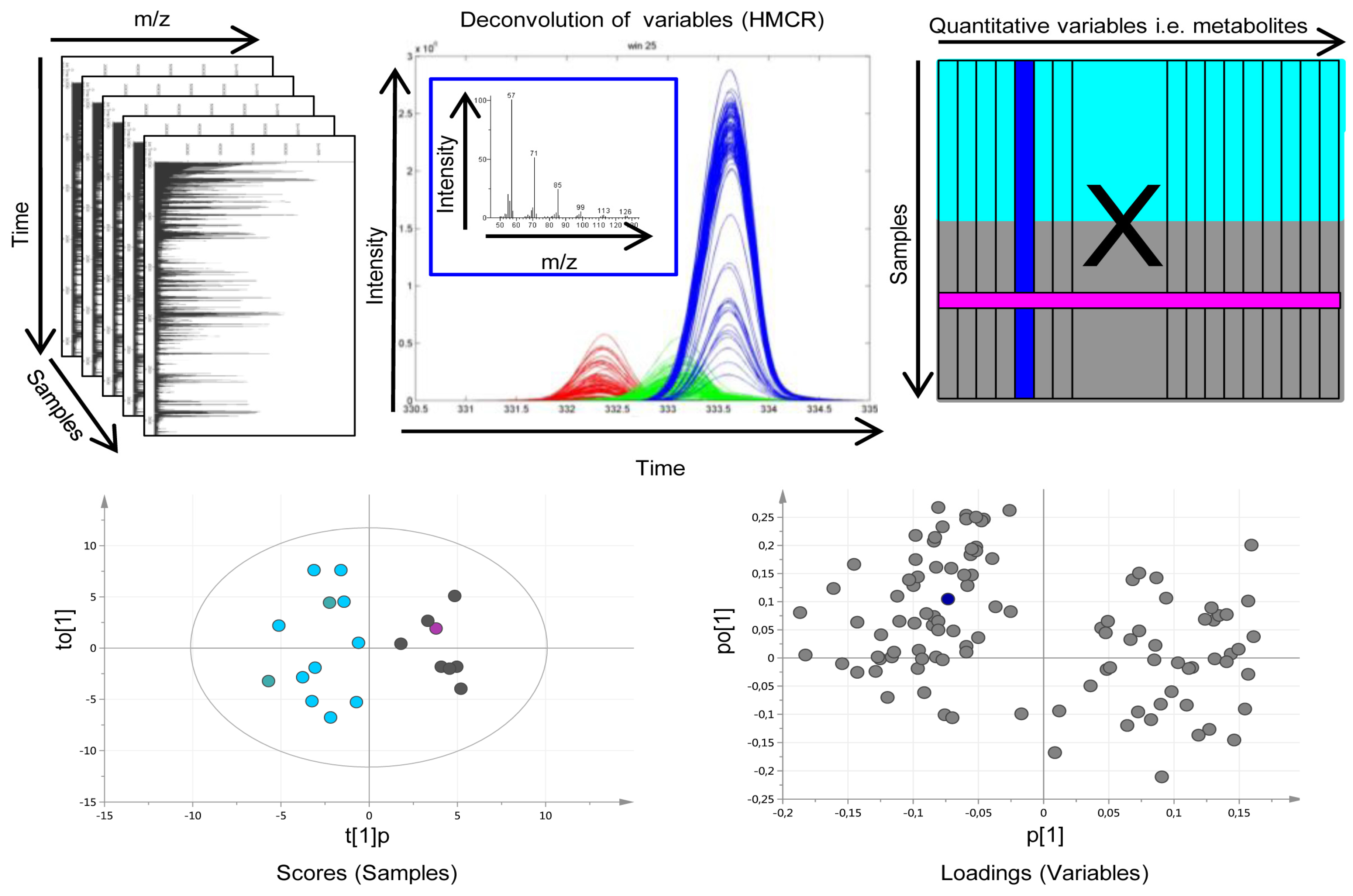
2.1. Data Processing and Curation
2.2. GBM and Oligodendroglioma Show Different Metabolic Patterns
| Tissue | Serum | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metabolite Id | RI | Corr. Diagnosis GBM vs. Oligo | Corr. Grade Oligo | Corr. Survival GBM | Corr. Survival Oligo | RI | Corr. Diagnosis GBM vs. Oligo | Corr. Grade Oligo | Corr. Survival GBM | Corr. Survival Oligo |
| 1-Monohexadecanoylglycerol | 2679 | ↓ * | ||||||||
| 2-Hydroxyglutaric acid | 1570.5 | ↓ * | ||||||||
| 2-Oxoisocaproic acid | - | ↓ | ||||||||
| 4-Aminobutyric acid (GABA) | 1525.3 | ↓ * | ||||||||
| Alanine | 1472.4 | ↑ | ||||||||
| Aminomalonic acid | 1465.0 | ↓ * | ||||||||
| Creatinine | 1548.3 | ↓ * | ||||||||
| Cystine | 2385.4 | ↑ * | ||||||||
| Fructose | 1858.8 | ↓ * | ↑ * | |||||||
| Glycerol-2-phosphate | 1714.6 | ↓ * | ||||||||
| Glycerol-3-phosphate | - | ↓ * | ↑ * | - | ||||||
| Glycine | 1305.5 | ↓ * | ||||||||
| Hexadecenoic acid | 2123.6 | ↑ | ||||||||
| Lauric acid | 1749.9 | ↓ | ||||||||
| Lysine | 2020.7 | ↓ | ||||||||
| Maltose | 2824.1 | ↑ | ↓ | |||||||
| Mannitol | 1917.5 | ↑ * | ↑* | 2029.0 | ↑* | |||||
| Myo-Inositol | - | ↓ * | ↑ * | ↑ | - | ↑ * | ||||
| Oxalic acid | 1118.3 | ↓* | ||||||||
| Phenylalanine | 1621.0 | ↑ * | 1722.0 | |||||||
| Ribitol | 1708.2 | ↓ * | ↑ * | ↑ * | ||||||
| Serine | 1358.4 | ↑ | ||||||||
| Spermidine | 2244.7 | ↑ * | ||||||||
| Sterol | 2864.5 | ↓ | ||||||||
| Threonic acid | 1551.6 | ↑ | ||||||||
| Threonic acid-1,4-lactone | 1472.2 | ↑ | ||||||||
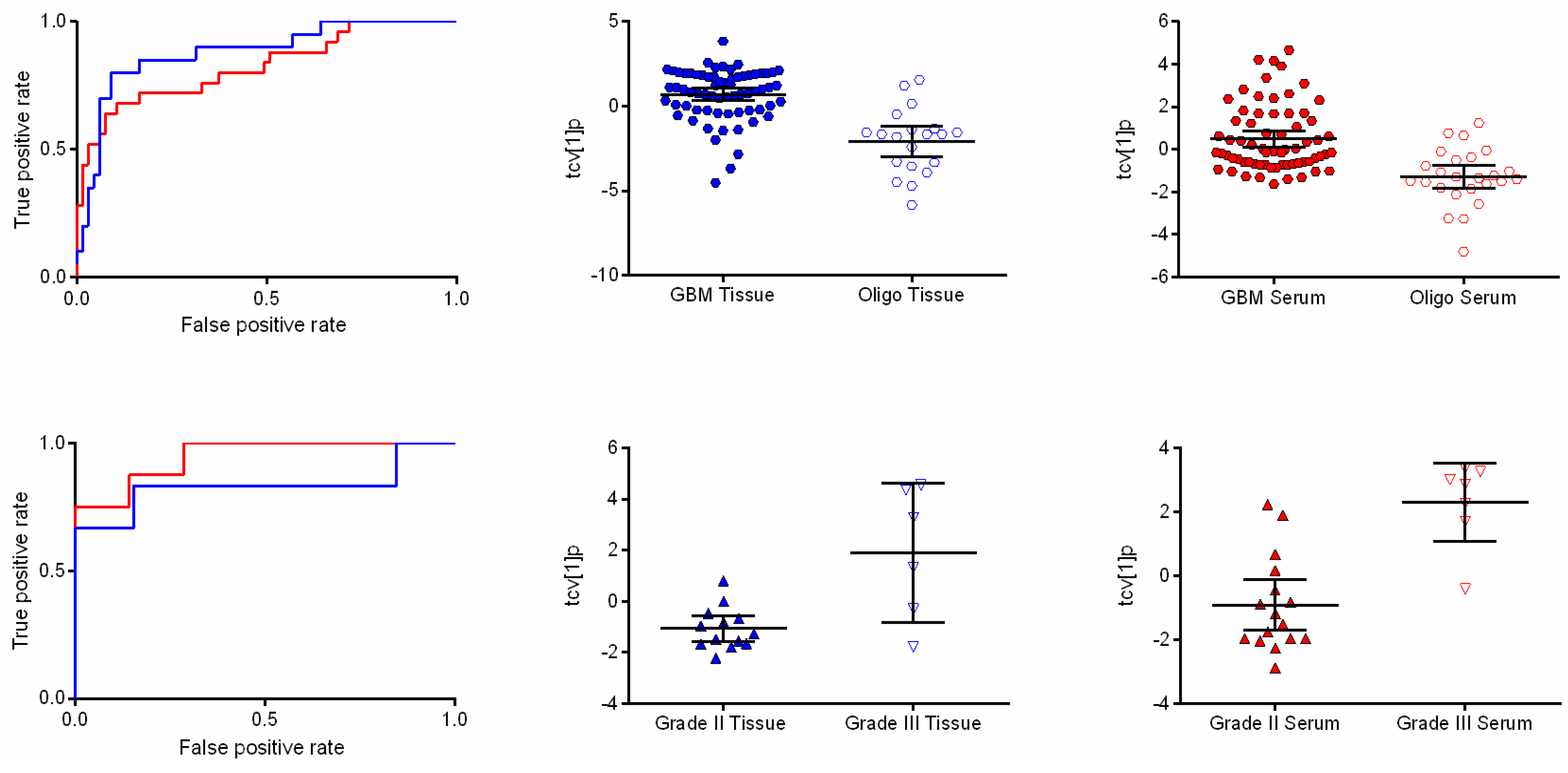
2.3. Metabolic Differences between Oligodendroglioma WHO Grade II and III
2.4. Metabolic Profiles Associated with Survival
2.5. Pathway Analysis
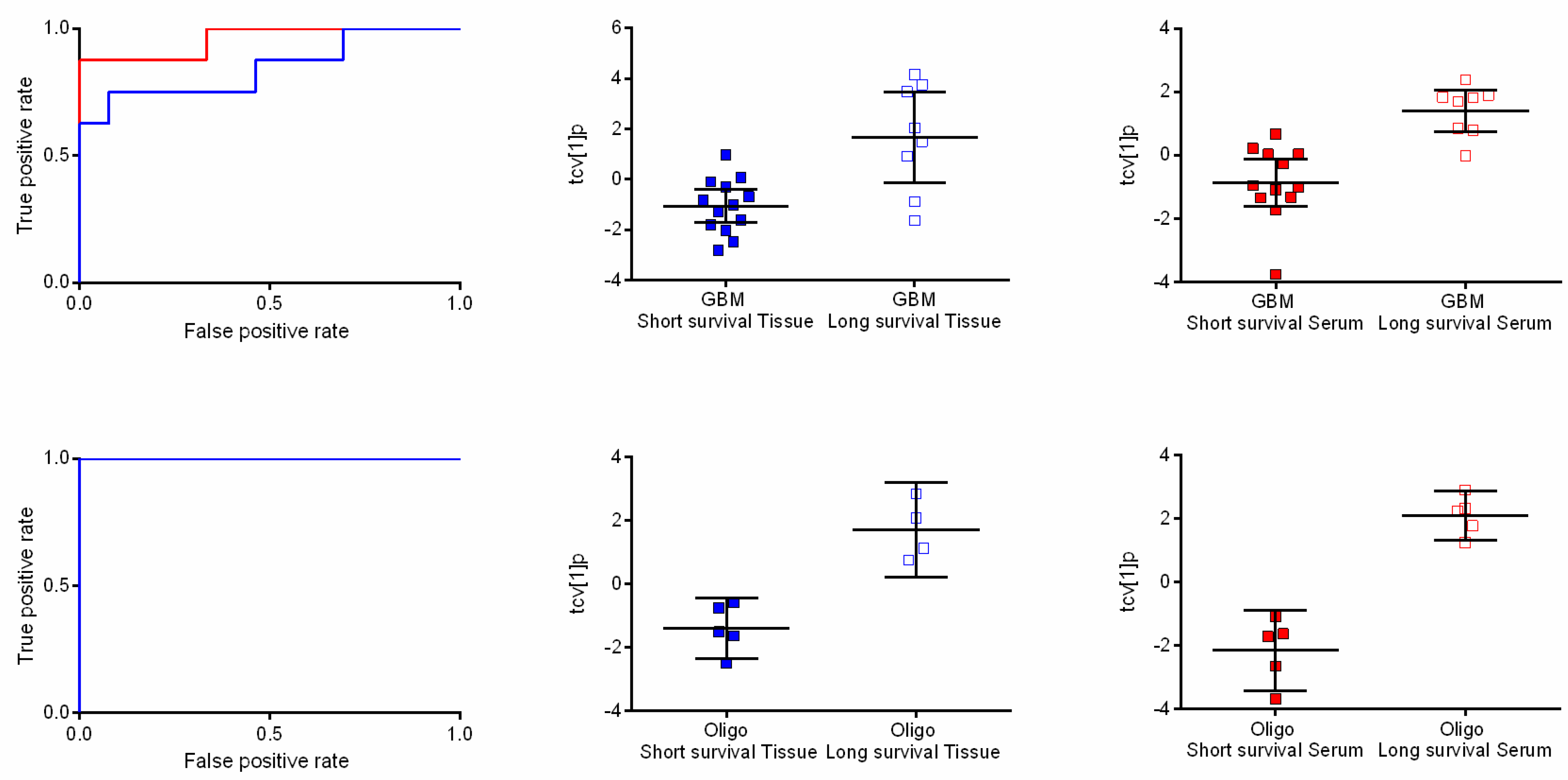
3. Discussion
3.1. Metabolomic Differences Associated with Diagnoses and Grading
3.2. The Metabolome as Prognostic Factor
3.3. Metabolic Pathways and Specific Metabolites of Interest
3.4. Multivariate Metabolic Patterns and Latent Biomarkers
4. Method
4.1. Samples
4.2. Sample Preparation and GC-TOFMS Analysis
4.3. Data Processing
4.4. Pattern Recognition and Statistical Analysis
4.5. Pathway Analysis
Acknowledgments
Authors Contributions
Conflicts of Interest
References
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K. WHO Classification of Tumours of the Central Nervous System; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, G.; Nakazato, Y.; Irie, T.; Okada, T.; Abe, M. Indeterminacy in the WHO classification of tumors: An example of the histopathological diagnosis of brain tumors. Brain Tumor pathol. 2011, 28, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Polley, M.Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.F.; Smith, J.S.; Chang, S.M.; Lamborn, K.R.; Prados, M.D.; Butowski, N.; Barbaro, N.M.; Parsa, A.T.; Berger, M.S.; McDermott, M.M. Preoperative prognostic classification system for hemispheric low-grade gliomas in adults. J. Neurosurg. 2008, 109, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Catalan-Uribarrena, G.; Bilbao-Barandica, G.; Pomposo-Gaztelu, I.; Undabeitia-Huertas, J.; Ruiz de Gopegui-Ruiz, E.; Galbarriatu-Gutierrez, L.; Canales-Llantada, M.; Aurrecoechea-Obieta, J.; Igartua-Azkune, A.; Carbayo-Lozano, G. Prognostic factors and survival in a prospective cohort of patients with high-grade glioma treated with carmustine wafers or temozolomide on an intention-to-treat basis. Acta Neurochir. 2012, 154, 211–222; discussion 222. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.R.; O’Neill, B.P. Glioblastoma survival in the United States before and during the temozolomide era. J. Neuro-oncol. 2012, 107, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Helseth, R.; Helseth, E.; Johannesen, T.B.; Langberg, C.W.; Lote, K.; Ronning, P.; Scheie, D.; Vik, A.; Meling, T.R. Overall survival, prognostic factors, and repeated surgery in a consecutive series of 516 patients with glioblastoma multiforme. Acta Neurol. Scand. 2010, 122, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, C.; Bowman, C.; Maurage, C.A.; Dubois, F.; Blond, S.; Porchet, N.; Escande, F. Loss of 1p, 19q, and 10q heterozygosity prospectively predicts prognosis of oligodendroglial tumors—Towards individualized tumor treatment? Neuro-oncology 2010, 12, 490–499. [Google Scholar] [PubMed]
- Weller, M.; Felsberg, J.; Hartmann, C.; Berger, H.; Steinbach, J.P.; Schramm, J.; Westphal, M.; Schackert, G.; Simon, M.; Tonn, J.C.; et al. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: A prospective translational study of the German Glioma Network. J. Clin. Oncol. 2009, 27, 5743–5750. [Google Scholar] [CrossRef] [PubMed]
- Cairncross, J.G.; Ueki, K.; Zlatescu, M.C.; Lisle, D.K.; Finkelstein, D.M.; Hammond, R.R.; Silver, J.S.; Stark, P.C.; Macdonald, D.R.; Ino, Y.; et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J. Natl. Cancer Inst. 1998, 90, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Robertson, T.; Koszyca, B.; Gonzales, M. Overview and recent advances in neuropathology. Part 1, Central nervous system tumours. Pathology 2011, 43, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Saraswathy, S.; Crawford, F.W.; Lamborn, K.R.; Pirzkall, A.; Chang, S.; Cha, S.; Nelson, S.J. Evaluation of MR markers that predict survival in patients with newly diagnosed GBM prior to adjuvant therapy. J. Neuro-oncol. 2009, 91, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Nakamizo, S.; Sasayama, T.; Shinohara, M.; Irino, Y.; Nishiumi, S.; Nishihara, M.; Tanaka, H.; Tanaka, K.; Mizukawa, K.; Itoh, T.; et al. GC/MS-based metabolomic analysis of cerebrospinal fluid (CSF) from glioma patients. J. Neuro-Oncol. 2013, 113, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Erb, G.; Elbayed, K.; Piotto, M.; Raya, J.; Neuville, A.; Mohr, M.; Maitrot, D.; Kehrli, P.; Namer, I.J. Toward improved grading of malignancy in oligodendrogliomas using metabolomics. Magn. Reson. Med. 2008, 59, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Baena, S.; Morales, J.M.; Martinetto, H.; Calvar, J.; Sevlever, G.; Castellano, G.; Cerda-Nicolas, M.; Celda, B.; Monleon, D. Comparative metabolic profiling of paediatric ependymoma, medulloblastoma and pilocytic astrocytoma. Int. J. Mol. Med. 2010, 26, 941–948. [Google Scholar] [PubMed]
- Jonsson, P.; Johansson, E.S.; Wuolikainen, A.; Lindberg, J.; Schuppe-Koistinen, I.; Kusano, M.; Sjostrom, M.; Trygg, J.; Moritz, T.; Antti, H. Predictive metabolite profiling applying hierarchical multivariate curve resolution to GC-MS datas—A potential tool for multi-parametric diagnosis. J. Proteome Res. 2006, 5, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Yang, S.; Wang, H.; Babb, J.S.; Johnson, G.; Cha, S.; Knopp, E.A.; Zagzag, D. Glioma grading: Sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am. J. Neuroradiol. 2003, 24, 1989–1998. [Google Scholar] [PubMed]
- Chinnaiyan, P.; Kensicki, E.; Bloom, G.; Prabhu, A.; Sarcar, B.; Kahali, S.; Eschrich, S.; Qu, X.; Forsyth, P.; Gillies, R. The metabolomic signature of malignant glioma reflects accelerated anabolic metabolism. Cancer Res. 2012, 72, 5878–5888. [Google Scholar] [CrossRef] [PubMed]
- Meixensberger, J.; Herting, B.; Roggendorf, W.; Reichmann, H. Metabolic patterns in malignant gliomas. J. Neuro-oncol. 1995, 24, 153–161. [Google Scholar] [CrossRef]
- Quon, H.; Brunet, B.; Alexander, A.; Murtha, A.; Abdulkarim, B.; Fulton, D.; Smerdely, M.; Johnson, M.; Urtasun, R.; Patel, S.; et al. Changes in serial magnetic resonance spectroscopy predict outcome in high-grade glioma during and after postoperative radiotherapy. Anticancer Res. 2011, 31, 3559–3565. [Google Scholar] [PubMed]
- Wibom, C.; Surowiec, I.; Moren, L.; Bergstrom, P.; Johansson, M.; Antti, H.; Bergenheim, A.T. Metabolomic patterns in glioblastoma and changes during radiotherapy: A clinical microdialysis study. J. Proteome Res. 2010, 9, 2909–2919. [Google Scholar] [CrossRef] [PubMed]
- Majos, C.; Bruna, J.; Julia-Sape, M.; Cos, M.; Camins, A.; Gil, M.; Acebes, J.J.; Aguilera, C.; Arus, C. Proton MR spectroscopy provides relevant prognostic information in high-grade astrocytomas. AJNR Am. J. Neuroradiol. 2011, 32, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Peeling, J.; Sutherland, G. High-resolution 1H NMR spectroscopy studies of extracts of human cerebral neoplasms. Magn. Reson. Med. 1992, 24, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [PubMed]
- Faria, A.V.; Macedo, F.C., Jr.; Marsaioli, A.J.; Ferreira, M.M.; Cendes, F. Classification of brain tumor extracts by high resolution (1)H MRS using partial least squares discriminant analysis. Braz. J. Med. Biol. Res. 2011, 44, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; De Micheli, E.; Bricolo, A.; Ballini, C.; Fattori, M.; Venturi, C.; Pedata, F.; Tipton, K.F.; Della Corte, L. Extracellular levels of amino acids and choline in human high grade gliomas: An intraoperative microdialysis study. Neurochem. Res. 2004, 29, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.S.; Thompson, C.B. Metabolic reprogramming: A cancer hallmark even warburg did not anticipate. Cancer Cell 2012, 21, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Sontheimer, H. A role for glutamate in growth and invasion of primary brain tumors. J. Neurochem. 2008, 105, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.C.; Sontheimer, H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999, 59, 4383–4391. [Google Scholar] [PubMed]
- Bergenheim, A.T.; Roslin, M.; Ungerstedt, U.; Waldenstrom, A.; Henriksson, R.; Ronquist, G. Metabolic manipulation of glioblastoma in vivo by retrograde microdialysis of L-2, 4 diaminobutyric acid (DAB). J. Neuro-oncol. 2006, 80, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Marcus, H.J.; Carpenter, K.L.; Price, S.J.; Hutchinson, P.J. In vivo assessment of high-grade glioma biochemistry using microdialysis: A study of energy-related molecules, growth factors and cytokines. J. Neuro-oncol. 2010, 97, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.J.; Fellows, G.A.; Griffiths, J.R.; Wilson, M.; Bell, B.A.; Howe, F.A. Ex-vivo HRMAS of adult brain tumours: Metabolite quantification and assignment of tumour biomarkers. Mol. Cancer 2010, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.; Smith, J.K.; Kwock, L. Correlation of myo-inositol levels and grading of cerebral astrocytomas. Am. J. Neuroradiol. 2000, 21, 1645–1649. [Google Scholar] [PubMed]
- Kallenberg, K.; Bock, H.C.; Helms, G.; Jung, K.; Wrede, A.; Buhk, J.H.; Giese, A.; Frahm, J.; Strik, H.; Dechent, P.; et al. Untreated glioblastoma multiforme: Increased myo-inositol and glutamine levels in the contralateral cerebral hemisphere at proton mr spectroscopy. Radiology 2009, 253, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Yokota, A. Absolute concentrations of metabolites in human brain tumors using in vitro proton magnetic resonance spectroscopy. NMR Biomed. 1997, 10, 2–12. [Google Scholar] [CrossRef]
- Da Rocha, A.B.; Mans, D.R.A.; Regner, A.; Schwartsmann, G. Targeting protein kinase C: New therapeutic opportunities against high-grade malignant gliomas? Oncologist 2002, 7, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Righi, V.; Andronesi, O.C.; Mintzopoulos, D.; Black, P.M.; Tzika, A.A. High-resolution magic angle spinning magnetic resonance spectroscopy detects glycine as a biomarker in brain tumors. Int. J. Oncol. 2010, 36, 301–306. [Google Scholar] [PubMed]
- Jonsson, P.; Gullberg, J.; Nordstrom, A.; Kusano, M.; Kowalczyk, M.; Sjostrom, M.; Moritz, T. A strategy for identifying differences in large series of metabolomic samples analyzed by GC/MS. Anal. Chem. 2004, 76, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, P.; Johansson, A.I.; Gullberg, J.; Trygg, J.A.J.; Grung, B.; Marklund, S.; Sjostrom, M.; Antti, H.; Moritz, T. High-throughput data analysis for detecting and identifying differences between samples in GC/MS-based metabolomic analyses. Anal. Chem. 2005, 77, 5635–5642. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Eriksson, L.; Trygg, J.; Wold, S. CV-ANOVA for significance testing of PLS and OPLS (R) models. J. Chemom. 2008, 22, 594–600. [Google Scholar] [CrossRef]
- ROCCET: ROC Curve Explorer & Tester. Available online: www.roccet.ca (accessed on 8 September 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mörén, L.; Bergenheim, A.T.; Ghasimi, S.; Brännström, T.; Johansson, M.; Antti, H. Metabolomic Screening of Tumor Tissue and Serum in Glioma Patients Reveals Diagnostic and Prognostic Information. Metabolites 2015, 5, 502-520. https://doi.org/10.3390/metabo5030502
Mörén L, Bergenheim AT, Ghasimi S, Brännström T, Johansson M, Antti H. Metabolomic Screening of Tumor Tissue and Serum in Glioma Patients Reveals Diagnostic and Prognostic Information. Metabolites. 2015; 5(3):502-520. https://doi.org/10.3390/metabo5030502
Chicago/Turabian StyleMörén, Lina, A. Tommy Bergenheim, Soma Ghasimi, Thomas Brännström, Mikael Johansson, and Henrik Antti. 2015. "Metabolomic Screening of Tumor Tissue and Serum in Glioma Patients Reveals Diagnostic and Prognostic Information" Metabolites 5, no. 3: 502-520. https://doi.org/10.3390/metabo5030502
APA StyleMörén, L., Bergenheim, A. T., Ghasimi, S., Brännström, T., Johansson, M., & Antti, H. (2015). Metabolomic Screening of Tumor Tissue and Serum in Glioma Patients Reveals Diagnostic and Prognostic Information. Metabolites, 5(3), 502-520. https://doi.org/10.3390/metabo5030502





