A Metabolomic Approach to Target Compounds from the Asteraceae Family for Dual COX and LOX Inhibition
Abstract
:1. Introduction
2. Results and Discussion
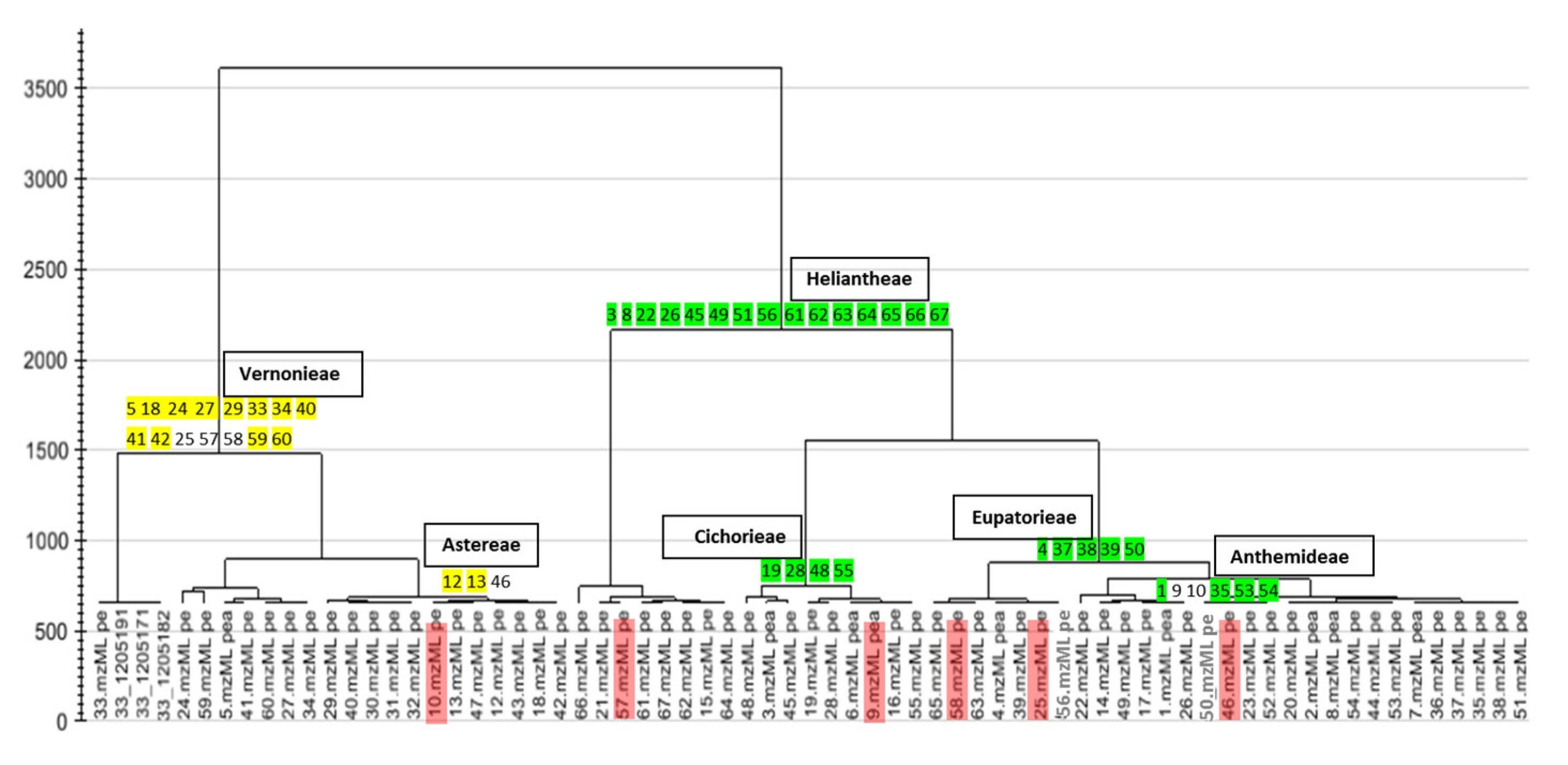
2.1. Chemical Profile (HPLC-ESI-HRMS) of the Extracts
| Species | Sample Codes | Chemistry Investigated/AI Evidence | Tribe * |
|---|---|---|---|
| Cichorium intybus L. (chicory) | 19 | Yes [37]/Yes [37] | Cichorieae Lam. & DC. |
| Minasia scapigera H. Rob. | 40 | No/No | Vernonieae Cass. |
| Piptolepis monticola Loeuille | 41 | No/No | Vernonieae Cass. |
| Prestelia eriopus Sch. Bip. | 42 | No/No | Vernonieae Cass. |
| Solidago microglossa DC. (arnica do campo) | 46 | Yes [68]/Yes [68,69] | Astereae Cass. |
| Sphagneticola trilobata (L.) Pruskei | 49 | Yes [70]/Yes [70] | Heliantheae Cass. |
| Tithonia diversifolia (Hemsl.) A. Gray (tree marigold) | 56 | Yes [71]/Yes [7] | Heliantheae Cass. |
| Vernonia herbacea (Vell.) Rusby | 57 | Yes [51]/No | Vernonieae Cass. |
| Vernonia platensis (Spreng.) Less. | 58 | Yes [51]/No | Vernonieae Cass. |
| Vernonia polyanthes Less. (assapeixe) | 59 | Yes/Yes [72] | Vernonieae Cass. |
| Vernonia rubriramea Mart. Ex DC. | 60 | No/No | Vernonieae Cass. |
| Viguiera robusta Gardner | 66 | Yes [73]/Yes [73] | Heliantheae Cass. |
| Viguiera trichophylla Dusén | 67 | No/No | Heliantheae Cass. |
| Peak Area | m/z | Retention Time | Molecular Formula | UV Peak at Maximum Absorbance (nm) | Dereplication | Literature * |
|---|---|---|---|---|---|---|
| 8.50 × 107 | 153.0195 (M−H)− | 11.2 | C7H6O4 | 255; 292 | protocatechuic acid | [74] |
| 9.12× 107 | 353.0883 (M−H)− | 13.9 | C16H18O9 | 300; 323 | 5-O-E-caffeoylquinic acid | [7] |
| 7.85× 107 | 355.1022 (M+H)+ | 13.9 | C16H18O9 | 300; 323 | 5-O-E-caffeoylquinic acid | [7] |
| 3.98× 108 | 515.1200 (M−H)− | 19.2 | C25H24O12 | 295; 326 | 3,5-di-O-E-caffeoylquinic acid | [74] |
| 3.44× 107 | 517.1340 (M+H)+ | 19.2 | C25H24O12 | 295; 326 | 5,3-di-O-E-caffeoylquinic acid | [74] |
| 3.32× 108 | 515.1200 (M−H)− | 19.7 | C25H24O12 | 295; 326 | 4,5-di-O-E-caffeoylquinic acid | ** |
| 2.05× 107 | 517.1340 (M+H)+ | 19.7 | C25H24O12 | 295; 326 | 5,4-di-O-E-caffeoylquinic acid | ** |
| 7.29× 107 | 367.1767 (M−H)− | 23.9 | C19H28O7 | 211 | tagitinin A | [7,71] |
| 2.41× 107 | 369.1906 (M+H)+ | 23.9 | C19H28O7 | 211 | tagitinin A | [7,71] |
| 2.51× 107 | 349.1644 (M−H)− | 27.0 | C19H24O6 | 254 | tagitinin C | [7,71] |
| 5.14× 106 | 349.1643 (M−H)− | 28.4 | C19H24O6 | 210 | tagitinin F | [71] |
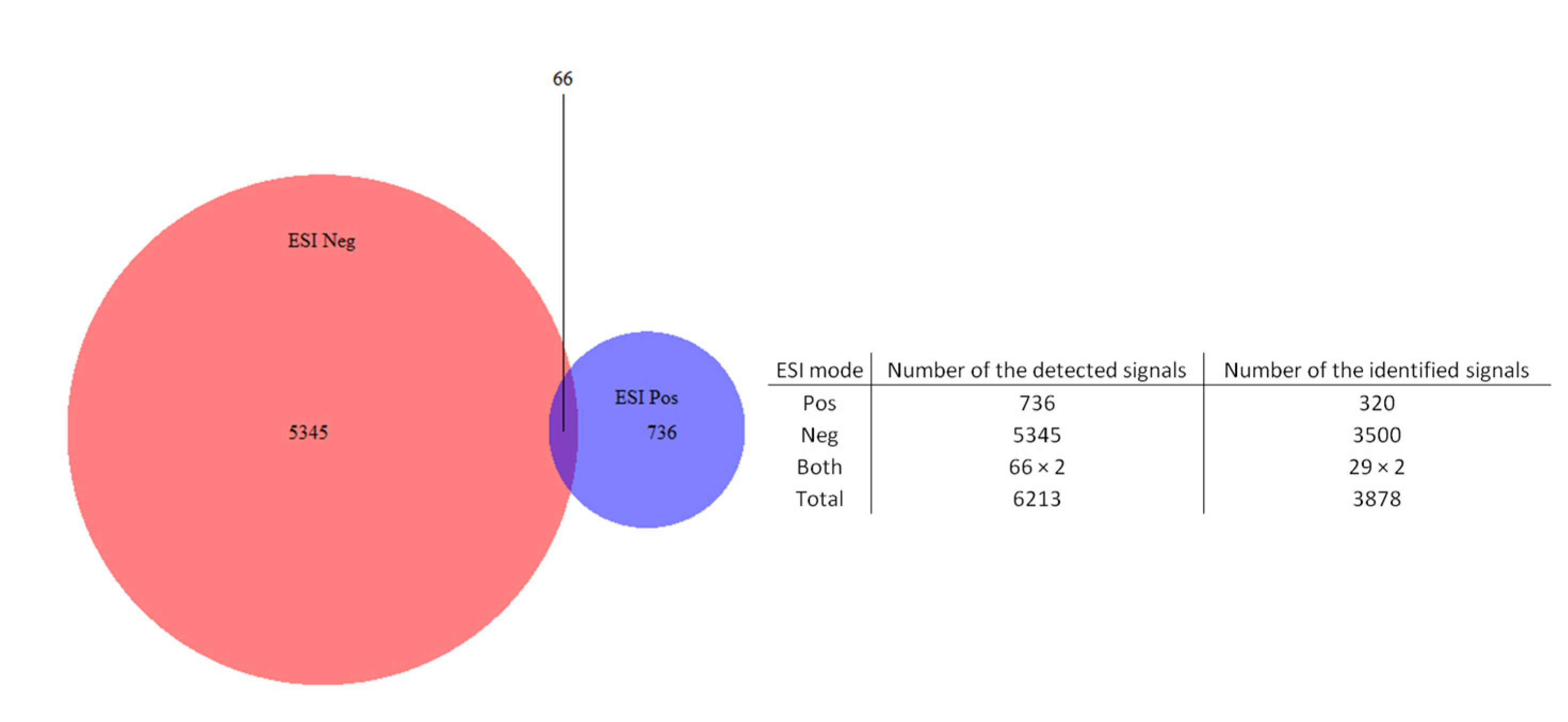
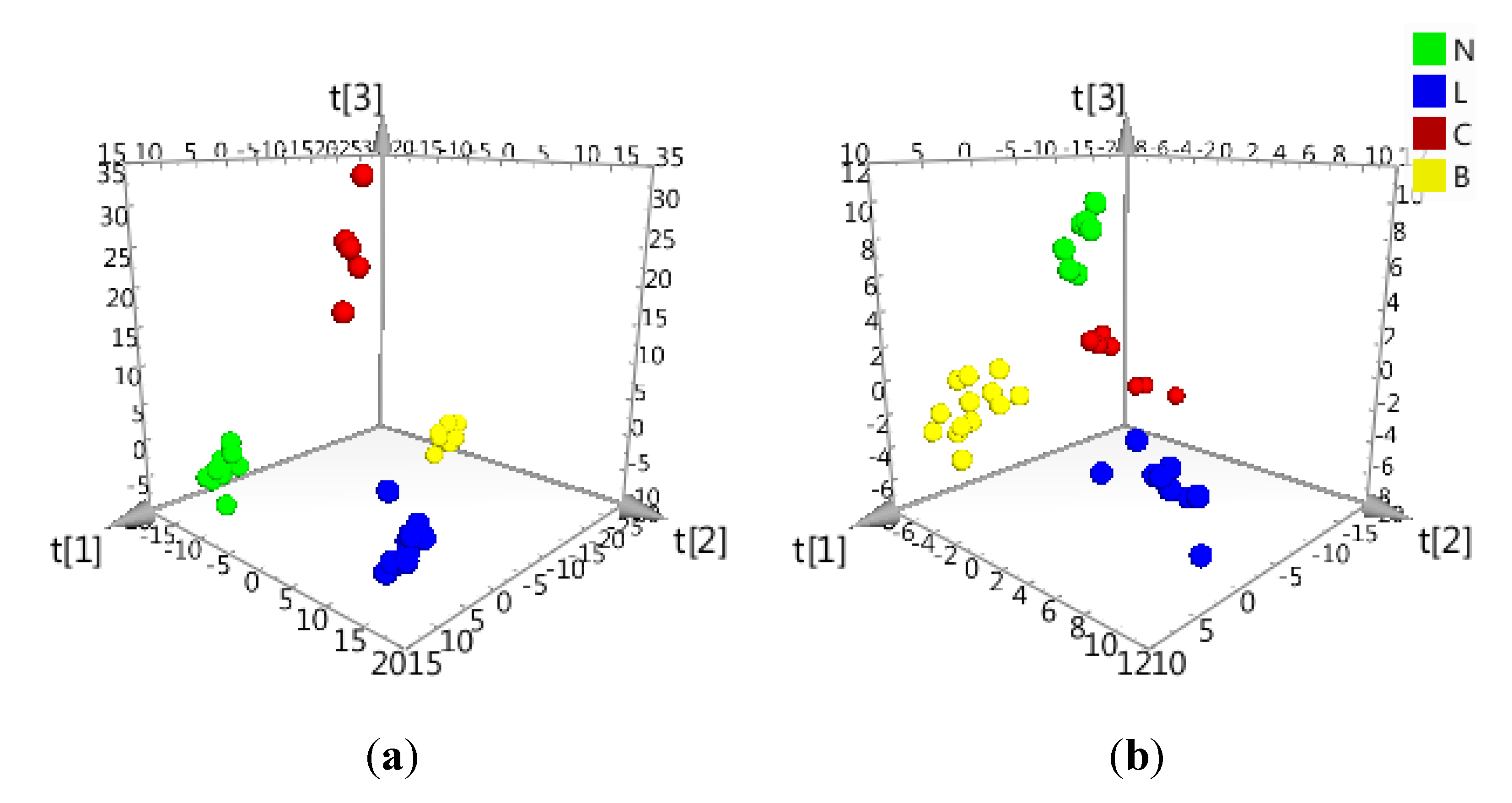
2.2. Data Treatment and PCA
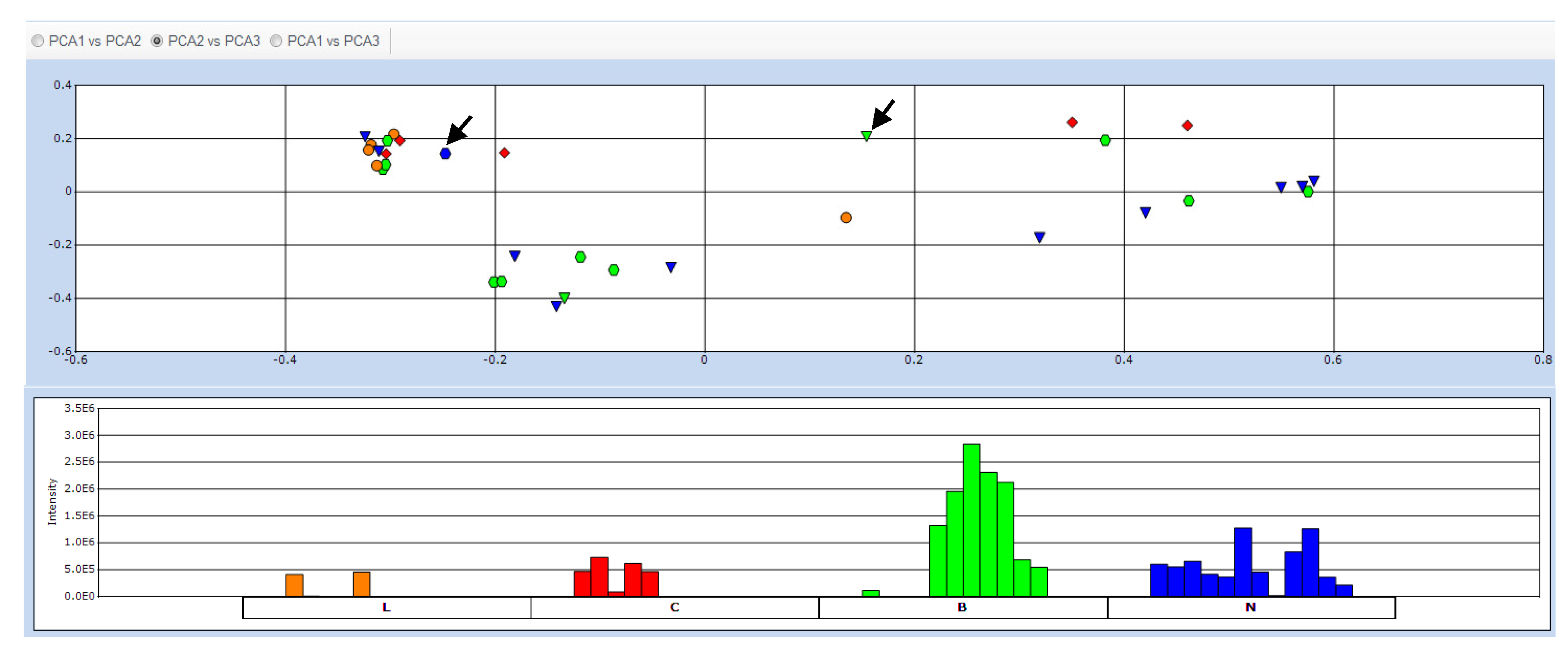
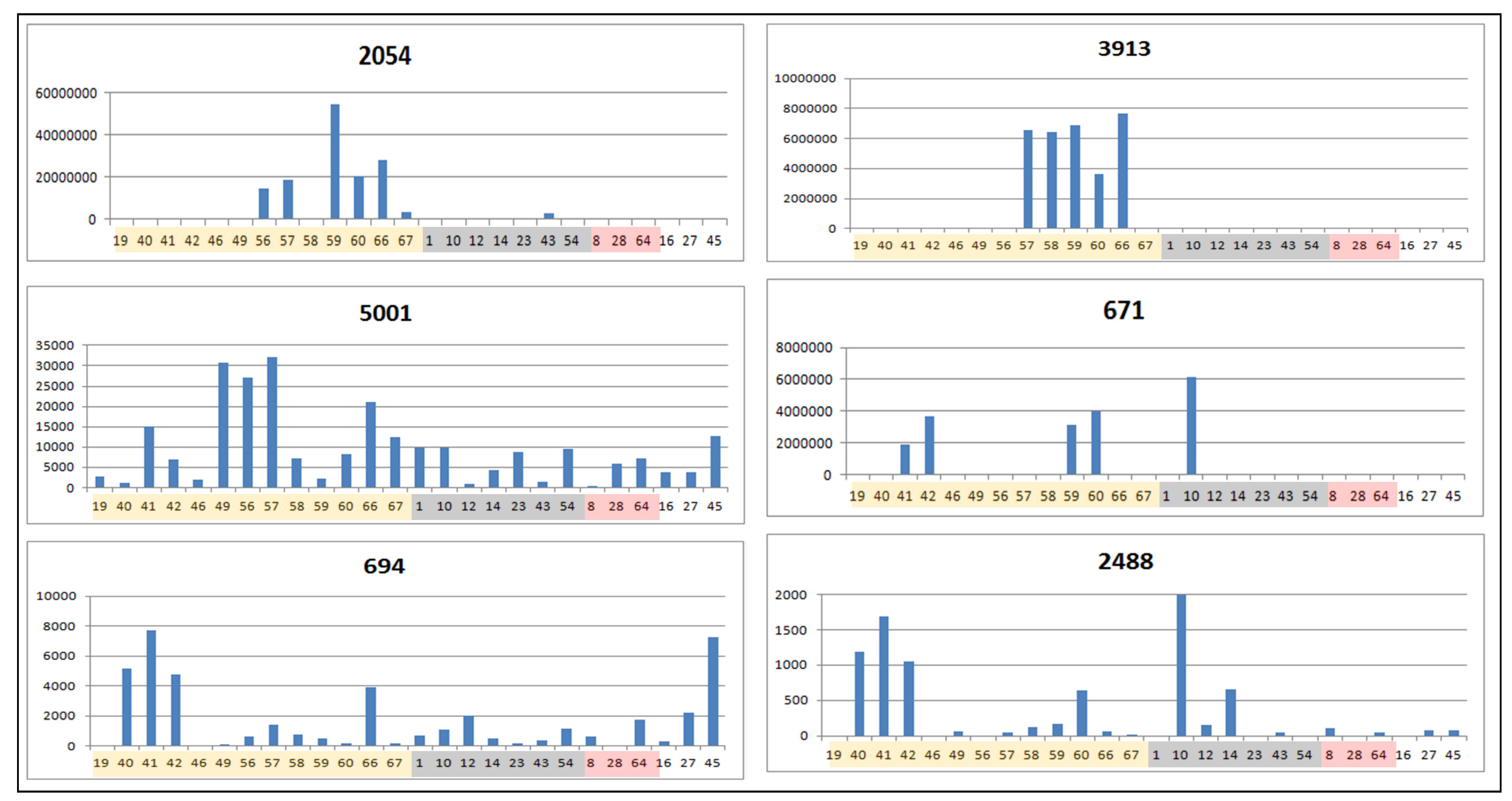
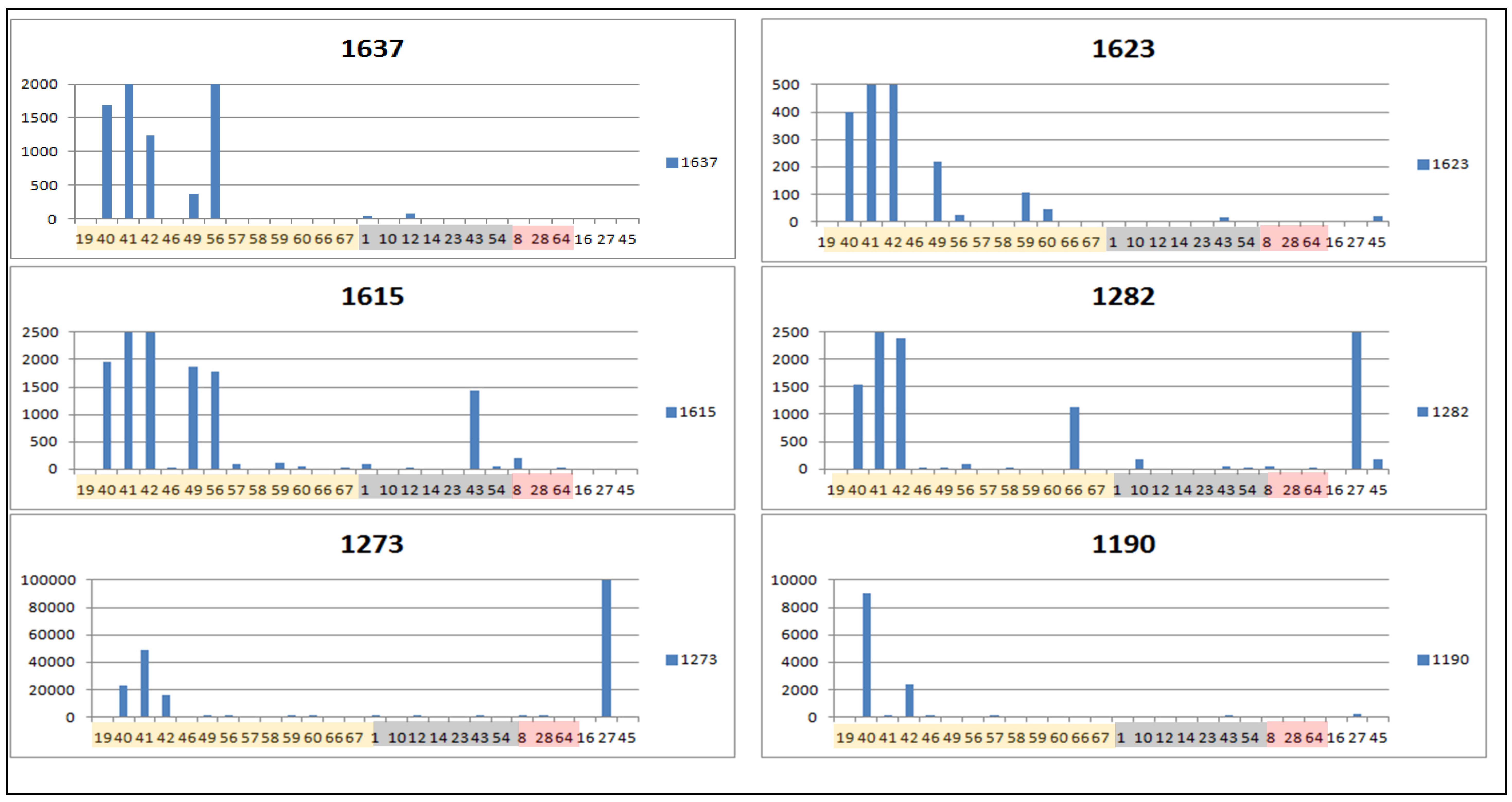
2.3. Determination of Biomarkers for Dual Inhibition by O2PLS
| ID | VIP Scores | m/z | RT | Mass Error (ppm) | MF | Hits (Number of Hits from SciFinder/DNP2015/AsterDB) a | |
|---|---|---|---|---|---|---|---|
| Negative Mode | |||||||
| 2054 | 1.92232 | 359.0778 | 14.2 | 1.656 | [M−H]− | C18H16O8 | F and PC (381/97/6) => Hexahydroxyflavone; TrMe ether, chrysosplenol D b |
| 3913 | 1.91038 | 341.0883 | 12.2 | 1.410 | [M−H]− | C15H18O9 | PC (162/11/1) |
| 2488 | 1.89577 | 679.1505 | 16.4 | 3.175 | [M−H]− | C30H32O18 | F (16/2/0) |
| 5001 | 1.78812 | 431.1931 | 18.7 | 1.912 | [M−H]− | C20H32O10 | PC and other classes (91/0/0) |
| 671 | 1.71425 | 487.1256 | 20.0 | 2.042 | [M−H]− | C24H24O11 | F and PC (101/14/1) => acacetin-7-O-β-d-(3′′-acetyl)-glucopyranoside c [78] |
| 2610 * | 1.57931 | 447.1306 | 17.5 | 2.051 | [M−H]− | C22H24O10 | F and PC (231/66/0) |
| 815* | 1.38122 | 509.2246 | 16.9 | 1.248 | [M−H]− | C22H38O13 | Saccharides (27/1/0) => β-D-glucopyranose, 1-[8-(β-d-glucopyranosyloxy)-2,6-dimethyl-2-octenoate] was found from the DNP |
| 694 | 1.30532 | 415.1255 | 15.6 | 0.958 | [M−H]− | C18H24O11 | PC (81/15/0) |
| 4582 * | 1.29768 | 470.9873 | 12.4 | 1.023 | [M−H]− | C23H6O11N | (0/0/0) |
| 3144 ** | 0.957026 | 513.2714 | 19.0 | 1.789 | [M−H]− | C26H42O10 | ST (80/15/0) |
| 829 ** | 0.834219 | 411.1797 | 25.3 | 1.413 | [M+Cl]− | C18H34O8 | d-glucopyranose,2,3-dihexanoate (107/1/0) |
| Positive Mode | |||||||
| 1273 | 1.61922 | 361.1643 | 32.0 | 0.774 | [M+H]+ | C20H24O6 | STL, D and PC (1850/219/27) d |
| 1282 | 1.60527 | 261.1118 | 32.1 | 1.077 | [M+H]+ | C15H16O4 | STL, PC and F (3723/158/10) |
| 1637 * | 1.56815 | 349.1642 | 28.2 | 0.897 | [M+H]+ | C19H24O6 | STL, D and PC (1424/164/12), identified as tagitinin F |
| 1116 * | 1.54159 | 600.2654 | 16.8 | 0.635 | [M+NH3]+ | C28H38O13 | (0/0/0) |
| 1190 | 1.42996 | 611.1400 | 20.0 | 0.717 | [M+H]+ | C30H26O14 | F (81/25/0) |
| 1623 | 1.36987 | 426.2120 | 27.9 | 0.511 | [M+NH3]+ | C21H29O8 | STL (0/0/0) |
| 1436 | 1.18345 | 418.1857 | 33.9 | 0.688 | [M+NH3]+ | C22H25O7 | D, F (6/0/0) |
| 1615 | 1.16202 | 363.1799 | 30.5 | 0.852 | [M+H]+ | C20H26O6 | STL, D and PC (1858/345/12) e |
| 692 * | 1.0815 | 449.1075 | 19.0 | 0.607 | [M+H]+ | C21H20O11 | F (258/101/2) ex.: Quercetrin occurs in Asteraceae (e.g., in the dual inhibitor extract of Solidago microglossa #46 [68]); luteolin, 7-β-d-glucopyranoside is widespread in plants, and also occurs in Asteraceae |
| 1207 ** | 0.88865 | 509.1290 | 20.6 | 0.160 | [M+H]+ | C23H24O13 | F (76/39/0) |
| 1333 ** | 0.862979 | 270.1697 | 29.1 | 1.112 | [M+NH3]+ | C14H20O4 | (6048/78/0) Butanoic acid, 3-methyl-,4-(1R,2S-dihydroxypropyl)phenyl ester |
| 276 ** | 0.818718 | 415.2112 | 34.5 | 0.634 | [M+H]+ | C24H30O6 | STL, D and PC (1438/43/0) |
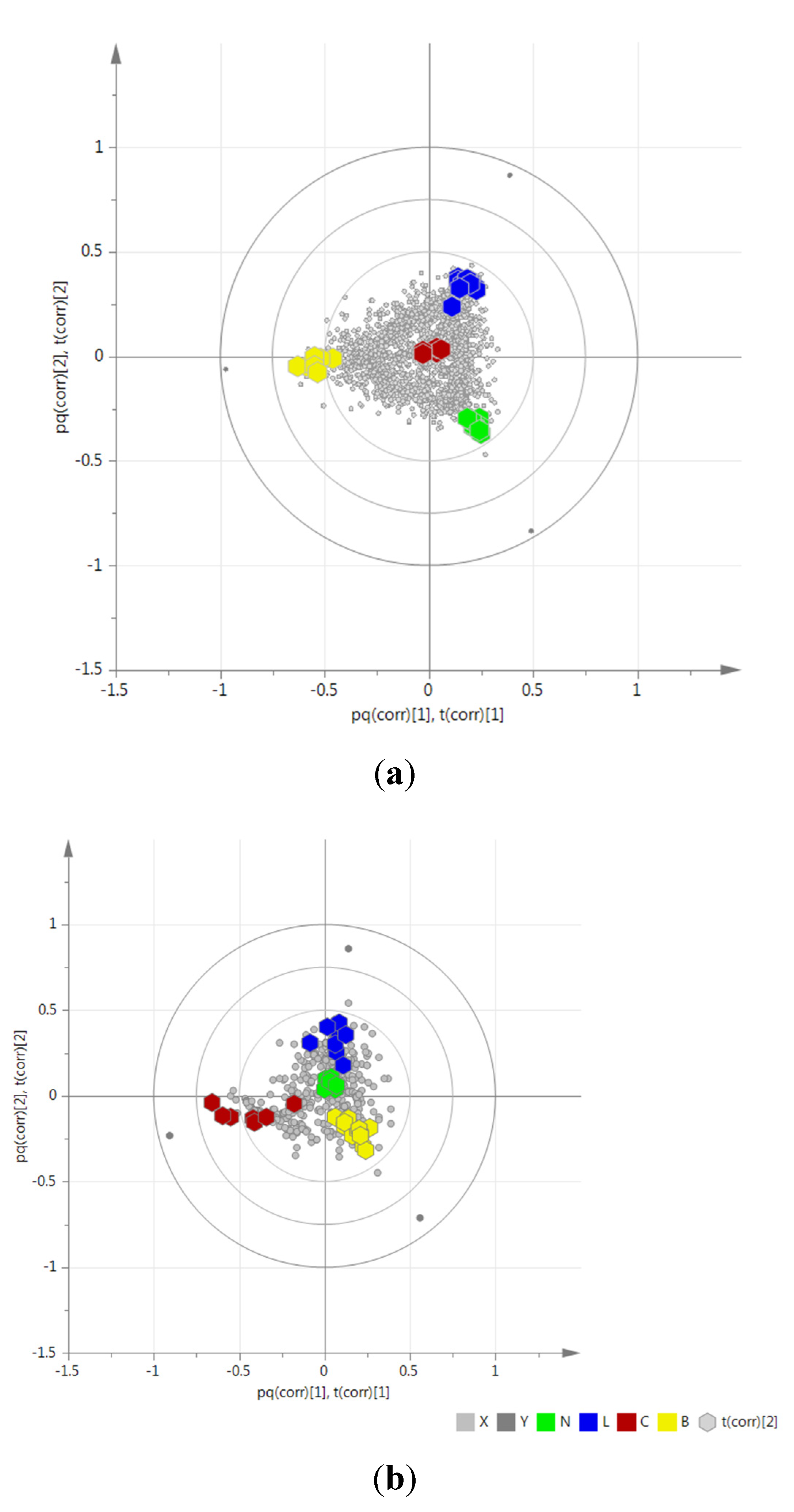
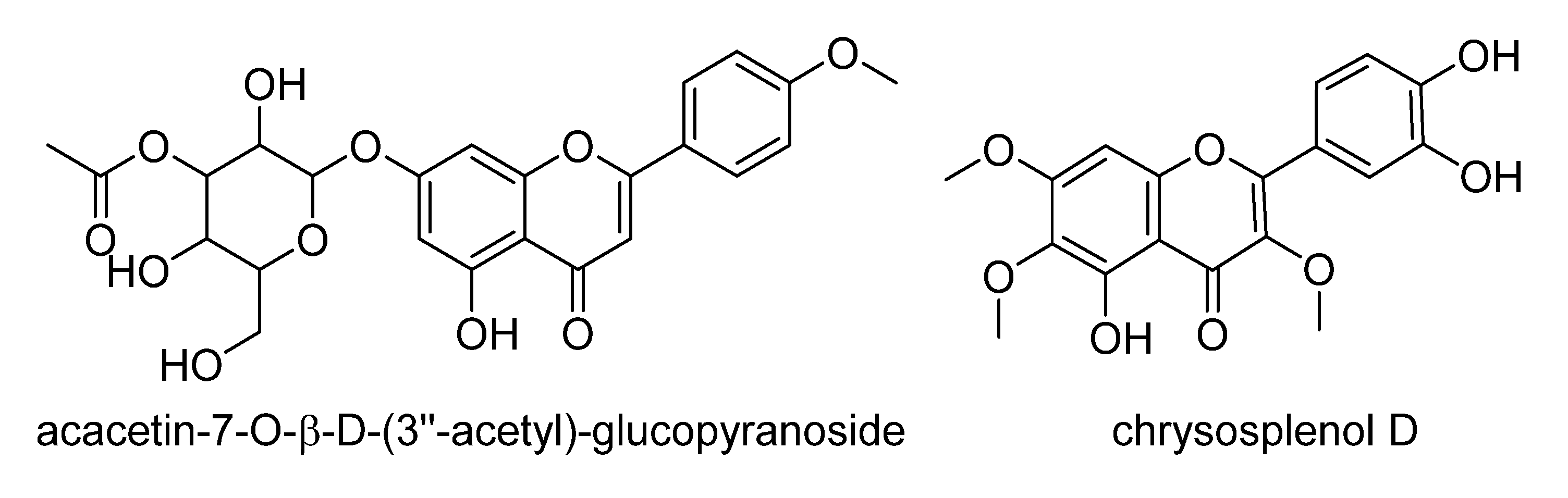
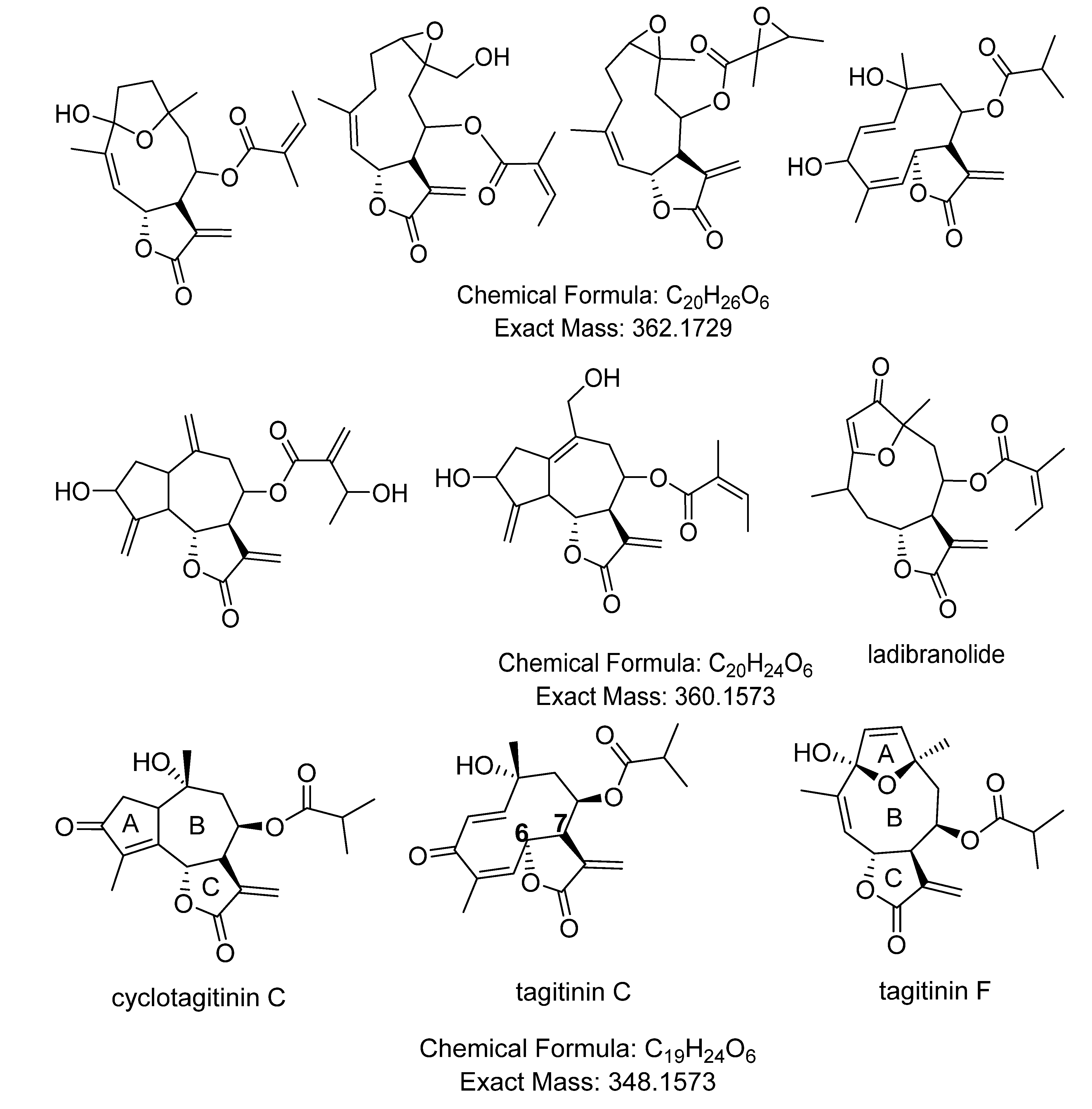
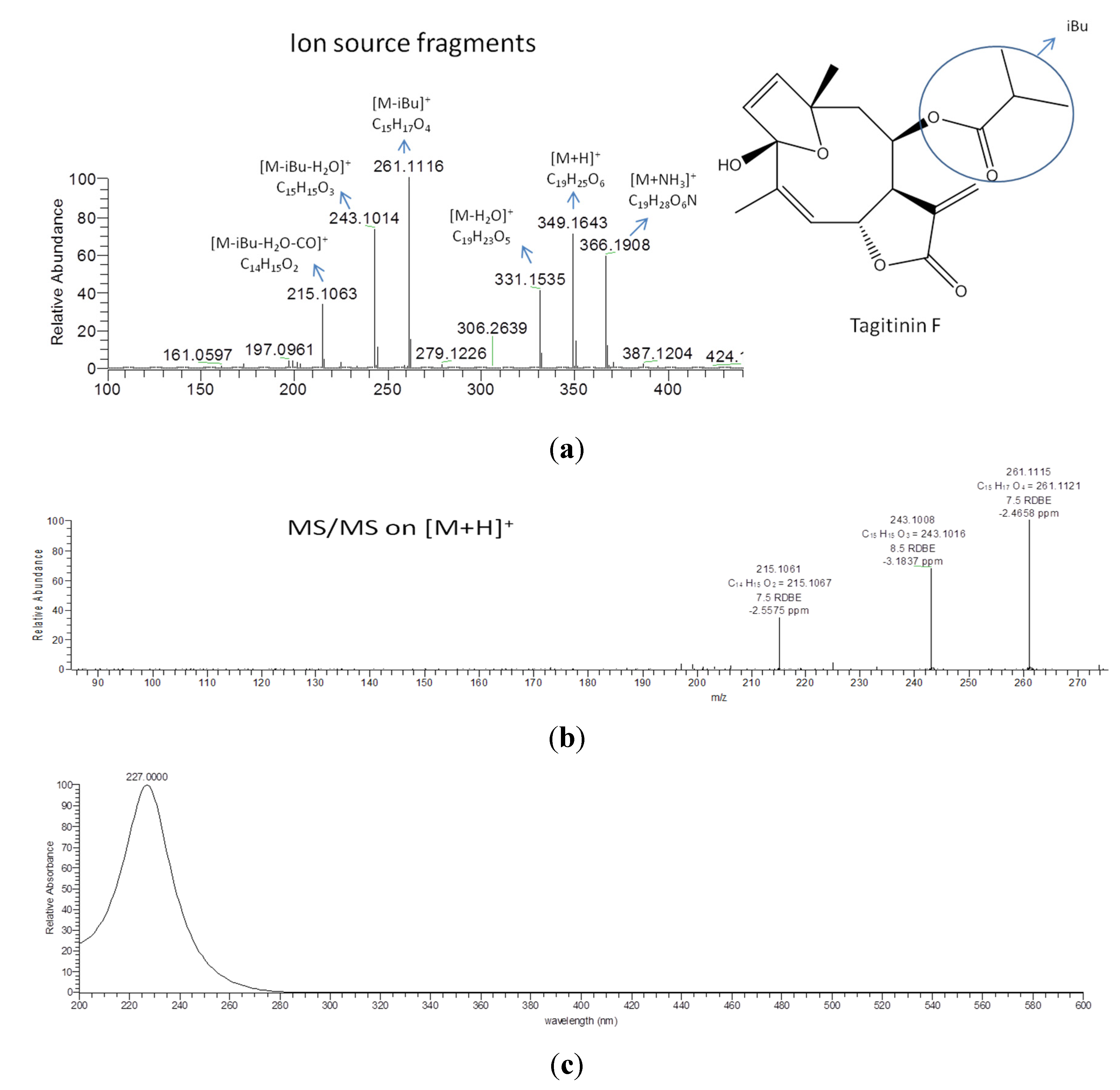
2.4. Validation of the O2PLS Model for the Predictions of Dual Inhibitors of COX-1 and 5-LOX
| MS Mode | R2 | Q2 | RMSECV | P2-group 1 ** | P2-group 2 ** | P2-group 3 *** | |
|---|---|---|---|---|---|---|---|
| O2PLS-DA | Negative mode | 1.00 | 0.84 | 0.19 | 0.70 | 0.80 | 0.80 |
| Positive mode | 1.00 | 0.70 | 0.26 | 0.60 | 0.60 | 0.60 |
3. Experimental Section
3.1. Plant Material
3.2. Extraction of Compounds from Plant Material
3.3. Chemicals and Materials
3.4. HPLC-ESI-HRMS Analysis
3.5. Data Treatment
3.6. MSA
3.7. Dereplication
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Newman, D.J. Natural products as leads to potential drugs: An old process or the new hope for drug discovery? J. Med. Chem. 2008, 51, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Chagas-Paula, D.A.; De Oliveira, R.B.; da Silva, V.C.; Gobbo-Neto, L.; Gasparoto, T.H.; Campanelli, A.P.; Faccioli, L.H.; Da Costa, F.B. Chlorogenic acids from Tithonia diversifolia demonstrate better anti-inflammatory effect than indomethacin and its sesquiterpene lactones. J. Ethnopharmacol. 2011, 136, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Verpoorte, R.; Choi, Y.H.; Kim, H.K. Ethnopharmacology and systems biology: A perfect holistic match. J. Ethnopharmacol. 2005, 100, 53–56. [Google Scholar] [CrossRef] [PubMed]
- De Donno, A.; Grassi, T.; Idolo, A.; Guido, M.; Papadia, P.; Caccioppola, A.; Villanova, L.; Merendino, A.; Bagordo, F.; Fanizzi, F.P. First-time comparison of the in vitro antimalarial activity of Artemisia annua herbal tea and artemisinin. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, J.D. Phytochemistry and medicinal plants. Phytochemistry 2001, 56, 237–243. [Google Scholar] [CrossRef]
- Wagner, H. Synergy research: Approaching a new generation of phytopharmaceuticals. Fitoterapia 2011, 82, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Halabalaki, M.; Skaltsounis, A. New concepts, experimental approaches, and dereplication strategies for the discovery of novel phytoestrogens from natural sources. Planta Med. 2013, 79, 514–532. [Google Scholar] [PubMed]
- Queiroz, E.F.; Wolfender, J.; Hostettmann, K. Modern approaches in the search for new lead antiparasitic compounds from higher plants. Curr. Drug Targets 2009, 10, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Saito, K. Metabolomics for unknown plant metabolites. Anal. Bioanal. Chem. 2013, 405, 5005–5011. [Google Scholar] [CrossRef] [PubMed]
- Yuliana, N.D.; Khatib, A.; Choi, Y.H.; Verpoorte, R. Metabolomics for bioactivity assessment of natural products. Phyther. Res. 2011, 25, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Mendes, P.; Dixon, R.A. Plant metabolomics: Large-scale phytochemistry in the functional genomics era. Phytochemistry 2003, 62, 817–836. [Google Scholar] [CrossRef]
- Lang, G.; Mayhudin, N.A.; Mitova, M.I.; Sun, L.; van der Sar, S.; Blunt, J.W.; Cole, A.L.J.; Ellis, G.; Laatsch, H.; Munro, M.H.G. Evolving trends in the dereplication of natural product extracts: New methodology for rapid, small-scale investigation of natural product extracts. J. Nat. Prod. 2008, 71, 1595–1599. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Lindon, J.C. Metabonomics. Nature 2008, 455, 1054–1056. [Google Scholar] [CrossRef] [PubMed]
- Shyur, L.F.; Yang, N. Metabolomics for phytomedicine research and drug development. Curr. Opin. Chem. Biol. 2008, 12, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Villas-Bôas, S.G.; Mas, S.; Akesson, M.; Smedsgaard, J.; Nielsen, J. Mass spectrometry in metabolome analysis. Mass Spectrom. Rev. 2005, 24, 613–646. [Google Scholar] [CrossRef] [PubMed]
- Weckwerth, W.; Morgenthal, K. Metabolomics: From pattern recognition to biological interpretation. Drug Discov. Today 2005, 10, 1551–1558. [Google Scholar] [CrossRef]
- Wolfender, J.; Marti, G.; Ferreira Queiroz, E. Advances in techniques for profiling crude extracts and for the rapid identificationof natural products: Dereplication, quality control and metabolomics. Curr. Org. Chem. 2010, 14, 1808–1832. [Google Scholar] [CrossRef]
- Bedair, M.; Sumner, L.W. Current and emerging mass-spectrometry technologies for metabolomics. Trends Anal. Chem. 2008, 27, 238–250. [Google Scholar] [CrossRef]
- Madsen, R.; Lundstedt, T.; Trygg, J. Chemometrics in metabolomics-a review in human disease diagnosis. Anal. Chim. Acta 2010, 659, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Trygg, J.; Holmes, E.; Lundstedt, T. Chemometrics in metabonomics. J. Proteome Res. 2007, 6, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Bylesjö, M.; Rantalainen, M.; Cloarec, O.; Nicholson, J.K.; Holmes, E.; Trygg, J. OPLS discriminant analysis: Combining the strengths of PLS-DA and SIMCA classification. J. Chemom. 2006, 20, 341–351. [Google Scholar] [CrossRef]
- Chagas-Paula, D.A.; Oliveira, T.B.; Zhang, T.; Edrada-Ebel, R.; da Costa, F.B. Prediction of Anti-inflammatory Plants and Discovery of Their Biomarkers by Machine Learning Algorithms and Metabolomic Studies. Planta Med. 2015, 81, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Chagas-Paula, D.A. A Metabolomic studies of Asteraceae by UHPLC-UV-HRFTMS, in vitro evaluation of the anti-inflammatory potential and their correlation by in silico methods. Ph.D. Thesis, Universitsssy of São Paulo, 2013. [Google Scholar]
- Calixto, J.B.; Otuki, M.F.; Santos, A.R.S. Anti-inflammatory compounds of plant origin. Part I. Action on arachidonic acid pathway, nitric oxide and nuclear factor kappa B (NF-kappaB). Planta Med. 2003, 69, 973–983. [Google Scholar] [PubMed]
- Werz, O. Inhibition of 5-lipoxygenase product synthesis by natural compounds of plant origin. Planta Med. 2007, 73, 1331–1357. [Google Scholar] [CrossRef] [PubMed]
- Schneider, I.; Bucar, F. Lipoxygenase inhibitors from natural plant sources. Part 1: Medicinal plants with inhibitory activity on arachidonate 5-lipoxygenase and 5-lipoxygenase[sol]cyclooxygenase. Phyther. Res. 2005, 19, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Knuesel, O.; Weber, M.; Suter, A. Arnica montana gel in osteoarthritis of the knee: An open, multicenter clinical trial. Adv. Ther. 2002, 19, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E.; Pittler, M.H. The efficacy and safety of feverfew (Tanacetum parthenium L.): An update of a systematic review. Public Health Nutr. 2000, 3, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Sumner, H.; Slan, U.; Knight, D.W.; Hoult, J.R.S. Inhibition of 5-lipoxygenase and cyclo-oxygenase in leukocytes by feverfew involvement of sesquiterpene lactones and other components. Biochem. Pharmacol. 1992, 43, 2313–2320. [Google Scholar] [CrossRef]
- Akihisa, T.; Yasukawa, K.; Oinuma, H.; Kasahara, Y.; Yamanouchi, S.; Takido, M.; Kumaki, K.; Tamura, T. Triterpene alcohols from the flowers of Compositae and their anti-inflammatory effects. Phytochemistry 1996, 43, 1255–1260. [Google Scholar] [CrossRef]
- Ripoll, C.; Schmidt, B.M.; Ilic, N.; Poulev, A.; Dey, M.; Kurmukov, A.G.; Raskin, I. Anti-inflammatory effects of a sesquiterpene lactone extract from chicory (Cichorium intybus L.) roots. Nat. Prod. Commun. 2007, 2, 717–722. [Google Scholar]
- Parente, L. Pros and cons of selective inhibition of cyclooxygenase-2 versus dual lipoxygenase/cyclooxygenase inhibition: Is two better than One? J. Rheumatol. 2001, 28, 2375–2382. [Google Scholar] [PubMed]
- Fiorucci, S.; Meli, R.; Bucci, M.; Cirino, G. Dual inhibitors of cyclooxygenase and 5-lipoxygenase. A new avenue in anti-inflammatory therapy? Biochem. Pharmacol. 2001, 62, 1433–1438. [Google Scholar] [CrossRef]
- Gaddi, A.; Cicero, A.F.G.; Pedro, E.J. Clinical perspectives of anti-inflammatory therapy in the elderly: The lipoxigenase (LOX)/cycloxigenase (COX) inhibition concept. Arch. Gerontol. Geriatr. 2004, 38, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.N.P.; Knaus, E.E. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): Cyclooxygenase (COX) inhibition and beyond. J. Pharm. Pharm. Sci. 2008, 11, 81–110. [Google Scholar]
- Celotti, F.; Durand, T. The metabolic effects of inhibitors of 5-lipoxygenase and of cyclooxygenase 1 and 2 are an advancement in the efficacy and safety of anti-inflammatory therapy. Prostaglandins Other Lipid Mediat. 2003, 71, 147–162. [Google Scholar] [CrossRef]
- Holčapek, M.; Jirásko, R.; Lísa, M. Recent developments in liquid chromatography-mass spectrometry and related techniques. J. Chromatogr. A 2012, 1259, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Zubarev, R.A.; Makarov, A. Orbitrap mass spectrometry. Anal. Chem. 2013, 85, 5288–5296. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Clasquin, M.F.; Melamud, E.; Amador-Noguez, D.; Caudy, A.A.; Rabinowitz, J.D. Metabolomic analysis via reversed-phase ion-pairing liquid chromatography coupled to a stand alone orbitrap mass spectrometer. Anal. Chem. 2010, 82, 3212–3221. [Google Scholar] [CrossRef] [PubMed]
- AsterDB. Available online: http://www.asterbiochem.org (accessed on 23 June 2015).
- Ernst, M.; Silva, D.B.; Silva, R.R.; Vêncio, R.Z.N.; Lopes, N.P. Mass spectrometry in plant metabolomics strategies: From analytical platforms to data acquisition and processing. Nat. Prod. Rep. 2014, 31, 784–806. [Google Scholar] [CrossRef] [PubMed]
- Corley, D.G.; Durley, R.C. Strategies for database dereplication of natural products. J. Nat. Prod. 1994, 57, 1484–1490. [Google Scholar] [CrossRef]
- Kind, T.; Fiehn, O. Metabolomic database annotations via query of elemental compositions: Mass accuracy is insufficient even at less than 1 ppm. BMC Bioinformatics 2006, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sandasi, M.; Kamatou, G.P.P.; Viljoen, A.M. An untargeted metabolomic approach in the chemotaxonomic assessment of two Salvia species as a potential source of α-bisabolol. Phytochemistry 2012, 84, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Martucci, M.E.P.; De Vos, R.C.H.; Carollo, C.A.; Gobbo-Neto, L. Metabolomics as a potential chemotaxonomical tool: Application in the genus Vernonia schreb. PLoS ONE 2014, 9, e93149. [Google Scholar] [CrossRef] [PubMed]
- Safer, S.; Cicek, S.S.; Pieri, V.; Schwaiger, S.; Schneider, P.; Wissemann, V.; Stuppner, H. Metabolic fingerprinting of Leontopodium species (Asteraceae) by means of 1H NMR and HPLC-ESI-MS. Phytochemistry 2011, 72, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Ahn, Y.G.; Kim, H.K.; Moon, B.C.; Lee, A.Y.; Ryu, D.H.; Hwang, G.S. Characterization of dandelion species using 1H NMR- and GC-MS-based metabolite profiling. Analyst 2011, 136, 4222–4231. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Deshpande, S.; Kuhnert, N. Profiling the chlorogenic acids of Rudbeckia hirta, Helianthus tuberosus, Carlina acaulis and Symphyotrichum novae-angliae leaves by LC-MS(n). Phytochem. Anal. 2011, 22, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Kirk, H.; Cheng, D.; Choi, Y.H.; Vrieling, K.; Klinkhamer, P.G.L. Transgressive segregation of primary and secondary metabolites in F(2) hybrids between Jacobaea aquatica and J. vulgaris. Metabolomics 2012, 8, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; El-ahmady, S.H.; Elian, F.S.; Wessjohann, L.A. Phytochemistry Metabolomics driven analysis of artichoke leaf and its commercial products via UHPLC—q-TOF-MS and chemometrics. Phytochemistry 2013, 95, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Modarai, M.; Yang, M.; Suter, A.; Kortenkamp, A.; Heinrich, M. Metabolomic profiling of liquid Echinacea medicinal products with in vitro inhibitory effects on cytochrome P450 3A4 (CYP3A4). Planta Med. 2010, 76, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Frédérich, M.; Jansen, C.; de Tullio, P.; Tits, M.; Demoulin, V.; Angenot, L. Metabolomic analysis of Echinacea spp. by 1H nuclear magnetic resonance spectrometry and multivariate data analysis technique. Phytochem. Anal. 2010, 21, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhi, H.; Zhang, F.S.; Sun, H.; Zhang, L.; Jia, J.; Xing, J.; Qin, X.M. Metabolomic profiling of the antitussive and expectorant plant Tussilago farfara L. by nuclear magnetic resonance spectroscopy and multivariate data analysis. J. Pharm. Biomed. Anal. 2013, 75, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Bórquez, J.; Kennelly, E.J.; Simirgiotis, M.J. Activity guided isolation of isoflavones and hyphenated HPLC-PDA-ESI-ToF-MS metabolome profiling of Azorella madreporica Clos. from northern Chile. Food Res. Int. 2013, 52, 288–297. [Google Scholar] [CrossRef]
- Inui, T.; Wang, Y.; Pro, S.M.; Franzblau, S.G.; Pauli, G.F. Fitoterapia Unbiased evaluation of bioactive secondary metabolites in complex matrices. Fitoterapia 2012, 83, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Ching, J.; Soh, W.L.; Tan, C.H.; Lee, J.; Tan, J.C.; Yang, J.; Yap, C.W.; Koh, H.L. Identification of active compounds from medicinal plant extracts using gas chromatography-mass spectrometry and multivariate data analysis. J. Sep. Sci. 2012, 35, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Yuliana, N.D.; Khatib, A.; Verpoorte, R.; Choi, Y.H. Comprehensive extraction method integrated with NMR metabolomics: A new bioactivity screening method for plants, adenosine A1 receptor binding compounds in Orthosiphon stamineus Benth. Anal. Chem. 2011, 83, 6902–6906. [Google Scholar] [CrossRef] [PubMed]
- Funk, V.A.; Susanna, A.; Stuessy, T.F.; Bayer, R.J. Systematics, Evolution, and Biogeography of Compositae; International Association for Plant Taxonomy: Vienna, Austria, 2009. [Google Scholar]
- Emerenciano, V.P.; Militão, J.S.L.T.; Campos, C.C.; Romoff, P.; Kaplan, M.A.C.; Zambon, M.; Brant, A.J.C. Flavonoids as chemotaxonomic markers for Asteraceae. Biochem. Syst. Ecol. 2001, 29, 947–957. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Oresic, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics 2010, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, L.; Zhang, T.; Viegelmann, C.; Martinez, I.J.; Cheng, C.; Dowdells, C.; Abdelmohsen, U.R.; Gernert, C.; Hentschel, U.; Edrada-Ebel, R. Metabolomic tools for secondary metabolite discovery from marine microbial symbionts. Mar. Drugs 2014, 12, 3416–3448. [Google Scholar] [CrossRef] [PubMed]
- Sabir, S.M.; Ahmad, S.D.; Hamid, A.; Khan, M.Q.; Athayde, M.L.; Santos, D.B.; Boligon, A.A.; Rocha, J.B.T. Antioxidant and hepatoprotective activity of ethanolic extract of leaves of Solidago microglossa containing polyphenolic compounds. Food Chem. 2012, 131, 741–747. [Google Scholar] [CrossRef]
- Tamura, E.K.; Jimenez, R.S.; Waismam, K.; Gobbo-Neto, L.; Lopes, N.P.; Malpezzi-Marinho, E.A.L.; Marinho, E.A.V.; Farsky, S.H.P. Inhibitory effects of Solidago chilensis Meyen hydroalcoholic extract on acute inflammation. J. Ethnopharmacol. 2009, 122, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Maldini, M.; Sosa, S.; Montoro, P.; Giangaspero, A.; Balick, M.J.; Pizza, C.; Della Loggia, R. Screening of the topical anti-inflammatory activity of the bark of Acacia cornigera Willdenow, Byrsonima crassifolia Kunth, Sweetia panamensis Yakovlev and the leaves of Sphagneticola trilobata Hitchcock. J. Ethnopharmacol. 2009, 122, 430–433. [Google Scholar] [PubMed]
- Chagas-Paula, D.A.; Oliveira, R.B.; Rocha, B.A.; da Costa, F.B. Ethnobotany, chemistry, and biological activities of the genus Tithonia (Asteraceae). Chem. Biodivers. 2012, 9, 210–235. [Google Scholar] [CrossRef] [PubMed]
- Temponi, V.D.S.; da Silva, J.B.; Alves, M.S.; Ribeiro, A.; de Jesus Ribeiro Gomes de Pinho, J.; Yamamoto, C.H.; Pinto, M.A.O.; Del-Vechio-Vieira, G.; de Sousa, O.V. Antinociceptive and Anti-Inflammatory Effects of Ethanol Extract from Vernonia polyanthes Leaves in Rodents. Int. J. Mol. Sci. 2012, 13, 3887–3899. [Google Scholar] [CrossRef] [PubMed]
- Valério, D.A.R.; Cunha, T.M.; Arakawa, N.S.; Lemos, H.P.; Da Costa, F.B.; Parada, C.A.; Ferreira, S.H.; Cunha, F.Q.; Verri, W.A. Anti-inflammatory and analgesic effects of the sesquiterpene lactone budlein A in mice: Inhibition of cytokine production-dependent mechanism. Eur. J. Pharmacol. 2007, 562, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.J.; Xi, Z.X.; Chen, W.S.; Li, X.; Sun, L.; Sun, L.N. Chemical constituents from Tithonia diversifolia and their chemotaxonomic significance. Biochem. Syst. Ecol. 2012, 44, 250–254. [Google Scholar] [CrossRef]
- Kim, H.K.; Saifullah; Khan, S.; Wilson, E.G.; Kricun, S.D.P.; Meissner, A.; Goraler, S.; Deelder, A.M.; Choi, Y.H.; Verpoorte, R. Metabolic classification of South American Ilex species by NMR-based metabolomics. Phytochemistry 2010, 71, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Scotti, M.T.; Emerenciano, V.; Ferreira, M.J.P.; Scotti, L.; Stefani, R.; da Silva, M.S.; Mendonça Junior, F.J.B. Self-organizing maps of molecular descriptors for sesquiterpene lactones and their application to the chemotaxonomy of the Asteraceae family. Molecules 2012, 17, 4684–4702. [Google Scholar] [CrossRef] [PubMed]
- Bailey, N.J.C.; Wang, Y.; Sampson, J.; Davis, W.; Whitcombe, I.; Hylands, P.J.; Croft, S.L.; Holmes, E. Prediction of anti-plasmodial activity of Artemisia annua extracts: Application of 1H NMR spectroscopy and chemometrics. J. Pharm. Biomed. Anal. 2004, 35, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Awale, S.; Tezuka, Y.; Ueda, J.; Tran, Q.L.; Kadota, S. Xanthine oxidase inhibitors from the flowers of Chrysanthemum sinense. Planta Med. 2006, 72, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Jachak, S. Cyclooxygenase Inhibitory Natural Products: Current Status. Curr. Med. Chem. 2006, 13, 659–678. [Google Scholar] [CrossRef] [PubMed]
- Babish, J.; Howell, T.; Pacioretty, L. Combinations of sesquiterpene lactones and ditepene triepoxide lactones for synergistic inhibition of cyclooxygenase-2. US20020077299, 2002. [Google Scholar]
- De Alvarenga, E.S.; Silva, S.A.; Barosa, L.C.A.; Demuner, A.J.; Parreira, A.G.; Ribeiro, R.I.M.A.; Marcussi, S.; Ferreira, J.M.S.; Resende, R.R.; Granjeiro, P.A.; et al. Synthesis and evaluation of sesquiterpene lactone inhibitors of phospholipase A2 from Bothrops jararacussu. Toxicon 2011, 57, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Rüngeler, P.; Castro, V.; Mora, G.; Gören, N.; Vichnewski, W.; Pahl, H.L.; Merfort, I.; Schmidt, T.J. Inhibition of transcription factor NF-kappaB by sesquiterpene lactones: A proposed molecular mechanism of action. Bioorg. Med. Chem. 1999, 7, 2343–2352. [Google Scholar] [CrossRef]
- Rüngeler, P.; Lyss, G.; Castro, V.; Mora, G.; Pahl, H.L.; Merfort, I. Study of three sesquiterpene lactones from Tithonia diversifolia on their anti-inflammatory activity using the transcription factor NF-kappa B and enzymes of the arachidonic acid pathway as targets. Planta Med. 1998, 64, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Siedle, B.; García-Piñeres, A.J.; Murillo, R.; Schulte-Mönting, J.; Castro, V.; Rüngeler, P.; Klaas, C.A.; da Costa, F.B.; Kisiel, W.; Merfort, I. Quantitative structure-activity relationship of sesquiterpene lactones as inhibitors of the transcription factor NF-kappaB. J. Med. Chem. 2004, 47, 6042–6054. [Google Scholar] [CrossRef] [PubMed]
- Kessner, D.; Chambers, M.; Burke, R.; Agus, D.; Mallick, P. ProteoWizard: Open source software for rapid proteomics tools development. Bioinformatics 2008, 24, 2534–2536. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Cheng, C.; Viegelmann, C.; Zhang, T.; Grkovic, T.; Ahmed, S.; Quinn, R.J.; Hentschel, U.; Edrada-Ebel, R. Dereplication strategies for targeted isolation of new antitrypanosomal actinosporins A and B from a marine sponge associated-Actinokineospora sp. EG49. Mar. Drugs 2014, 12, 1220–1244. [Google Scholar] [CrossRef] [PubMed]
- Viegelmann, C.; Margassery, L.M.; Kennedy, J.; Zhang, T.; O’Brien, C.; O’Gara, F.; Morrissey, J.P.; Dobson, A.D.W.; Edrada-Ebel, R. Metabolomic profiling and genomic study of a marine sponge-associated Streptomyces sp. Mar. Drugs 2014, 12, 3323–3351. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chagas-Paula, D.A.; Zhang, T.; Da Costa, F.B.; Edrada-Ebel, R. A Metabolomic Approach to Target Compounds from the Asteraceae Family for Dual COX and LOX Inhibition. Metabolites 2015, 5, 404-430. https://doi.org/10.3390/metabo5030404
Chagas-Paula DA, Zhang T, Da Costa FB, Edrada-Ebel R. A Metabolomic Approach to Target Compounds from the Asteraceae Family for Dual COX and LOX Inhibition. Metabolites. 2015; 5(3):404-430. https://doi.org/10.3390/metabo5030404
Chicago/Turabian StyleChagas-Paula, Daniela A., Tong Zhang, Fernando B. Da Costa, and RuAngelie Edrada-Ebel. 2015. "A Metabolomic Approach to Target Compounds from the Asteraceae Family for Dual COX and LOX Inhibition" Metabolites 5, no. 3: 404-430. https://doi.org/10.3390/metabo5030404
APA StyleChagas-Paula, D. A., Zhang, T., Da Costa, F. B., & Edrada-Ebel, R. (2015). A Metabolomic Approach to Target Compounds from the Asteraceae Family for Dual COX and LOX Inhibition. Metabolites, 5(3), 404-430. https://doi.org/10.3390/metabo5030404







