1. Introduction
Nasopharyngeal carcinoma (NPC) is a type of head and neck cancer that originates from the epithelial cells lining the nasopharynx, a region anatomically close to the upper airway and nasal passages [
1,
2,
3,
4,
5]. NPC is particularly notable for its association with Epstein–Barr virus (EBV) infection, genetic predispositions, and environmental factors such as dietary habits and exposure to carcinogens [
6]. Clinical features of NPC include nonspecific symptoms like nasal congestion, epistaxis, hearing loss, and cervical lymphadenopathy [
7,
8,
9]. Early detection and accurate staging are critical for NPC management, as the disease can metastasize to regional lymph nodes and distant organs.
Currently, there are no widely available non-invasive screening tests for NPC. The primary screening method, EBV serology, while helpful, has limitations in terms of sensitivity and specificity. Moreover, nasoendoscopy, the gold standard for NPC detection, is not easily accessible outside of specialist settings, which complicates early detection efforts. The lack of effective non-invasive tests underscores a critical gap in NPC diagnosis and screening.
Accurate diagnosis of NPC involves a multidisciplinary approach. This typically includes nasopharyngoscopy for visual examination of the nasopharynx, imaging modalities such as magnetic resonance imaging (MRI) and computed tomography (CT) scans for tumor localization and staging, and biopsy for histological confirmation of malignancy [
10,
11,
12]. Molecular and genetic tests may also play a role in identifying specific biomarkers and prognostic indicators in NPC patients.
However, the diagnostic workup for NPC, including nasopharyngoscopy, imaging studies, and biopsy, can be invasive, time-consuming, and costly. Imaging modalities like MRI and CT scans expose patients to ionizing radiation and require skilled interpretation for accurate tumor localization and staging. Biopsy procedures carry risks of bleeding, infection, and sampling errors, necessitating careful consideration of clinical indications and the potential benefits versus harms.
Volatile organic compounds (VOCs) are a diverse group of organic chemicals present in exhaled breath, originating from various metabolic processes within the body [
13,
14]. Detected in trace amounts, these compounds can serve as potential biomarkers for a wide range of diseases, including nasopharyngeal carcinoma (NPC). Biomarkers are measurable indicators of a biological condition, and in the context of breath analysis, they offer a non-invasive method for disease detection and monitoring. Multiple studies have demonstrated differences in breath VOCs between patients with and without cancer. A recent systematic review [
15] summarized more than a hundred candidate VOCs across cancer studies, with commonly reported examples including acetone, 2-butanone, isoprene, nonanal, and tetradecane, which have been repeatedly linked to altered metabolism in cancer patients.
Although breath VOC analysis has not yet been widely applied to NPC, previous studies on head and neck squamous cell carcinoma [
16] and other cancers [
15] have consistently demonstrated the value of VOCs as biomarkers of tumor-associated metabolic changes. These findings support the biological plausibility of our approach and provide a strong rationale for extending this line of research to NPC. To date, no study has specifically examined breath analysis in NPC, despite it being one of the most prevalent head and neck cancers in Asian populations [
17].
Breath tests are a compelling diagnostic approach because they are non-invasive and easy to administer and allow for rapid analysis [
18]. The process involves collecting exhaled breath from patients, which is then analyzed using advanced techniques such as proton transfer reaction mass spectrometry (PTR-MS). PTR-MS is a highly sensitive method that enables real-time detection and quantification of VOCs in breath samples, making it a valuable tool for identifying disease-specific biomarkers.
Breath analysis using PTR-MS and the detection of VOC features represent a promising frontier in medical diagnostics. This approach not only improves our understanding of the metabolic changes associated with disease but also creates opportunities for personalized medicine, where treatments may be tailored to the individual patient’s VOC profile.
4. Discussion
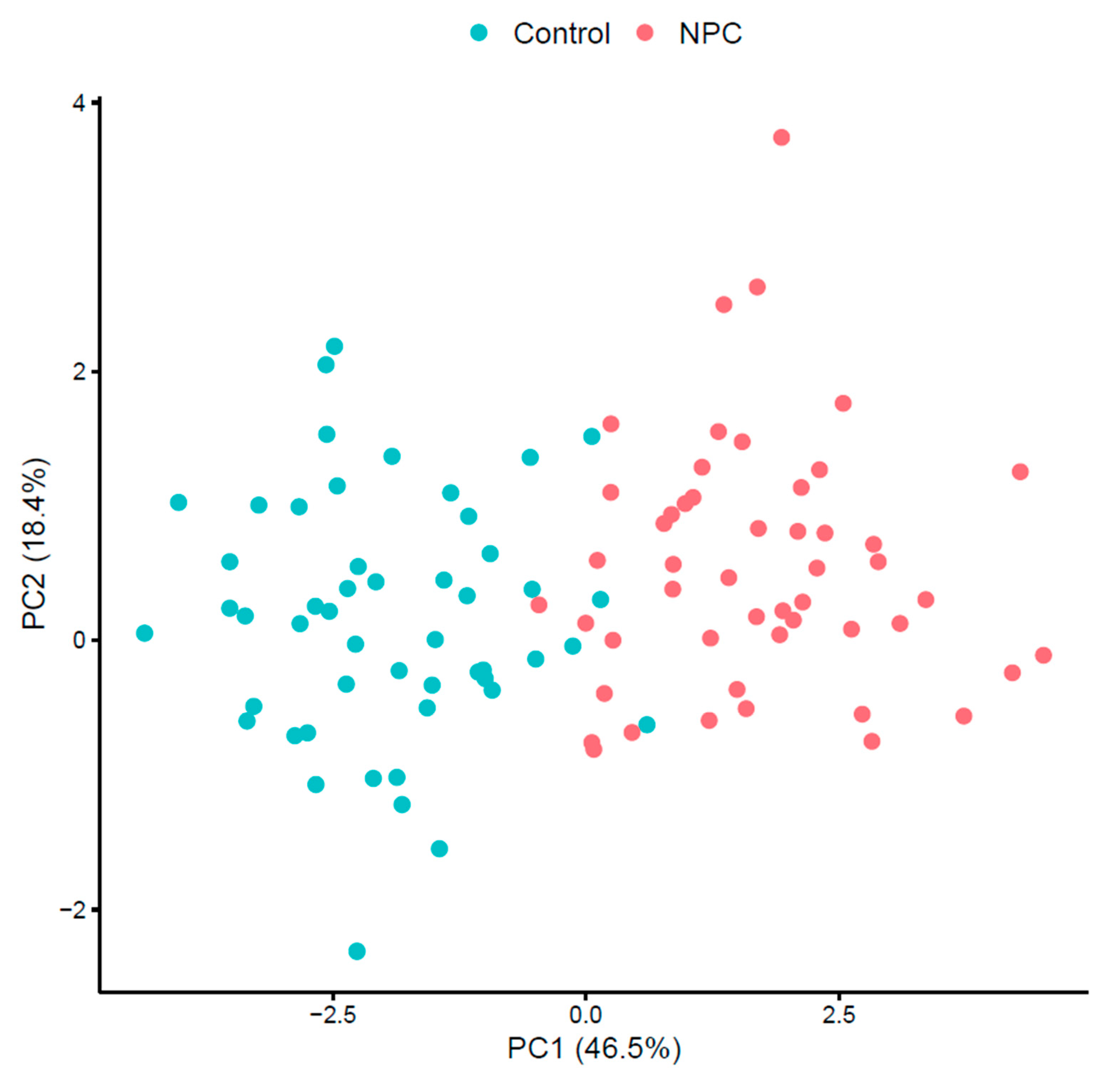
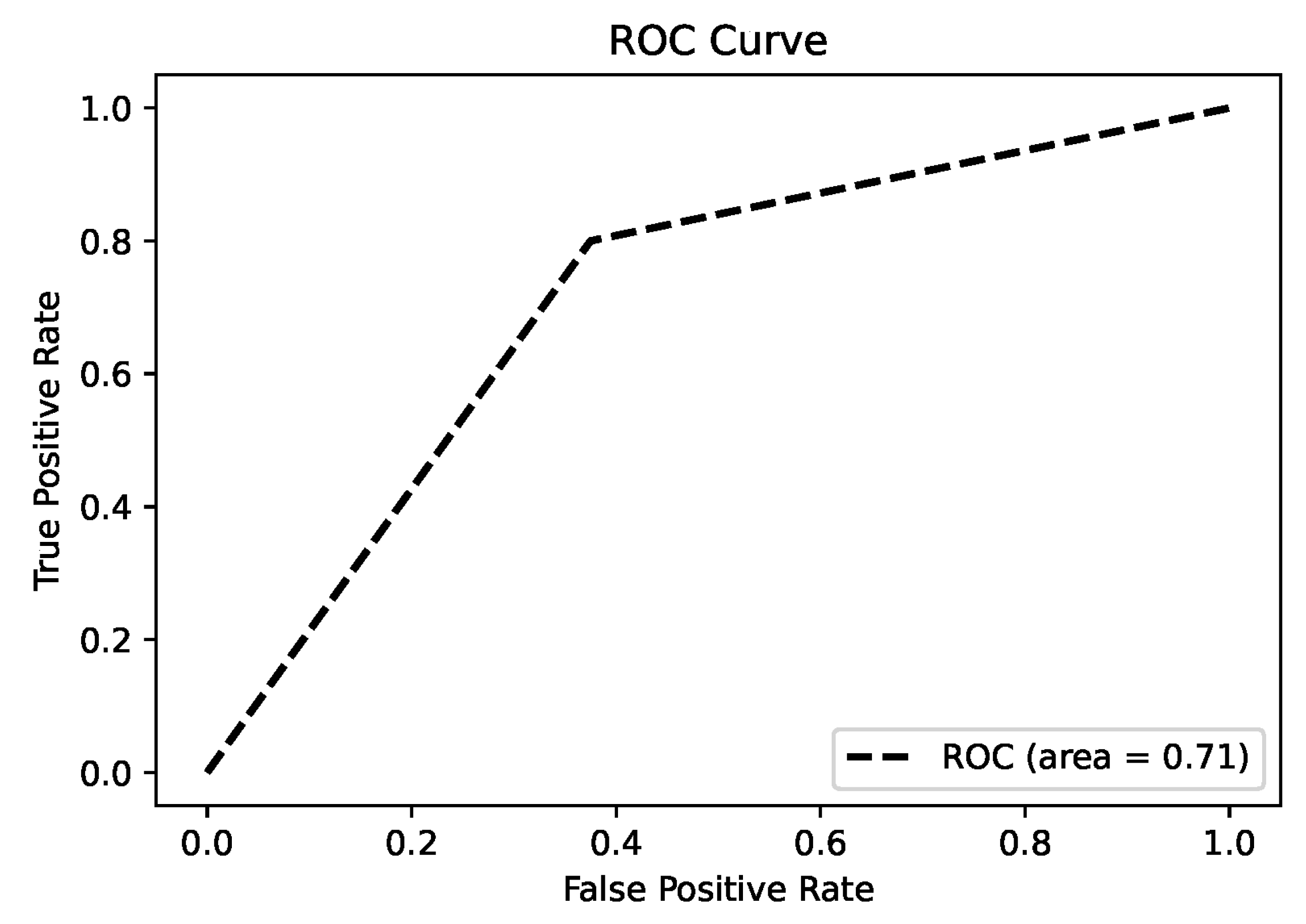
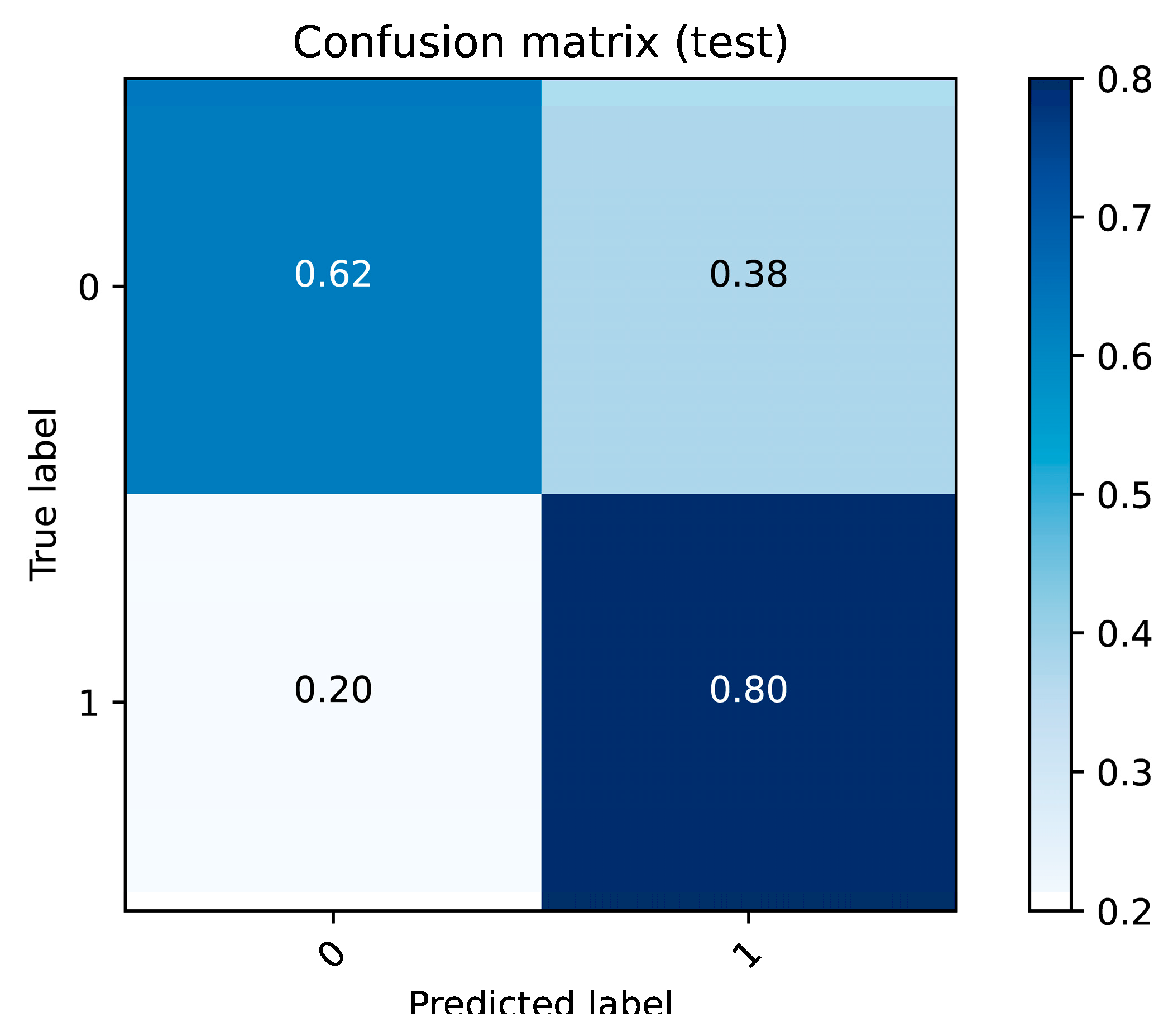
The results of this study underscore the potential of proton transfer reaction mass spectrometry (PTR-MS) in identifying volatile organic compound (VOC) biomarkers for diagnosing nasopharyngeal carcinoma (NPC). Significant differences in VOC profiles between the NPC patients and control subjects were observed, identifying specific biomarkers that can effectively distinguish between diseased and healthy individuals.
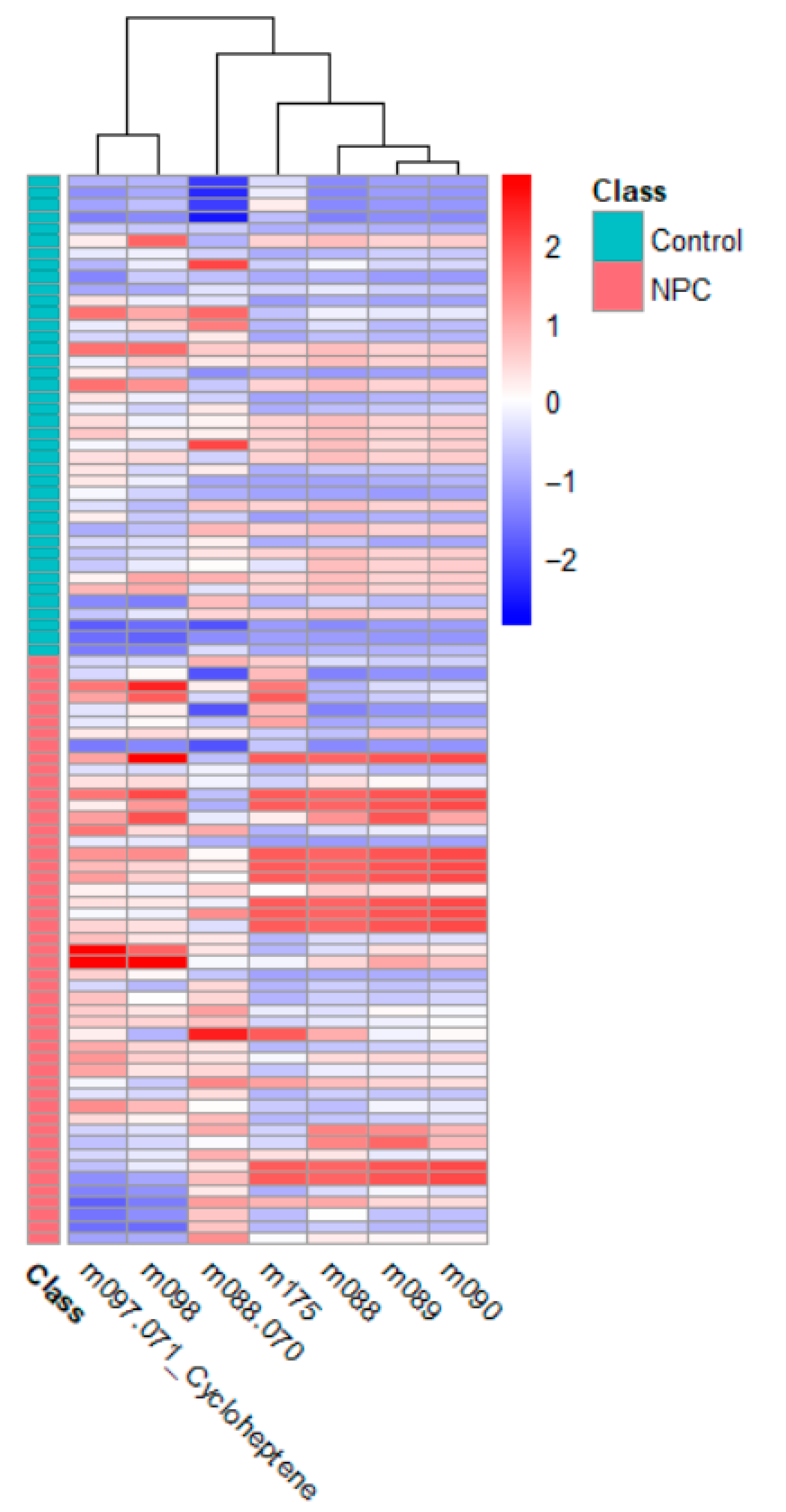
In the discussion of the potential identities of m089 and m175, it is essential to hypothesize the possible compounds based on their mass-to-charge (m/z) ratios and their relevance to nasopharyngeal carcinoma (NPC). Given that PTR-MS assigns a VOC ID that is one unit larger than the molecular weight due to proton attachment, these m/z values can provide insights into their molecular identities and their roles in NPC.
M089 and m175 likely represent VOCs that are products of altered metabolic processes in NPC. These compounds may serve as biomarkers for the disease, reflecting the oxidative stress and lipid metabolism disruptions that are critical in the progression of NPC. Further research is required to confirm the exact identities of these VOCs and elucidate their roles in NPC pathophysiology, but their significant presence suggests that they could be valuable for early diagnosis and monitoring.
M089 likely corresponds to a molecule with a molecular weight of approximately 88 Da, with butyric acid as a potential candidate. Butyric acid is a short-chain fatty acid involved in essential metabolic processes, including inflammation and immune regulation [
24]. Elevated levels may indicate dysregulated fatty acid metabolism, a phenomenon frequently associated with cancer. Ji et al. (2025) showed that the exhaled breath profiles of colorectal cancer patients exhibited significant alterations in VOCs related to short-chain fatty acid metabolism, including butyric acid [
25], underscoring the role of short-chain fatty acid dysregulation in malignancy. In the context of NPC, butyric acid may therefore serve as a marker of altered energy metabolism or an inflammatory response associated with tumor progression.
Regarding m175, we were unable to conclusively determine the corresponding compound. The m/z value of 175 represented a wide range of molecules, possibly related to fatty acid derivatives or other metabolic byproducts. However, without a clear chemical identification, it remains speculative. Further analysis, such as detailed mass spectrometry or database matching, is needed to accurately characterize m175 and assess its potential relevance in NPC. Identifying this compound could provide more insight into the metabolic alterations occurring in NPC patients.
A key contribution of this research is its novel application of PTR-MS for the non-invasive detection of NPC. Traditional diagnostic methods, such as nasopharyngoscopy for NPC, are invasive, time-consuming, and costly. In contrast, PTR-MS breath analysis offers a rapid, non-invasive, and cost-effective alternative suitable for clinical settings. Detection of significant VOC features, such as
m/
z 89 and
m/
z 175 in NPC patients, highlights the potential sensitivity and specificity of this method [
26].
This study also enhances the understanding of the metabolic changes associated with NPC. The correlation analyses reveal potential metabolic pathways involved in this disease, providing insights for future research and therapeutic strategies. The identified VOCs associated with NPC could inform the development of targeted therapies and improve patient outcomes.
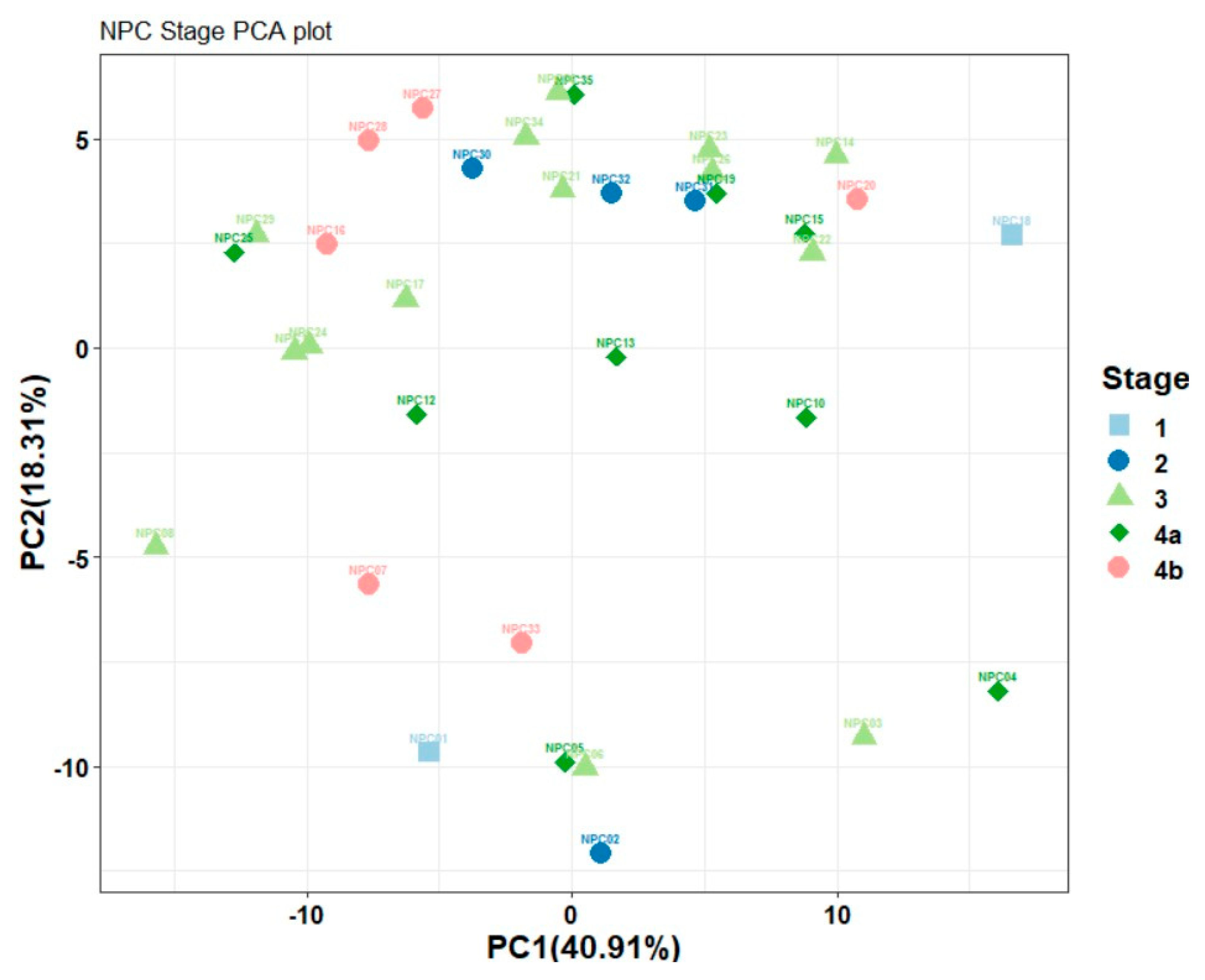
Improvements over previous studies include a comprehensive analysis of multiple VOCs and the application of advanced statistical techniques to validate the findings. Principal component analysis (PCA), heat maps, and correlation maps provide a robust framework for visualizing and interpreting the data, enhancing the reliability of the results.
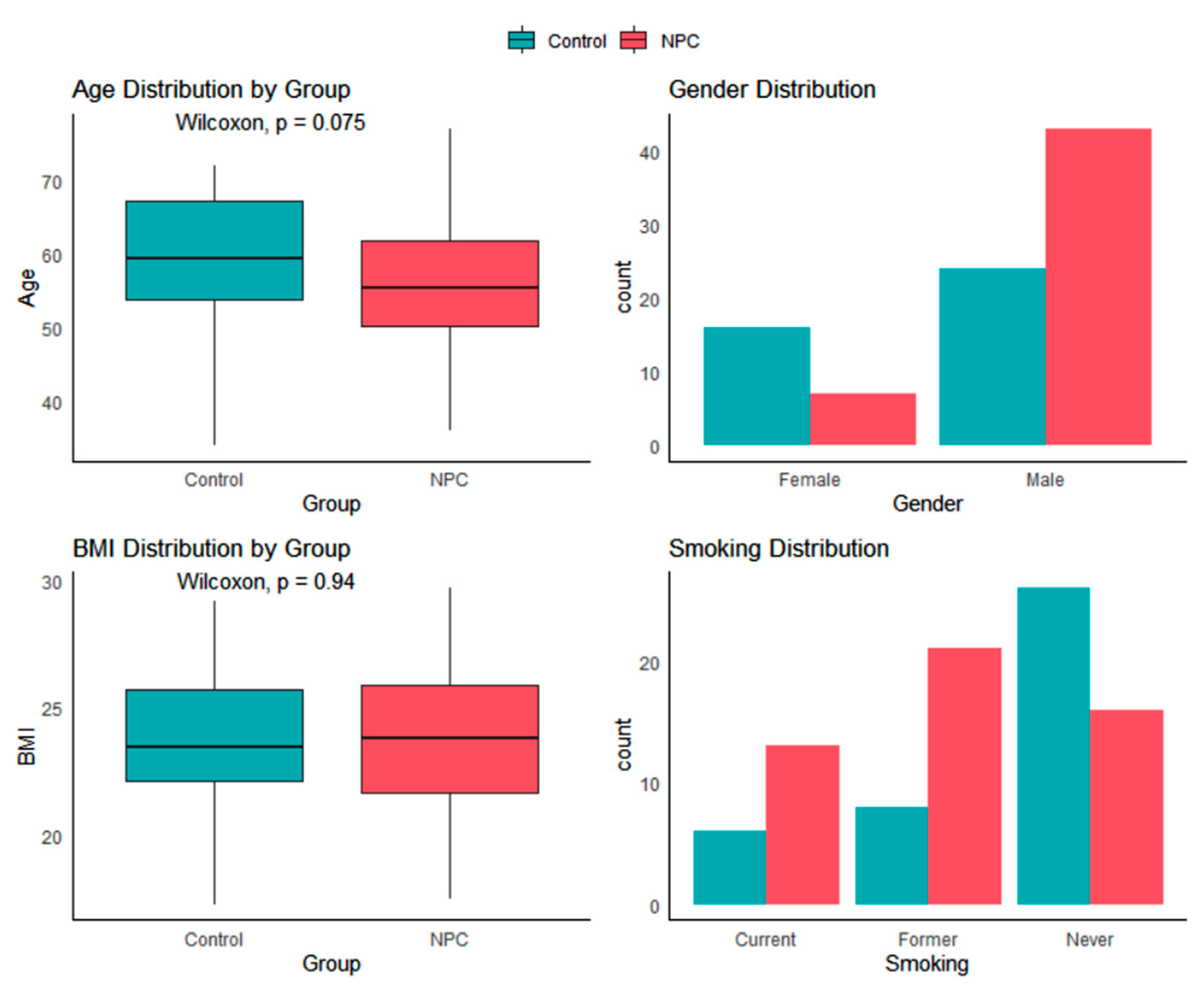
The demographics of our NPC group closely resemble those of other studies, with a mean age at diagnosis ranging from 55 to 60 years [
27,
28], and over 70% of patients being diagnosed at advanced stages (Stage III and IV) [
27]. Although there are notable differences in gender and age between the NPC and control groups, other studies have indicated that variations in exhaled breath VOC content are not significantly affected by these factors [
29,
30]. Although most patients in our cohort had advanced-stage NPC, consistent with regional epidemiology, the scientific and clinical value of PTR-MS breath analysis lies in its potential to detect NPC at earlier stages (I/II). Early detection is critical for improving survival, and PTR-MS offers a non-invasive screening approach that could complement existing diagnostic pathways. Larger future studies with sufficient early-stage patients are needed to validate this application.
The identification of VOCs with consistent diagnostic value across different populations is essential for the clinical translation of PTR-MS breath analysis [
31]. In the context of NPC, our findings highlight m089 and m175 as promising candidates, but validation in larger and more diverse cohorts is needed to confirm their reliability. Future studies should also investigate the integration of VOC profiling with established diagnostic modalities such as EBV serology and nasoendoscopy to enhance accuracy and improve patient outcomes. In addition, evaluating the temporal stability of these biomarkers and their response to treatment could provide important insights into NPC progression and therapeutic efficacy [
23,
31].
A limitation of this study is that the putative biomarkers m089 and m175 were not chemically confirmed. In future work, we plan to analyze pure standard compounds and compare their PTR-MS spectra with breath measurements to definitively establish compound identities.
In summary, this study demonstrates the feasibility and potential of PTR-MS for the non-invasive screening of NPC. This approach offers a promising tool for the early detection and monitoring of this condition, with significant implications for clinical practice and patient care.
Author Contributions
Conceptualization, Z.J., A.C.T., M.Y.L. and C.H.G.; methodology, Z.J. and C.H.G.; formal analysis, C.H.G. and F.Z.; investigation, A.C.T., K.B.C., E.W.F., J.Y.G., H.L., Y.K.C., A.S.Q.C. and M.Y.L.; writing—original draft preparation, Z.J. and C.H.G.; writing—review and editing, Z.J., C.H.G., Z.X. and F.Z.; supervision, Z.J., A.C.T., K.B.C., M.Y.L. and C.H.G.; administration, Z.J., A.C.T. and C.H.G.; funding acquisition, C.H.G. and F.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study protocol was approved by the NHG Domain Specific Review Board (DSRB) on 2 September 2019; approval number 2019/00345.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
Zhunan Jia, Zihao Xing, Fuchang Zhang, and Fang Du are employees of Breathonix Pte Ltd. The paper reflects the views of the scientists, and not the company.
Abbreviations
The following abbreviations are used in this manuscript:
| NPC | Nasopharyngeal Carcinoma |
| PTR-MS | Proton Transfer Reaction Mass Spectrometry |
| VOC | Volatile Organic Compound |
| EBV | Epstein–Barr Virus |
| MRI | Magnetic Resonance Imaging |
| CT | Computed Tomography |
| AJCC | American Joint Committee on Cancer |
| TOF-MS | Time-of-Flight Mass Spectrometer |
References
- Chen, Y.-P.; Chan, A.T.C.; Le, Q.-T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef]
- Wu, L.; Li, C.; Pan, L. Nasopharyngeal carcinoma: A review of current updates. Exp. Ther. Med. 2018, 15, 3687–3692. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.; Lin, Y.; Kim, J.; You, L.; Xu, Z.; He, B.; Jablons, D.M. Nasopharyngeal carcinoma—Review of the molecular mechanisms of tumorigenesis. Head Neck 2008, 30, 946–963. [Google Scholar] [CrossRef]
- Jeyakumar, A.; Brickman, T.M.; Jeyakumar, A.; Doerr, T. Review of nasopharyngeal carcinoma. Ear Nose Throat J. 2006, 85, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.T.; Ye, W.; Zeng, Y.-X.; Adami, H.-O. The evolving epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1035–1047. [Google Scholar] [CrossRef]
- Chua, M.L.K.; Wee, J.T.S.; Hui, E.P.; Chan, A.T.C. Nasopharyngeal carcinoma. Lancet 2016, 387, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.-J.; Hsu, W.-L.; Chen, C.-J.; Chiu, Y.-L. Feature reviews of the molecular mechanisms of nasopharyngeal carcinoma. Biomedicines 2023, 11, 1528. [Google Scholar] [CrossRef]
- Adham, M.; Kurniawan, A.N.; Muhtadi, A.I.; Roezin, A.; Hermani, B.; Gondhowiardjo, S.; Tan, I.B.; Middeldorp, J.M. Nasopharyngeal carcinoma in Indonesia: Epidemiology, incidence, signs, and symptoms at presentation. Chin. J. Cancer 2012, 31, 185–196. [Google Scholar] [CrossRef]
- Lee, A.W.; Ma, B.B.; Ng, W.T.; Chan, A.T. Management of nasopharyngeal carcinoma: Current practice and future perspective. J. Clin. Oncol. 2015, 33, 3356–3364. [Google Scholar] [CrossRef]
- Tabuchi, K.; Nakayama, M.; Nishimura, B.; Hayashi, K.; Hara, A. Early Detection of Nasopharyngeal Carcinoma. Int. J. Otolaryngol. 2011, 2011, 638058. [Google Scholar] [CrossRef]
- Razek, A.A.K.A.; King, A. MRI and CT of nasopharyngeal carcinoma. Am. J. Roentgenol. 2012, 198, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Chan, A.; Licitra, L.; Trama, A.; Orlandi, E.; Hui, E.; Halámková, J.; Mattheis, S.; Baujat, B.; Hardillo, J.; et al. Nasopharyngeal carcinoma: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 452–465. [Google Scholar] [CrossRef]
- Davies, S.J.; Španěl, P.; Smith, D. Breath analysis of ammonia, volatile organic compounds and deuterated water vapor in chronic kidney disease and during dialysis. Bioanalysis 2014, 6, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Capone, S.; Tufariello, M.; Forleo, A.; Longo, V.; Giampetruzzi, L.; Radogna, A.V.; Casino, F.; Siciliano, P. Chromatographic analysis of VOC patterns in exhaled breath from smokers and nonsmokers. Biomed. Chromatogr. 2017, 32, e4132. [Google Scholar] [CrossRef]
- Moura, P.C.; Raposo, M.; Vassilenko, V. Breath volatile organic compounds (vocs) as biomarkers for the diagnosis of pathological conditions: A Review. Biomed. J. 2023, 46, 100623. [Google Scholar] [CrossRef]
- Dharmawardana, N.; Goddard, T.; Woods, C.; Watson, D.I.; Ooi, E.H.; Yazbeck, R. Development of a non-invasive exhaled breath test for the diagnosis of head and neck cancer. Br. J. Cancer 2020, 123, 1775–1781. [Google Scholar] [CrossRef]
- Mahdavifar, N.; Ghoncheh, M.; Mohammadian-Hafshejani, A.; Khosravi, B.; Salehiniya, H. Epidemiology and inequality in the incidence and mortality of nasopharynx cancer in Asia. Osong Public Health Res. Perspect. 2016, 7, 360–372. [Google Scholar] [CrossRef]
- Pereira, J.; Porto-Figueira, P.; Cavaco, C.; Taunk, K.; Rapole, S.; Dhakne, R.; Nagarajaram, H.; Câmara, J.S. Breath analysis as a potential and non-invasive frontier in disease diagnosis: An overview. Metabolites 2015, 5, 3–55. [Google Scholar] [CrossRef]
- Hanna, G.B.; Boshier, P.R.; Markar, S.R.; Romano, A. Accuracy and methodologic challenges of volatile organic compound–based exhaled breath tests for cancer diagnosis. JAMA Oncol. 2019, 5, e182815. [Google Scholar] [CrossRef]
- Zanoni, D.K.; Patel, S.G.; Shah, J.P. Changes in the 8th edition of the American Joint Committee on Cancer (AJCC) staging of head and neck cancer: Rationale and implications. Curr. Oncol. Rep. 2019, 21, 52. [Google Scholar] [CrossRef] [PubMed]
- Herbig, J.; Müller, M.; Schallhart, S.; Titzmann, T.; Graus, M.; Hansel, A. On-line breath analysis with PTR-TOF. J. Breath Res. 2009, 3, 027004. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Ong, W.Q.; Zhang, F.; Du, F.; Thavasi, V.; Thirumalai, V. A study of 9 common breath vocs in 504 healthy subjects using PTR-TOF-MS. Metabolomics 2024, 20, 79. [Google Scholar] [CrossRef]
- Jia, Z.; Patra, A.; Kutty, V.K.; Venkatesan, T. Critical review of volatile organic compound analysis in breath and in vitro cell culture for detection of lung cancer. Metabolites 2019, 9, 52. [Google Scholar] [CrossRef]
- Siddiqui, M.T.; Cresci, G.A.M. The Immunomodulatory Functions of Butyrate. J. Inflamm. Res. 2021, 14, 6025–6041. [Google Scholar] [CrossRef]
- Ji, Y.; Chen, J.; Liu, Y.; Li, X. Breathomics profiling facilitates personalized screening for colorectal cancer. Talanta 2026, 297, 128646. [Google Scholar] [CrossRef] [PubMed]
- Issitt, T.; Wiggins, L.; Veysey, M.; Sweeney, S.T.; Brackenbury, W.J.; Redeker, K. Volatile compounds in human breath: Critical review and meta-analysis. J. Breath Res. 2022, 16, 024001. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-G.; Liao, X.-L.; He, Z.-Y.; Tang, L.-Y.; Chen, X.-T.; Wang, Y.; Lin, Q. Demographic and clinicopathological characteristics of nasopharyngeal carcinoma and survival outcomes according to age at diagnosis: A population-based analysis. Oral Oncol. 2017, 73, 83–87. [Google Scholar] [CrossRef]
- Colaco, R.; Betts, G.; Donne, A.; Swindell, R.; Yap, B.; Sykes, A.; Slevin, N.; Homer, J.; Lee, L. Nasopharyngeal carcinoma—A retrospective review of demographics, treatment and patient outcome in a single centre. Clin. Oncol. 2013, 25, 171–177. [Google Scholar] [CrossRef]
- Blanchet, L.; Smolinska, A.; Baranska, A.; Tigchelaar, E.; Swertz, M.; Zhernakova, A.; Dallinga, J.W.; Wijmenga, C.; van Schooten, F.J. Factors that influence the volatile organic compound content in human breath. J. Breath Res. 2017, 11, 016013. [Google Scholar] [CrossRef]
- Dragonieri, S.; Quaranta, V.N.; Carratu, P.; Ranieri, T.; Resta, O. Influence of age and gender on the profile of exhaled volatile organic compounds analyzed by an electronic nose. J. Bras. Pneumol. 2016, 42, 143–145. [Google Scholar] [CrossRef]
- Jia, Z.; Thavasi, V.; Venkatesan, T.; Lee, P. Breath analysis for lung cancer early detection—A clinical study. Metabolites 2023, 13, 1197. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).