Metabolomic Profiling Reveals Distinct Signatures in Primary and Secondary Polycythemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection
2.3. Metabolite Extraction
2.4. LC/MS Analysis
2.5. Data Processing
2.6. Statistical Analysis
3. Results
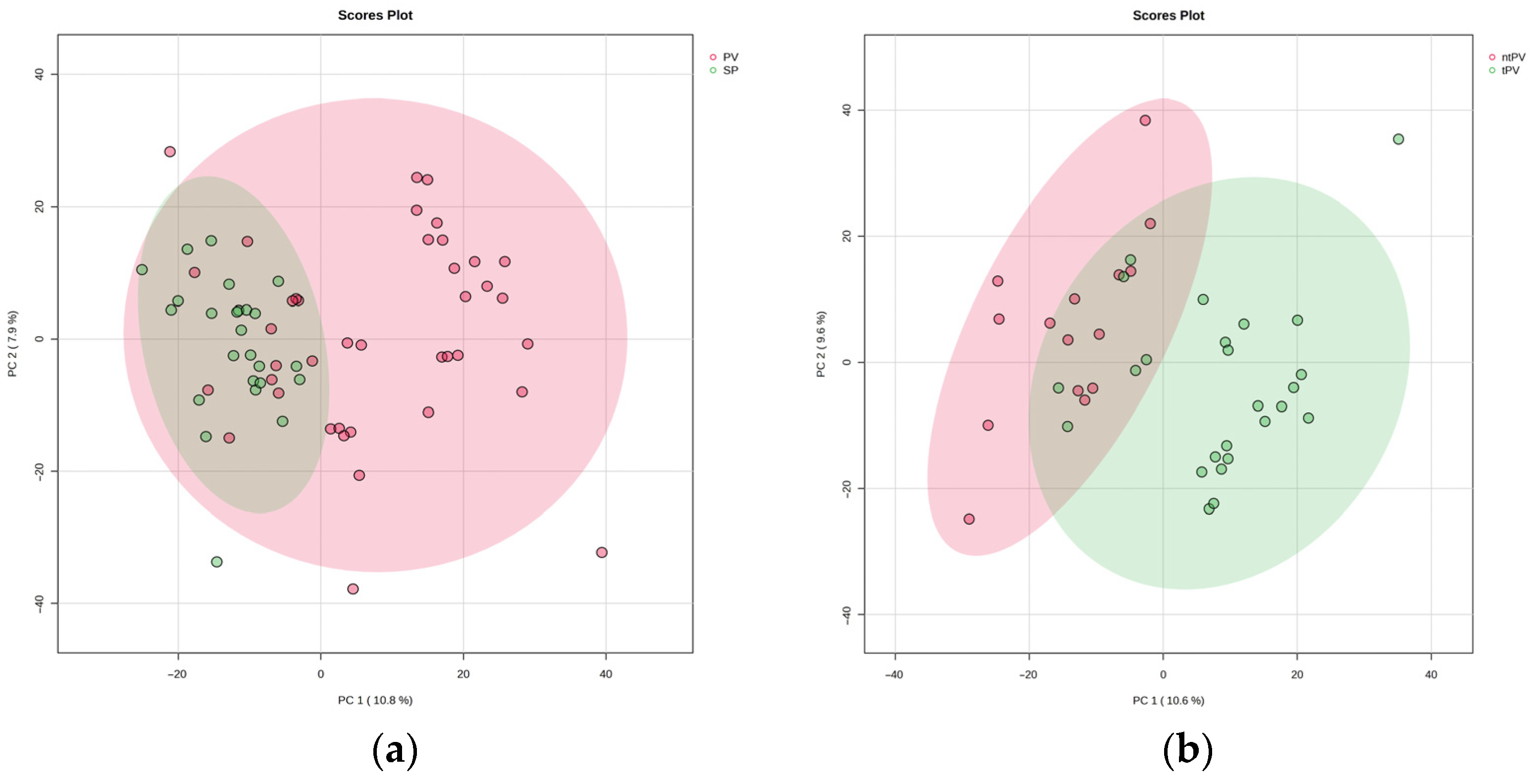
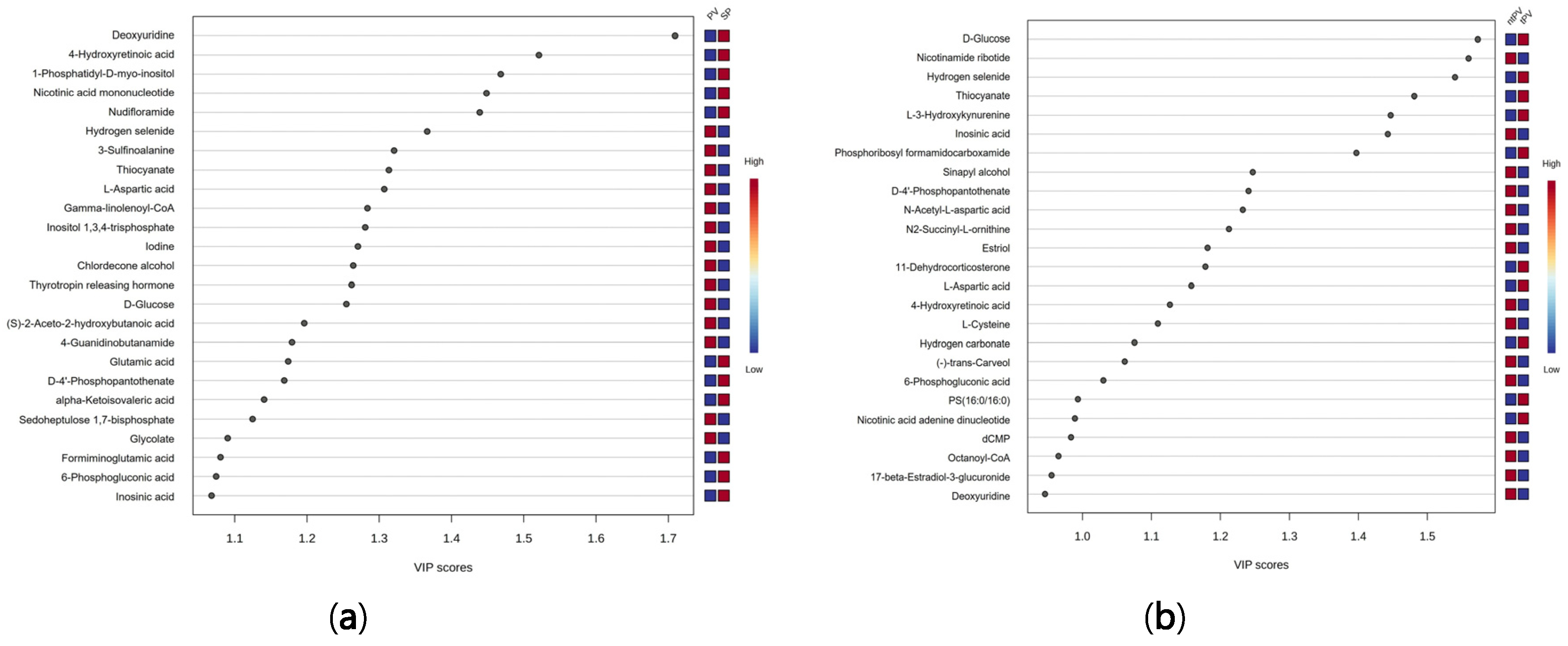
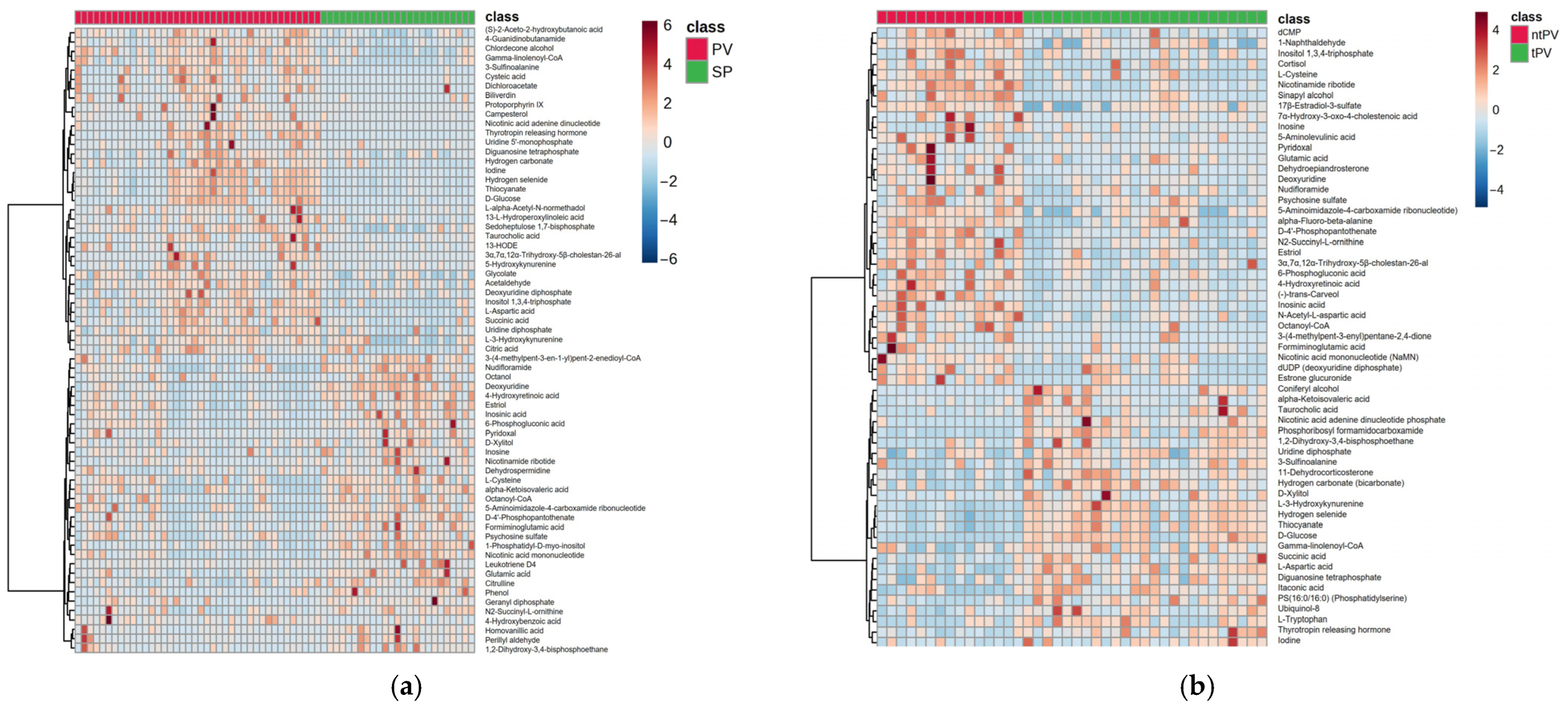
3.1. Comparison Between Secondary Polycythemia and Polycythemia Vera Patients
3.2. Subgroup Analysis of Treated and Untreated Polycythemia Vera Patients
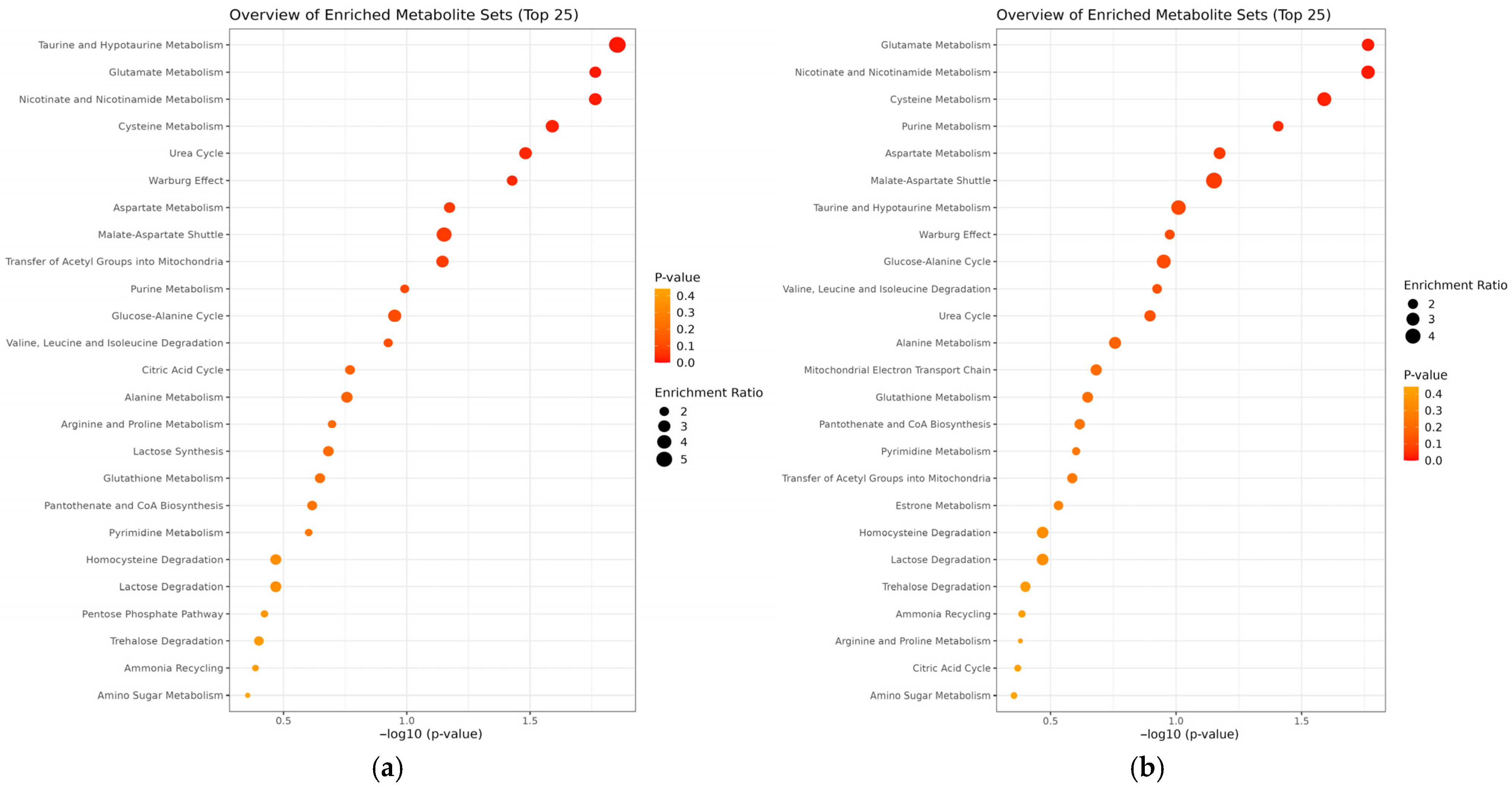
3.3. Metabolomic Distinctions Between Disease Subgroups: PV vs. SP and tPV vs. ntPV
3.4. Cross-Comparison Analysis
4. Discussion
4.1. Metabolomic Differentiation Between SP and PV
4.1.1. Central Carbon Metabolism and Energy Production
4.1.2. Amino Acid Metabolism and Cellular Signaling
4.1.3. Nucleotide Metabolism and Cell Proliferation
4.1.4. Hormonal and Neuroendocrine Perturbations
4.1.5. Alterations in Redox-Active Metabolism and Antioxidant Defense
4.1.6. Impaired Retinoic Acid Metabolism and Megakaryocytic Dysfunction
4.2. Impact of Cytoreductive Therapy on Metabolic Profiles
4.2.1. Therapeutic Normalization of Glucose Metabolism
4.2.2. B-Vitamin Metabolism and Co-Factor Availability
4.2.3. Lipid Metabolism and Cellular Membrane Composition
4.3. Shared Metabolic Pathways and Disease Mechanisms
4.3.1. Glutamate Metabolism
4.3.2. Purine Metabolism and DNA Synthesis
4.4. Clinical Implications and Diagnostic Potential
4.4.1. Biomarker Development
4.4.2. Therapeutic Target Identification
4.5. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| GPX | Glutathione peroxidase |
| Hgb | Hemoglobin |
| Hct | Hematocrit |
| IP3 | Inositol 1,3,4-trisphosphate |
| JAK/STAT | Janus kinase/signal transducer and activator of transcription protein |
| LC/MS | Liquid chromatography/mass spectrometer |
| MAPK/ERK | Mitogen-activated protein kinases/extracellular signal-regulated kinases |
| MPN | Myeloproliferative neoplasm |
| NAAD | Nicotinamide adenine dinucleotide nicotinic acid adenine dinucleotide (NAAD) |
| ntPV | Not receiving polcytemia vera |
| OXPHOS | Oxidative phosphorylation |
| Q-TOF LC/MS | Quadrupole-time of flight liquid chromatography/mass spectrometer |
| PCA | Principal component analysis |
| PLS-DA | Partial least squares discriminant analysis |
| PLT | Platelet |
| PV | Polycytemia vera |
| RAR | retinoid receptors |
| SD | Standard deviation |
| SP | Secondary polycytemia |
| TAUT | Taurine transporter |
| TCA | Tricarboxylic acid |
| TRH | Thyrotropin releasing hormone |
| tPV | Treated polyctemia vera |
| VIP | Variable importance in projection |
| WBC | White Blood Cell |
References
- Tefferi, A.; Vannucchi, A.M.; Barbui, T. Polycythemia vera: Historical oversights, diagnostic details, and therapeutic views. Leukemia 2021, 35, 3339–3351. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Qi, J.; Babon, J.J.; Cao, L.; Fan, G.; Lang, J.; Zhang, J.; Mi, P.; Kobe, B.; Wang, F. The JAK-STAT pathway: From structural biology to cytokine engineering. Signal Transduct. Target. Ther. 2024, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.Z.; Anwer, F. Secondary Polycythemia; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Dalamaga, M. Clinical metabolomics: Useful insights, perspectives and challenges. Metab. Open 2024, 22, 100290. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhou, X.; Wang, X. Metabolic reprogramming in hematologic malignancies: Advances and clinical perspectives. Cancer Res. 2022, 82, 2955–2963. [Google Scholar] [CrossRef] [PubMed]
- Cacemiro, M.; Cominal, J.; Almeida, F.; Oliveira, M.; Sorgi, C.; Figueiredo-Pontes, L.; Faccioli, L.; Gardinassi, L.; Castro, F. Metabolic profile of bone marrow plasma in myeloproliferative neoplasms. Hematol. Transfus. Cell Ther. 2021, 43, S137. [Google Scholar] [CrossRef]
- Li, X.; Xu, M.; Chen, Y.; Zhai, Y.; Li, J.; Zhang, N.; Yin, J.; Wang, L. Metabolomics for hematologic malignancies: Advances and perspective. Medicine 2024, 103, e39782. [Google Scholar] [CrossRef] [PubMed]
- Morganti, C.; Cabezas-Wallscheid, N.; Ito, K. Metabolic regulation of hematopoietic stem cells. Hemasphere 2022, 6, e740. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Zhao, Y.; Xia, Z.; Hakemi, M.G.; Bazhin, A.V. The importance of cellular metabolic pathways in pathogenesis and selective treatments of hematological malignancies. Front. Oncol. 2021, 11, 767026. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Laranjeira, A.B.; Kong, T.; Lin, S.; Ashworth, K.J.; Liu, A.; Lasky, N.M.; Fisher, D.A.; Cox, M.J.; Fulbright, M.C. Multiomic profiling reveals metabolic alterations mediating aberrant platelet activity and inflammation in myeloproliferative neoplasms. J. Clin. Investig. 2024, 134, e172256. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Gao, W.-Q.; Liu, Y. Metabolic heterogeneity in cancer: An overview and therapeutic implications. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2020, 1874, 188421. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.N.; Hansen, N.; Hilfiker, J.; Rai, S.; Majewska, J.-M.; Leković, D.; Gezer, D.; Andina, N.; Galli, S.; Cassel, T. JAK2-mutant hematopoietic cells display metabolic alterations that can be targeted to treat myeloproliferative neoplasms. Blood J. Am. Soc. Hematol. 2019, 134, 1832–1846. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lv, Y.; Yang, E.; Li, Y.; Wang, D.; Hu, G.; Li, Y.; Wang, M.; Liu, W.; Sun, M. Metabolic Biomarkers Affecting Cell Proliferation and Prognosis in Polycythemia Vera. Cancers 2022, 14, 4913. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Zhang, C.; Le, A. Glucose metabolism in cancer: The Warburg effect and beyond. Adv. Exp. Med. Biol. 2021, 1311, 3–15. [Google Scholar] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Wright, K.L.; Epling-Burnette, P.K.; Reuther, G.W. Metabolic vulnerabilities and epigenetic dysregulation in myeloproliferative neoplasms. Front. Immunol. 2020, 11, 604142. [Google Scholar] [CrossRef] [PubMed]
- Czegle, I.; Gray, A.L.; Wang, M.; Liu, Y.; Wang, J.; Wappler-Guzzetta, E.A. Mitochondria and Their Relationship with Common Genetic Abnormalities in Hematologic Malignancies. Life 2021, 11, 1351. [Google Scholar] [CrossRef] [PubMed]
- Farge, T.; Saland, E.; de Toni, F.; Aroua, N.; Hosseini, M.; Perry, R.; Bosc, C.; Sugita, M.; Stuani, L.; Fraisse, M.; et al. Chemotherapy-Resistant Human Acute Myeloid Leukemia Cells Are Not Enriched for Leukemic Stem Cells but Require Oxidative Metabolism. Cancer Discov. 2017, 7, 716–735. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.G.; Thompson, C.B. Tumor suppressors and cell metabolism: A recipe for cancer growth. Genes Dev. 2009, 23, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Durmus, A.; Mentese, A.; Yilmaz, M.; Sumer, A.; Akalin, I.; Topal, C.; Alver, A. The thrombotic events in polycythemia vera patients may be related to increased oxidative stress. Med. Princ. Pract. 2014, 23, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Stipanuk, M.H.; Ueki, I.; Dominy, J.E.; Simmons, C.R.; Hirschberger, L.L. Cysteine dioxygenase: A robust system for regulation of cellular cysteine levels. Amino Acids 2009, 37, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Vener, C.; Novembrino, C.; Bamonti Catena, F.; Fracchiolla, N.S.; Gianelli, U.; Savi, F.; Radaelli, F.; Fermo, E.; Cortelezzi, A.; Lonati, S.; et al. Oxidative stress is increased in primary and post-polycythemia vera myelofibrosis. Exp. Hematol. 2010, 38, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Kopacz, A.; Jozkowicz, A.; Grochot-Przeczek, A. Chapter 2—Cysteine thiol modifications—Oxidative (eu)stress perspective. In Modulation of Oxidative Stress; Saso, L., Giuffrè, A., Valacchi, G., Maccarrone, M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 17–27. [Google Scholar]
- Gu, S.X.; Stevens, J.W.; Lentz, S.R. Regulation of thrombosis and vascular function by protein methionine oxidation. Blood 2015, 125, 3851–3859. [Google Scholar] [CrossRef] [PubMed]
- Lentz, S.R. Mechanisms of homocysteine-induced atherothrombosis. J. Thromb. Haemost. 2005, 3, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.S.; Tauro, L.; Joshi, H.B.; Makhal, A.; Sobczak, T.; Goret, J.; Dewitte, A.; Kaveri, S.; Chakrapani, H.; Matsuda, M.M.; et al. Influence of homocysteine on regulating immunothrombosis: Mechanisms and therapeutic potential in management of infections. Inflamm. Res. 2025, 74, 86. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Smith, B.C. Cysteine and methionine oxidation in thrombotic disorders. Curr. Opin. Chem. Biol. 2023, 76, 102350. [Google Scholar] [CrossRef] [PubMed]
- Ripps, H.; Shen, W. Review: Taurine: A “very essential” amino acid. Mol. Vis. 2012, 18, 2673–2686. [Google Scholar] [PubMed] [PubMed Central]
- Schaffer, S.; Takahashi, K.; Azuma, J. Role of osmoregulation in the actions of taurine. Amino Acids 2000, 19, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Jong, C.J.; Azuma, J.; Schaffer, S.W. Role of mitochondrial permeability transition in taurine deficiency-induced apoptosis. Exp. Clin. Cardiol. 2011, 16, 125–128. [Google Scholar] [PubMed]
- Sharma, S.; Rodems, B.J.; Baker, C.D.; Kaszuba, C.M.; Franco, E.I.; Smith, B.R.; Ito, T.; Swovick, K.; Welle, K.; Zhang, Y.; et al. Taurine from tumour niche drives glycolysis to promote leukaemogenesis. Nature 2025, 644, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.H.; Kim, D.; Kesavan, R.; Brown, H.; Dey, T.; Soflaee, M.H.; Vu, H.S.; Tasdogan, A.; Guo, J.; Bezwada, D.; et al. De novo and salvage purine synthesis pathways across tissues and tumors. Cell 2024, 187, 3602–3618. [Google Scholar] [CrossRef] [PubMed]
- Mullen, N.J.; Singh, P.K. Nucleotide metabolism: A pan-cancer metabolic dependency. Nat. Rev. Cancer 2023, 23, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.D.; Savani, M.R.; Abdullah, K.G.; McBrayer, S.K. Emerging roles of nucleotide metabolism in cancer. Trends Cancer 2023, 9, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Austin, W.R.; Armijo, A.L.; Campbell, D.O.; Singh, A.S.; Hsieh, T.; Nathanson, D.; Herschman, H.R.; Phelps, M.E.; Witte, O.N.; Czernin, J.; et al. Nucleoside salvage pathway kinases regulate hematopoiesis by linking nucleotide metabolism with replication stress. J. Exp. Med. 2012, 209, 2215–2228. [Google Scholar] [CrossRef] [PubMed]
- Cocco, L.; Follo, M.Y.; Manzoli, L.; Suh, P.G. Phosphoinositide-specific phospholipase C in health and disease. J. Lipid Res. 2015, 56, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Laubach, J.P.; Fu, P.; Jiang, X.; Salter, K.H.; Potti, A.; Arcasoy, M.O. Polycythemia vera erythroid precursors exhibit increased proliferation and apoptosis resistance associated with abnormal RAS and PI3K pathway activation. Exp. Hematol. 2009, 37, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Buks, R.; Dagher, T.; Rotordam, M.G.; Monedero Alonso, D.; Cochet, S.; Gautier, E.-F.; Chafey, P.; Cassinat, B.; Kiladjian, J.-J.; Becker, N.; et al. Altered Ca2+ Homeostasis in Red Blood Cells of Polycythemia Vera Patients Following Disturbed Organelle Sorting during Terminal Erythropoiesis. Cells 2022, 11, 49. [Google Scholar] [CrossRef]
- Bhuria, V.; Franz, T.; Baldauf, C.; Böttcher, M.; Chatain, N.; Koschmieder, S.; Brümmendorf, T.H.; Mougiakakos, D.; Schraven, B.; Kahlfuß, S.; et al. Activating mutations in JAK2 and CALR differentially affect intracellular calcium flux in store operated calcium entry. Cell Commun. Signal 2024, 22, 186. [Google Scholar] [CrossRef]
- Gómez-Cebrián, N.; Rojas-Benedicto, A.; Albors-Vaquer, A.; Bellosillo, B.; Besses, C.; Martínez-López, J.; Pineda-Lucena, A.; Puchades-Carrasco, L. Polycythemia Vera and Essential Thrombocythemia Patients Exhibit Unique Serum Metabolic Profiles Compared to Healthy Individuals and Secondary Thrombocytosis Patients. Cancers 2021, 13, 482. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, K.; Rattigan, K.; Helgason, V.; Yan, F. Metabolic Profiling of Myeloproliferative Neoplasms Reveals Subtype Specific Metabolic Traits. In Myeloproliferative Syndromes and Chronic Myeloid Leukemia: Basic and Translational; Ash Publications: Washington, DC, USA, 2022; Volume 140, (Suppl. S1). [Google Scholar] [CrossRef]
- Bhattacharya, S.; Goyal, A.; Kaur, P.; Singh, R.; Kalra, S. Anticancer Drug-induced Thyroid Dysfunction. Eur. Endocrinol. 2020, 16, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Joseph-Bravo, P.; Jaimes-Hoy, L.; Uribe, R.-M.; Charli, J.-L. 60 years of neuroendocrinology: TRH, the first hypophysiotropic releasing hormone isolated: Control of the pituitary–thyroid axis. J. Endocrinol. 2015, 226, T85–T100. [Google Scholar] [CrossRef] [PubMed]
- De Luca, R.; Davis, P.J.; Lin, H.-Y.; Gionfra, F.; Percario, Z.A.; Affabris, E.; Pedersen, J.Z.; Marchese, C.; Trivedi, P.; Anastasiadou, E. Thyroid hormones interaction with immune response, inflammation and non-thyroidal illness syndrome. Front. Cell Dev. Biol. 2021, 8, 614030. [Google Scholar] [CrossRef] [PubMed]
- Bellosillo, B.; Doubek, M.; Tomuleasa, C.; Griesshammer, M.; Marchetti, M.; Sacha, T.; Gisslinger, H. JAK2 mutations in polycythemia vera: From molecular origins to inflammatory pathways and clinical implications. Memo-Mag. Eur. Med. Oncol. 2025, 17, 79–93. [Google Scholar] [CrossRef]
- Jiang, S.-H.; Zhang, X.-X.; Hu, L.-P.; Wang, X.; Li, Q.; Zhang, X.-L.; Li, J.; Gu, J.-R.; Zhang, Z.-G. Systemic regulation of cancer development by neuro-endocrine-immune signaling network at multiple levels. Front. Cell Dev. Biol. 2020, 8, 586757. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Liu, C.-H.; Lei, M.; Zeng, Q.; Li, L.; Tang, H.; Zhang, N. Metabolic regulation of the immune system in health and diseases: Mechanisms and interventions. Signal Transduct. Target. Ther. 2024, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Palandri, F.; Mora, B.; Gangat, N.; Catani, L. Is there a gender effect in polycythemia vera? Ann. Hematol. 2021, 100, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Mayer, D. Interaction of JAK with steroid receptor function. Jakstat 2013, 2, e24911. [Google Scholar] [CrossRef] [PubMed]
- Marty, C.; Lacout, C.; Droin, N.; Le Couédic, J.P.; Ribrag, V.; Solary, E.; Vainchenker, W.; Villeval, J.L.; Plo, I. A role for reactive oxygen species in JAK2V617F myeloproliferative neoplasm progression. Leukemia 2013, 27, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Pioggia, G.; Tonacci, A.; Casciaro, M.; Musolino, C.; Gangemi, S. Synergic Crosstalk between Inflammation, Oxidative Stress, and Genomic Alterations in BCR–ABL-Negative Myeloproliferative Neoplasm. Antioxidants 2020, 9, 1037. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.; Skouta, R. The Selenoprotein Glutathione Peroxidase 4: From Molecular Mechanisms to Novel Therapeutic Opportunities. Biomedicines 2022, 10, 891. [Google Scholar] [CrossRef] [PubMed]
- Ingold, I.; Berndt, C.; Schmitt, S.; Doll, S.; Poschmann, G.; Buday, K.; Roveri, A.; Peng, X.; Porto Freitas, F.; Seibt, T.; et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 2018, 172, 409–422.e421. [Google Scholar] [CrossRef] [PubMed]
- Krakowiak, A.; Pietrasik, S. New Insights into Oxidative and Reductive Stress Responses and Their Relation to the Anticancer Activity of Selenium-Containing Compounds as Hydrogen Selenide Donors. Biology 2023, 12, 875. [Google Scholar] [CrossRef] [PubMed]
- Marill, J.; Idres, N.; Capron, C.C.; Nguyen, E.; Chabot, G.G. Retinoic acid metabolism and mechanism of action: A review. Curr. Drug Metab. 2003, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Napoli, J.L. Physiological insights into all-trans-retinoic acid biosynthesis. Biochim. Biophys. Acta 2012, 1821, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Wallscheid, N.; Buettner, F.; Sommerkamp, P.; Klimmeck, D.; Ladel, L.; Thalheimer, F.B.; Pastor-Flores, D.; Roma, L.P.; Renders, S.; Zeisberger, P.; et al. Vitamin A-Retinoic Acid Signaling Regulates Hematopoietic Stem Cell Dormancy. Cell 2017, 169, 807–823.e819. [Google Scholar] [CrossRef] [PubMed]
- Shivdasani, R.A.; Fujiwara, Y.; McDevitt, M.A.; Orkin, S.H. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. Embo J. 1997, 16, 3965–3973. [Google Scholar] [CrossRef] [PubMed]
- Makita, T.; Duncan, S.A.; Sucov, H.M. Retinoic acid, hypoxia, and GATA factors cooperatively control the onset of fetal liver erythropoietin expression and erythropoietic differentiation. Dev. Biol. 2005, 280, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Zhao, W.; Shi, Y.; Li, Y.N.; Zhang, L.S.; Zhang, H.Q.; Wang, D. Retinoic acid amide inhibits JAK/STAT pathway in lung cancer which leads to apoptosis. Tumour Biol. 2015, 36, 8671–8678. [Google Scholar] [CrossRef] [PubMed]
- Walkley, C.R.; Olsen, G.H.; Dworkin, S.; Fabb, S.A.; Swann, J.; McArthur, G.A.; Westmoreland, S.V.; Chambon, P.; Scadden, D.T.; Purton, L.E. A Microenvironment-Induced Myeloproliferative Syndrome Caused by Retinoic Acid Receptor γ Deficiency. Cell 2007, 129, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Marchioli, R.; Finazzi, G.; Specchia, G.; Cacciola, R.; Cavazzina, R.; Cilloni, D.; De Stefano, V.; Elli, E.; Iurlo, A.; Latagliata, R. Cardiovascular events and intensity of treatment in polycythemia vera. N. Engl. J. Med. 2013, 368, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, S.; Santoro, M.; Accurso, V.; Agliastro, G.; Raso, S.; Di Piazza, F.; Perez, A.; Bono, M.; Russo, A.; Siragusa, S. Cardiovascular Risk in Polycythemia Vera: Thrombotic Risk and Survival: Can Cytoreductive Therapy Be Useful in Patients with Low-Risk Polycythemia Vera with Cardiovascular Risk Factors? Oncol. Res. Treat. 2020, 43, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Brierley, C.K.; Psaila, B. Sugar thieves and addicts: Nutrient subversion in JAK2 MPNs. Blood J. Am. Soc. Hematol. 2019, 134, 1778–1780. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.N.; Higashi, R.M.; Fan, T.W. Metabolic reprogramming in tumors: Contributions of the tumor microenvironment. Genes. Dis. 2020, 7, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, H.C. Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: Is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood J. Am. Soc. Hematol. 2012, 119, 3219–3225. [Google Scholar] [CrossRef] [PubMed]
- Castro-Portuguez, R.; Sutphin, G.L. Kynurenine pathway, NAD(+) synthesis, and mitochondrial function: Targeting tryptophan metabolism to promote longevity and healthspan. Exp. Gerontol. 2020, 132, 110841. [Google Scholar] [CrossRef] [PubMed]
- Bogan, K.L.; Brenner, C. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu. Rev. Nutr. 2008, 28, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Locasale, J.W. Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nat. Rev. Cancer 2013, 13, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Pernes, G.; Flynn, M.C.; Lancaster, G.I.; Murphy, A.J. Fat for fuel: Lipid metabolism in haematopoiesis. Clin. Transl. Immunol. 2019, 8, e1098. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Ciano, K.; Dong, K.; Zucker, S. Targeting glutamine metabolism in myeloproliferative neoplasms. Blood Cells Mol. Dis. 2015, 55, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Coen, C.; Yan, J.; Saygin, C.; Arellano, N.; Nauman, M.; Zawieracz, K.; Vanni, D.; Catricalà, S.; Casetti, I.C.; Borsani, O. Glutamine Metabolism Is Altered in Myeloproliferative Neoplasms and Represents a Potential Novel Therapeutic Target. Blood 2023, 142, 6350. [Google Scholar] [CrossRef]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer 2016, 16, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.M.; Rebel, V.I. JAK2 and genomic instability in the myeloproliferative neoplasms: A case of the chicken or the egg? Am. J. Hematol. 2012, 87, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Putter, J.S.; Seghatchian, J. Polycythaemia vera: Molecular genetics, diagnostics and therapeutics. Vox Sang. 2021, 116, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Stetka, J.; Gursky, J.; Liñan Velasquez, J.; Mojzikova, R.; Vyhlidalova, P.; Vrablova, L.; Bartek, J.; Divoky, V. Role of DNA Damage Response in Suppressing Malignant Progression of Chronic Myeloid Leukemia and Polycythemia Vera: Impact of Different Oncogenes. Cancers 2020, 12, 903. [Google Scholar] [CrossRef] [PubMed]
- Regimbeau, M.; Mary, R.; Hermetet, F.; Girodon, F. Genetic Background of Polycythemia Vera. Genes 2022, 13, 637. [Google Scholar] [CrossRef] [PubMed]
- Chatree, S.; Thongmaen, N.; Tantivejkul, K.; Sitticharoon, C.; Vucenik, I. Role of Inositols and Inositol Phosphates in Energy Metabolism. Molecules 2020, 25, 5079. [Google Scholar] [CrossRef] [PubMed]
- Rezuchova, I.; Hudecova, S.; Soltysova, A.; Matuskova, M.; Durinikova, E.; Chovancova, B.; Zuzcak, M.; Cihova, M.; Burikova, M.; Penesova, A.; et al. Type 3 inositol 1,4,5-trisphosphate receptor has antiapoptotic and proliferative role in cancer cells. Cell Death Dis. 2019, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Rosa, N.; Sneyers, F.; Parys, J.B.; Bultynck, G. Chapter Four—Type 3 IP3 receptors: The chameleon in cancer. In International Review of Cell and Molecular Biology; Spetz, J.K.E., Galluzzi, L., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 351, pp. 101–148. [Google Scholar] [CrossRef] [PubMed]
| SP (n = 25) | PV (n = 40) | p-Value | |
|---|---|---|---|
| Mean Age (years) ± SD | 45.8 (±13.6) | 54.3 (±13.1) | 0.659 |
| Gender (n) (%) | |||
| Male | 18 (72%) | 27 (67.5%) | 0.704 |
| Female | 7 (28%) | 13 (32.5%) | |
| Mean WBC (mm3) ± SD | 9222 (±2507.5.1) | 8232.8 (±2653.3) | 0.337 |
| Mean Hgb (g/dL) ± SD | 16.9 (±1.3) | 14.7 (±2.7) | 0.00 |
| Mean Plt (mm3) ± SD | 257,560 (±73,158.5) | 365,907.5 (±170,619.6) | 0.00 |
| tPV (n = 25) | ntPV (n = 15) | p-Value | |
|---|---|---|---|
| Mean Age (years) ± SD | 58.2 (±13.7) | 47.6 (±9.0) | 0.021 |
| Gender (n) (%) | |||
| Male | 15 (60%) | 12 (80%) | 0.191 |
| Female | 10 (40%) | 3 (20%) | |
| Mean Duration of PV (year) ± SD | 4.8 ± 4.0 | 4.8 ± 5.8 | 0.476 |
| Mean WBC (mm3) ± SD | 7487.7 (±2811.0) | 9474.7 (±1849.6) | 0.528 |
| Mean Hgb (g/dL) ± SD | 14.1 (±3.1) | 15.6 (±1.1) | 0.030 |
| Mean Plt (mm3) ± SD | 320,640 (±14,762) | 441,353.3 (±184,326) | 0.384 |
| Genetic Mutation Status (%) | 0.679 | ||
| JAK-2 V617F mutation | 24 (96%) | 15 (100%) | |
| JAK-2 Exon 12 mutation | 1 (4%) | - | |
| Therapeutic Agent (%) | |||
| Hydroxyurea | 21 (84%) | ||
| Interferon | 2 (8%) | ||
| Ruxolitinib | 2 (8%) | ||
| Treatment Indications (%) | |||
| Age > 60 | 7 (28%) | ||
| History of thrombosis | 9 (36%) | ||
| Persistent leukocytosis, inadequate hematocrit control, | 4 (16%) | ||
| Intolerance to phlebotomy | 4 (16%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yıldırım, M.; Erdoğdu, B.; Sayın, S.; Kaplan, O.; Koç, E.; Karadeniz, M.; Karakaya, B.; Güney, M.; Çelebier, M.; Aylı, M. Metabolomic Profiling Reveals Distinct Signatures in Primary and Secondary Polycythemia. Metabolites 2025, 15, 630. https://doi.org/10.3390/metabo15090630
Yıldırım M, Erdoğdu B, Sayın S, Kaplan O, Koç E, Karadeniz M, Karakaya B, Güney M, Çelebier M, Aylı M. Metabolomic Profiling Reveals Distinct Signatures in Primary and Secondary Polycythemia. Metabolites. 2025; 15(9):630. https://doi.org/10.3390/metabo15090630
Chicago/Turabian StyleYıldırım, Murat, Batuhan Erdoğdu, Selim Sayın, Ozan Kaplan, Emine Koç, Mine Karadeniz, Bülent Karakaya, Mustafa Güney, Mustafa Çelebier, and Meltem Aylı. 2025. "Metabolomic Profiling Reveals Distinct Signatures in Primary and Secondary Polycythemia" Metabolites 15, no. 9: 630. https://doi.org/10.3390/metabo15090630
APA StyleYıldırım, M., Erdoğdu, B., Sayın, S., Kaplan, O., Koç, E., Karadeniz, M., Karakaya, B., Güney, M., Çelebier, M., & Aylı, M. (2025). Metabolomic Profiling Reveals Distinct Signatures in Primary and Secondary Polycythemia. Metabolites, 15(9), 630. https://doi.org/10.3390/metabo15090630






