Analysis of FsTyDC1 Gene from Forsythia suspensa in Response to Drought and Salt Stress Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatment
2.2. Bioinformatics Analysis of FsTyDC1
2.3. Cloning of the FsTyDC1 Gene
2.4. Analysis of the Gene Expression Patterns of FsTyDC1
2.5. Analysis of Subcellular Localization
2.6. Construction of FsTyDC1 Gene Silencing Vector and Expression Analysis
2.7. Statistical Analysis
3. Results
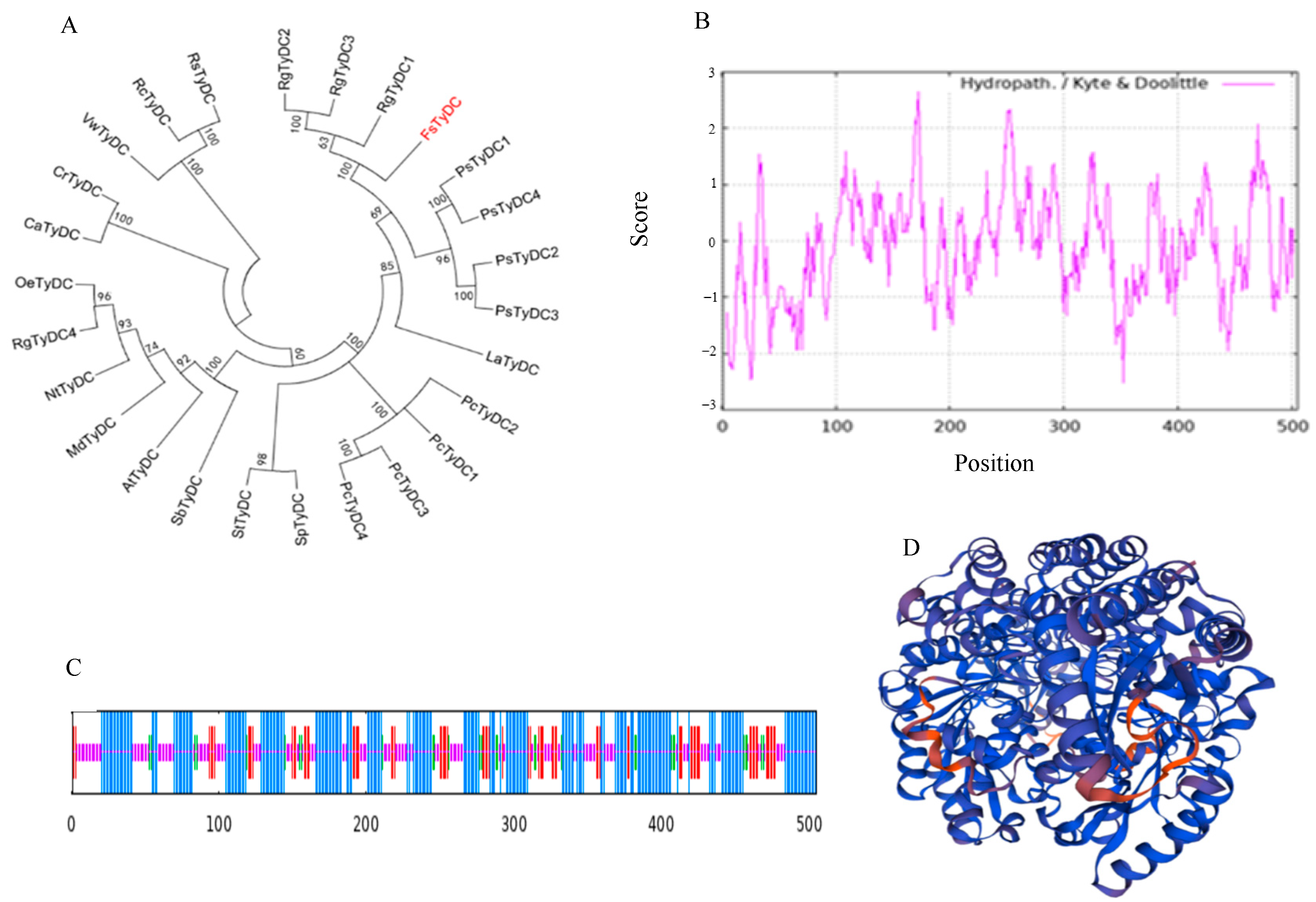
3.1. Identification of TyDC from F. suspensa
3.2. Cloning and Vector Construction of FsTyDC1
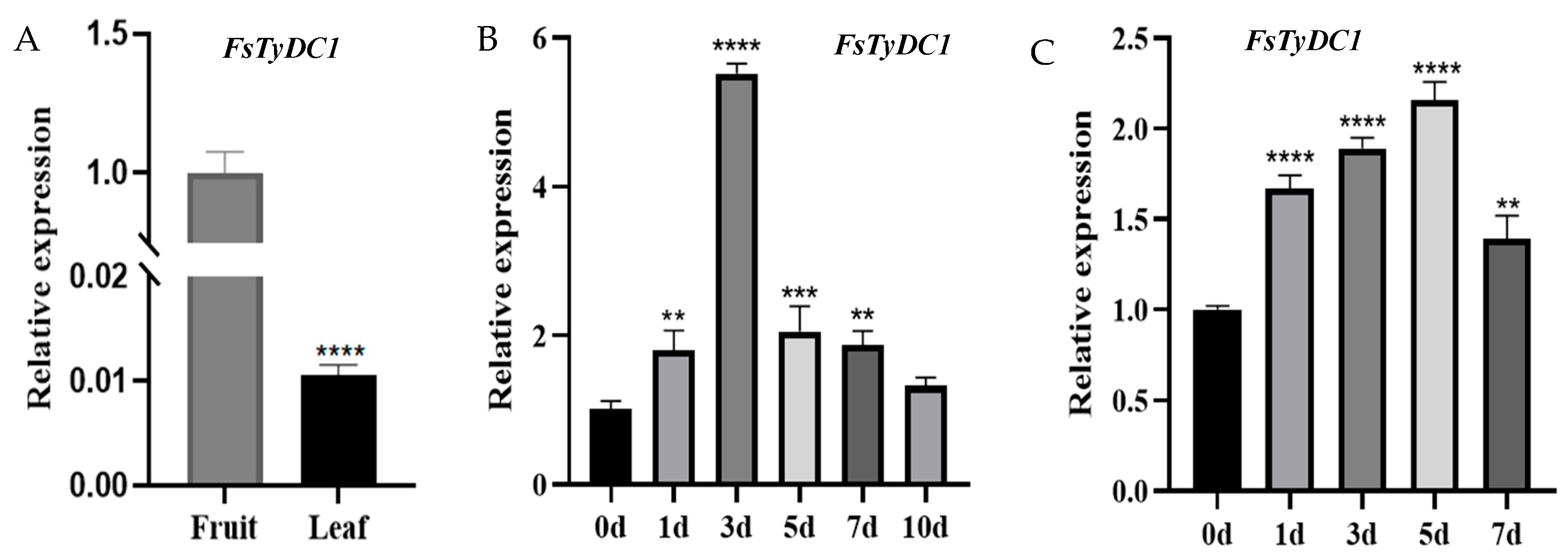
3.3. Spatiotemporal Expression Patterns and Drought and Salt Response of FsTyDC1
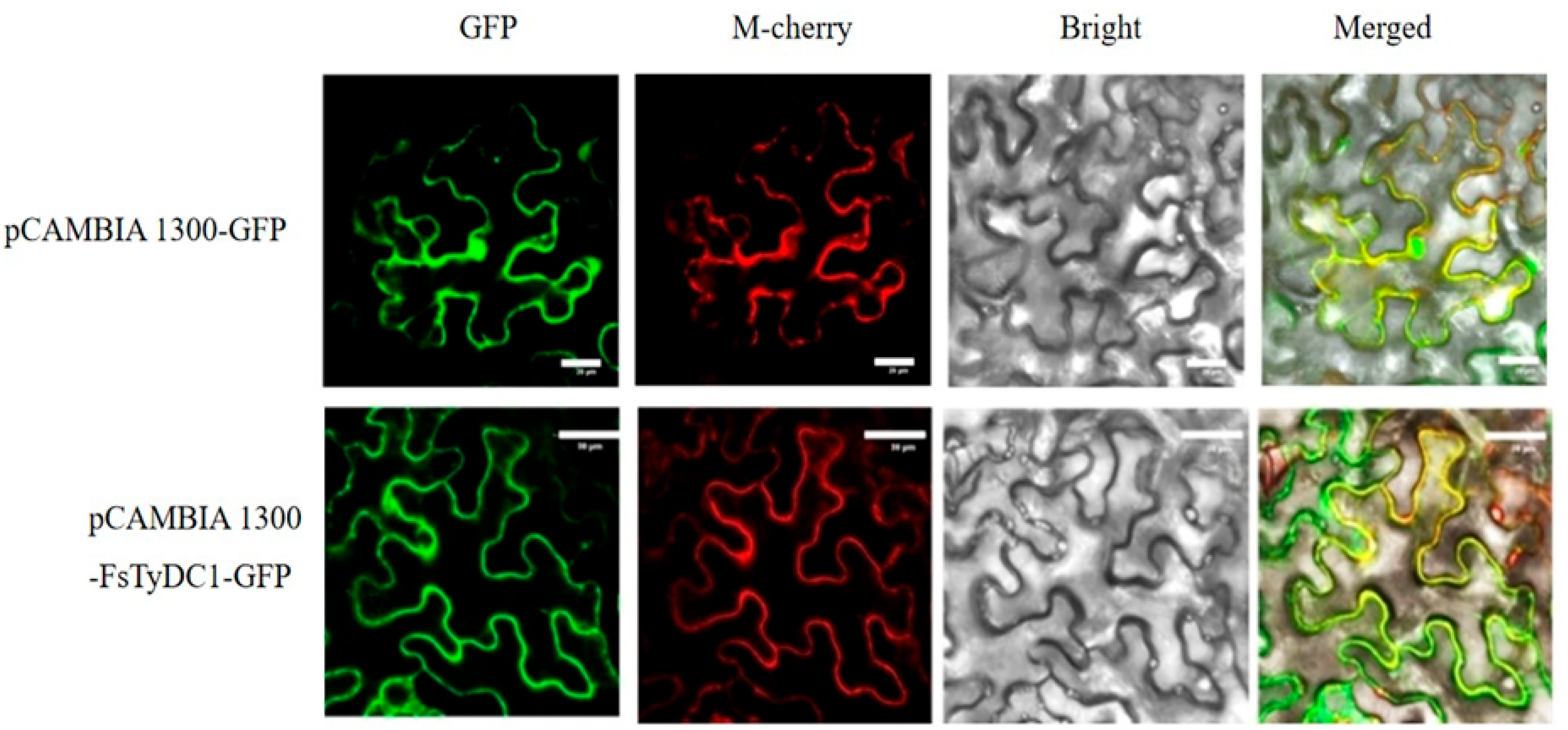
3.4. Subcellular Localization Analysis of FsTyDC1 Proteins
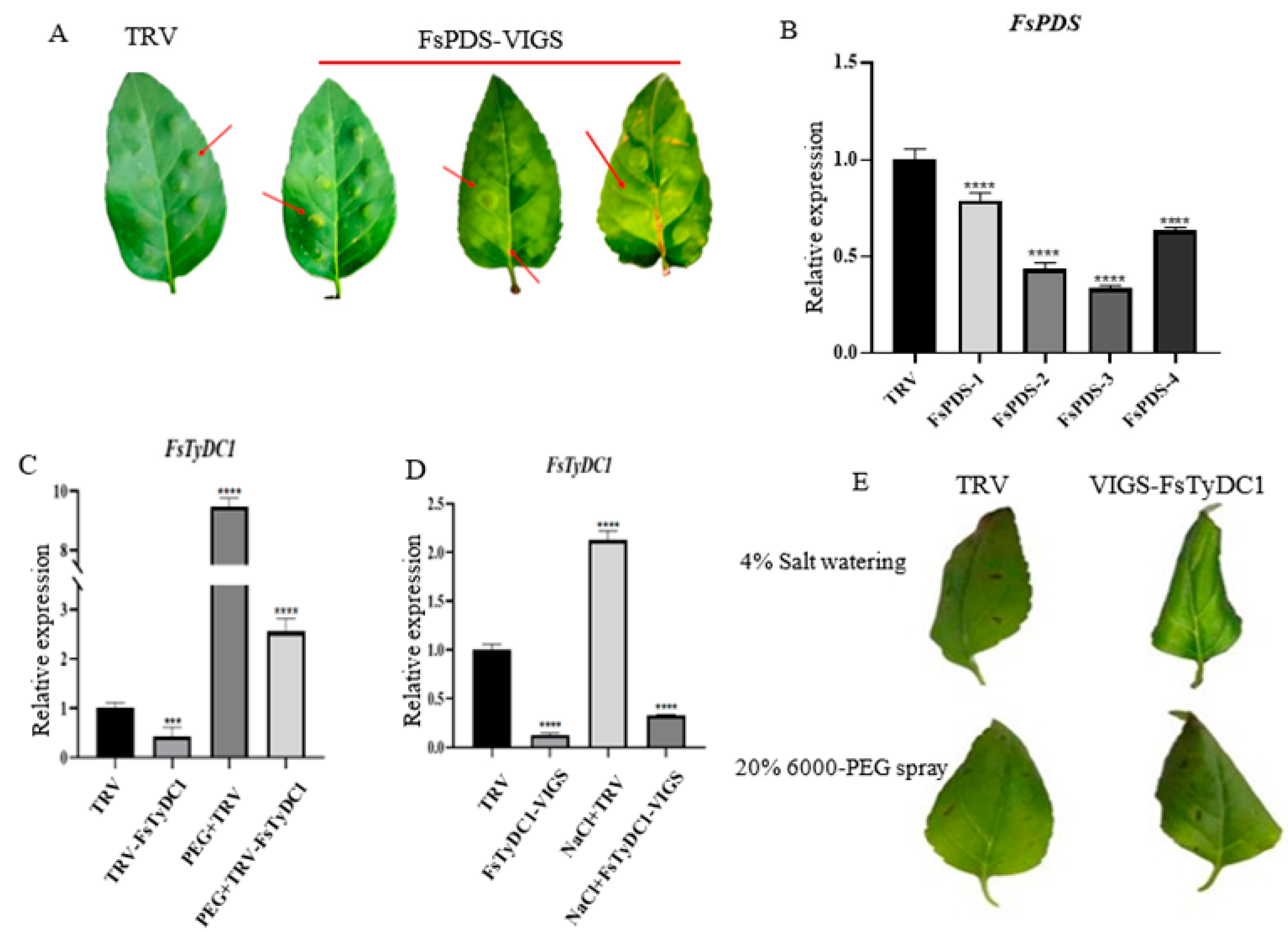
3.5. Effects of FsTyDC1 Silencing on Drought and Stress Responses in F. suspensa
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TyDC | Tyrosine decarboxylase |
| qRT-PCR | Quantitative reverse transcription |
| ORF | Open reading frame |
| DA | Dopamine |
| ABA | Abscisic acid |
| ROS | Reactive oxygen species |
| VIGS | Virus-induced gene silencing |
| TRV | Tobacco rattle |
References
- Liu, X.; Zhang, J.; Liu, H.; Shang, H.; Zhao, X.; Xu, H.; Zhang, H.; Hou, D. Comparative Transcriptome Analysis of MeJA Responsive Enzymes Involved in Phillyrin Biosynthesis of Forsythia suspensa. Metabolites 2022, 12, 1143. [Google Scholar] [CrossRef] [PubMed]
- Jing, F.; Li, F.; Zhang, T.; Lv, X.; Zhao, T.; Feng, S. Recent advances in chemical composition and biological activity of Forsythia suspensa. J. Chin. Med. Mater. 2023, 46, 242–251. [Google Scholar]
- Pharmacopoeia Committee of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China: Part I; China Pharmaceutical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Wang, C.; Chen, S.; Guo, H.; Jiang, H.; Liu, H.; Fu, H.; Wang, D. Forsythoside A Mitigates Alzheimer’s-like Pathology by Inhibiting Ferroptosis-mediated Neuroinflammation via Nrf2/GPX4 Axis Activation. Int. J. Biol. Sci. 2022, 18, 2075–2090. [Google Scholar] [CrossRef] [PubMed]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef]
- Arpiwi, N.; Muksin, I.; Nio, S. Drought Stress Decreases Morphophysiological Characteristics of Pongamia pinnata (L.) Pierre a Biodiesel Tree. Pak. J. Biol. Sci. 2023, 26, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant. Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, H.; Yang, Y.; Feng, X.; Chen, X.; Xiao, F.; Lin, H.; Guo, Y. Insights into plant salt stress signaling and tolerance. J. Genet. Genomics. 2024, 51, 16–34. [Google Scholar] [CrossRef]
- Li, C.; Duan, C.; Zhang, H.; Zhao, Y.; Meng, Z.; Zhao, Y.; Zhang, Q. Adaptative Mechanisms of Halophytic Eutrema salsugineum Encountering Saline Environment. Front Plant Sci. 2022, 13, 909527. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.K. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Gao, B.; Zhu, H.; Liu, Z.; He, X.; Sun, J.; Li, Y.; Wu, X.; Pehrsson, P.; Zhang, Y.; Yao, Y.; et al. Chemical Compositions of Lianqiao (Forsythia suspensa) Extracts and Their Potential Health Benefits. Pharmaceuticals 2024, 17, 740. [Google Scholar] [CrossRef]
- He, N.; Dong, Y.; Zhu, S. A Study on optimization of extraction process and antioxidant and antibacteria activity of polysaccharides from Forsythia suspensa. J. Shangluo Univ. 2024, 38, 59–65. [Google Scholar]
- Qi, L.; Chen, X.; Jin, H.; Li, J.; He, J.; Chang, Y. Research progress on the chemical constituents and pharmacological activities of Forsythiae fructus. J. Tianjin Univ. Trad. Chin. Med. 2021, 40, 168–175. [Google Scholar]
- Xu, Y.; Zhao, Z.; Wu, Y.; Shen, J.; Zhang, Q.; Cheng, T.; Wang, J.; Pan, H. Evaluation of cold resistance of Forsythia germplasm resources. J. North. For. Univ. 2023, 51, 33–39. [Google Scholar]
- Liu, S. Analysis of the key points of Forsythia suspensa cultivation management. Seed Sci. Technol. 2024, 42, 73–75. [Google Scholar]
- Liang, B.; Li, C.; Ma, C.; Wei, Z.; Wang, Q.; Huang, D.; Chen, Q.; Li, C.; Ma, F. Dopamine alleviates nutrient deficiency-induced stress in Malus hupehensis. Plant Physiol. Bioch. 2017, 119, 346–359. [Google Scholar] [CrossRef]
- Zhou, K.; Hu, L.; Li, Y.T.; Chen, X.; Zhang, Z.; Liu, B.; Li, P.; Gong, X.; Ma, F. MdUGT88F1-mediated phloridzin biosynthesis regulates apple development and Valsa canker resistance. Plant physiol. 2019, 180, 2290–2305. [Google Scholar] [CrossRef]
- Hu, L.; Zhou, K.; Ren, G.; Yang, S.; Liu, Y.; Zhang, Z.; Li, Y.; Gong, X.; Ma, F. Myo-inositol mediates reactive oxygen species-induced programmed cell death via salicylic acid-dependent and ethylene-dependent pathways in apple. Hortic. Res. 2020, 7, 138. [Google Scholar] [CrossRef]
- Gichuki, D.K.; Li, Q.; Hou, Y.; Liu, Y.; Ma, M.; Zhou, H.; Xu, C.; Zhu, Z.; Wang, L.; Musila, F.M.; et al. Characterization of Flavonoids and Transcripts Involved in Their Biosynthesis in Different Organs of Cissus rotundifolia Lam. Metabolites 2021, 11, 741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Tang, Z.; Wang, Y.; Gao, T.; Liu, X.; Ma, F.; Li, C. Tissue distribution and changes in dopamine during development and stress responses in Malus germplasm. J. Integr. Agric. 2022, 21, 710–724. [Google Scholar] [CrossRef]
- Gao, T.; Liu, X.; Shan, L.; Wu, Q.; Liu, Y.; Zhang, Z.; Ma, F.; Li, C. Exogenous dopamine and overexpression of the dopamine synthase gene MdTYDC alleviated apple replant disease. Environ. Exp. Bot. 2020, 178, 104159. [Google Scholar] [CrossRef]
- Jiao, C.; Lan, G.; Sun, Y.; Wang, G.; Sun, Y. Dopamine alleviates chilling stress in watermelon seedlings via modulation of proline content, antioxidant enzyme activity, and polyamine metabolism. J. Plant Grow. Reg. 2021, 40, 277–292. [Google Scholar] [CrossRef]
- Liu, X.; Gao, T.; Zhang, Z.; Tan, K.; Jin, Y.; Zhao, Y.; Ma, F.; Li, C. The mitigation effects of exogenous dopamine on low nitrogen stress in Malus hupehensis. J. Integr. Agric. 2020, 19, 2709–2724. [Google Scholar] [CrossRef]
- Wang, Y. Study on the Function and Mechanism of Apple Tyrosine Decarboxylase (MdTyDC) in Drought and Salt Tolerance. Ph.D. Thesis, Northwest A&F University, Yangling, China, 2021; pp. 84–85. [Google Scholar]
- Tang, Z. Function of Dopamine in Regulating Apple Heavy Metal Cadmium and Copper Stress. Ph.D. Thesis, Northwest A&F University, Yangling, China, 2022; pp. 1–9. [Google Scholar]
- Chen, Y. Effects of Dopamine on Root Development and Physiological Characteristics of Apple Under Salt Stress. Ph.D. Thesis, Shandong Agricultural University, Tai’an, China, 2022; pp. 1–7. [Google Scholar]
- Xiong, Z.; Sun, J.; Wang, L.; Huang, X.L.; Sun, H.; Qiao, F.; Jiang, X. Cloning and expression analysis of tyrosine decarboxylase gene in Gloriosa superba L. Mol. Plant Breed. 2023, 21, 1811–1819. [Google Scholar]
- Liu, X.; Wang, Y.; Ma, X.; Zhang, H.; Zhou, Y.; Ma, F.; Li, C. MdbHLH93 confers drought tolerance by activating MdTyDC expression and promoting dopamine biosynthesis. Int. J. Biol. Macromol. 2024, 258 Pt 2, 129003. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yuan, X.; Zhang, Z.; Wang, Y.; Ma, F.; Li, C. Dopamine Enhances the Resistance of Apple to Valsa mali Infection. Phytopathology 2022, 112, 1141–1151. [Google Scholar] [CrossRef]
- Jiang, M. Soluble Expression of Recombinant Tyrosine Decarboxylase and Application in Synthesis of Tyramine. Master’s Thesis, Jiangnan University, Wuxi, China, 2018; pp. 1–12. [Google Scholar]
- Zhang, Z.; Tang, Z.; Jing, G.; Gao, S.; Liu, C.; Ai, S.; Liu, Y.; Liu, Q.; Li, C.; Ma, F. Dopamine confers cadmium tolerance in apples by improving growth, reducing reactive oxygen species, and changing secondary metabolite levels. Environ. Exp. Bot. 2023, 208, 105264. [Google Scholar] [CrossRef]
- Sumer, Z.; Muhammad, A.; Zhao, T.; Wang, P.; Javaria, T.; Wang, Y.; Zhao, J.; Chen, X.; Shen, S.; Gu, A. Virus-Induced Gene Silencing (VIGS): A Powerful tool for crop improvement and its advancement towards epigenetics. Int. J. Mol. Sci. 2023, 24, 5608. [Google Scholar]
- Li, J.; Yu, Z.; Feng, B. Advances in research and application of virus induced gene silencing in plants. Mol. Plant Breed. 2019, 17, 1537–1542. [Google Scholar]
- Li, R.; Li, Y.; Tang, M.; Qu, Z.; Zheng, P.; Hou, W. Construction of TRV-mediated gene silencing system in Panax ginseng. Chin. Herb. Med. 2024, 55, 232–239. [Google Scholar]
- Zhang, X.; Gui, M.; Liu, D.; Zhang, J.; Liu, Y. Phenotypic comparison of CaDXS and CaPDS Gene Silencing in Capsicum annuum L. J. Yunnan Agric. Univ. 2024, 39, 125–131. [Google Scholar]
- Zhou, Y.C. Preliminary Mapping of QTL for Wheat Powdery Mildew Resistance and Functional Analysis of Related Genes. Master’s Thesis, Northwest A&F University, Yangling, China, 2023; pp. 72–73. [Google Scholar]
- Chen, N.; Li, X.; Liu, J.; Hao, Q. Cloning, expression, and function analysis under ralstonia solanacearum stress of pathogenesis-related protein SlPR1b gene in tomato (Solanum lycopersicum). J. Agric. Biotechnol. 2023, 31, 2477–2489. [Google Scholar]
- Liu, X.; He, L.; Wu, Z.; Teng, N. GAMYB transcription factor LoMYB65 from lily plays a vital role in pollen development. Hortic. Plant J. 2024, 10, 223–238. [Google Scholar] [CrossRef]
- Wu, N.; Lan, H.; Huang, M.; Wang, W.; Liu, J. GmAGB1 plays a positive regulatory role in soybean defense responses. Chin. J. Biot. 2024, 40, 1050–1064. [Google Scholar]
- Nie, H.; Wang, Y.; Wei, C.; Grover, C.; Su, Y.; Wendel, J.; Hua, J. Embryogenic Calli Induction and Salt Stress Response Revealed by RNA-Seq in Diploid Wild Species Gossypium sturtianum and Gossypium raimondii. Front. Plant. Sci. 2021, 12, 715041. [Google Scholar] [CrossRef]
- Zhang, X.F. Study on the Regulation Mechanism of Nitric Oxide on Endogenous Hormones and MicroRNA of Alfalfa Seedlings Under PEG Stress. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2020; pp. 10–12. [Google Scholar]
- Liu, H. Study on salt tolerance of Forsythia suspensa. Tech. Soil Water Conser. 2013, 1, 1–3. [Google Scholar]
- Shen, J.; Wu, Y.; Jiang, Z.; Xu, Y.; Zheng, T.; Wang, J.; Cheng, T.; Zhang, Q.; Pan, H. Selection and validation of appropriate reference genes for gene expression studies in Forsythia. Physiol. Mol. Biol. Plants 2019, 26, 173–188. [Google Scholar] [CrossRef]
- Tan, X.; Chen, J.; Zhang, J.; Guo, G.; Zhang, H.; Zhao, X.; Lv, S.; Xu, H.; Hou, D. Gene expression and interaction analysis of FsWRKY4 and FsMAPK3 in Forsythia suspensa. Plants 2023, 12, 3415. [Google Scholar] [CrossRef]
- Shen, J.; Si, W.; Wu, Y.; Xu, Y.; Wang, Y.; Cheng, T.; Zhang, Q.; Pan, H. Establishment and verification of an efficient virus-induced gene silencing system in Forsythia. Hortic. Plant J. 2021, 7, 81–88. [Google Scholar]
- Wang, Y.; Chen, Q.; Zheng, J.; Zhang, Z.; Gao, T.; Li, C.; Ma, F. Overexpression of the tyrosine decarboxylase gene MdTyDC in apple enhances long-term moderate drought tolerance and WUE. Plant Sci. 2021, 313, 111064. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Qiao, H.; Ma, M.; Wang, X.; Tian, Y.; Bai, S.; Hasi, A. Genome-Wide Identification and Characterization of Melon bHLH Transcription Factors in Regulation of Fruit Development. Plants 2021, 10, 2721. [Google Scholar]
- Rao, Y.; Lin, Y.; Wang, J.; Liu, G.; Zhang, W.; Peng, T. Positional effects of the expression of tyrosine decarboxylase gene in pansy (Viola × wittrockiana). J. Nucl. Agric. Sci. 2023, 37, 1720–1728. [Google Scholar]
- Pang, Q.; Zhao, G.; Wang, D.; Zhu, X.; Xie, L.; Zuo, D.; Wang, L.; Tian, L.; Peng, F.; Xu, B.; et al. Water periods impact the structure and metabolic potential of the nitrogen-cycling microbial communities in rivers of arid and semi-arid regions. Water Res. 2024, 267, 122472. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, L.; Pei, N.; Cushman, S.; Si, Y. Transcriptomic responses to drought stress among natural populations provide insights into local adaptation of weeping forsythia. BMC. Plant. Biol. 2021, 21, 273. [Google Scholar] [CrossRef]
- Yan, H.; Mu, R.; Jian, Y.; Ke, W.; Yan, J.; Rui, F.; Lei, Z.; Chang, F. Functional characterization of tyrosine decarboxylase genes that contribute to acteoside biosynthesis in Rehmannia glutinosa. Planta 2022, 255, 64. [Google Scholar] [CrossRef]
- Thomas, L.; Stephan, P. Gene expression and characterization of a stress-induced tyrosine decarboxylase from Arabidopsis thaliana. FEBS Lett. 2009, 583, 1895–1900. [Google Scholar]
- Pan, D.; Zhang, S.; Liu, F.; Tian, Q.; Yang, X.; Wang, L.; Yue, Y. Application of virus-induced gene silencing technology to investigate the phytochrome metabolism mechanism: A review. Chin. J. Biotechnol. 2023, 39, 2579–2599. [Google Scholar]
- Eunsook, C.; Eunsoo, S.; Yeoung, C.; Eun, J.; Sang, K.; Sanghyeob, L.; Jeong, M.; Young, H.; Doil, C. A method of high frequency virus-induced gene silencing in chili pepper (Capsicum annuum L. cv. Bukang). Mol. Cells 2004, 17, 377–380. [Google Scholar]
- Liu, N. Cloning and Functional Analysis of Drought Tolerance Related Genes GhHSP70-26 and GhVHA-A in Gossypium hirsutum L. Ph.D. Thesis, Xinjiang Agricultural University, Urumqi, China, 2017; pp. 81–86. [Google Scholar]

| Primer Name | Primer Sequence (5′-3′) | Annealing Temperature (°C) | Product Sizes (bp) |
|---|---|---|---|
| FsTyDC1-F | ACCCTCATTCACAGGTAGCAA | 56 | 1500 |
| FsTyDC1-R | CAAAACACGATACAGCAAAGATT | ||
| qPCR-FsUKN1-F | CAGACCAGCTTTGAGGAGTATC | 60 | 90 |
| qPCR-FsUKN1-R | GGCCAGAAACCAGTAGTCAATA | ||
| qPCR-FsTyDC1-F | CCGAGCAGTCTCAACGACAA | 60 | 108 |
| qPCR-FsTyDC1-R | CGCAAAGAAATAATGGAACCAG | ||
| GFP-FsTyDC1-F | cgggggactgagctcggtaccATGGAAACTACGACTCGATGCTC | 56 | 1515 |
| GFP-FsTyDC1-R | catgtcgactctagaggatccTAAGATAGCTTCTGGAAGTCTCGGT | ||
| VIGS-FsTyDC1-F | gtgagtaaggttaccgaattcCACCATCGGAACCACGTCA | 56 | 370 |
| VIGS-FsTyDC1-R | cgtgagctcggtaccggatccATAGCTACGAAGCACCAGCCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Chen, J.; Yuan, M.; Wang, P.; Li, W.; Li, Y.; Yang, C.; Lv, S.; Ma, Z.; Zhang, H.; et al. Analysis of FsTyDC1 Gene from Forsythia suspensa in Response to Drought and Salt Stress Treatment. Metabolites 2025, 15, 628. https://doi.org/10.3390/metabo15090628
Xu J, Chen J, Yuan M, Wang P, Li W, Li Y, Yang C, Lv S, Ma Z, Zhang H, et al. Analysis of FsTyDC1 Gene from Forsythia suspensa in Response to Drought and Salt Stress Treatment. Metabolites. 2025; 15(9):628. https://doi.org/10.3390/metabo15090628
Chicago/Turabian StyleXu, Jiaqi, Jiaxi Chen, Meng Yuan, Panpan Wang, Wenwen Li, Yilong Li, Chong Yang, Shufang Lv, Zhanqiang Ma, Hongxiao Zhang, and et al. 2025. "Analysis of FsTyDC1 Gene from Forsythia suspensa in Response to Drought and Salt Stress Treatment" Metabolites 15, no. 9: 628. https://doi.org/10.3390/metabo15090628
APA StyleXu, J., Chen, J., Yuan, M., Wang, P., Li, W., Li, Y., Yang, C., Lv, S., Ma, Z., Zhang, H., Xu, H., Zhao, X., Wang, T., & Hou, D. (2025). Analysis of FsTyDC1 Gene from Forsythia suspensa in Response to Drought and Salt Stress Treatment. Metabolites, 15(9), 628. https://doi.org/10.3390/metabo15090628








