Essential Amino Acid Supplementation May Attenuate Systemic Inflammation and Improve Hypoalbuminemia in Subacute Hemiplegic Stroke Patients
Abstract
1. Introduction
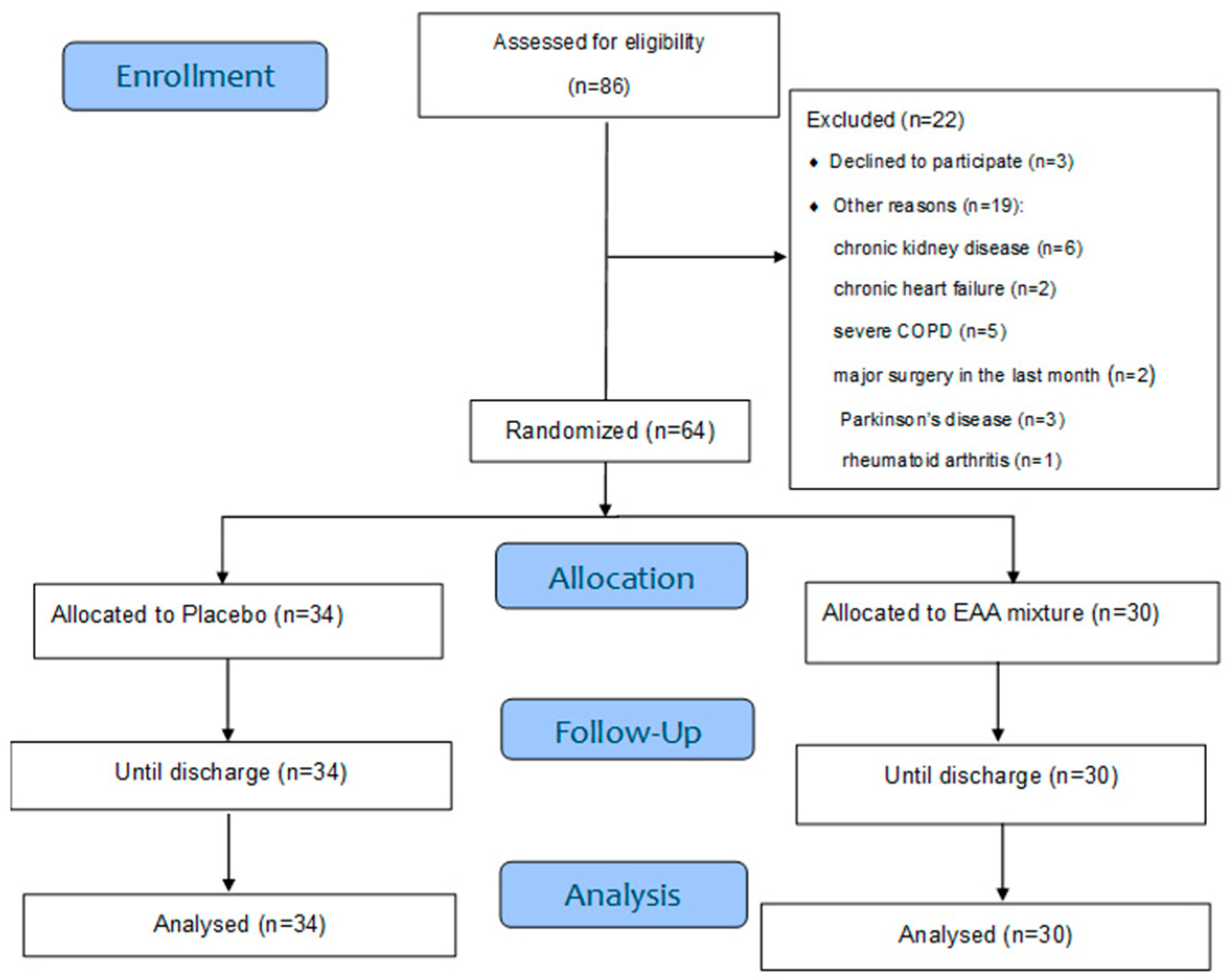
2. Materials and Methods
2.1. Population
2.2. Procedures
- Variables of routine assessment
- •
- C-Reactive Protein (CRP): A primary marker of inflammation (normal value < 0.5 mg/dL).
- •
- Serum proteins:
- ○
- Negative acute-phase proteins:
- ▪
- Albumin (normal value: 3.5–4.76 g/dL)
- ▪
- Prealbumin (normal value: 18–32 mg/dL)
- ▪
- Transferrin (normal value: 202–364 mg/dL)
- ○
- Positive acute-phase protein:
- ▪
- Fibrinogen (normal value: 230–550 mg/dL).
- •
- White Blood Cell (WBC) profile: Total white blood cell count (TWBC), as well as neutrophil and monocyte counts, were evaluated as non-specific indicators of inflammation. The neutrophil-to-lymphocyte ratio (N/L ratio), with a laboratory reference range of 1.5–3.0, was used as an indicator of the balance between innate and adaptive immune responses [73].
- •
- Albumin/CRP ratio: This ratio was calculated to adjust for the inflammatory component influencing serum albumin levels and to provide a more specific index of nutritional and inflammatory status.
- 2.
- Functional status
- •
- Motor FIM (M-FIM): Assesses activities such as feeding, grooming, dressing, toileting, and mobility. Score range: 13 to 91.
- •
- Cognitive FIM (C-FIM): Assesses cognitive functions including communication and social cognition. Score range: 5 to 35.
- 3.
- Patient randomization
- 4.
- Rehabilitation protocol
- •
- Passive, active, and active-assistive range of motion exercises;
- •
- Coordination and facilitation techniques targeting the paretic (contralateral) limbs;
- •
- Trunk stabilization and strengthening exercises;
- •
- Active exercises for the unaffected limbs;
- •
- Ambulation training using assistive devices or therapist support as needed.
2.3. Objectives of the Study
2.4. Statistical Analysis
2.4.1. Sample Size Estimation
2.4.2. Between-Group Comparisons
3. Results
4. Discussion
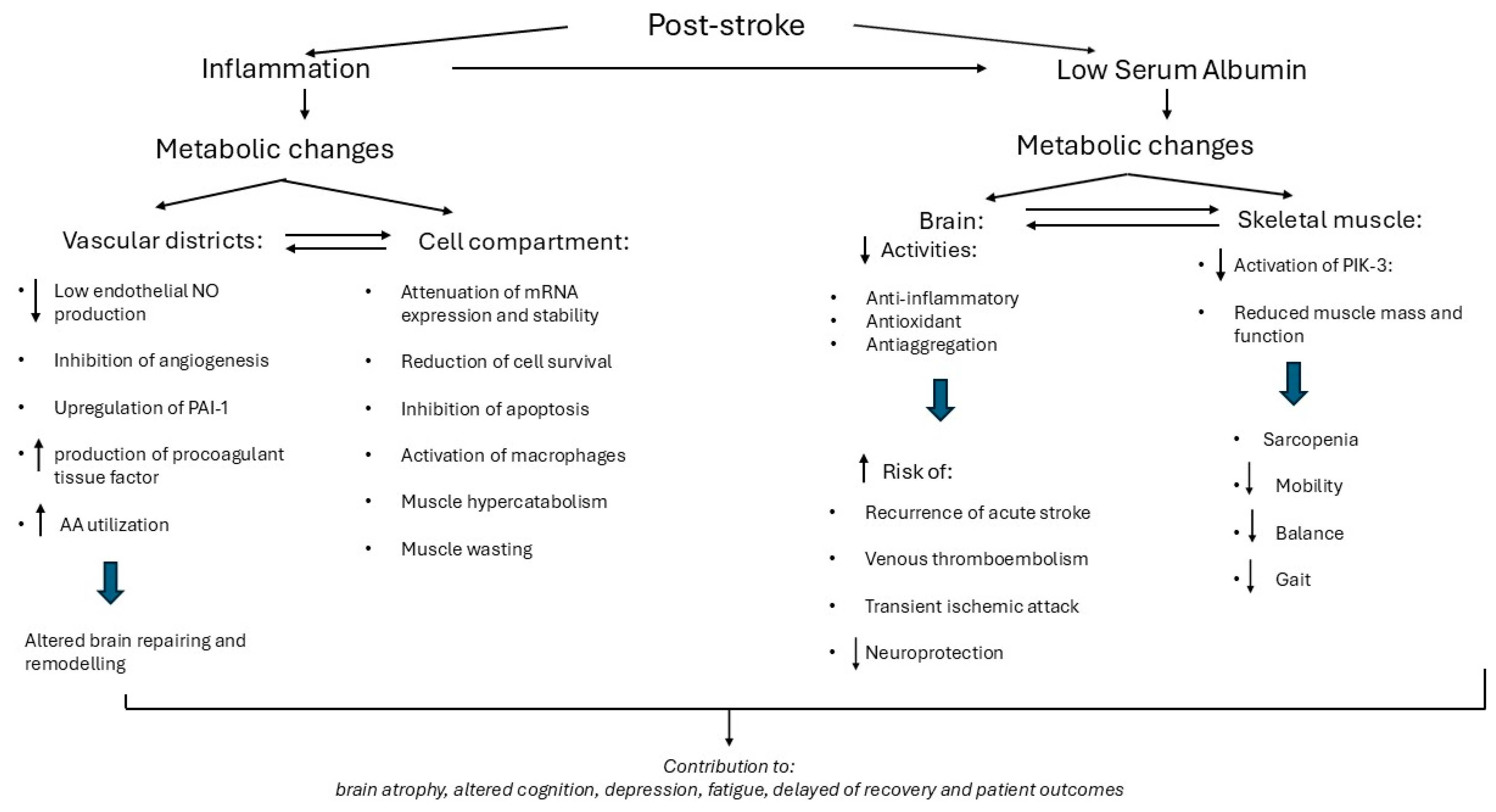
4.1. Baseline Inflammation and Low Alb Levels: Mechanisms and Potential Negative Influence on Functional Recovery
4.1.1. Inflammation
4.1.2. Low Circulating Alb
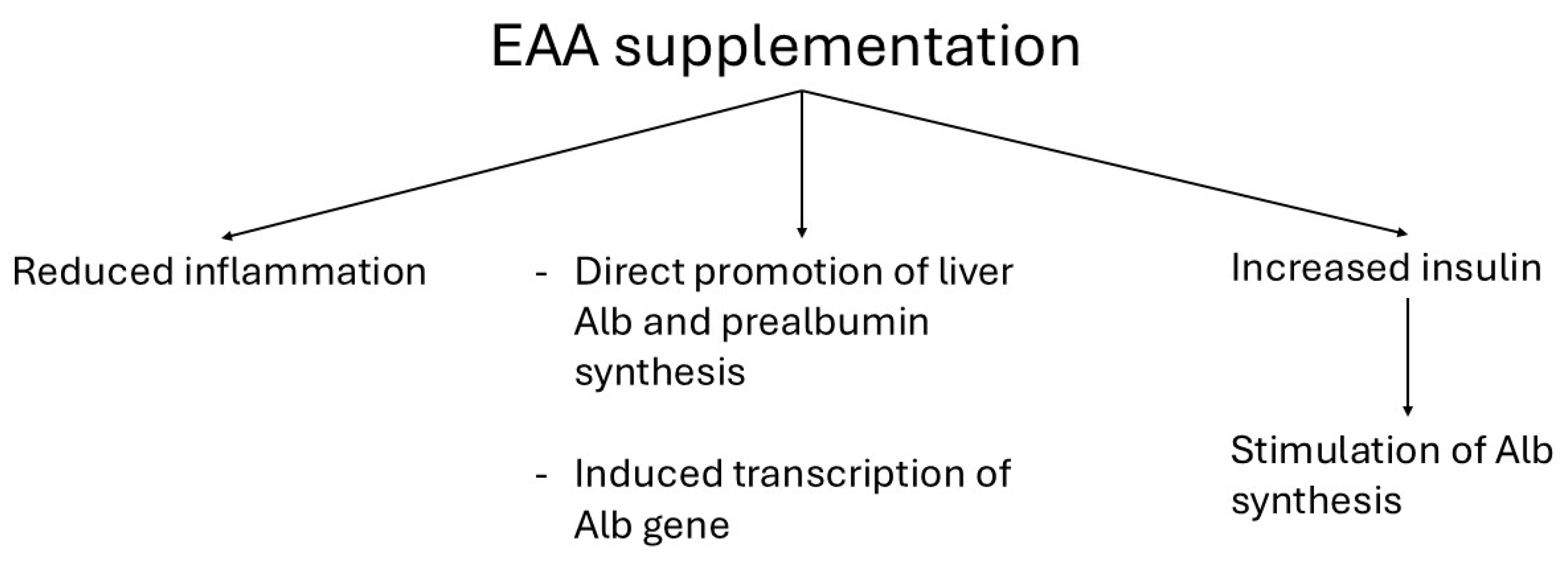
4.2. EAA-Induced Attenuation of Inflammation and Improvement of Hypoalbuminemia
4.2.1. Attenuated Inflammation
4.2.2. Improvement of Hypoalbuminemia
4.3. After 2-Month Rehabilitation: Relationship Between Metabolic Variables and Functional Recovery
4.4. Potential Factors Limiting a Full Response to EAA Supplementation
4.5. Limitations
4.6. Future Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Alb | Albumin |
| FIM | Functional Independence Measure |
| EAAs | Essential Amino Acids |
| BCAAs | Branched Chain Amino Acids |
| BMI | Body Mass Index |
| BW | Body Weight |
| N.V. | Normal Value |
| T0 | Time 0 |
| T1 | Time 1 |
| CRP | C-Reactive Protein |
| TWBC | Total White Blood Cell Count |
| T-FIM | Total Functional Independence Measure |
| M-FIM | Motor Functional Independence Measure |
| C-FIM | Cognitive Functional Independence Measure |
| Plac | Placebo group |
| N/L ratio | Neutrophil/lymphocyte ratio |
| PSI | Post stroke inflammation |
References
- SPREAD, Stroke Prevention and Educational Awareness. Diffusion. In Ictus Cerebrale: Linee Guida Italiane di Prevenzione e Trattamento; Pubblicazioni Catel-Hyperphar Group: Milano, Italy, 2005. [Google Scholar]
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Adams, R.J.; Berry, J.D.; Brown, T.M.; Carnethon, M.R.; Dai, S.; De Simone, G.; Ford, E.S.; et al. Heart disease and stroke statistics—2011 update: A report from the American Heart Association. Circulation 2011, 123, e18–e209. [Google Scholar] [CrossRef]
- Hacke, W.; Donnan, G.; Fieschi, C.; Kaste, M.; von Kummer, R.; Broderick, J.P.; Brott, T.; Frankel, M.; Grotta, J.C.; Haley, E.C., Jr.; et al. Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004, 363, 768–774. [Google Scholar] [CrossRef]
- Zamparo, P.; Francescato, M.P.; De Luca, G.; Lovati, L.; di Prampero, P.E. The energy cost of level walking in patients with hemiplegia. Scand. J. Med. Sci. Sports 1995, 5, 348–352. [Google Scholar] [CrossRef]
- Detrembleur, C.; Dierick, F.; Stoquart, G.; Chantraine, F.; Lejeune, T. Energy cost, mechanical work, and efficiency of hemiparetic walking. Gait Posture 2003, 18, 47–55. [Google Scholar] [CrossRef]
- Stinear, C.M.; Lang, C.E.; Zeiler, S.; Byblow, W.D. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020, 19, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, R.; Scocchi, M.; Iadarola, P.; Franciscone, P.; Verri, M.; Boschi, F.; Pasini, E.; Viglio, S. Protein supplementation may enhance the spontaneous recovery of neurological alterations in patients with ischaemic stroke. Clin. Rehabil. 2008, 22, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Shin, Y.I. Nutritional supplementation in stroke rehabilitation: A narrative review. Brain Neurorehabil. 2022, 15, e3. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, R.; Baiardi, P.; Scocchi, M.; Iadarola, P.; Verri, M.; Sessarego, P.; Boschi, F.; Pasini, E.; Pastoris, O.; Viglio, S. Normalization of zinc intake enhances neurological retrieval of patients suffering from ischemic strokes. Nutr. Neurosci. 2009, 12, 219–225. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Bise, T.; Shimazu, S.; Tanoue, M.; Tomioka, Y.; Araki, M.; Nishino, T.; Kuzuhara, A.; Takatsuki, F. Effects of a leucine-enriched amino acid supplement on muscle mass, muscle strength, and physical function in post-stroke patients with sarcopenia: A randomized controlled trial. Nutrition 2019, 58, 1–6. [Google Scholar] [CrossRef]
- Ikeda, T.; Morotomi, N.; Kamono, A.; Ishimoto, S.; Miyazawa, R.; Kometani, S.; Sako, R.; Kaneko, N.; Iida, M.; Kawate, N. The Effects of Timing of a Leucine-Enriched Amino Acid Supplement on Body Composition and Physical Function in Stroke Patients: A Randomized Controlled Trial. Nutrients 2020, 12, 1928. [Google Scholar] [CrossRef]
- Chen, H.; Fu, C.; Fang, W.; Wang, Z.; Zhang, D.; Zhang, H. Influence of nutritional status on rehabilitation efficacy of patients after stroke-a scoping review. Front. Neurol. 2025, 16, 1502772. [Google Scholar] [CrossRef]
- Aquilani, R.; Boselli, M.; D’Antona, G.; Baiardi, P.; Boschi, F.; Viglio, S.; Iadarola, P.; Pasini, E.; Barbieri, A.; Dossena, M.; et al. Unaffected arm muscle hypercatabolism in dysphagic subacute stroke patients: The effects of essential amino acid supplementation. BioMed Res. Int. 2014, 2014, 964365. [Google Scholar] [CrossRef]
- Scherbakov, N.; Ebner, N.; Sandek, A.; Meisel, A.; Haeusler, K.G.; von Haehling, S.; Anker, S.D.; Dirnagl, U.; Joebges, M.; Doehner, W. Influence of essential amino acids on muscle mass and muscle strength in patients with cerebral stroke during early rehabilitation: Protocol and rationale of a randomized clinical trial (AMINO-Stroke Study). BMC Neurol. 2016, 16, 10. [Google Scholar] [CrossRef]
- Sakai, K.; Niimi, M.; Momosaki, R.; Hoshino, E.; Yoneoka, D.; Nakayama, E.; Masuoka, K.; Maeda, T.; Takahashi, N.; Sakata, N. Nutritional therapy for reducing disability and improving activities of daily living in people after stroke. Cochrane Database Syst. Rev. 2024, 8, CD014852. [Google Scholar] [CrossRef]
- Iadecola, C.; Buckwalter, M.S.; Anrather, J. Immune responses to stroke: Mechanisms, modulation, and therapeutic potential. J. Clin. Investig. 2020, 130, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, J.L.; Becker, K.J.; Kim, J.S.; Choi-Kwon, S.; Saban, K.L.; McNair, N.; Mead, G.E. Poststroke Fatigue: Emerging Evidence and Approaches to Management: A Scientific Statement for Healthcare Professionals From the American Heart Association. Stroke 2017, 48, e159–e170. [Google Scholar] [CrossRef] [PubMed]
- Towfighi, A.; Ovbiagele, B.; El Husseini, N.; Hackett, M.L.; Jorge, R.E.; Kissela, B.M.; Mitchell, P.H.; Skolarus, L.E.; Whooley, M.A.; Williams, L.S. Poststroke Depression: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2017, 48, e30–e43. [Google Scholar] [CrossRef] [PubMed]
- Choi-Kwon, S.; Han, S.W.; Kwon, S.U.; Kim, J.S. Poststroke fatigue: Characteristics and related factors. Cerebrovasc. Dis. 2005, 19, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Scottish Intercollegiate Guidelines Network (SIGN). Management of Patients with Stroke: Rehabilitation, Prevention and Management of Complications, and Discharge Planning: A National Clinical Guideline. 2010. Available online: https://collections.nlm.nih.gov/catalog/nlm:nlmuid-101609293-pdf (accessed on 5 August 2025).
- Glader, E.L.; Stegmayr, B.; Asplund, K. Poststroke fatigue: A 2-year follow-up study of stroke patients in Sweden. Stroke 2002, 33, 1327–1333. [Google Scholar] [CrossRef]
- Staub, F.; Bogousslavsky, J. Fatigue after stroke: A major but neglected issue. Cerebrovasc. Dis. 2001, 12, 75–81. [Google Scholar] [CrossRef]
- Naess, H.; Lunde, L.; Brogger, J. The effects of fatigue, pain, and depression on quality of life in ischemic stroke patients: The Bergen Stroke Study. Vasc. Health Risk Manag. 2012, 8, 407–413. [Google Scholar] [CrossRef]
- Norheim, K.B.; Harboe, E.; Gøransson, L.G.; Omdal, R. Interleukin-1 inhibition and fatigue in primary Sjögren’s syndrome: A double blind, randomised clinical trial. PLoS ONE 2012, 7, e30123. [Google Scholar] [CrossRef]
- Tookman, A.J.; Jones, C.L.; DeWitte, M.; Lodge, P.J. Fatigue in patients with advanced cancer: A pilot study of an intervention with infliximab. Support. Care Cancer 2008, 16, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Baranauskaite, A.; Raffayová, H.; Kungurov, N.V.; Kubanova, A.; Venalis, A.; Helmle, L.; Srinivasan, S.; Nasonov, E.; Vastesaeger, N.; RESPOND Investigators. Infliximab plus methotrexate is superior to methotrexate alone in the treatment of psoriatic arthritis in methotrexate-naive patients: The RESPOND study. Ann. Rheum. Dis. 2012, 71, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, M.; Suzuki, T.; Tsuboi, H.; Ito, S.; Mamura, M.; Goto, D.; Matsumoto, I.; Tsutsumi, A.; Sumida, T. Anti-interleukin-6 receptor antibody (tocilizumab) treatment of multicentric Castleman’s disease. Intern. Med. 2007, 46, 771–774. [Google Scholar] [CrossRef]
- Strand, V.; Burmester, G.R.; Ogale, S.; Devenport, J.; John, A.; Emery, P. Improvements in health-related quality of life after treatment with tocilizumab in patients with rheumatoid arthritis refractory to tumour necrosis factor inhibitors: Results from the 24-week randomized controlled RADIATE study. Rheumatology 2012, 51, 1860–1869. [Google Scholar] [CrossRef]
- Burmester, G.R.; Feist, E.; Kellner, H.; Braun, J.; Iking-Konert, C.; Rubbert-Roth, A. Effectiveness and safety of the interleukin 6-receptor antagonist tocilizumab after 4 and 24 weeks in patients with active rheumatoid arthritis: The first phase IIIb real-life study (TAMARA). Ann. Rheum. Dis. 2011, 70, 755–759. [Google Scholar] [CrossRef]
- Loubinoux, I.; Kronenberg, G.; Endres, M.; Schumann-Bard, P.; Freret, T.; Filipkowski, R.K.; Kaczmarek, L.; Popa-Wagner, A. Post-stroke depression: Mechanisms, translation and therapy. J. Cell. Mol. Med. 2012, 16, 1961–1969. [Google Scholar] [CrossRef]
- Parikh, R.M.; Robinson, R.G.; Lipsey, J.R.; Starkstein, S.E.; Fedoroff, J.P.; Price, T.R. The impact of poststroke depression on recovery in activities of daily living over a 2-year follow-up. Arch. Neurol. 1990, 47, 785–789. [Google Scholar] [CrossRef]
- Robinson, R.G.; Bolla-Wilson, K.; Kaplan, E.; Lipsey, J.R.; Price, T.R. Depression influences intellectual impairment in stroke patients. Br. J. Psychiatry 1986, 148, 541–547. [Google Scholar] [CrossRef]
- Alexandrova, M.L.; Danovska, M.P. Cognitive impairment one year after ischemic stroke: Predictors and dynamics of significant determinants. Turk. J. Med. Sci. 2016, 46, 1366–1373. [Google Scholar] [CrossRef]
- Kuźma, E.; Lourida, I.; Moore, S.F.; Levine, D.A.; Ukoumunne, O.C.; Llewellyn, D.J. Stroke and dementia risk: A systematic review and meta-analysis. Alzheimer’s Dement. 2018, 14, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Wolf, P.A.; Beiser, A.; Elias, M.F.; Au, R.; Kase, C.S.; D’Agostino, R.B.; DeCarli, C. Stroke risk profile, brain volume, and cognitive function: The Framingham Offspring Study. Neurology 2004, 63, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Firbank, M.J.; Wiseman, R.M.; Burton, E.J.; Saxby, B.K.; O’Brien, J.T.; Ford, G.A. Brain atrophy and white matter hyperintensity change in older adults and relationship to blood pressure. Brain atrophy, WMH change and blood pressure. J. Neurol. 2007, 254, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Hoshide, S.; Ishikawa, J.; Shimada, K.; Kario, K. Insular cortex atrophy as an independent determinant of disrupted diurnal rhythm of ambulatory blood pressure in elderly hypertension. Am. J. Hypertens. 2009, 22, 723–729. [Google Scholar] [CrossRef]
- Knecht, S.; Oelschläger, C.; Duning, T.; Lohmann, H.; Albers, J.; Stehling, C.; Heindel, W.; Breithardt, G.; Berger, K.; Ringelstein, E.B.; et al. Atrial fibrillation in stroke-free patients is associated with memory impairment and hippocampal atrophy. Eur. Heart J. 2008, 29, 2125–2132. [Google Scholar] [CrossRef]
- Lane, C.A.; Barnes, J.; Nicholas, J.M.; Sudre, C.H.; Cash, D.M.; Parker, T.D.; Malone, I.B.; Lu, K.; James, S.-N.; Keshavan, A.; et al. Associations between blood pressure across adulthood and late-life brain structure and pathology in the neuroscience substudy of the 1946 British birth cohort (Insight 46): An epidemiological study. Lancet Neurol. 2019, 18, 942–952. [Google Scholar] [CrossRef]
- Simats, A.; Liesz, A. Systemic inflammation after stroke: Implications for post-stroke comorbidities. EMBO Mol. Med. 2022, 14, e16269. [Google Scholar] [CrossRef]
- Zaremba, J.; Losy, J. Early TNF-alpha levels correlate with ischaemic stroke severity. Acta Neurol. Scand. 2001, 104, 288–295. [Google Scholar] [CrossRef]
- Waje-Andreassen, U.; Kråkenes, J.; Ulvestad, E.; Thomassen, L.; Myhr, K.M.; Aarseth, J.; Vedeler, C.A. IL-6: An early marker for outcome in acute ischemic stroke. Acta Neurol. Scand. 2005, 111, 360–365. [Google Scholar] [CrossRef]
- Basic Kes, V.; Simundic, A.M.; Nikolac, N.; Topic, E.; Demarin, V. Pro-inflammatory and anti-inflammatory cytokines in acute ischemic stroke and their relation to early neurological deficit and stroke outcome. Clin. Biochem. 2008, 41, 1330–1334. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Di Sciacca, R.; Di Raimondo, D.; Serio, A.; D’Aguanno, G.; La Placa, S.; Pecoraro, R.; Arnao, V.; Marino, L.; Monaco, S.; et al. Plasma levels of inflammatory and thrombotic/fibrinolytic markers in acute ischemic strokes: Relationship with TOAST subtype, outcome and infarct site. J. Neuroimmunol. 2009, 215, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.S.; Berry, K.; Beneyto, M.M.; Gaudilliere, D.; Ganio, E.A.; Culos, A.; Ghaemi, M.S.; Choisy, B.; Djebali, K.; Einhaus, J.F.; et al. A year-long immune profile of the systemic response in acute stroke survivors. Brain 2019, 142, 978–991. [Google Scholar] [CrossRef]
- Oto, J.; Suzue, A.; Inui, D.; Fukuta, Y.; Hosotsubo, K.; Torii, M.; Nagahiro, S.; Nishimura, M. Plasma proinflammatory and anti-inflammatory cytokine and catecholamine concentrations as predictors of neurological outcome in acute stroke patients. J. Anesth. 2008, 22, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Liesz, A.; Rüger, H.; Purrucker, J.; Zorn, M.; Dalpke, A.; Möhlenbruch, M.; Englert, S.; Nawroth, P.P.; Veltkamp, R. Stress mediators and immune dysfunction in patients with acute cerebrovascular diseases. PLoS ONE 2013, 8, e74839. [Google Scholar] [CrossRef] [PubMed]
- Stanne, T.M.; Angerfors, A.; Andersson, B.; Brännmark, C.; Holmegaard, L.; Jern, C. Longitudinal Study Reveals Long-Term Proinflammatory Proteomic Signature After Ischemic Stroke Across Subtypes. Stroke 2022, 53, 2847–2858. [Google Scholar] [CrossRef]
- Garlichs, C.D.; Kozina, S.; Fateh-Moghadam, S.; Handschu, R.; Tomandl, B.; Stumpf, C.; Eskafi, S.; Raaz, D.; Schmeisser, A.; Yilmaz, A.; et al. Upregulation of CD40-CD40 ligand (CD154) in patients with acute cerebral ischemia. Stroke 2003, 34, 1412–1418. [Google Scholar] [CrossRef]
- Ladenvall, C.; Jood, K.; Blomstrand, C.; Nilsson, S.; Jern, C.; Ladenvall, P. Serum C-reactive protein concentration and genotype in relation to ischemic stroke subtype. Stroke 2006, 37, 2018–2023. [Google Scholar] [CrossRef]
- Aquilani, R.; Boselli, M.; Paola, B.; Pasini, E.; Iadarola, P.; Verri, M.; Viglio, S.; Condino, A.; Boschi, F. Is stroke rehabilitation a metabolic problem? Brain Inj. 2014, 28, 161–173. [Google Scholar] [CrossRef]
- Aquilani, R.; Emilio, B.; Dossena, M.; Baiardi, P.; Testa, A.; Boschi, F.; Viglio, S.; Iadarola, P.; Pasini, E.; Verri, M. Correlation of deglutition in subacute ischemic stroke patients with peripheral blood adaptive immunity: Essential amino acid improvement. Int. J. Immunopathol. Pharmacol. 2015, 28, 576–583. [Google Scholar] [CrossRef]
- Holmegaard, L.; Stanne, T.M.; Andreasson, U.; Zetterberg, H.; Blennow, K.; Blomstrand, C.; Jood, K.; Jern, C. Proinflammatory protein signatures in cryptogenic and large artery atherosclerosis stroke. Acta Neurol. Scand. 2021, 143, 303–312. [Google Scholar] [CrossRef]
- Benakis, C.; Brea, D.; Caballero, S.; Faraco, G.; Moore, J.; Murphy, M.; Sita, G.; Racchumi, G.; Ling, L.; Pamer, E.G.; et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med. 2016, 22, 516–523. [Google Scholar] [CrossRef]
- Liu, Q.; Johnson, E.M.; Lam, R.K.; Wang, Q.; Ye, H.B.; Wilson, E.N.; Minhas, P.S.; Liu, L.; Swarovski, M.S.; Tran, S.; et al. Peripheral TREM1 responses to brain and intestinal immunogens amplify stroke severity. Nat. Immunol. 2019, 20, 1023–1034. [Google Scholar] [CrossRef]
- Boselli, M.; Aquilani, R.; Baiardi, P.; Dioguardi, F.S.; Guarnaschelli, C.; Achilli, M.P.; Arrigoni, N.; Iadarola, P.; Verri, M.; Viglio, S.; et al. Supplementation of essential amino acids may reduce the occurrence of infections in rehabilitation patients with brain injury. Nutr. Clin. Pract. 2012, 27, 99–113. [Google Scholar] [CrossRef]
- Zhuang, H.-H.; Chen, Q.-H.; Wang, W.; Qu, Q.; Xu, W.-X.; Hu, Q.; Wu, X.-L.; Chen, Y.; Wan, Q.; Xu, T.-T.; et al. The efficacy of polymyxin B in treating stroke-associated pneumonia with carbapenem-resistant Gram-negative bacteria infections: A multicenter real-world study using propensity score matching. Front. Pharmacol. 2025, 16, 1413563. [Google Scholar] [CrossRef]
- Iadecola, C.; Fisher, M.; Sacco, R.L. Introduction to the Compendium on Stroke and Neurocognitive Impairment. Circ. Res. 2022, 130, 1073–1074. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, N.; Ren, L.; Yan, Y.; Sun, N.; Li, Y.-J.; Han, W.; Xue, R.; Liu, Q.; Hao, J.; et al. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc. Natl. Acad. Sci. USA 2014, 11, 18315–18320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, L.; Zhu, B.; Yang, Y.; Cai, C.; Wang, X.; Deng, L.; He, B.; Cui, Y.; Zhou, W. A comparative study of the neuroprotective effects of dl-3-n-butylphthalide and edaravone dexborneol on cerebral ischemic stroke rats. Eur. J. Pharmacol. 2023, 951, 175801. [Google Scholar] [CrossRef] [PubMed]
- Rittig, N.; Bach, E.; Thomsen, H.H.; Johannsen, M.; Jørgensen, J.O.; Richelsen, B.; Jessen, N.; Møller, N. Amino acid supplementation is anabolic during the acute phase of endotoxin-induced inflammation: A human randomized crossover trial. Clin. Nutr. 2016, 35, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Solerte, S.B.; Gazzaruso, C.; Bonacasa, R.; Rondanelli, M.; Zamboni, M.; Basso, C.; Locatelli, E.; Schifino, N.; Giustina, A.; Fioravanti, M. Nutritional supplements with oral amino acid mixtures increases whole-body lean mass and insulin sensitivity in elderly subjects with sarcopenia. Am. J. Cardiol. 2008, 101, 69E–77E. [Google Scholar] [CrossRef]
- Aquilani, R.; Zuccarelli, G.C.; Maestri, R.; Boselli, M.; Dossena, M.; Baldissarro, E.; Boschi, F.; Buonocore, D.; Verri, M. Essential amino acid supplementation is associated with reduced serum C-reactive protein levels and improved circulating lymphocytes in post-acute inflamed elderly patients. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211036823. [Google Scholar] [CrossRef]
- Aquilani, R.; Bolasco, P.; Murtas, S.; Maestri, R.; Iadarola, P.; Testa, C.; Deiana, M.L.; Esposito, M.P.; Contu, R.; Cadeddu, M.; et al. Effects of a Metabolic Mixture on Gut Inflammation and Permeability in Elderly Patients with Chronic Kidney Disease: A Proof-of-Concept Study. Metabolites 2022, 12, 987. [Google Scholar] [CrossRef]
- Evans, D.C.; Corkins, M.R.; Malone, A.; Miller, S.; Mogensen, K.M.; Guenter, P.; Jensen, G.L.; ASPEN Malnutrition Committee. The Use of Visceral Proteins as Nutrition Markers: An ASPEN Position Paper. Nutr. Clin. Pract. 2021, 36, 22–28. [Google Scholar] [CrossRef]
- Rondanelli, M.; Guido, D.; Faliva, M.A.; Gasparri, C.; Peroni, G.; Iannell, G.; Nichetti, M.; Naso, M.; Infantino, V.; Spadaccini, D.; et al. Effects of essential amino acid supplementation on pain in the elderly with hip fractures: A pilot, double-blind, placebo-controlled, randomised clinical trial. J. Biol. Regul. Homeost. Agents 2020, 34, 721–731. [Google Scholar] [CrossRef]
- Belayev, L.; Busto, R.; Zhao, W.; Clemens, J.A.; Ginsberg, M.D. Effect of delayed albumin hemodilution on infarction volume and brain edema after transient middle cerebral artery occlusion in rats. J. Neurosurg. 1997, 87, 595–601. [Google Scholar] [CrossRef]
- Belayev, L.; Zhao, W.; Pattany, P.M.; Weaver, R.G.; Huh, P.W.; Lin, B.; Busto, R.; Ginsberg, M.D. Diffusion-weighted magnetic resonance imaging confirms marked neuroprotective efficacy of albumin therapy in focal cerebral ischemia. Stroke 1998, 29, 2587–2599. [Google Scholar] [CrossRef] [PubMed]
- Belayev, L.; Liu, Y.; Zhao, W.; Busto, R.; Ginsberg, M.D. Human albumin therapy of acute ischemic stroke: Marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke 2001, 32, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Belayev, L.; Saul, I.; Huh, P.W.; Finotti, N.; Zhao, W.; Busto, R.; Ginsberg, M.D. Neuroprotective effect of high-dose albumin therapy against global ischemic brain injury in rats. Brain Res. 1999, 845, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, M.D.; Zhao, W.; Belayev, L.; Alonso, O.F.; Liu, Y.; Loor, J.Y.; Busto, R. Diminution of metabolism/blood flow uncoupling following traumatic brain injury in rats in response to high-dose human albumin treatment. J. Neurosurg. 2001, 94, 499–509. [Google Scholar] [CrossRef]
- Chumlea, W.C.; Roche, A.F.; Steinbaugh, M.L. Estimating stature from knee height for persons 60 to 90 years of age. J. Am. Geriatr. Soc. 1985, 33, 116–120. [Google Scholar] [CrossRef]
- Haines, R.W.; Harrois, A.; Prowle, J.R.; Søvik, S.; Beitland, S. Deserved attention for acute kidney injury after major trauma. Intensiv. Care Med. 2019, 45, 907–908. [Google Scholar] [CrossRef]
- Shaafi, S.; Ebrahimpour-Koujan, S.; Khalili, M.; Shamshirgaran, S.M.; Hashemilar, M.; Taheraghdam, A.; Shakouri, S.K.; Hokmabadi, E.S.; Ahmadi, Y.; Farhoudi, M.; et al. Effects of Alpha Lipoic Acid Supplementation on Serum Levels of Oxidative Stress, Inflammatory Markers and Clinical Prognosis among Acute Ischemic Stroke Patients: A Randomized, Double Blind, TNS Trial. Adv. Pharm. Bull. 2020, 10, 284–289. [Google Scholar] [CrossRef]
- Hashemilar, M.; Khalili, M.; Rezaeimanesh, N.; Hokmabadi, E.S.; Rasulzade, S.; Shamshirgaran, S.M.; Taheraghdam, A.; Farhoudi, M.; Shaafi, S.; Shakouri, S.K.; et al. Effect of Whey Protein Supplementation on Inflammatory and Antioxidant Markers, and Clinical Prognosis in Acute Ischemic Stroke (TNS Trial): A Randomized, Double Blind, Controlled, Clinical Trial. Adv. Pharm. Bull. 2020, 10, 135–140. [Google Scholar] [CrossRef]
- Uchino, Y.; Watanabe, M.; Takata, M.; Amiya, E.; Tsushima, K.; Adachi, T.; Hiroi, Y.; Funazaki, T.; Komuro, I. Effect of Oral Branched-Chain Amino Acids on Serum Albumin Concentration in Heart Failure Patients with Hypoalbuminemia: Results of a Preliminary Study. Am. J. Cardiovasc. Drugs 2018, 18, 327–332. [Google Scholar] [CrossRef]
- Ho, K.M.; Lipman, J. An update on C-reactive protein for intensivists. Anaesth. Intensiv. Care 2009, 37, 234–241. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandris, C.; Lauro, R.; Presta, I.; Sesti, G. C-reactive protein induces phosphorylation of insulin receptor substrate-1 on Ser307 and Ser 612 in L6 myocytes, thereby impairing the insulin signalling pathway that promotes glucose transport. Diabetologia 2007, 50, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.S.; Clark, J.; Wagenmakers, A.J.M. The effect of exercise and nutrition on intramuscular fat metabolism and insulin sensitivity. Annu. Rev. Nutr. 2010, 30, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Sjöholm, A.; Nyström, T. Endothelial inflammation in insulin resistance. Lancet 2005, 365, 610–612. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Esper, D.H.; Coplin, W.M.; Carhuapoma, J.R. Energy expenditure in patients with nontraumatic intracranial hemorrhage. JPEN J. Parenter. Enter. Nutr. 2006, 30, 71–75. [Google Scholar] [CrossRef]
- Bisoendial, R.J.; Kastelein, J.J.P.; Levels, J.H.M.; Zwaginga, J.J.; van den Bogaard, B.; Reitsma, P.H.; Meijers, J.C.M.; Hartman, D.; Levi, M.; Stroes, E.S.G. Activation of inflammation and coagulation after infusion of C-reactive protein in humans. Circ. Res. 2005, 96, 714–716. [Google Scholar] [CrossRef]
- Elliott, P.; Chambers, J.C.; Zhang, W.; Clarke, R.; Hopewell, J.C.; Peden, J.F.; Erdmann, J.; Braund, P.; Engert, J.C.; Bennett, D.; et al. Genetic Loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA 2009, 302, 37–48. [Google Scholar] [CrossRef]
- Iida, K.; Tani, S.; Atsumi, W.; Yagi, T.; Kawauchi, K.; Matsumoto, N.; Hirayama, A. Association of plasminogen activator inhibitor-1 and low-density lipoprotein heterogeneity as a risk factor of atherosclerotic cardiovascular disease with triglyceride metabolic disorder: A pilot cross-sectional study. Coron. Artery Dis. 2017, 28, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.Y.; Yao, Y.M. The Clinical Significance and Potential Role of C-Reactive Protein in Chronic Inflammatory and Neurodegenerative Diseases. Front. Immunol. 2018, 9, 1302. [Google Scholar] [CrossRef] [PubMed]
- Suliman, M.E.; Qureshi, A.R.; Stenvinkel, P.; Pecoits-Filho, R.; Bárány, P.; Heimbürger, O.; Anderstam, B.; Ayala, E.R.; Filho, J.C.D.; Alvestrand, A.; et al. Inflammation contributes to low plasma amino acid concentrations in patients with chronic kidney disease. Am. J. Clin. Nutr. 2005, 82, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Reeds, P.J.; Fjeld, C.R.; Jahoor, F. Do the differences between the amino acid compositions of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states? J. Nutr. 1994, 124, 906–910. [Google Scholar] [CrossRef]
- Zidorio, A.P.; Togo, C.; Jones, R.; Dutra, E.; de Carvalho, K. Resting Energy Expenditure and Protein Balance in People with Epidermolysis Bullosa. Nutrients 2019, 11, 1257. [Google Scholar] [CrossRef]
- Chen, Y.F.; Lu, H.C.; Hou, P.C.; Lin, Y.C.; Aala, W., Jr.; Onoufriadis, A.; McGrath, J.A.; Chen, Y.L.; Hsu, C.K. Plasma metabolomic profiling reflects the malnourished and chronic inflammatory state in recessive dystrophic epidermolysis bullosa. J. Dermatol. Sci. 2022, 107, 82–88. [Google Scholar] [CrossRef]
- Martindale, R.G. Novel nutrition strategies to enhance recovery after surgery. JPEN J. Parenter. Enter. Nutr. 2023, 47, 476–481. [Google Scholar] [CrossRef]
- Aquilani, R.; Verri, M.; Iadarola, P.; Arcidiaco, P.; Boschi, F.; Dossena, M.; Sessarego, P.; Scocchi, M.; Arrigoni, N.; Pastoris, O. Plasma precursors of brain catecholaminergic and serotonergic neurotransmitters in rehabilitation patients with ischemic stroke. Arch. Phys. Med. Rehabil. 2004, 85, 779–784. [Google Scholar] [CrossRef]
- Schmidt, K.C.; Cook, M.P.; Qin, M.; Kang, J.; Burlin, T.V.; Smith, C.B. Measurement of regional rates of cerebral protein synthesis with L-[1-11C]leucine and PET with correction for recycling of tissue amino acids: I. Kinetic modeling approach. J. Cereb. Blood Flow Metab. 2005, 25, 617–628. [Google Scholar] [CrossRef]
- Smith, C.B.; Schmidt, K.C.; Qin, M.; Burlin, T.V.; Cook, M.P.; Kang, J.; Saunders, R.C.; Bacher, J.D.; Carson, R.E.; Channing, M.A.; et al. Measurement of regional rates of cerebral protein synthesis with L-[1-11C]leucine and PET with correction for recycling of tissue amino acids: II. Validation in rhesus monkeys. J. Cereb. Blood Flow Metab. 2005, 25, 629–640. [Google Scholar] [CrossRef]
- Dallman, P.R.; Spirito, R.A. Brain response to protein undernutrition. Mechanism of preferential protein retention. J. Clin. Investig. 1972, 51, 2175–2180. [Google Scholar] [CrossRef]
- Burns, J.M.; Johnson, D.K.; Watts, A.; Swerdlow, R.H.; Brooks, W.M. Reduced lean mass in early Alzheimer disease and its association with brain atrophy. Arch. Neurol. 2010, 67, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Beavers, K.M.; Brinkley, T.E.; Nicklas, B.J. Effect of exercise training on chronic inflammation. Clin. Chim. Acta 2010, 411, 785–793. [Google Scholar] [CrossRef]
- Bistrian, B.R. Hypoalbuminemic malnutrition. JPEN J. Parenter. Enter. Nutr. 2023, 47, 824–826. [Google Scholar] [CrossRef] [PubMed]
- Bretschera, C.; Boesiger, F.; Kaegi-Braun, N.; Hersberger, L.; Lobo, D.N.; Evans, D.C.; Tribolet, P.; Gomes, F.; Hoess, C.; Pavlicek, V.; et al. Admission serum albumin concentrations and response to nutritional therapy in hospitalised patients at malnutrition risk: Secondary analysis of a randomised clinical trial. eClinicalMedicine 2022, 45, 101301. [Google Scholar] [CrossRef]
- Wiedermann, C.J. Anti-inflammatory activity of albumin. Crit. Care Med. 2007, 35, 981–982, author reply 982–983. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Fernández-Gajardo, R.; Gutiérrez, R.; Matamala, J.M.; Carrasco, R.; Miranda-Merchak, A.; Feuerhake, W. Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS Neurol. Disord. Drug Targets 2013, 12, 698–714. [Google Scholar] [CrossRef]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef]
- Anraku, M.; Chuang, V.T.G.; Maruyama, T.; Otagiri, M. Redox properties of serum albumin. Biochim. Biophys. Acta 2013, 1830, 5465–5472. [Google Scholar] [CrossRef]
- Lam, F.W.; Cruz, M.A.; Leung, H.C.E.; Parikh, K.S.; Wayne Smith, C.; Rumbaut, R.E. Histone induced platelet aggregation is inhibited by normal albumin. Thromb. Res. 2013, 132, 69–76. [Google Scholar] [CrossRef]
- Zhang, Q.; Lei, Y.X.; Wang, Q.; Jin, Y.P.; Li Fu, R.; Geng, H.H.; Huang, L.L.; Wang, X.X.; Wang, P.X. Serum albumin level is associated with the recurrence of acute ischemic stroke. Am. J. Emerg. Med. 2016, 34, 1812–1816. [Google Scholar] [CrossRef]
- Folsom, A.R.; Lutsey, P.L.; Heckbert, S.R.; Cushman, M. Serum albumin and risk of venous thromboembolism. Thromb. Haemost. 2010, 104, 100–104. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Seidu, S.; Katechia, D.T.; Laukkanen, J.A. Inverse association between serum albumin and future risk of venous thromboembolism: Interrelationship with high sensitivity C-reactive protein. Ann. Med. 2018, 50, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, A.; Meng, X.; Lin, J.; Jiang, Y.; Jing, J.; Zuo, Y.; Wang, Y.; Zhao, X.; Li, H.; et al. Low serum albumin levels predict poor outcome in patients with acute ischaemic stroke or transient ischaemic attack. Stroke Vasc. Neurol. 2021, 6, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Thuemmler, R.J.; Pana, T.A.; Carter, B.; Mahmood, R.; Bettencourt-Silva, J.H.; Metcalf, A.K.; Mamas, M.A.; Potter, J.F.; Myint, P.K. Serum Albumin and Post-Stroke Outcomes: Analysis of UK Regional Registry Data, Systematic Review, and Meta-Analysis. Nutrients 2024, 16, 1486. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.B.; Moldawer, L.L.; Bistrian, B.R.; Blackburn, G.L. Biological measures for the formulation of a hospital prognostic index. Am. J. Clin. Nutr. 1981, 34, 2013–2022. [Google Scholar] [CrossRef]
- Reinhardt, G.F.; Myscofski, J.W.; Wilkens, D.B.; Dobrin, P.B.; Mangan, J.E., Jr.; Stannard, R.T. Incidence and mortality of hypoalbuminemic patients in hospitalized veterans. JPEN J. Parenter. Enter. Nutr. 1980, 4, 357–359. [Google Scholar] [CrossRef]
- Acharya, R.; Poudel, D.; Bowers, R.; Patel, A.; Schultz, E.; Bourgeois, M.; Paswan, R.; Stockholm, S.; Batten, M.; Kafle, S.; et al. Low serum albumin predicts severe outcomes in COVID-19 Infection: A single-center retrospective case-control study. J. Clin. Med. Res. 2021, 13, 258–267. [Google Scholar] [CrossRef]
- Kudsk, K.; Tolley, E.; DeWitt, R.; Janu, P.G.; Blackwell, A.P.; Yeary, S.; King, B.K. Preoperative albumin and surgical site identify surgical risk for major postoperative complications. JPEN J. Parenter. Enter. Nutr. 2003, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nipper, C.A.; Lim, K.; Riveros, C.; Hsu, E.; Ranganathan, S.; Xu, J.; Brooks, M.; Esnaola, N.; Klaassen, Z.; Jerath, A.; et al. The association between serum albumin and post-operative outcomes among patients undergoing common surgical procedures: An analysis of a multi-specialty surgical cohort from the national surgical quality improvement program(NSQIP). J. Clin. Med. 2022, 11, 6543. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Koehler, K.M.; Romero, L.; Garry, P.J. Serum albumin is associated with skeletal muscle in elderly men and women. Am. J. Clin. Nutr. 1996, 64, 552–558. [Google Scholar] [CrossRef]
- Golden, M.H.N. Waterlow, J.C., Stephen, J.M., Eds.; Metabolism of branched chain amino acids. In Nitrogen Metabolism in Man; Applied Science: London, UK, 1981; p. 109. [Google Scholar]
- Holeček, M. Relation between glutamine, branched-chain amino acids, and protein metabolism. Nutrition 2002, 18, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.L.; Yu, T.T.; Kang, K.; Zhao, J. Effects of glutamine on markers of intestinal inflammatory response and mucosal permeability in abdominal surgery patients: A meta-analysis. Exp. Ther. Med. 2016, 12, 3499–3506. [Google Scholar] [CrossRef]
- Jimenez, R.V.; Kuznetsova, V.; Connelly, A.N.; Hel, Z.; Szalai, A.J. C-Reactive Protein Promotes the Expansion of Myeloid Derived Cells With Suppressor Functions. Front. Immunol. 2019, 10, 2183. [Google Scholar] [CrossRef]
- Aquilani, R.; Zuccarelli, G.C.; Condino, A.M.; Catani, M.; Rutili, C.; Del Vecchio, C.; Pisano, P.; Verri, M.; Iadarola, P.; Viglio, S.; et al. Despite Inflammation, Supplemented Essential Amino Acids May Improve Circulating Levels of Albumin and Haemoglobin in Patients after Hip Fractures. Nutrients 2017, 9, 637. [Google Scholar] [CrossRef]
- Coëffier, M.; Miralles-Barrachina, O.; Le Pessot, F.; Lalaude, O.; Daveau, M.; Lavoinne, A.; Lerebours, E.; Déchelotte, P. Influence of glutamine on cytokine production by human gut in vitro. Cytokine 2001, 13, 148–154. [Google Scholar] [CrossRef]
- Lightfoot, A.; McArdle, A.; Griffiths, R.D. Muscle in defense. Crit. Care Med. 2009, 37, S384–S390. [Google Scholar] [CrossRef]
- Hu, Y.M.; Hsiung, Y.C.; Pai, M.H.; Yeh, S.L. Glutamine Administration in Early or Late Septic Phase Downregulates Lymphocyte PD-1/PD-L1 Expression and the Inflammatory Response in Mice With Polymicrobial Sepsis. JPEN J. Parenter. Enter. Nutr. 2018, 42, 538–549. [Google Scholar] [CrossRef]
- Chen, Y.; Tseng, S.H.; Yao, C.L.; Li, C.; Tsai, Y.H. Distinct Effects of Growth Hormone and Glutamine on Activation of Intestinal Stem Cells. JPEN J. Parenter. Enter. Nutr. 2018, 42, 642–651. [Google Scholar] [CrossRef]
- Hsieh, L.C.; Chien, S.L.; Huang, M.S.; Tseng, H.F.; Chang, C.K. Anti-inflammatory and anticatabolic effects of short-term beta-hydroxy-beta-methylbutyrate supplementation on chronic obstructive pulmonary disease patients in intensive care unit. Asia Pac. J. Clin. Nutr. 2006, 15, 544–550. [Google Scholar] [PubMed]
- Nunes, E.A.; Kuczera, D.; Brito, G.A.P.; Bonatto, S.J.R.; Yamazaki, R.K.; Tanhoffer, R.A.; Mund, R.C.; Kryczyk, M.; Fernandes, L.C. Beta-hydroxy-beta-methylbutyrate supplementation reduces tumor growth and tumor cell proliferation ex vivo and prevents cachexia in Walker 256 tumor-bearing rats by modifying nuclear factor-kappaB expression. Nutr. Res. 2008, 28, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Mishima, E.; Fukuda, S.; Mukawa, C.; Yuri, A.; Kanemitsu, Y.; Matsumoto, Y.; Akiyama, Y.; Fukuda, N.N.; Tsukamoto, H.; Asaji, K.; et al. Evaluation of the impact of gut microbiota on uremic solute accumulation by a CE-TOFMS-based metabolomics approach. Kidney Int. 2017, 92, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Flaim, K.E.; Liao, W.S.; Peavy, D.E.; Taylor, J.M.; Jefferson, L.S. The role of amino acids in the regulation of protein synthesis in perfused rat liver. II. Effects of amino acid deficiency on peptide chain initiation, polysomal aggregation, and distribution of albumin mRNA. J. Biol. Chem. 1982, 257, 2939–2946. [Google Scholar] [CrossRef]
- Flaim, K.E.; Peavy, D.E.; Everson, W.V.; Jefferson, L.S. The role of amino acids in the regulation of protein synthesis in perfused rat liver. I. Reduction in rates of synthesis resulting from amino acid deprivation and recovery during flow-through perfusion. J. Biol. Chem. 1982, 257, 2932–2938. [Google Scholar] [CrossRef]
- Wada, A.; Nakamura, M.; Kobayashi, K.; Kuroda, A.; Harada, D.; Kido, S.; Kuwahata, M. Effects of amino acids and albumin administration on albumin metabolism in surgically stressed rats: A basic nutritional study. JPEN J. Parenter. Enter. Nutr. 2023, 47, 399–407. [Google Scholar] [CrossRef]
- De Feo, P.; Volpi, E.; Lucidi, P.; Cruciani, G.; Reboldi, G.; Siepi, D.; Mannarino, E.; Santeusanio, F.; Brunetti, P.; Bolli, G.B. Physiological increments in plasma insulin concentrations have selective and different effects on synthesis of hepatic proteins in normal humans. Diabetes 1993, 42, 995–1002. [Google Scholar] [CrossRef]
- Rothschild, M.A.; Oratz, M.; Mongelli, J.; Fishman, L.; Schreiber, S.S. Amino acid regulation of albumin synthesis. J. Nutr. 1969, 98, 395–403. [Google Scholar] [CrossRef]
- Iwata, M.; Kuzuya, M.; Kitagawa, Y.; Iguchi, A. Prognostic value of serum albumin combined with serum C-reactive protein levels in older hospitalized patients: Continuing importance of serum albumin. Aging Clin. Exp. Res. 2006, 18, 307–311. [Google Scholar] [CrossRef]
- Bucci, T.; Pastori, D.; Pignatelli, P.; Ntaios, G.; Abdul-Rahim, A.H.; Violi, F.; Lip, G.Y.H. Albumin Levels and Risk of Early Cardiovascular Complications After Ischemic Stroke: A Propensity-Matched Analysis of a Global Federated Health Network. Stroke 2024, 55, 604–612. [Google Scholar] [CrossRef]
- Cirillo, C.; Brihmat, N.; Castel-Lacanal, E.; Le Friec, A.; Barbieux-Guillot, M.; Raposo, N.; Pariente, J.; Viguier, A.; Simonetta-Moreau, M.; Albucher, J.F.; et al. Post-stroke remodeling processes in animal models and humans. J. Cereb. Blood Flow Metab. 2020, 40, 3–22. [Google Scholar] [CrossRef]
- Aquilani, R.; Scocchi, M.; Iadarola, P.; Viglio, S.; Pasini, E.; Condello, S.; Boschi, F.; Pastoris, O.; Bongiorno, A.I.; Verri, M. Spontaneous neurocognitive retrieval of patients with sub-acute ischemic stroke is associated with dietary protein intake. Nutr. Neurosci. 2010, 13, 129–134. [Google Scholar] [CrossRef]
- Fernstrom, J.D. Dietary amino acids and brain function. J. Am. Diet. Assoc. 1994, 94, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Mason, L.J.; Mackin, K.E.; Srikhanta, Y.N.; Lyras, D.; Prakash, M.D.; Nurgali, K.; Venegas, A.; Hill, M.D.; Moore, R.J.; et al. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat. Med. 2016, 22, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Doweiko, J.P.; Nompleggi, D.J. The role of albumin in human physiology and pathophysiology, Part III: Albumin and disease states. JPEN J. Parenter. Enter. Nutr. 1991, 15, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, G.J.; Martin, G.S.; Evans, T.W. Albumin: Biochemical properties and therapeutic potential. Hepatology 2005, 41, 1211–1219. [Google Scholar] [CrossRef]
- Kawakami, A.; Kubota, K.; Yamada, N.; Tagami, U.; Takehana, K.; Sonaka, I.; Suzuki, E.; Hirayama, K. Identification and characterization of oxidized human serum albumin. A slight structural change impairs its ligand-binding and antioxidant functions. FEBS J. 2006, 273, 3346–3357. [Google Scholar] [CrossRef]
- Das, S.; Maras, J.S.; Hussain, M.S.; Sharma, S.; David, P.; Sukriti, S.; Shasthry, S.M.; Maiwall, R.; Trehanpati, N.; Singh, T.P.; et al. Hyperoxidized albumin modulates neutrophils to induce oxidative stress and inflammation in severe alcoholic hepatitis. Hepatology 2017, 65, 631–646. [Google Scholar] [CrossRef]
- Alcaraz-Quiles, J.; Casulleras, M.; Oettl, K.; Titos, E.; Flores-Costa, R.; Duran-Güell, M.; López-Vicario, C.; Pavesi, M.; Stauber, R.E.; Arroyo, V.; et al. Oxidized albumin triggers a cytokine storm in leukocytes through P38 mitogen-activated protein kinase: Role in systemic inflammation in decompensated cirrhosis. Hepatology 2018, 68, 1937–1952. [Google Scholar] [CrossRef]


| Mixture Substrates: | 5.1 g |
|---|---|
| L-leucine § | 1200 mg |
| L-lysine § | 900 mg |
| L-threonine § | 700 mg |
| L-isoleucine § | 600 mg |
| L-valine § | 600 mg |
| L-cystine | 150 mg |
| L-histidine | 150 mg |
| L-phenylalanine § | 100 mg |
| L-methionine § | 50 mg |
| L-tryptophan § | 50 mg |
| Vitamin B6 | 0.85 mg |
| Vitamin B1 | 0.70 mg |
| Citric acid | 409 mg |
| Succinic acid | 102.5 mg |
| Malic acid | 102.5 mg |
| Variable | Placebo (N = 34) | EAA (N = 30) | p Value |
|---|---|---|---|
| Demographic | |||
| Age (yrs) * | 71.1 ± 13.7 | 76.4 ± 9.9 | 0.09 |
| Sex (Males,%) § | 18 (52.9%) | 12 (40.0%) | 0.30 |
| Anthropometric | |||
| Body weight (kg) * | 65.8 ± 12.0 | 66.5 ± 17.4 | 0.84 |
| Body Mass Index (BMI, kg/m2) * | 24.0 ± 3.8 | 24.1 ± 4.9 | 0.97 |
| Comorbidities | |||
| Hypertension § | 27 (79.4%) | 23 (76.7%) | 0.79 |
| Atrial fibrillation § | 11 (32.4%) | 9 (30.0%) | 0.84 |
| Ischemic heart disease § | 4 (11.8%) | 5 (16.7%) | 0.57 |
| Dyslipidemia § | 12 (35.3%) | 10 (33.3%) | 0.87 |
| Diabetes mellitus § | 8 (23.5%) | 9 (30.0%) | 0.56 |
| Drugs taken upon admission or during hospitalization | |||
| ACE inhibitors § | 20 (58.8%) | 17 (56.7%) | 0.86 |
| Calcium antagonists § | 10 (29.4%) | 13 (43.3%) | 0.25 |
| Beta-blockers § | 14 (41.2%) | 11 (36.7%) | 0.71 |
| Antihypertensives § | 27 (79.4%) | 23 (76.7%) | 0.79 |
| Antiaggregants or anticoagulants § | 22 (64.7%) | 21 (70.0%) | 0.65 |
| Lipid-lowering agents § | 14 (41.2%) | 12 (40.0%) | 0.92 |
| Hypoglycemic agents § | 6 (17.6%) | 8 (26.7%) | 0.38 |
| Antidepressants § | 15 (44.1%) | 17 (56.7%) | 0.32 |
| Neuroleptics (quetiapine) § | 8 (23.5%) | 9 (30.0%) | 0.56 |
| Antidepressant + quetiapine § | 12 (35.3%) | 9 (30.0%) | 0.65 |
| Antibiotics (at admission) § | 20 (58.8%) | 11 (36.7%) | 0.08 |
| Variable | Stroke Population T0 (N = 64) |
|---|---|
| Clinical | |
| Aetiology (% patients) | |
| Ischemic | 82.8 |
| Haemorrhagic | 17.2 |
| Ischemic stroke location (% patients) | |
| LACI | 15.1 |
| PACI | 30.2 |
| POCI | 20.8 |
| TACI | 34.0 |
| Route of feeding (% patients) | |
| Oral | 79.7 |
| PEG | 20.3 |
| Biohumoral variables | |
| Erythrosedimentation rate (NV: <15 mm/1st h) | 49.40 ± 27.21 |
| Haemoglobin (NV: M 13.2–17.3 g/dL; F 11.7–15.5 g/dL) | 12.31 ± 1.82 |
| Blood urea (NV: 16–40 mg/dL) | 47.63 ± 25.92 |
| Creatinine (NV: M 0.73–1.18 mg/dL; F 0.55–1.02 mg/dL) | 1.00 ± 0.39 |
| Glucose (NV: 70–115 mg/dL) | 104.4 ± 28.1 |
| Total white blood cells (TWBC, NV: 4000–10,000/µL) | 7949 ± 2065 |
| Neutrophils % TWBC | 64.85 ± 10.81 |
| Neutrophil count (NV: 2000–8000/µL) | 6102 ± 1727 |
| Lymphocytes % TWBC | 22.59 ± 9.45 |
| Lymphocyte count (NV: 1500–4000/µL) | 1677 ± 468 |
| Monocytes % TWBC | 8.93 ± 2.08 |
| Monocyte count (NV: 100–1000/µL) | 735.1 ± 238.5 |
| Neutrophil/Lymphocyte ratio (NV: 1.5–3) | 3.50 ± 1.67 |
| Albumin (NV: 3.5–4.76 g/dL) | 3.08 ± 0.51 |
| Prealbumin (NV: 18–32 mg/dL) | 17.67 ± 5.33 |
| Fibrinogen (NV: 230–550 mg/dL) | 455.7 ± 114.8 |
| Transferrin (NV: 202–364 mg/dL) | 200.4 ± 38.8 |
| C-Reactive Protein (CRP: NV < 0.5 mg/dL) | 2.48 ± 1.99 |
| Albumin/CRP ratio (g/mg) | 2.35 ± 1.72 |
| Total-FIM (NV: 18–126 points) | 38.73 ± 19.90 |
| Motor-FIM (NV: 13–91 points) | 21.97 ± 12.36 |
| Cognitive-FIM (NV: 5–35 points) | 16.73 ± 10.65 |
| Variable | Placebo (N = 34) | EAA (N = 30) | p-Value |
|---|---|---|---|
| Bio humoral | |||
| C-Reactive Protein (CRP: NV < 0.5 mg/dL) | 2.13 ± 1.82 | 2.89 ± 2.12 | 0.13 |
| Albumin (NV: 3.5–4.76 g/dL) | 3.10 ± 0.46 | 3.07 ± 0.57 | 0.82 |
| Prealbumin (NV: 18–32 mg/dL) | 18.3 ± 6.2 | 16.9 ± 3.9 | 0.28 |
| Albumin/CRP ratio (g/mg) | 2.58 ± 1.68 | 2.03 ± 1.72 | 0.20 |
| Body Mass Index (BMI, kg/m2) | 24.0 ± 3.8 | 24.1 ± 4.9 | 0.97 |
| Body weight (kg) | 65.8 ± 12.0 | 66.5 ± 17.4 | 0.84 |
| Haemoglobin (NV: M 13.2–17.3 g/dL; F 11.7–15.5 g/dL) | 12.3 ± 1.6 | 12.4 ± 2.0 | 0.83 |
| Blood urea (NV: 16–40 mg/dL) | 51.2 ± 23.9 | 43.2 ± 27.4 | 0.22 |
| Creatinine (NV: M 0.73–1.18 mg/dL; F 0.55–1.02 mg/dL) | 1.06 ± 0.46 | 0.93 ± 0.29 | 0.19 |
| Glucose (NV: 70–115 mg/dL) | 101.5 ± 17.3 | 115.1 ± 44.1 | 0.10 |
| Erythrosedimentation rate (NV: <15 mm/1st h) | 49.5 ± 25.2 | 52.3 ± 32.2 | 0.72 |
| Total white blood cells (TWBC, NV: 4000–10,000/µL) | 7900 ± 2306 | 8004 ± 1792 | 0.84 |
| Neutrophils % TWBC | 66.0 ± 9.9 | 63.5 ± 11.7 | 0.35 |
| Neutrophil count (NV: 2000–8000/µL) | 6529 ± 1771 | 5675 ± 1643 | 0.23 |
| Lymphocytes % TWBC | 21.6 ± 9.3 | 23.8 ± 9.6 | 0.36 |
| Lymphocyte count (NV: 1500–4000/µL) | 1652 ± 501 | 1701 ± 453 | 0.80 |
| Monocytes % TWBC | 8.48 ± 2.04 | 9.44 ± 2.04 | 0.065 |
| Monocyte count (NV: 100–1000/µL) | 836 ± 295 | 657 ± 161 | 0.14 |
| Fibrinogen (NV: 230–550 mg/dL) | 445 ± 111 | 476 ± 124 | 0.31 |
| Total-FIM (NV: 18–126 points) | 42.3 ± 20.5 | 34.7 ± 18.7 | 0.13 |
| Motor-FIM (NV: 13–91 points) | 24.0 ± 13.9 | 19.7 ± 10.2 | 0.17 |
| Cognitive-FIM (NV: 5–35 points) | 18.2 ± 10.3 | 15.0 ± 11.0 | 0.23 |
| Variable | Δ Placebo (N = 34) | Δ EAA (N = 30) | p-Value |
|---|---|---|---|
| Bio humoral | |||
| Albumin (NV: 3.5–4.76 g/dL) | 0.17 ± 0.44 | 0.36 ± 0.38 | 0.033 |
| C-Reactive Protein (CRP: NV < 0.5 mg/dL) | −0.86 ± 2.25 | −2.04 ± 2.15 | 0.036 |
| Albumin/CRP ratio (g/mg) | 7.21 ± 9.59 | 10.28 ± 12.44 | 0.27 |
| Body Mass Index (BMI, kg/m2) | −0.36 ± 1.15 | −0.27 ± 1.14 | 0.75 |
| Body weight (kg) | −1.07 ± 3.33 | −0.87 ± 3.14 | 0.81 |
| Haemoglobin (NV: M 13.2–17.3 g/dL; F 11.7–15.5 g/dL) | −0.63 ± 1.66 | 0.05 ± 1.32 | 0.08 |
| Blood urea (NV: 16–40 mg/dL) | −7.9 ± 20.1 | −2.8 ± 28.5 | 0.41 |
| Creatinine (NV: M 0.73–1.18 mg/dL; F 0.55–1.02 mg/dL) | −0.00 ± 0.26 | −0.01 ± 0.19 | 0.92 |
| Glucose (NV: 70–115 mg/dL) | −6.3 ± 12.8 | −15.5 ± 28.2 | 0.12 |
| Erythrosedimentation rate (NV: <15 mm/1st h) | −8.5 ± 25.2 | −14.2 ± 20.2 | 0.44 |
| Total white blood cells (TWBC, NV: 4000–10,000/µL) | −1676 ± 1852 | −1256 ± 2562 | 0.45 |
| Neutrophils % TWBC | −7.60 ± 7.84 | −6.62 ± 10.86 | 0.68 |
| Neutrophil count (NV: 2000–8000/µL) | −2061 ± 1864 | −1085 ± 2864 | 0.33 |
| Lymphocytes % TWBC | 7.13 ± 7.63 | 7.08 ± 9.43 | 0.98 |
| Lymphocyte count (NV: 1500–4000/µL) | 305 ± 314 | 177 ± 189 | 0.24 |
| Monocytes % TWBC | −0.04 ± 2.09 | −0.11 ± 2.36 | 0.90 |
| Monocyte count (NV: 100–1000/µL) | −159 ± 321 | 33 ± 369 | 0.29 |
| Prealbumin (NV: 18–32 mg/dL) | 0.61 ± 4.19 | 3.08 ± 5.65 | 0.05 |
| Fibrinogen (NV: 230–550 mg/dL) | −58.8 ± 93.8 | −62.8 ± 122.3 | 0.90 |
| Total-FIM (NV: 18–126 points) | 26.4 ± 19.1 | 23.0 ± 17.3 | 0.47 |
| Motor-FIM (NV: 13–91 points) | 21.6 ± 16.9 | 18.8 ± 16.2 | 0.51 |
| Cognitive-FIM (NV: 5–35 points) | 4.88 ± 5.93 | 4.23 ± 6.34 | 0.67 |
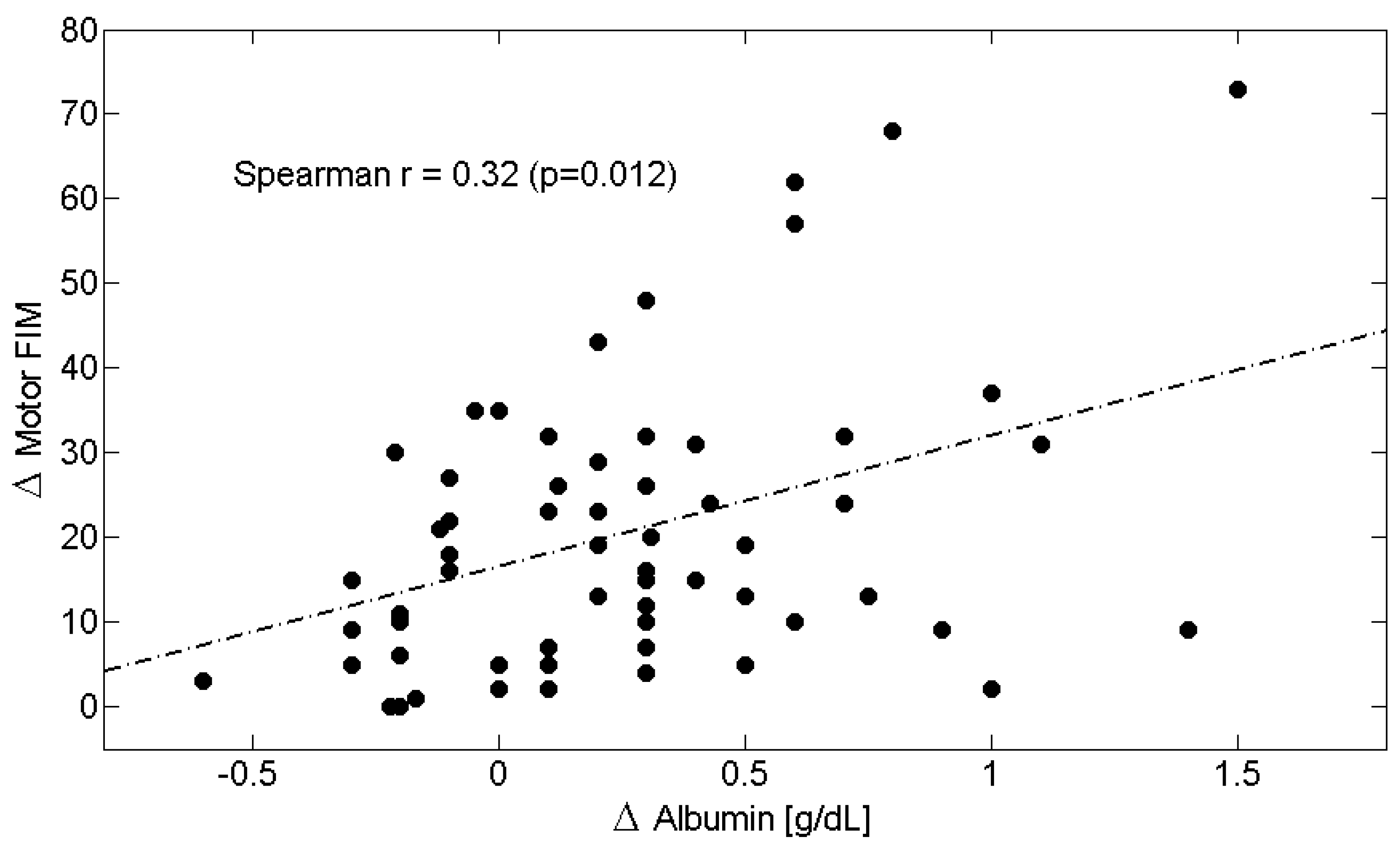
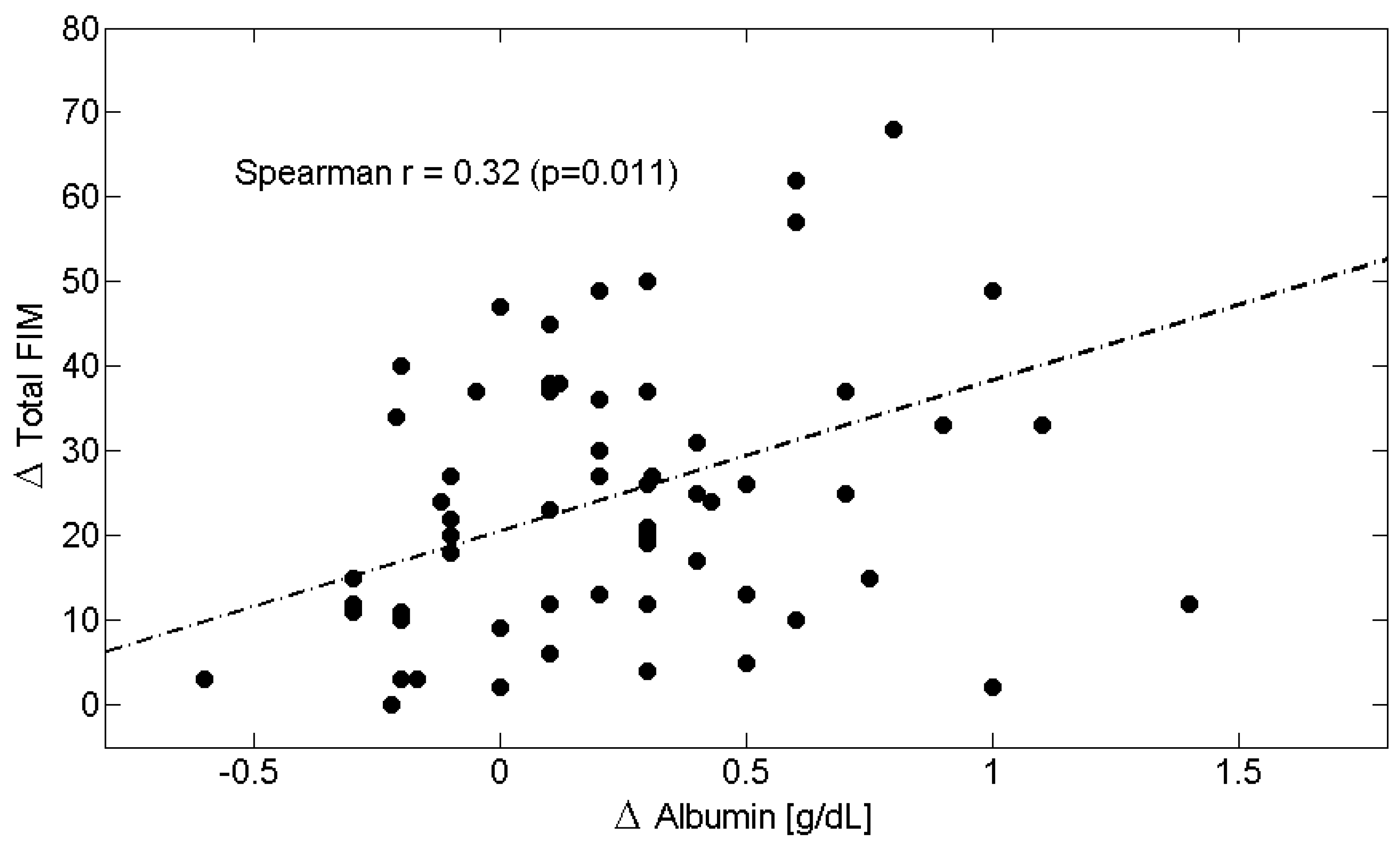
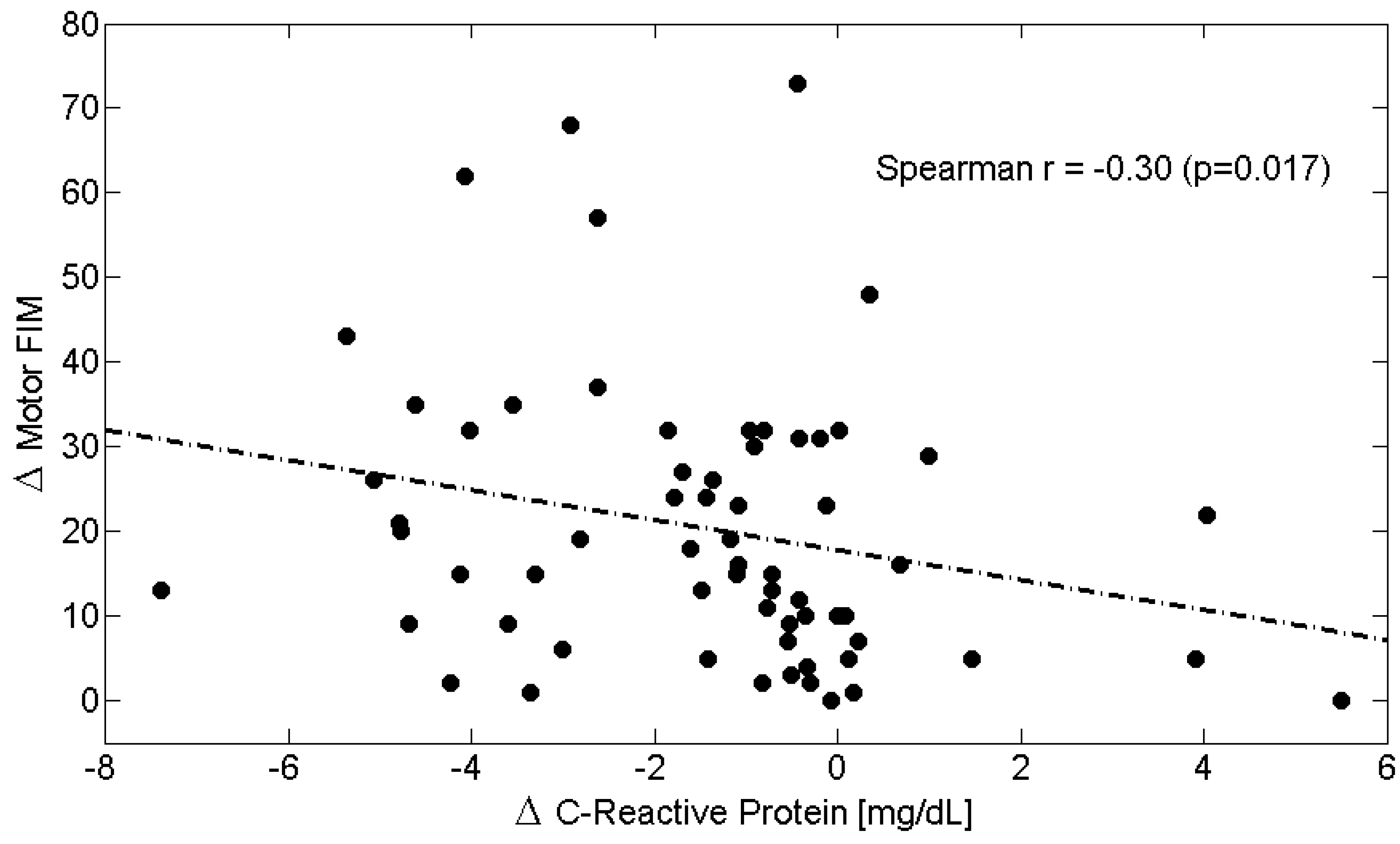
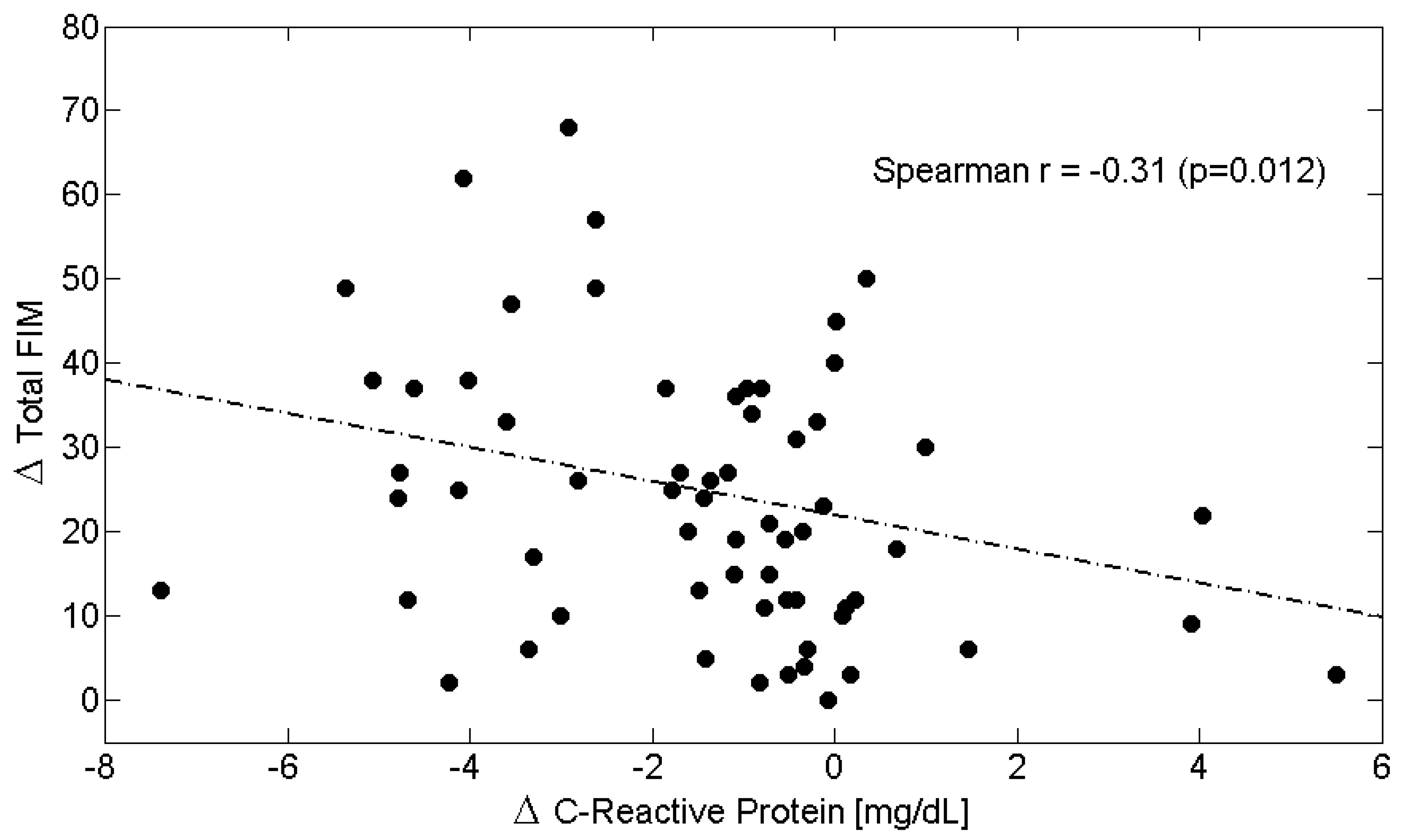
| Δs | C-Reactive Protein (CRP) | Albumin | Albumin/CRP Ratio | Total-FIM | Motor-FIM | Cognitive-FIM |
|---|---|---|---|---|---|---|
| C-Reactive Protein (CRP) | 1.00 | −0.32 † | −0.55 ‡ | −0.31 ^ | −0.30 ^ | −0.11 |
| Albumin | −0.32 † | 1.00 | 0.32 ^ | 0.32 ^ | 0.32 ^ | 0.03 |
| Albumin/CRP ratio | −0.55 ‡ | 0.32 ^ | 1.00 | 0.28 ^ | 0.32 ^ | −0.01 |
| Total-FIM | −0.31 ^ | 0.32 ^ | 0.28 ^ | 1.00 | 0.93 ‡ | 0.39 † |
| Motor-FIM | −0.30 ^ | 0.32 ^ | 0.32 ^ | 0.93 ‡ | 1.00 | 0.13 |
| Cognitive-FIM | −0.11 | 0.03 | −0.01 | 0.39 † | 0.13 | 1.00 |
| Variable | Placebo (N = 34) | EAA (N = 30) | p-Value |
|---|---|---|---|
| Bio humoral | |||
| Albumin (NV: 3.5–4.76 g/dL) | 3.26 ± 0.42 | 3.42 ± 0.47 | 0.18 |
| C-Reactive Protein (CRP: NV < 0.5 mg/dL) | 1.26 ± 2.40 | 0.85 ± 1.05 | 0.38 |
| Albumin/CRP ratio (g/mg) | 9.8 ± 9.8 | 12.3 ± 12.4 | 0.36 |
| Body Mass Index (BMI, kg/m2) | 23.6 ± 4.0 | 23.8 ± 4.6 | 0.90 |
| Body weight (kg) | 64.7 ± 12.0 | 65.6 ± 16.2 | 0.79 |
| Haemoglobin (NV: M 13.2–17.3 g/dL; F 11.7–15.5 g/dL) | 11.6 ± 1.1 | 12.4 ± 1.8 | 0.041 |
| Blood urea (NV: 16–40 mg/dL) | 43.3 ± 20.9 | 40.6 ± 19.8 | 0.60 |
| Creatinine (NV: M 0.73–1.18 mg/dL; F 0.55–1.02 mg/dL) | 1.06 ± 0.38 | 0.92 ± 0.25 | 0.10 |
| Glucose (NV: 70–115 mg/dL) | 92.7 ± 13.5 | 95.4 ± 20.8 | 0.55 |
| Erythrosedimentation rate (NV: <15 mm/1st h) | 40.5 ± 23.9 | 33.0 ± 23.9 | 0.29 |
| Total white blood cells (TWBC, NV: 4000–10,000/µL) | 6223 ± 2062 | 6748 ± 2461 | 0.36 |
| Neutrophils % TWBC | 58.4 ± 9.0 | 56.9 ± 10.4 | 0.52 |
| Neutrophil count (NV: 2000–8000/µL) | 4469 ± 2249 | 4590 ± 2628 | 0.90 |
| Lymphocytes % TWBC | 28.7 ± 9.2 | 30.8 ± 9.9 | 0.37 |
| Lymphocyte count (NV: 1500–4000/µL) | 1957 ± 630 | 1878 ± 602 | 0.76 |
| Monocytes % TWBC | 8.44 ± 2.09 | 9.32 ± 1.86 | 0.08 |
| Monocyte count (NV: 100–1000/µL) | 677 ± 374 | 690 ± 507 | 0.96 |
| Prealbumin (NV: 18–32 mg/dL) | 19.1 ± 5.4 | 19.8 ± 5.6 | 0.58 |
| Fibrinogen (NV: 230–550 mg/dL) | 380 ± 59 | 408 ± 103 | 0.23 |
| Total-FIM (NV: 18–126 points) | 68.6 ± 27.7 | 57.8 ± 28.6 | 0.13 |
| Motor-FIM (NV: 13–91 points) | 45.5 ± 21.3 | 38.5 ± 20.0 | 0.18 |
| Cognitive-FIM (NV: 5–35 points) | 23.1 ± 9.4 | 19.3 ± 14.6 | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boselli, M.; Aquilani, R.; Maestri, R.; Iadarola, P.; Magistroni, A.; Ferretti, C.; Pierobon, A.; Cotta Ramusino, M.; Costa, A.; Buonocore, D.; et al. Essential Amino Acid Supplementation May Attenuate Systemic Inflammation and Improve Hypoalbuminemia in Subacute Hemiplegic Stroke Patients. Metabolites 2025, 15, 626. https://doi.org/10.3390/metabo15090626
Boselli M, Aquilani R, Maestri R, Iadarola P, Magistroni A, Ferretti C, Pierobon A, Cotta Ramusino M, Costa A, Buonocore D, et al. Essential Amino Acid Supplementation May Attenuate Systemic Inflammation and Improve Hypoalbuminemia in Subacute Hemiplegic Stroke Patients. Metabolites. 2025; 15(9):626. https://doi.org/10.3390/metabo15090626
Chicago/Turabian StyleBoselli, Mirella, Roberto Aquilani, Roberto Maestri, Paolo Iadarola, Alessandro Magistroni, Chiara Ferretti, Antonia Pierobon, Matteo Cotta Ramusino, Alfredo Costa, Daniela Buonocore, and et al. 2025. "Essential Amino Acid Supplementation May Attenuate Systemic Inflammation and Improve Hypoalbuminemia in Subacute Hemiplegic Stroke Patients" Metabolites 15, no. 9: 626. https://doi.org/10.3390/metabo15090626
APA StyleBoselli, M., Aquilani, R., Maestri, R., Iadarola, P., Magistroni, A., Ferretti, C., Pierobon, A., Cotta Ramusino, M., Costa, A., Buonocore, D., Peviani, M., Boschi, F., & Verri, M. (2025). Essential Amino Acid Supplementation May Attenuate Systemic Inflammation and Improve Hypoalbuminemia in Subacute Hemiplegic Stroke Patients. Metabolites, 15(9), 626. https://doi.org/10.3390/metabo15090626








