Yigong San Extract Modulates Metabolism, Antioxidant Status, and Immune Function to Improve Health in Diarrheic Calves
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Chemical Composition Analysis of YGS
2.2. Animals
2.3. Drug Administration
2.4. Therapeutic Efficacy Observation
2.5. Analysis of Serum Biochemical Indices
2.6. Oxidative Stress Indicator Detection
2.7. Cytokine Detection
2.8. Preparation of Serum Samples for Metabolomic Analysis
2.9. Metabolomics Analysis of Serum Samples
2.10. Data Processing and Statistical Analysis
3. Results
3.1. Identification of the Main Chemical Components in YGS by UPLC-MS/MS
3.2. The Therapeutic Effects of YGS on Diarrheal Calves
3.3. The Effects of YGS on the Biochemical Blood Indexes
3.4. The Effects of YGS on the Oxidative Stress Indicators
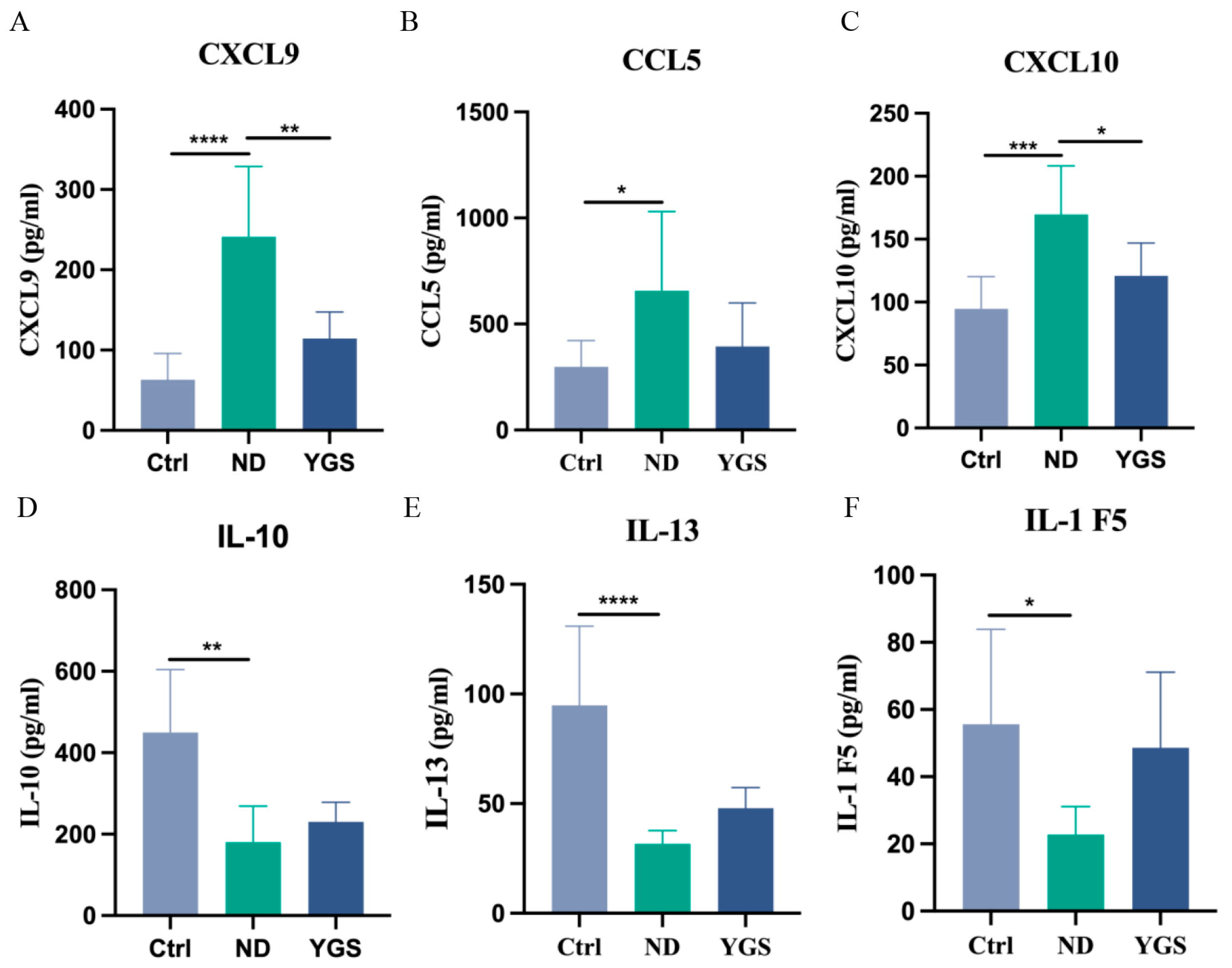
3.5. The Effects of YGS on the Cytokine Levels
3.6. The Effects of YGS on Metabolomics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| ALP | alkaline phosphatase |
| ALT | alanine aminotransferase |
| AMY | amylase |
| AST | aspartate aminotransferase |
| Ca | calcium |
| CAT | catalase |
| CHOL | total cholesterol |
| CK | creatine kinase |
| Cl | chloride |
| ELISA | enzyme-linked immunosorbent assays |
| Fe | iron |
| GLB | globulin |
| GSH-PX | glutathione peroxidase |
| IBD | inflammatory bowel disease |
| K | potassium |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LDH | lactate dehydrogenase |
| MDA | malondialdehyde |
| Mg | magnesium |
| NAHMS | National Animal Health Monitoring System |
| OPLS-DA | orthogonal partial least squares-discriminant analysis |
| P | phosphorus |
| PCA | principal component analysis |
| PLS-DA | partial least squares-discriminant analysis |
| QC | quality control |
| SD | standard deviation |
| SOD | superoxide dismutase |
| TBA | total bile acids |
| TCM | Traditional Chinese medicines |
| TP | total protein |
| UA | uric acid |
| VIP | Variable Importance in Projection |
| YGS | Yigong San |
References
- Kim, H.S.; Whon, T.W.; Sung, H.; Jeong, Y.S.; Jung, E.S.; Shin, N.R.; Hyun, D.W.; Kim, P.S.; Lee, J.Y.; Lee, C.H. Longitudinal evaluation of fecal microbiota transplantation for ameliorating calf diarrhea and improving growth performance. Nat. Commun. 2021, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Abuelo, A.; Cullens, F.; Brester, J.L. Effect of preweaning disease on the reproductive performance and first-lactation milk production of heifers in a large dairy herd. J. Dairy Sci. 2021, 104, 7008–7017. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Córdoba, C.; Branco-Lopes, R.; Latorre-Segura, L.; de Barros-Abreu, M.; Fausak, E.D.; Silva-Del-Río, N. Use of antimicrobials in the treatment of calf diarrhea: A systematic review. Anim. Health Res. Rev. 2022, 23, 101–112. [Google Scholar] [CrossRef]
- Yong-Il, C.; Kyoung-Jin, Y. An overview of calf diarrhea-infectious etiology, diagnosis, and intervention. J. Vet. Sci. 2014, 15, 1. [Google Scholar]
- Jia, J.; Chen, J.; Wang, G.; Li, M.; Zheng, Q.; Li, D. Progress of research into the pharmacological effect and clinical application of the traditional Chinese medicine Rehmanniae Radix. Biomed. Pharmacother. 2023, 168, 115809. [Google Scholar] [CrossRef]
- Wang, M.; Huang, H.; Hu, Y.; Liu, Y.; Zeng, X.; Zhuang, Y.; Yang, H.; Wang, L.; Chen, S.; Yin, L.; et al. Effects of dietary supplementation with herbal extract mixture on growth performance, organ weight and intestinal morphology in weaning piglets. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1462–1470. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, Y.; Yang, W.; Huang, C.; Ou, Z.; He, J.; Yang, M.; Wu, J.; Yao, H.; Yang, Y.; et al. Viola yedoensis Makino alleviates lipopolysaccharide-induced intestinal oxidative stress and inflammatory response by regulating the gut microbiota and NF-κB-NLRP3/ Nrf2-MAPK signaling pathway in broiler. Ecotoxicol. Environ. Saf. 2024, 282, 116692. [Google Scholar] [CrossRef]
- Bonelli, F.; Turini, L.; Sarri, G.; Serra, A.; Mele, M. Oral administration of chestnut tannins to reduce the duration of neonatal calf diarrhea. BMC Vet. Res. 2018, 14, 227. [Google Scholar] [CrossRef]
- Wang, S.; Cui, D.; Lv, Y.; Yan, Z.; Zhang, J. Cangpu Oral Liquid as a Possible Alternative to Antibiotics for the Control of Undifferentiated Calf Diarrhea. Front. Vet. Sci. 2022, 9, 879857. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Liu, K.; Wang, Y.; Song, M.; Lian, H.; Gao, T.; Zhang, L.; Fu, T. Supplementation of the probiotic Lactobacillus rhamnosus GG to pre-weaning calves decreases diarrhea incidence by modulating gut bacteria and associated metabolites. Anim. Nutr. 2025, 21, 234–244. [Google Scholar] [CrossRef]
- Van Treuren, W.; Dodd, D. Microbial Contribution to the Human Metabolome: Implications for Health and Disease. Annu. Rev. Pathol. 2020, 15, 345–369. [Google Scholar] [CrossRef]
- Cao, P.P.; Hu, C.L.; Li, M.J.; An, Y.H.; Feng, X.; Ma, X.H.; Wang, D.Z.; Song, Z.H.; Ji, G.S.; Yang, D.; et al. 16S rRNA and metabolomics reveal the key microbes and key metabolites that regulate diarrhea in Holstein male calves. Front. Microbiol. 2024, 15, 1521719. [Google Scholar] [CrossRef]
- Kim, S.J.; Shin, H.J.; Lee, B.J.; Kim, D.S.; Lee, J.H.; Jeong, M.Y.; Kim, H.L.; Park, J.; Lim, H.; Kim, S.H.; et al. The antiinflammatory mechanism of Igongsan in mouse peritoneal macrophages via suppression of NF-κB/Caspase-1 activation. Phytother. Res. 2014, 28, 736. [Google Scholar] [CrossRef]
- Zheng, Q.; Guan, Y.; Xia, L.; Wang, Z.; Jiang, Y.; Zhang, X.; Wang, J.; Wang, G.; Pu, Y.; Xia, J.; et al. Effect of Yi Gong San Decoction on Iron Homeostasis in a Mouse Model of Acute Inflammation. Evid.-Based Complement. Altern. Med. 2016, 2016, 2696480. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; Luo, J.; Chen, T.; Xi, Q.; Sun, J.; Wei, L.; Zhang, Y. Synergism of fermented feed and ginseng polysaccharide on growth performance, intestinal development, and immunity of Xuefeng black-bone chickens. BMC Vet. Res. 2024, 20, 13. [Google Scholar] [CrossRef]
- Zhu, J.; Lian, J.; Deng, H.; Luo, J.; Chen, T.; Sun, J.; Zhang, Y.; Yang, Y.; Liu, P.; Xi, Q. Effects of Spinach Extract and Licorice Extract on Growth Performance, Antioxidant Capacity, and Gut Microbiota in Weaned Piglets. Animals 2024, 14, 321. [Google Scholar] [CrossRef]
- Lv, J.; Fu, Y.; Ga, Y.; Han, C.; Fan, Y.; Wei, Y.; Hao, S.; Hao, Z. Lianweng Granules Alleviate Intestinal Barrier Damage via the IL-6/STAT3/PI3K/AKT Signaling Pathway with Dampness-Heat Syndrome Diarrhea. Antioxidants 2024, 13, 661. [Google Scholar] [CrossRef]
- Shen, L.; Shen, Y.; You, L.; Zhang, Y.; Su, Z.; Peng, G.; Deng, J.L.; Zhong, Z.; Yu, S.; Zong, X.; et al. Blood metabolomics reveals the therapeutic effect of Pueraria polysaccharide on calf diarrhea. BMC Vet. Res. 2023, 19, 98. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.L.; Yang, Y.; Ma, L.; Malmuthuge, N.; Guan, L.L.; Bu, D.P. Dynamics of oxidative stress and immune responses in neonatal calves during diarrhea. J. Dairy Sci. 2024, 107, 1286–1298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, M.; Zhang, M.; Zhang, H.; Huang, Q.; Jian, F.; Yin, F.; Zhang, L.; Du, Z.; Yang, R.; et al. Effects of Sodium Butyrate and Ampelopsis grossedentata Flavonoidson Production Performance, Diarrhea and Serum Indicators in Australian-Canadian F1 Holstein Weaned Calves. Chin. Anim. Husb. Vet. Med. 2025, 52, 3651–3660. [Google Scholar]
- Zhang, Y.T.; Yang, Y.; Bu, D.P.; Ma, L. The effects of gamma-aminobutyric acid on growth performance, diarrhoea, ruminal fermentation, and antioxidant capacity in pre-weaned calves. Animal 2025, 19, 101493. [Google Scholar] [CrossRef] [PubMed]
- Sammari, H.; Jedidi, S.; Selmi, H.; Rtibi, K.; Jabri, M.A.; Jridi, M.; Zouari, N.; Toumi, L.; Sebai, H. Protective effects of Crataegus azarolus L. berries aqueous extract against castor oil–induced diarrhea, oxidative stress, and inflammation in rat. Neurogastroenterol. Motil. 2020, 33, e14065. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wang, X.; Xiong, H.; Deng, Z.; Peng, X.; Xiao, L.; Jiang, L.; Sun, Y. Bioactives and their metabolites from Tetrastigma hemsleyanum leaves ameliorate DSS-induced colitis via protecting the intestinal barrier, mitigating oxidative stress and regulating the gut microbiota. Food Funct. 2021, 12, 11760–11776. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Ding, B.; Liu, Y.; Zhang, Y.; Wang, Y.; Yang, L.; Yang, Y.; Liu, X. Blueberry anthocyanins ameliorate arsenic-induced cognitive impairment in rats: Mitigating mitochondrial damage and dysregulation. Phytomedicine 2025, 145, 157062. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, G.; Zhou, K.; Wen, J.; Zheng, F.; Sun, L.; Ren, X. Structural characterization, antioxidant activity, and the effects of Codonopsis pilosula polysaccharides on the solubility and stability of flavonoids. J. Pharm. Biomed. Anal. 2023, 229, 115368. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, Q.L.; Hua, J.W.; Cheng, W.L.; Qin, L.P. The traditional uses, phytochemistry, and pharmacology of Atractylodes macrocephala Koidz.: A review. J. Ethnopharmacol. 2018, 226, 143–167. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.; Sun, M.; Duan, Y.; Wang, L.; Yu, N.; Peng, D.; Chen, W.; Wang, Y. Preparation, characterization, antioxidant and antianemia activities of Poria cocos polysaccharide iron (III) complex. Heliyon 2023, 9, e12819. [Google Scholar] [CrossRef]
- Mastrodi Salgado, J.; Baroni Ferreira, T.R.; de Oliveira Biazotto, F.; Dos Santos Dias, C.T. Increased antioxidant content in juice enriched with dried extract of pomegranate (Punica granatum) peel. Plant Foods Hum. Nutr. 2012, 67, 39–43. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, S.; Geng, X.; Jiang, H.; Dai, Y.; Wang, P.; Hua, M.; Gao, Q.; Lang, S.; Hou, L.; et al. Antioxidant Effects of Roasted Licorice in a Zebrafish Model and Its Mechanisms. Molecules 2022, 27, 7743. [Google Scholar] [CrossRef]
- Yang, B.; Huang, Z.; He, Z.; Yue, Y.; Zhou, Y.; Ross, R.P.; Stanton, C.; Zhang, H.; Zhao, J.; Chen, W. Protective effect of Bifidobacterium bifidum FSDJN7O5 and Bifidobacterium breve FHNFQ23M3 on diarrhea caused by enterotoxigenic Escherichia coli. Food Funct. 2021, 12, 7271–7282. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, M.; Dabak, M.; Tumer, K.C. The relationship between serum cytokine profile and vitamin D in calves with neonatal diarrhea. Cytokine 2023, 165, 156173. [Google Scholar] [CrossRef]
- Jiang, B.C.; Liu, T.; Gao, Y.J. Chemokines in chronic pain: Cellular and molecular mechanisms and therapeutic potential. Pharmacol. Ther. 2020, 212, 107581. [Google Scholar] [CrossRef]
- Yu, L.; Dong, J.; Wang, Y.; Zhang, P.; Liu, Y.; Zhang, L.; Liang, P.; Wang, L.; Song, C. Porcine epidemic diarrhea virus nsp4 induces pro-inflammatory cytokine and chemokine expression inhibiting viral replication in vitro. Arch. Virol. 2019, 164, 1147–1157. [Google Scholar] [CrossRef]
- Xu, W.; Fang, S.; Wang, Y.; Chi, X.; Ma, X.; Zhang, T.; Hu, S. Receptor and signaling pathway involved in bovine lymphocyte activation by Atractylodis macrocephalae polysaccharides. Carbohydr. Polym. 2020, 234, 115906. [Google Scholar] [CrossRef]
- He, L.; Wang, C.; Simujide, H.; Aricha, H.; Zhang, J.; Liu, B.; Zhang, C.; Cui, Y.; Aorigele, C. Effect of Early Pathogenic Escherichia coli Infection on the Intestinal Barrier and Immune Function in Newborn Calves. Front. Cell. Infect. Microbiol. 2022, 12, 818276. [Google Scholar] [CrossRef]
- Ong, E.S. Urine Metabolites and Bioactive Compounds from Functional Food: Applications of Liquid Chromatography Mass Spectrometry. Crit. Rev. Anal. Chem. 2024, 54, 3196–3211. [Google Scholar] [CrossRef]
- Ferreira, N.S.; Tostes, R.C.; Paradis, P.; Schiffrin, E.L. Aldosterone, Inflammation, Immune System, and Hypertension. Am. J. Hypertens. 2021, 34, 15–27. [Google Scholar] [CrossRef]
- Wang, T.; Wu, H.; Shi, X.; Dai, M.; Liu, Y. Aminoadipic acid aggravates atherosclerotic vascular inflammation through ROS/TXNIP/NLRP3 pathway, a harmful microbial metabolite reduced by paeonol. Int. J. Biochem. Cell Biol. 2024, 177, 106678. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Y.; Zhao, Z.; Chu, Y.; Yang, W. The role of amino acid metabolism in inflammatory bowel disease and other inflammatory diseases. Front. Immunol. 2023, 14, 1284133. [Google Scholar] [CrossRef]
- Song, G.; Gan, Q.; Qi, W.; Wang, Y.; Xu, M.; Li, Y. Fructose Stimulated Colonic Arginine and Proline Metabolism Dysbiosis, Altered Microbiota and Aggravated Intestinal Barrier Dysfunction in DSS-Induced Colitis Rats. Nutrients 2023, 15, 782. [Google Scholar] [CrossRef]
- Ding, Y.; Koda, Y.; Shashni, B.; Takeda, N.; Zhang, X.; Tanaka, N.; Nishikawa, Y.; Nagasaki, Y. An orally deliverable ornithine-based self-assembling polymer nanomedicine ameliorates hyperammonemia in acetaminophen-induced acute liver injury. Acta Biomater. 2023, 168, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yuan, H.; Xu, E.; Liu, J. Toxicology of paraquat and pharmacology of the protective effect of 5-hydroxy-1-methylhydantoin on lung injury caused by paraquat based on metabolomics. Sci. Rep. 2020, 10, 1790. [Google Scholar] [CrossRef] [PubMed]
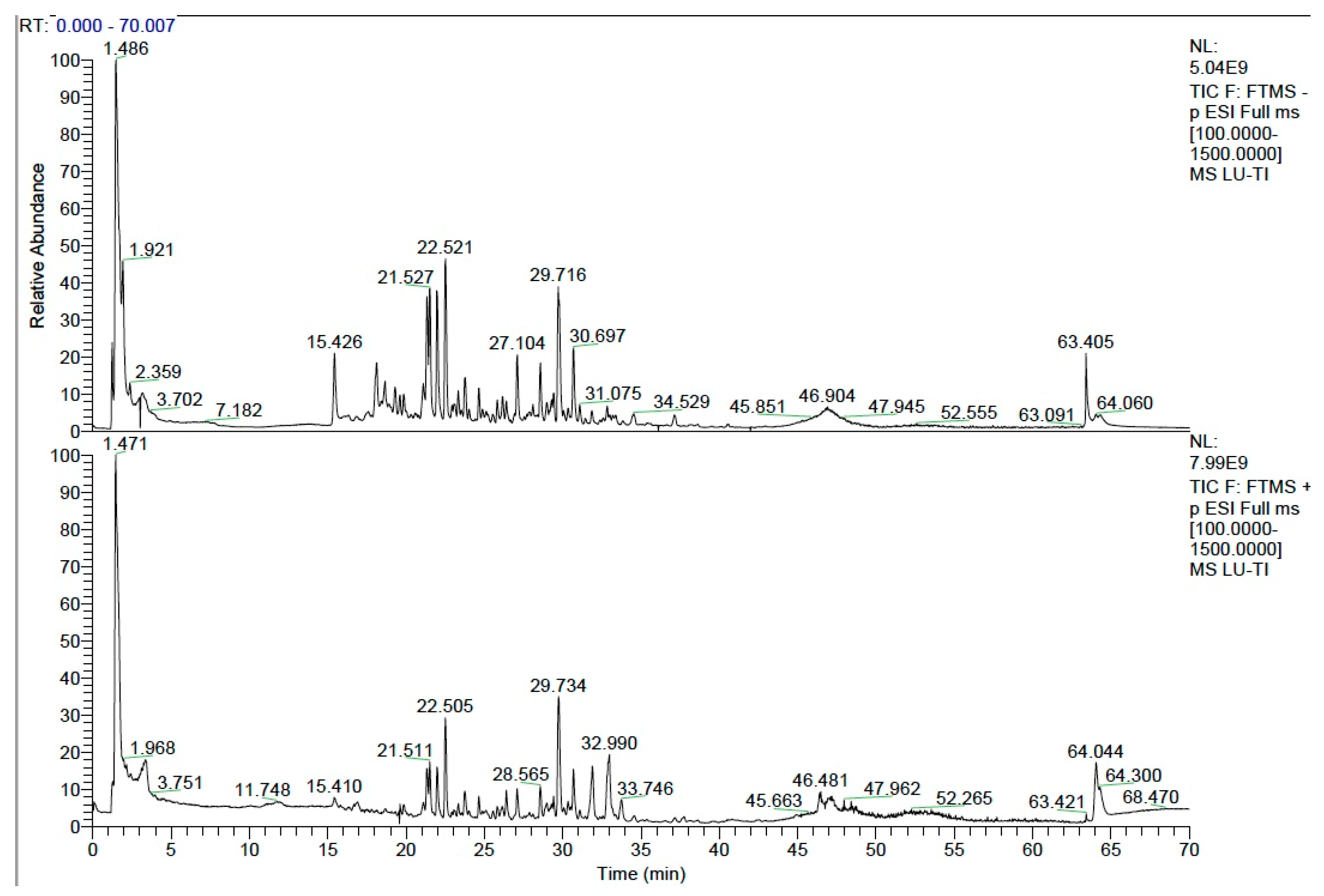





| Chinese Name | Latin Name | Batch Number | Ratio |
|---|---|---|---|
| Dang Shen | Codonopsis pilosula (Franch.) Nannf. | 220925002 | 1 |
| Bai Zhu | Atractylodes macrocephala Koidz. | 220911004 | 1 |
| Fu Ling | Poria cocos (Schw.) Wolf | 221221002 | 1 |
| Chen Pi | Citrus reticulata Blanco | 230615001 | 1 |
| Gan Cao | Glycyrrhiza uralensis Fisch. ex DC. | 230627001 | 1 |
| Items | Treatment | Group | p-Value | ||
|---|---|---|---|---|---|
| Ctrl | ND | YGS | |||
| Fecal scores | |||||
| D0 | 0.10 ± 0.32 b | 3.70 ± 0.48 a | 3.50 ± 0.53 a | <0.001 | |
| D1 | 0.00 ± 0.00 b | 3.60 ± 0.52 a | 3.50 ± 0.53 a | <0.001 | |
| D2 | 0.10 ± 0.32 b | 3.60 ± 0.52 a | 3.20 ± 0.42 a | <0.001 | |
| D3 | 0.00 ± 0.00 b | 3.00 ± 0.00 a | 2.70 ± 0.48 a | <0.001 | |
| D4 | 0.20 ± 0.42 b | 3.00 ± 0.82 a | 2.20 ± 0.63 a | <0.001 | |
| D5 | 0.00 ± 0.00 b | 2.60 ± 0.52 a | 1.50 ± 0.53 a | <0.001 | |
| D6 | 0.10 ± 0.32 b | 2.50 ± 0.53 a | 0.20 ± 0.42 b | <0.001 | |
| D7 | 0.10 ± 0.32 b | 2.60 ± 0.52 a | 0.30 ± 0.48 b | <0.001 | |
| Dehydration scores | |||||
| D0 | 0.00 ± 0.00 b | 3.60 ± 0.52 a | 3.60 ± 0.52 a | <0.001 | |
| D1 | 0.00 ± 0.00 b | 3.40 ± 0.52 a | 3.00 ± 0.47 a | <0.001 | |
| D2 | 0.00 ± 0.00 b | 3.40 ± 0.52 a | 2.70 ± 0.48 a | <0.001 | |
| D3 | 0.00 ± 0.00 c | 3.50 ± 0.53 a | 2.20 ± 0.63 b | <0.001 | |
| D4 | 0.00 ± 0.00 c | 2.80 ± 0.42 a | 1.60 ± 0.52 b | <0.001 | |
| D5 | 0.00 ± 0.00 b | 2.40 ± 0.52 a | 0.90 ± 0.57 b | <0.001 | |
| D6 | 0.00 ± 0.00 b | 2.60 ± 0.52 a | 0.20 ± 0.42 b | <0.001 | |
| D7 | 0.00 ± 0.00 b | 2.70 ± 0.67 a | 0.00 ± 0.00 b | <0.001 | |
| Mental scores | |||||
| D0 | 0.00 ± 0.00 b | 3.50 ± 0.53 a | 3.60 ± 0.52 a | <0.001 | |
| D1 | 0.30 ± 0.48 b | 3.50 ± 0.53 a | 2.90 ± 0.32 a | <0.001 | |
| D2 | 0.10 ± 0.32 b | 3.70 ± 0.48 a | 2.60 ± 0.52 a | <0.001 | |
| D3 | 0.30 ± 0.48 b | 3.00 ± 0.00 a | 2.00 ± 0.47 a | <0.001 | |
| D4 | 0.10 ± 0.32 b | 3.00 ± 0.00 a | 1.60 ± 0.52 a | <0.001 | |
| D5 | 0.00 ± 0.00 b | 2.40 ± 0.52 a | 0.70 ± 0.48 b | <0.001 | |
| D6 | 0.00 ± 0.00 b | 2.40 ± 0.52 a | 0.20 ± 0.42 b | <0.001 | |
| D7 | 0.10 ± 0.32 b | 2.50 ± 0.53 a | 0.00 ± 0.00 b | <0.001 | |
| Items | Group | p-Value | ||
|---|---|---|---|---|
| Ctrl | ND | YGS | ||
| ALT (U/L) | 11.16 ± 3.17 a | 6.30 ± 0.55 b | 8.49 ± 1.58 a | <0.001 |
| AST (U/L) | 46.34 ± 6.65 a | 50.52 ± 6.46 a | 47.18 ± 5.62 a | 0.341 |
| TP (g/dL) | 5.40 ± 0.56 b | 6.65 ± 0.61 a | 5.21 ± 0.65 b | <0.001 |
| GLB (g/dL) | 2.54 ± 0.50 b | 3.97 ± 0.48 a | 2.34 ± 0.57 b | <0.001 |
| ALP (U/L) | 446.43 ± 187.82 a | 230.13 ± 65.38 b | 512.96 ± 198.97 a | 0.001 |
| LDH (U/L) | 779.71 ± 76.95 a | 744.27 ± 90.07 a | 768.66 ± 64.00 a | 0.381 |
| CK (U/L) | 75.70 ± 19.62 c | 188.13 ± 47.30 a | 127.27 ± 36.79 b | <0.001 |
| TBA (μmol/L) | 19.43 ± 6.39 a | 17.76 ± 9.05 a | 18.78 ± 3.66 a | 0.869 |
| UREA (mg/dL) | 10.44 ± 2.12 b | 19.16 ± 5.29 a | 13.64 ± 1.95 b | <0.001 |
| UA (mg/dL) | 1.06 ± 0.21 a | 1.03 ± 0.19 a | 0.89 ± 0.10 a | 0.129 |
| CHOL (mg/dL) | 83.09 ± 4.31 b | 42.26 ± 12.02 c | 105.29 ± 19.47 a | <0.001 |
| AMY (U/L) | 21.20 ± 6.74 b | 38.06 ± 3.69 a | 24.50 ± 5.01 b | <0.001 |
| P (mmol/L) | 10.86 ± 1.30 a | 10.78 ± 1.09 a | 11.33 ± 1.15 a | 0.573 |
| CL (mmol/L) | 94.93 ± 2.01 a | 97.76 ± 3.81 a | 96.90 ± 1.47 a | 0.085 |
| K (mmol/L) | 6.22 ± 0.80 a | 6.20 ± 0.34 a | 6.39 ± 0.52 a | 0.744 |
| Fe (μg/dL) | 140.98 ± 73.35 a | 157.00 ± 110.06 a | 172.13 ± 100.02 a | 0.862 |
| Ca (mg/dL) | 11.30 ± 0.77 a | 10.01 ± 0.27 b | 10.82 ± 0.31 a | 0.003 |
| Mg (mmol/L) | 1.05 ± 0.10 a | 0.84 ± 0.05 b | 0.97 ± 0.03 b | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Han, C.; Lv, J.; Zhang, X.; Ni, X.; Wang, X.; Wang, J.; Ma, Y.; Hao, Z. Yigong San Extract Modulates Metabolism, Antioxidant Status, and Immune Function to Improve Health in Diarrheic Calves. Metabolites 2025, 15, 618. https://doi.org/10.3390/metabo15090618
Huang S, Han C, Lv J, Zhang X, Ni X, Wang X, Wang J, Ma Y, Hao Z. Yigong San Extract Modulates Metabolism, Antioxidant Status, and Immune Function to Improve Health in Diarrheic Calves. Metabolites. 2025; 15(9):618. https://doi.org/10.3390/metabo15090618
Chicago/Turabian StyleHuang, Sijuan, Chao Han, Jianyu Lv, Xiaosong Zhang, Xuan Ni, Xin Wang, Jianfei Wang, Yunfei Ma, and Zhihui Hao. 2025. "Yigong San Extract Modulates Metabolism, Antioxidant Status, and Immune Function to Improve Health in Diarrheic Calves" Metabolites 15, no. 9: 618. https://doi.org/10.3390/metabo15090618
APA StyleHuang, S., Han, C., Lv, J., Zhang, X., Ni, X., Wang, X., Wang, J., Ma, Y., & Hao, Z. (2025). Yigong San Extract Modulates Metabolism, Antioxidant Status, and Immune Function to Improve Health in Diarrheic Calves. Metabolites, 15(9), 618. https://doi.org/10.3390/metabo15090618






