Ethnomedicinal Applications, Phytochemistry, and Pharmacological Properties of Zanthoxylum caribaeum Lam.: A Comprehensive Review
Abstract
1. Introduction
2. Nomenclature and Taxonomy
3. Traditional Uses of Zanthoxylum caribaeum in Medicine
4. Germination Challenges and Transplanting Strategies for Z. caribaeum
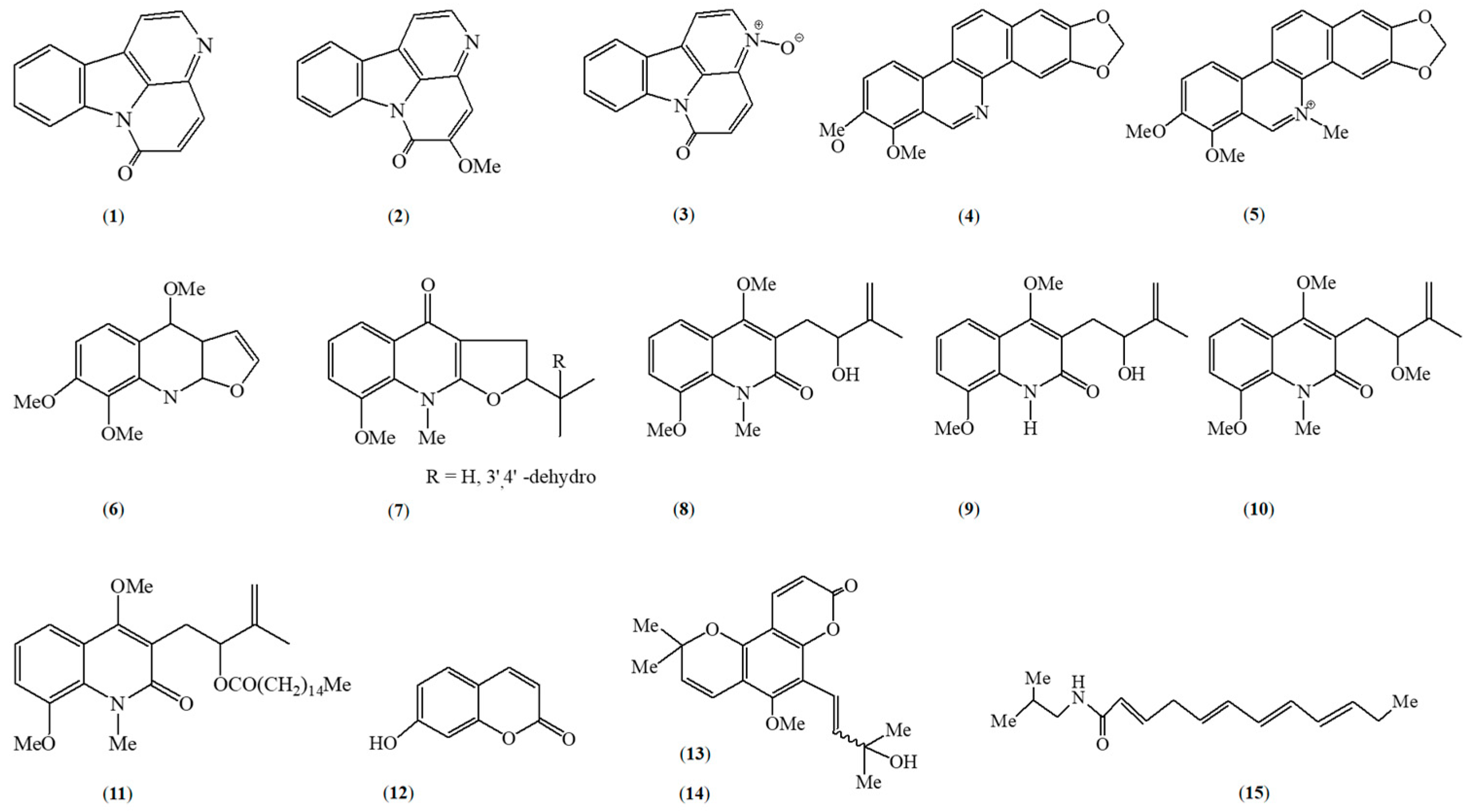
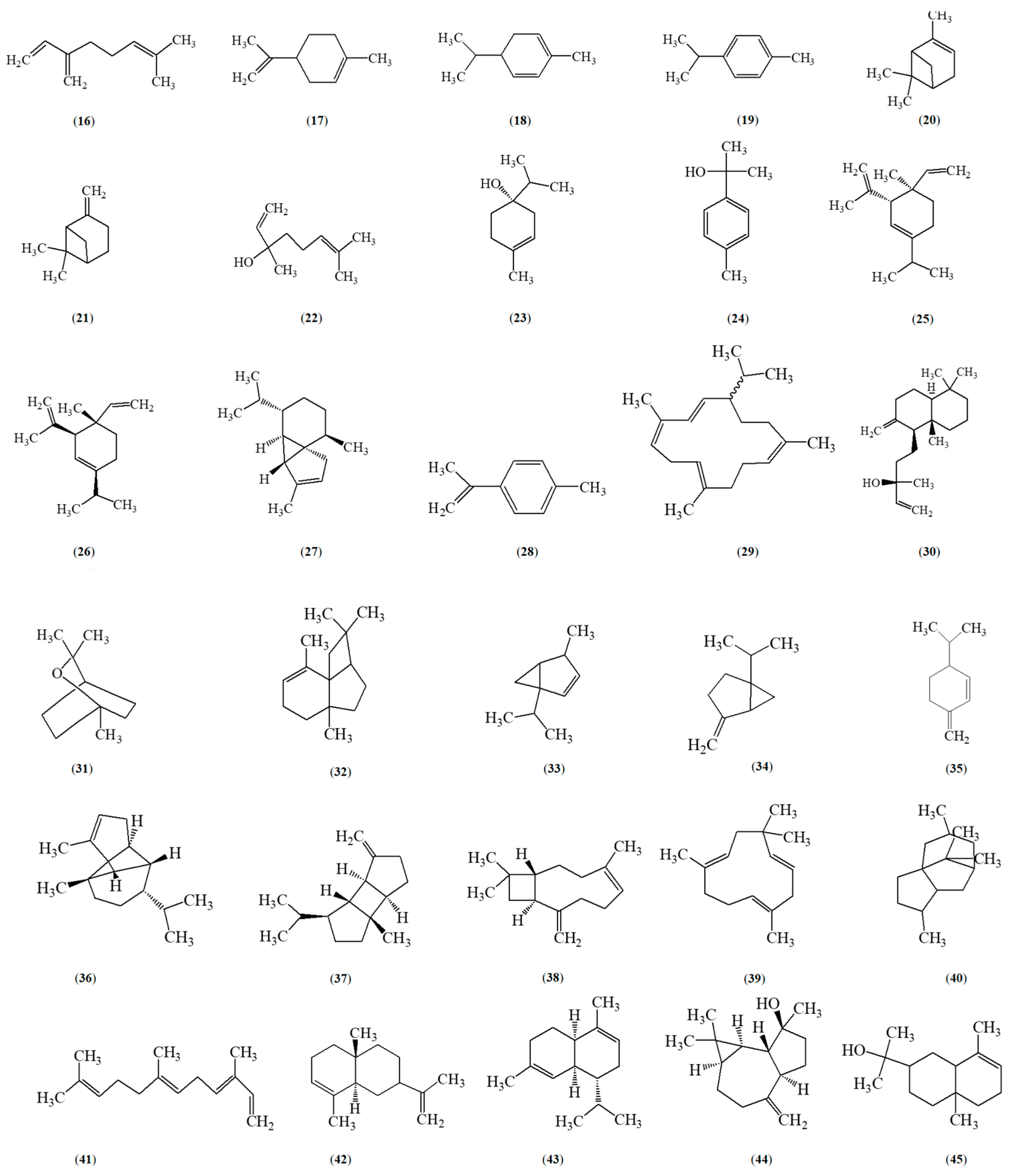
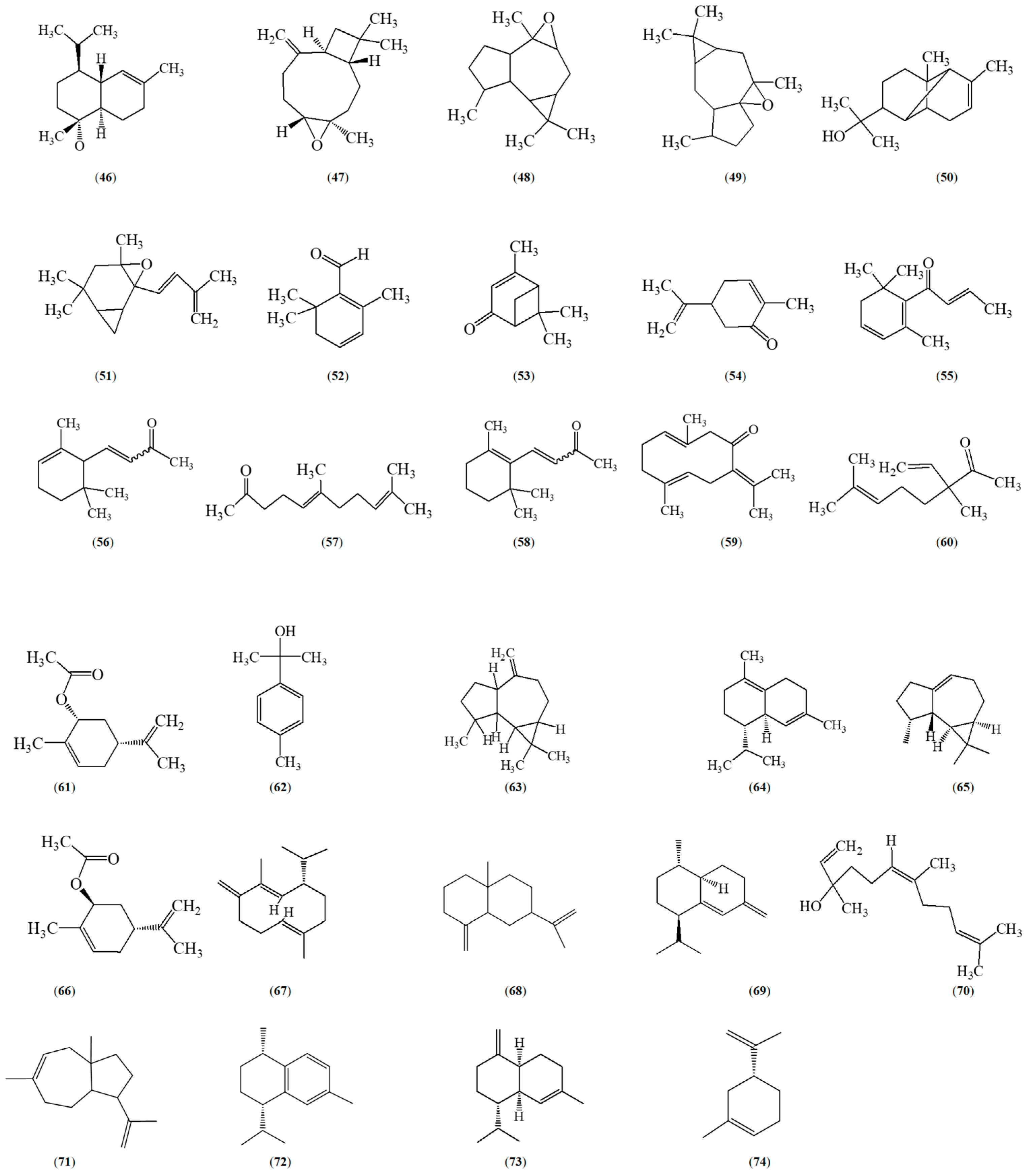
5. Phytochemical Constituents of Z. caribaeum
| Metabolite Family | Extracting Solvent a | ||||||
|---|---|---|---|---|---|---|---|
| Me2CO | AcOEt | EtOH | Hex | MeOH | CH2Cl2 | Aq | |
| Alkaloids | − | − | − | − | − | − | − |
| Anthocyanidins | − | − | − | − | − | − | − |
| Aurones | − | − | − | − | − | − | − |
| Chalcones | − | − | − | − | − | − | − |
| Coumarins | − | − | − | − | − | − | − |
| Steroids | + | + | + | + | + | ++ | − |
| Flavones | + | + | + | + | + | + | − |
| Flavonols | + | + | + | + | + | + | + |
| Saponins | − | − | − | − | − | − | + |
| Condensed tannins | − | − | + | − | ++ | − | + |
| Triterpenoids | + | + | + | + | + | + | + |
| Xanthones | + | + | + | + | + | + | + |
6. Exploring the Bioactive Properties of Z. Caribaeum
| Compounds | Family | Biological Activities | Plant Part | Reference |
|---|---|---|---|---|
| Leishmanicidal activity | Stem bark | [32] | ||
| Canthin-6-one (1) | Alkaloid | Trypanosomalactivity | Stem bark | [28] |
| Antifungal activity | Stem bark | [40] | ||
| Leishmanicidal activity | Stem bark | [32] | ||
| 5-methoxycanthin-6-one (2) | Alkaloid | Trypanosomal activity | Stem bark | [28] |
| Antifungal activity | Stem bark | [40] | ||
| Canthin-6-one-N- oxyde (3) | Alkaloid | Trypanosomal activity | Stem bark | [28] |
| Chelerythrine (5) | Quinolone Alkaloid | Not specified | Root | [21,33] |
| Skimmianine (6) | Quinolone Alkaloid | Not specified | Root | [21,41] |
| Neoacutifolidine (7) | Quinolone Alkaloid | Not specified | Leaves | [31] |
| Acutifoline (8) | Quinolone Alkaloid | Not specified | Leaves | [31] |
| Acutifolidine (9) | Quinolone Alkaloid | Not specified | Leaves | [31] |
| O-methylacutifoline (10) | Quinolone Alkaloid | Not specified | Leaves | [31] |
| Acutifolidine palmitate (11) | Quinolone Alkaloid | Not specified | Leaves | [31] |
| Umbelliforine (12) | Coumarin | Antiproliferative activity | Root bark | [18] |
| Trans-avicennol (14) | Coumarin | Antiproliferative activity | Root bark | [29] |
6.1. Antioxidant Activities of Z. caribaeum
| Extracting Solvent | Test Solution a | % Capture DPPH b | IC50 (μg/mL) c |
| EtOH | 71. 12 | 24.39 | |
| Me2CO | 66.16 | 29.67 | |
| MeOH | 61.99 | 27.98 | |
| ACOEt | 48.56 | 29.67 | |
| BHT (positive control) | 92.80 | 7.93 |
6.2. Leishmanicidal Activity of Z. caribaeum
6.3. Antichagasic Effects of Z. caribaeum Against Trypanosoma cruzi
6.4. Antimicrobial Activities of Z. caribaeum
6.5. Acaricidal Activity of Z. caribaeum
6.6. Antimalarial Activity of Z. caribaeum
| Compounds | P. falciparum Strain IC50 (μg/mL) | |||
|---|---|---|---|---|
| F32 | K1 | PFB | FcB1 | |
| Trans-avicennol (1) | 0.5 | 2.7 | 1.2 | 2.2 |
| Canthin-6-one (2) | 2.0 | 5.3 | 3.2 | 4.0 |
| 5-methoxycanthin-6-one (3) | 10.4 | 5.1 | Nt b | Nt |
| Extracts | ||||
| DCM | 8.9 | 8.9 | Nt | Nt |
| EtOH | 10.5 | 9.3 | Nt | Nt |
| MeOH | 89.5 | >100 | Nt | Nt |
| Chloroquine a | 2.6 | 77.4 | 27.8 | 62.8 |
6.7. Anti-Inflammatory Activity of Z. caribaeum
6.8. Antiproliferative Activity of Z. caribaeum
6.9. Effects Against Cardiovascular Disease
6.10. Larvicidal Activity
7. Toxicological Aspects of Z. caribaeum
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rohwer, J.G.; Kubitzki, K.; Rohwer, J.G.; Bittrich, V. The Families and Genera of Vascular Plants; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Simpson, M.G. Plant Systematics; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-812628-8. [Google Scholar]
- Fournet, J. Flore Illustrée des Phanérogames de Guadeloupe & Martinique, 1st ed.; INRA Édition Gondwana; CIRAD: Paris, France, 2002; Volume 2. [Google Scholar]
- Morton, C.M.; Telmer, C. New Subfamily Classification for the Rutaceae. Ann. Mo. Bot. Gard. 2014, 99, 620–641. [Google Scholar] [CrossRef]
- Reichelt, N.; Wen, J.; Pätzold, C.; Appelhans, M.S. Target Enrichment Improves Phylogenetic Resolution in the Genus Zanthoxylum (Rutaceae) and Indicates Both Incomplete Lineage Sorting and Hybridization Events. Ann. Bot. 2021, 128, 497–510. [Google Scholar] [CrossRef]
- Orel, H.K.; McLay, T.G.B.; Neal, W.C.; Forster, P.I.; Bayly, M.J. Plastid Phylogenomics of the Eriostemon Group (Rutaceae; Zanthoxyloideae): Support for Major Clades and Investigation of a Backbone Polytomy. Aust. Syst. Bot. 2023, 36, 355–385. [Google Scholar] [CrossRef]
- Heywood, V.H. Les Plantes à Fleurs; Nathan: Paris, France, 1996. [Google Scholar]
- Bourlière, F.; Mabberley, D.J. The Plant Book. A Portable Dictionary of the Higher Plants; Revue d’Écologie (La Terre et La Vie); Cambridge University Press: Cambridge, UK, 1987; Volume 3, pp. 198–199. [Google Scholar]
- Waterman, P.G. New Combinations in Zanthoxylum L. (1753). Taxon 1975, 24, 361–366. [Google Scholar] [CrossRef]
- Wen, J.; Xiang, Q.; Guo, J.; Zhang, J.; Yang, N.; Huang, Y.; Chen, Y.; Hu, T.; Rao, C. Pharmacological Activities of Zanthoxylum L. Plants and Its Exploitation and Utilization. Heliyon 2024, 10, e33207. [Google Scholar] [CrossRef]
- Appelhans, M.S.; Reichelt, N.; Groppo, M.; Paetzold, C.; Wen, J. Phylogeny and Biogeography of the Pantropical Genus Zanthoxylum and Its Closest Relatives in the Proto-Rutaceae Group (Rutaceae). Mol. Phylogenet. Evol. 2018, 126, 31–44. [Google Scholar] [CrossRef]
- Engler, A. Rutaceae. In Naturlichen Pflanzenfamilie III; Engelmann: Leipzig, Germany, 1896; Volume 4, p. 95. [Google Scholar]
- Beurton, C. Gynoecium and Perianth in Zanthoxylum Sl (Rutaceae). Plant Syst. Evol. 1994, 189, 165–191. [Google Scholar] [CrossRef]
- Brizicky, G.K. Taxonomic and Nomenclatural Notes on Zanthoxylum and Glycosmis (Rutaceae). J. Arnold Arbor. 1962, 43, 80–93. [Google Scholar] [CrossRef]
- Brizicky, G.K. The Genera of Rutaceae in the Southeastern United States. J. Arnold Arbor. 1962, 43, 1–22. [Google Scholar] [CrossRef]
- Hartley, T.G. A Revision of the Malesian Species of Zanthoxylum (Rutaceae). J. Arnold Arbor. 1966, 47, 171–221. [Google Scholar] [CrossRef]
- Fish, F.; Gray, A.I.; Waterman, P.G. Alkaloids and Coumarins of Zanthoxylum Elephantiasis [Proceedings]. J. Pharm. Pharmacol. 1976, 28, 69P. [Google Scholar] [PubMed]
- Zepernick, B.; Timler, F.K. Zanthoxylum Zanthoxyloides (Lam.) Zepernick and Timler Comb. nova (Rutaceae). Willdenowia 1981, 11, 361–362. [Google Scholar]
- Escalante, M.G. El Género Fagara En La Argentina. Bol. Soc. Argent. Bot. 1961, 9, 291–317. [Google Scholar]
- Museum National d’Histoire Zanthoxylum Caribaeum Lam. 1786. Available online: https://inpn.mnhn.fr/espece/cd_nom/630913 (accessed on 7 July 2024).
- Diehl, E.E.; von Poser, G.L.; Henriques, A.T. Constituents of Zanthoxylum Rugosum St.-Hil & Tul. Biochem. Syst. Ecol. 2000, 28, 275–277. [Google Scholar] [CrossRef]
- Spichiger, R.; Stutz de Ortega, L. Flora de Paraguay: Rutaceae; Missouri Botanical Garden: St. Louis, MO, USA, 1987.
- Schnee, L. Plantas Comunes de Venezuela; Universidad Central de Venezuela: Caracas, Venezuela, 1973. [Google Scholar]
- Soriano-Agatón, F.; Lagoutte, D.; Poupon, E.; Roblot, F.; Fournet, A.; Gantier, J.-C.; Hocquemiller, R. Extraction, Hemisynthesis, and Synthesis of Canthin-6-One Analogues. Evaluation of Their Antifungal Activities. J. Nat. Prod. 2005, 68, 1581–1587. [Google Scholar] [CrossRef]
- Márquez, L.; Agüero, J.; Hernández, I.; Garrido, G.; Martínez, I.; Diéguez, R.; Prieto, S.; Rivas, Y.; Molina-Torres, J.; Curini, M.; et al. Anti-Inflammatory Evaluation and Phytochemical Characterization of Some Plants of the Zanthoxylum Genus. Acta Farm. Bonaer. 2005, 24, 325. [Google Scholar]
- Cheng, M.-J.; Wu, C.-C.; Tsai, I.-L.; Chen, I.-S. Chemical and Antiplatelet Constituents from the Stem of Zanthoxylum Beecheyanum. J. Chin. Chem. Soc. 2004, 51, 1065–1072. [Google Scholar] [CrossRef]
- Mutinda, E.S.; Kimutai, F.; Mkala, E.M.; Waswa, E.N.; Odago, W.O.; Nanjala, C.; Ndungu, C.N.; Gichua, M.K.; Njire, M.M.; Gituru, R.W.; et al. Ethnobotanical Uses, Phytochemistry and Pharmacology of Pantropical Genus Zanthoxylum L. (Rutaceae): An Update. J. Ethnopharmacol. 2023, 303, 115895. [Google Scholar] [CrossRef]
- Ferreira, M.E.; Nakayama, H.; de Arias, A.R.; Schinini, A.; de Bilbao, N.V.; Serna, E.; Lagoutte, D.; Soriano-Agatón, F.; Poupon, E.; Hocquemiller, R.; et al. Effects of Canthin-6-One Alkaloids from Zanthoxylum Chiloperone on Trypanosoma Cruzi-Infected Mice. J. Ethnopharmacol. 2007, 109, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Cebrián-Torrejón, G.; Assad Kahn, S.; Lagarde, N.; Castellano, F.; Leblanc, K.; Rodrigo, J.; Molinier-Frenkel, V.; Rojas de Arias, A.; Ferreira, M.E.; Thirant, C.; et al. Antiproliferative Activity of Trans-Avicennol from Zanthoxylum Chiloperone Var. Angustifolium against Human Cancer Stem Cells. J. Nat. Prod. 2012, 75, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Rollet, B.; Fiard, J.P.; Huc, R. Arbres des Petites Antilles; Office National des Forêts (ONF): Maisons-Alfort, France, 2010. [Google Scholar]
- Arruda, M.S.P.; Fernandes, J.B.; da Silva, M.F.G.F.; Vieira, P.C.; Pirani, J.R. Quinolone Alkaloids from Zanthoxylum Acutifolium. Phytochemistry 1992, 31, 3617–3619. [Google Scholar] [CrossRef]
- Ferreira, M.E.; Rojas de Arias, A.; Torres de Ortiz, S.; Inchausti, A.; Nakayama, H.; Thouvenel, C.; Hocquemiller, R.; Fournet, A. Leishmanicidal Activity of Two Canthin-6-One Alkaloids, Two Major Constituents of Zanthoxylum Chiloperone Var. Angustifolium. J. Ethnopharmacol. 2002, 80, 199–202. [Google Scholar] [CrossRef]
- Krane, B.D.; Fagbule, M.O.; Shamma, M.; Gözler, B. The Benzophenanthridine Alkaloids. J. Nat. Prod. 1984, 47, 1–43. [Google Scholar] [CrossRef]
- Souza, J.G.D.L.D.; Pinto, F.G.D.S.; Toledo, A.G.; Alves, L.F.A.; Alves, D.S. Biological Activities and Phytochemical Screening of Leaf Extracts from Zanthoxylum Caribaeum L. (Rutaceae). Biosci. J. 2020, 36, 223–234. [Google Scholar] [CrossRef]
- Farouil, L.; Dias, R.P.; Popotte-Julisson, G.; Bibian, G.; Adou, A.I.; de la Mata, A.P.; Sylvestre, M.; Harynuk, J.J.; Cebrián-Torrejón, G. The Metabolomic Profile of the Essential Oil from Zanthoxylum Caribaeum (Syn. Chiloperone) Growing in Guadeloupe FWI Using GC × GC-TOFMS. Metabolites 2022, 12, 1293. [Google Scholar] [CrossRef]
- Guy, I.; Charles, B.; Guinaudeau, H.; Ferreira, M.E.; Rojas De Arias, A.; Fournet, A. Essential Oils from Zanthoxylum Chiloperone and Z. Riedelianum Growing in Paraguay. Pharm. Biol. 2001, 39, 152–154. [Google Scholar] [CrossRef]
- Nooreen, Z.; Tandon, S.; Yadav, N.P.; Kumar, P.; Xuan, T.D.; Ahmad, A. Zanthoxylum: A Review of Its Traditional Uses, Naturally Occurring Constituents and Pharmacological Properties. Curr. Org. Chem. 2019, 23, 1307–1341. [Google Scholar] [CrossRef]
- Lv, J.; Yang, S.; Zhou, W.; Liu, Z.; Tan, J.; Wei, M. Microbial Regulation of Plant Secondary Metabolites: Impact, Mechanisms and Prospects. Microbiol. Res. 2024, 283, 127688. [Google Scholar] [CrossRef] [PubMed]
- Thesnor, V.; Molinié, R.; Giebelhaus, R.T.; de la Mata Espinosa, A.P.; Harynuk, J.J.; Bénimélis, D.; Vanhoye, B.; Dunyach-Rémy, C.; Sylvestre, M.; Cheremond, Y.; et al. Antibacterial Activity and Untargeted Metabolomics Profiling of Acalypha Arvensis Poepp. Molecules 2023, 28, 7882. [Google Scholar] [CrossRef] [PubMed]
- Thouvenel, C.; Gantier, J.-C.; Duret, P.; Fourneau, C.; Hocquemiller, R.; Ferreira, M.-E.; de Arias, A.R.; Fournet, A. Antifungal Compounds from Zanthoxylum Chiloperone Var. Angustifolium. Phytother. Res. 2003, 17, 678–680. [Google Scholar] [CrossRef]
- Couillerot, E.; Caron, C.; Comoe, L.; Audran, J.-C.; Molinatti, P.; Zeches, M.; Le Men-Olivier, L.; Jardillier, J.-C.; Chenieux, J.-C. Benzophenanthridine and Furoquinoline Accumulation in Cell Suspension Cultures of Fagara Zanthoxyloides. Phytochemistry 1994, 37, 425–428. [Google Scholar] [CrossRef]
- De Lara De Souza, J.G.; Toledo, A.G.; Walerius, A.H.; Jann Favreto, W.A.; Da Costa, W.F.; Da Silva Pinto, F.G. Chemical Composition, Antimicrobial, Repellent and Antioxidant Activity of Essential Oil of Zanthoxylum Caribaeum Lam. J. Essent. Oil Bear. Plants 2019, 22, 380–390. [Google Scholar] [CrossRef]
- Sikkema, J.; De Bont, J.A.; Poolman, B. Interactions of Cyclic Hydrocarbons with Biological Membranes. J. Biol. Chem. 1994, 269, 8022–8028. [Google Scholar] [CrossRef]
- Shahidi, F.; Janitha, P.K.; Wanasundara, P.D. Phenolic Antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef]
- Elva, S.; Marisel, M.; Nilsa, G. Determination of the in Vivo Activity of Leaves Extract of Zanthoxylum Chiloperone Var. Angustifolium (Tembetary Hú) Orally and Intralesionally Administered to BALB/c Mice Experimentally Infected with Leishmania. Arch. Cancer Sci. Ther. 2022, 6, 038–043. [Google Scholar] [CrossRef]
- Rassi, A.; de Rezende, J.M. American Trypanosomiasis (Chagas Disease). Infect. Dis. Clin. 2012, 26, 275–291. [Google Scholar] [CrossRef]
- Ferreira, M.E.; Cebrián-Torrejón, G.; Corrales, A.S.; de Bilbao, N.V.; Rolón, M.; Gomez, C.V.; Leblanc, K.; Yaluf, G.; Schinini, A.; Torres, S. Zanthoxylum Chiloperone Leaves Extract: First Sustainable Chagas Disease Treatment. J. Ethnopharmacol. 2011, 133, 986–993. [Google Scholar] [CrossRef] [PubMed]
- de Bilbao, N.V.; Giebelhaus, R.T.; Dias, R.P.; Ferreira, M.E.; Martínez, M.; Velasco-Carneros, L.; Nam, S.L.; de la Mata, A.P.; Maréchal, J.-D.; Adou, A.I. Exploring the Anti-Chagas Activity of Zanthoxylum Chiloperone’s Seedlings Through Metabolomics and Protein–Ligand Docking. Plants 2025, 14, 954. [Google Scholar] [CrossRef] [PubMed]
- Diéguez-Hurtado, R.; Garrido-Garrido, G.; Prieto-González, S.; Iznaga, Y.; González, L.; Molina-Torres, J.; Curini, M.; Epifano, F.; Marcotullio, M.C. Antifungal Activity of Some Cuban Zanthoxylum Species. Fitoterapia 2003, 74, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Garrido, J.; Salinas-Sandoval, M.; Díaz-López, A.; Jácquez-Ríos, P.; Arriaga-Alba, M.; Ordaz-Pichardo, C. In Vitro and in Vivo Antifungal Activity, Liver Profile Test, and Mutagenic Activity of Five Plants Used in Traditional Mexican Medicine. Rev. Bras. Farmacogn. 2015, 25, 22–28. [Google Scholar] [CrossRef]
- Ortega-Buitrago, O.; Chaves-Bedoya, G.; Ortiz-Rojas, L.Y. Chemical Composition and Antimicrobial Activity of Ethanolic Bark and Leave Extract of Zanthoxylum Caribaeum Lam. from Norte de Santander, Colombia. Rev. Chim. 2021, 72, 152–161. [Google Scholar] [CrossRef]
- Chaves-Bedoya, G.; Vera, E.J.; Ortiz-Rojas, L.Y. Phytochemical Characterization of Ethanolic Extracts of the Leaves of Zanthoxylum Caribaeum Lam and Evaluation of Antimicrobial Activity Against Burkholderia Glumae. Rev. Chim. 2022, 73, 51–61. [Google Scholar] [CrossRef]
- Nogueira, J.; Vinturelle, R.; Mattos, C.; Tietbohl, L.A.C.; Santos, M.G.; Da Silva Vaz, I.; Mourão, S.C.; Rocha, L.; Folly, E. Acaricidal Properties of the Essential Oil from Zanthoxylum Caribaeum against Rhipicephalus Microplus. J. Med. Entomol. 2014, 51, 971–975. [Google Scholar] [CrossRef]
- Cebrian-Torrejon, G.; Spelman, K.; Leblanc, K.; Munoz-Durango, K.; Gutierrez, S.T.; Ferreira, M.E.; de Arias, A.R.; Figadere, B.; Fournet, A.; Maciuk, A.; et al. The Antiplasmodium Effects of a Traditional South American Remedy: Zanthoxylum Chiloperone Var. Angustifolium against Chloroquine Resistant and Chloroquine Sensitive Strains of Plasmodium Falciparum. Rev. Bras. Farmacogn. 2011, 21, 652–661. [Google Scholar] [CrossRef][Green Version]
- Villalba, M.A.; Carmo, M.I.; Leite, M.N.; Sousa, O.V. Atividades farmacológicas dos extratos de Zanthoxylum chiloperone (Rutaceae). Rev. Bras. Farmacogn. 2007, 17, 236–241. [Google Scholar] [CrossRef]
- Doménech-Carbó, A.; Rodrigo, R.; Maréchal, J.-D.; Poupon, E.; Fournet, A.; Figadère, B.; Cebrián-Torrejón, G. Bioelectrochemical Monitoring of Soluble Guanylate Cyclase Inhibition by the Natural β-Carboline Canthin-6-One. J. Mol. Struct. 2017, 1134, 661–667. [Google Scholar] [CrossRef]
- Sanabria, L.; Segovia, E.A.; González, N.; Alcaraz, P.; Vera de Bilbao, N. Larvicidal Activity of Aqueous Plants Extracts on Aedes Aegypti Larva (First Trials). Mem. Inst. Investig. Cienc. Salud 2009, 7, 26–31. [Google Scholar]
- Nogueira, J.; Mourão, S.C.; Dolabela, I.B.; Santos, M.G.; Mello, C.B.; Kelecom, A.; Mexas, R.; Feder, D.; Fernandes, C.P.; Gonzalez, M.S.; et al. Zanthoxylum Caribaeum (Rutaceae) Essential Oil: Chemical Investigation and Biological Effects on Rhodnius Prolixus Nymph. Parasitol. Res. 2014, 113, 4271–4279. [Google Scholar] [CrossRef]
| Name | Reference |
|---|---|
| Fagara caribaea (Lam.) Krug&Urb. | [3] |
| Fagara (Martius ex Engler) Engler | [21] |
| Fagara chiloperone var. angustifolia (Engl.) Engl. Ex Chodat & Hassl. | [22] |
| Zanthoxylum chiloperone Martius ex Engler | [21] |
| Zanthoxylum chiloperone var. angustifolium Engl. | [22] |
| Zanthoxylum rugosum A. St. -Hill. &Tul. | [21] |
| Zanthoxylum aromaticum sensu Duss | [3] |
| Part Used | Country | Traditional Applications | References |
|---|---|---|---|
| Root bark | Paraguay, Cuba | Antirheumatic | [25,28] |
| Stem bark, leaves | Paraguay | Skin diseases | [24,27] |
| Stem bark | Paraguay | Emmenagogue | [29] |
| Stem bark | Paraguay | Antimalarial | [29] |
| Root bark | Paraguay | Anticancer | [29] |
| Stem bark | Cuba | Antiasthmatic | [25,27] |
| Stem bark | Cuba | Anti-ulcer | [25,27] |
| Leaves | Latin America | Stomach ailments | [27] |
| Extract and Compound | L. braziliensis | L. amazonensis | L. donovani |
|---|---|---|---|
| N-methylglucamine | >100 | >100 | >100 |
| Pentamidine a | 5 | 5 | 5 |
| Alkaloidal extract (CH2CL2) | 100 | 100 | 100 |
| Methanolic extract | >100 | >100 | >100 |
| Canthin-6-one | 100 | 100 | 100 |
| 5-methoxycanthin-6-one | 100 | 100 | 100 |
| Treatment | n b | Negative Parasitaemia/Number of Survivors (Days Post-Infection) | ||||
|---|---|---|---|---|---|---|
| Post-Infection Time | 0 Days | 18 Days | 32 Days | 45 Days | 56 Days | 68 Days |
| Control | 20 | 0/19 | 4/17 | 6/14 | 0/12 | 3/11 |
| Benznidazole | 20 | 14/20 | 12/20 | 10/19 | 11/19 | 17/19 |
| 1 oral | 20 | 5/20 | 11/20 | 15/20 | 17/20 | 19/20 |
| 1 s.c | 21 | 6/21 | 6/21 | 12/21 | 10/21 | 19/21 |
| 2 oral | 9 | 8/9 | 2/7 | 1/7 | 4/7 | 3/7 |
| 2 s.c. a | 8 | 6/8 | 5/8 | 3/8 | 4/8 | 6/8 |
| 3 orally administered | 10 | 0/10 | 0/7 | 0/6 | 6/6 | 6/6 |
| Crude Zanthoxylum caribaeum alkaloid oral | 12 | 1/12 | 0/10 | 5/9 | 5/9 | 8/9 |
| Crude Zanthoxylum caribaeum alkaloid s.c | 12 | 1/11 | 1/11 | 10/11 | 10/11 | 5/10 |
| Species | Canthin-6-One | 5-Methoxycanthin-6-One | Ketoconazole |
|---|---|---|---|
| Candida albicans | 56.1 | 190.3 | 46 |
| Candida glabrata | 12.8 | 90.8 | 5.3 |
| Candida tropicalis | 12.8 | 45.4 | 5.3 |
| Aspergillus fumigatus | 56.1 | 97.5 | 45.9 |
| Aspergillus niger | 12.8 | 22.7 | 5.3 |
| Aspergillus terreus | 12.8 | >181.6 | 5.3 |
| Cryptococcus neoformans | 12.8 | 45.4 | 5.3 |
| Geotrichum candidum | 12.8 | >181.6 | 5.3 |
| Saccharomyces cerevisiae | 12.8 | >181.6 | 5.3 |
| Trichophyton mentagrophytes var. interdigitale | >216.2 | 12.3 | 5.8 |
| Trichosporon beigelii | 12.8 | 22.7 | 5.3 |
| Trichosporon cutaneum | 12.8 | 90.8 | 5.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adou, A.I.; Fleurima, E.; Thesnor, V.; Urrutia, A.; Fournet, A.; Sylvestre, M.-N.; Sylvestre, M.; Benfodda, Z.; Cebrián-Torrejón, G. Ethnomedicinal Applications, Phytochemistry, and Pharmacological Properties of Zanthoxylum caribaeum Lam.: A Comprehensive Review. Metabolites 2025, 15, 614. https://doi.org/10.3390/metabo15090614
Adou AI, Fleurima E, Thesnor V, Urrutia A, Fournet A, Sylvestre M-N, Sylvestre M, Benfodda Z, Cebrián-Torrejón G. Ethnomedicinal Applications, Phytochemistry, and Pharmacological Properties of Zanthoxylum caribaeum Lam.: A Comprehensive Review. Metabolites. 2025; 15(9):614. https://doi.org/10.3390/metabo15090614
Chicago/Turabian StyleAdou, Ahissan Innocent, Ebed Fleurima, Valendy Thesnor, Ander Urrutia, Alain Fournet, Marie-Noëlle Sylvestre, Muriel Sylvestre, Zohra Benfodda, and Gerardo Cebrián-Torrejón. 2025. "Ethnomedicinal Applications, Phytochemistry, and Pharmacological Properties of Zanthoxylum caribaeum Lam.: A Comprehensive Review" Metabolites 15, no. 9: 614. https://doi.org/10.3390/metabo15090614
APA StyleAdou, A. I., Fleurima, E., Thesnor, V., Urrutia, A., Fournet, A., Sylvestre, M.-N., Sylvestre, M., Benfodda, Z., & Cebrián-Torrejón, G. (2025). Ethnomedicinal Applications, Phytochemistry, and Pharmacological Properties of Zanthoxylum caribaeum Lam.: A Comprehensive Review. Metabolites, 15(9), 614. https://doi.org/10.3390/metabo15090614








