Time-Resolved Metabolomics Reveals Mitochondrial Protection in Septic Liver Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Sepsis Model
2.2. Metabolomic Analyses
2.2.1. Chemicals
2.2.2. Pretreatment of Tissue Samples
2.2.3. LC Condition
2.2.4. MS Conditions
2.2.5. Data Processing
2.3. Measurement of MDA via TBARS Assay
2.4. Western Blot Analysis
2.5. Statistical Analysis
3. Results
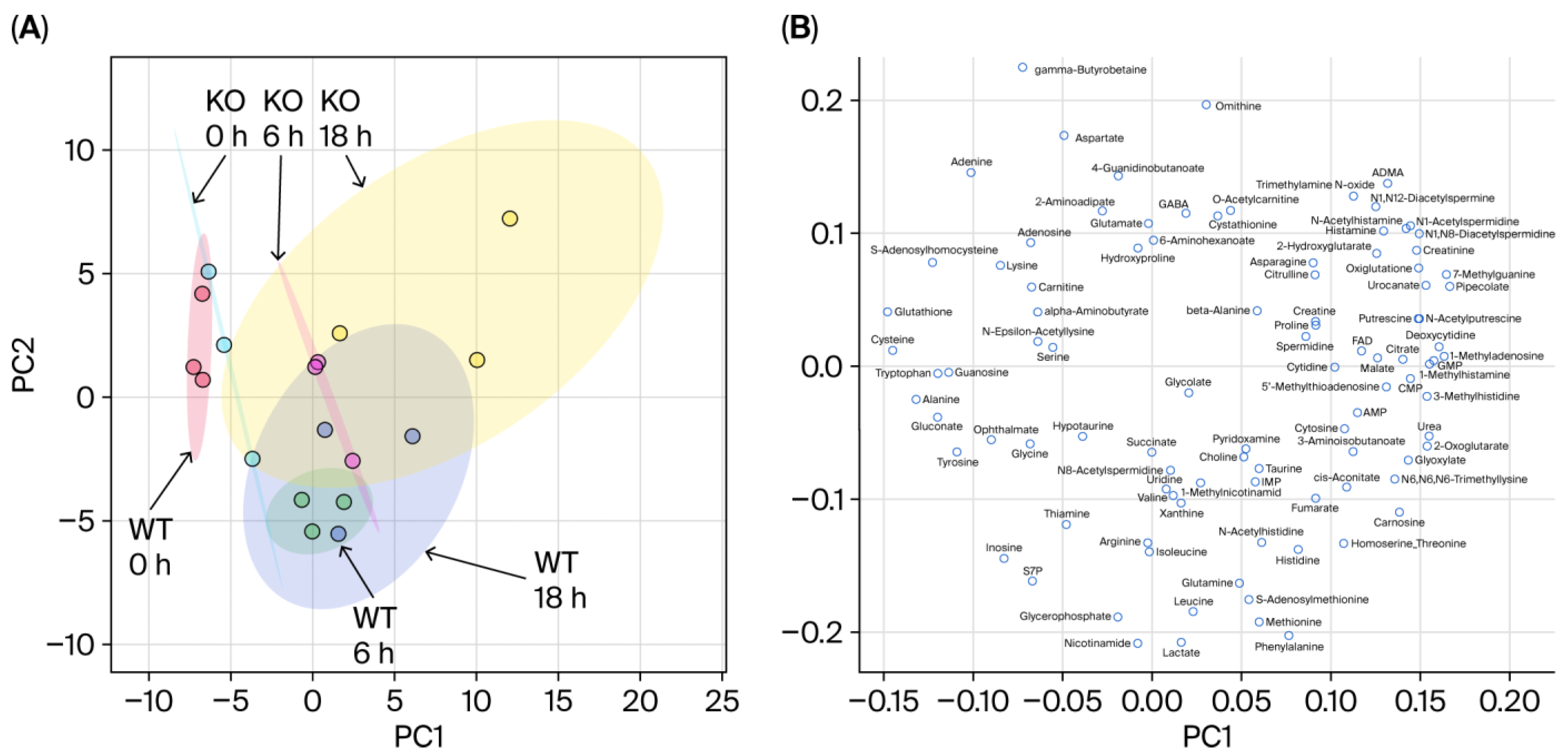
3.1. Overview of the Metabolomic Profile
3.2. Differences in Metabolomic Profiles Between WT and KO
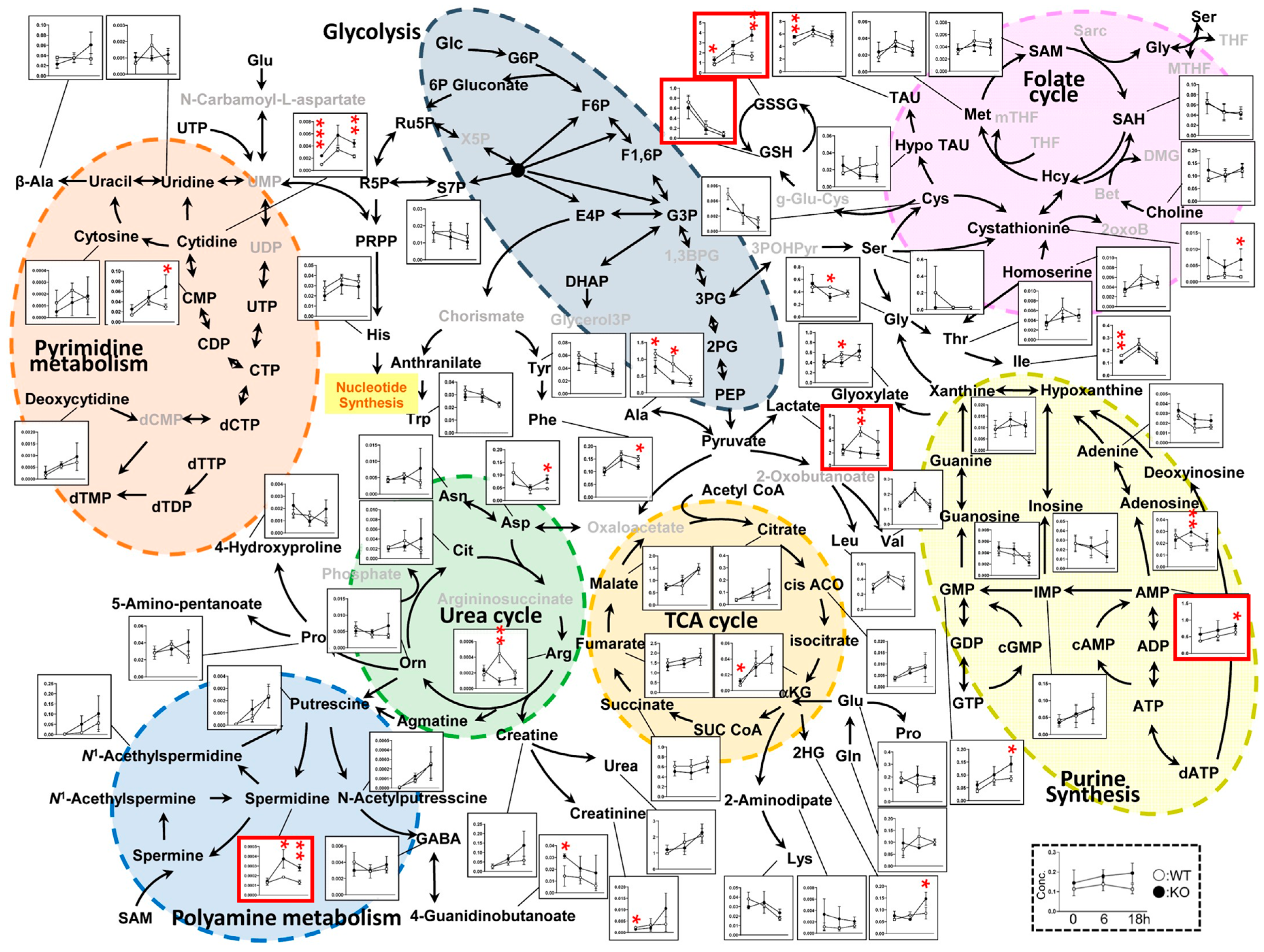
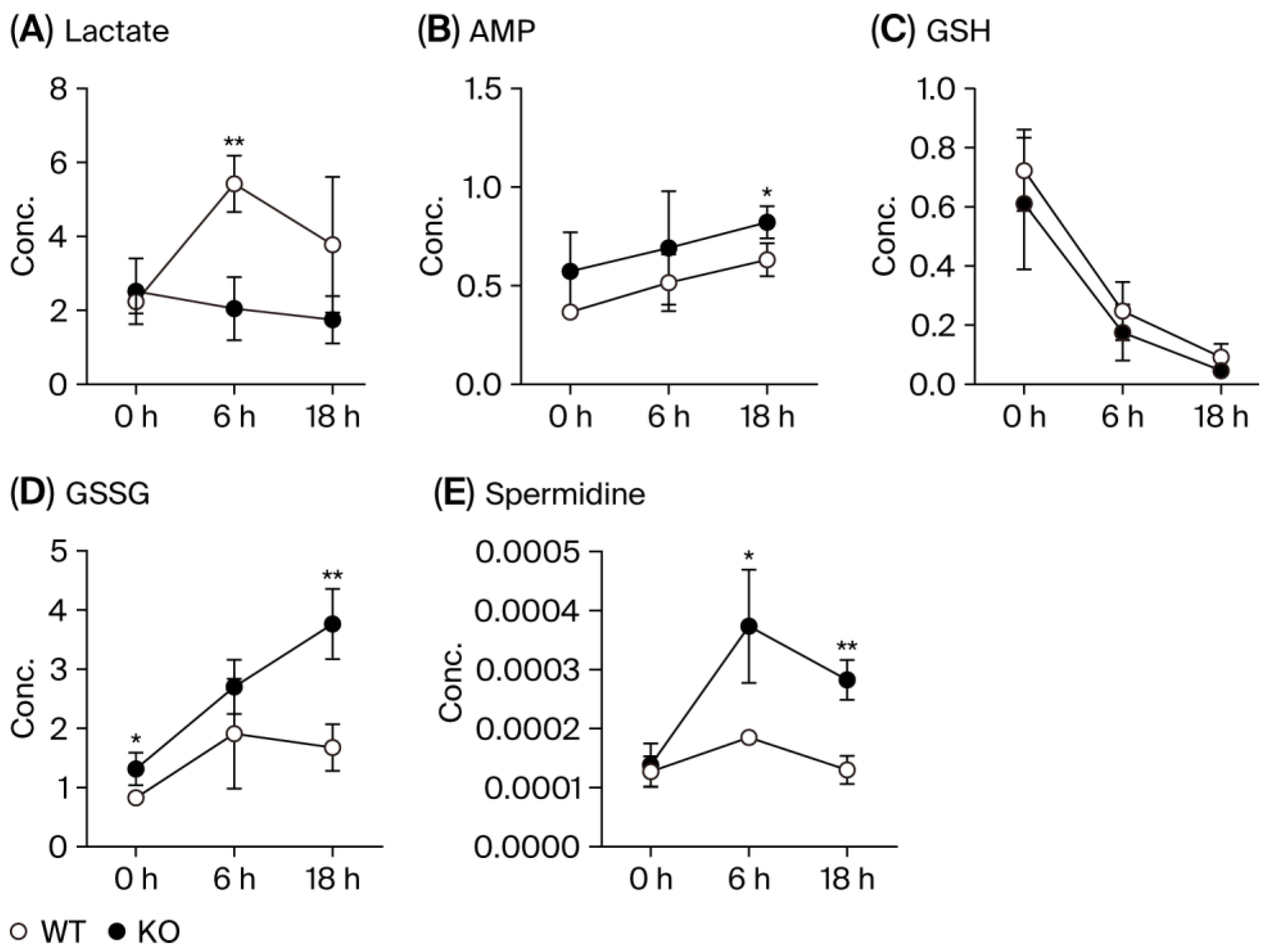
3.3. Metabolic Pathways
3.4. MDA Production
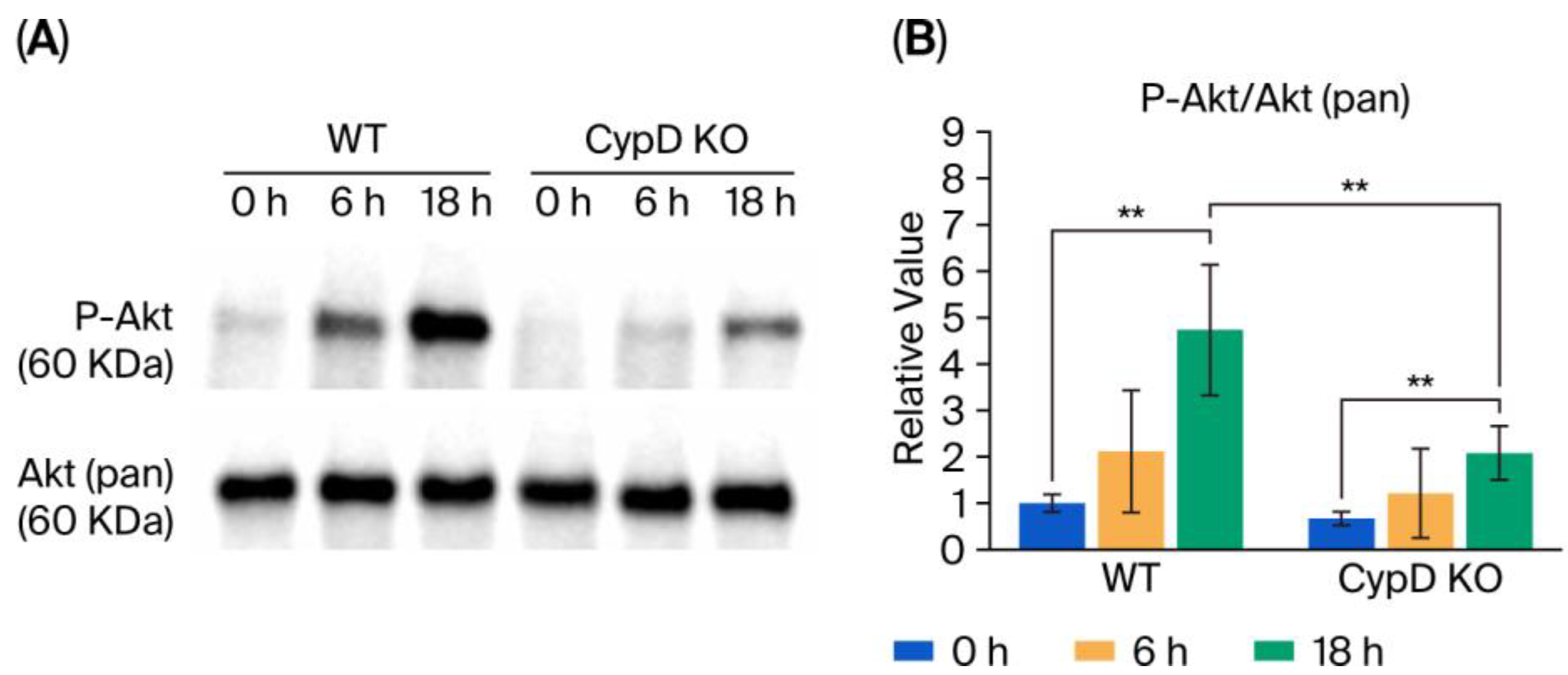
3.5. Akt Phosphorylation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| ADP | Adenosine Diphosphate |
| ATP | Adenosine Triphosphate |
| CLP | Cecal Ligation and Puncture |
| GSH | Reduced Glutathione |
| GSSG | Oxidized Glutathione |
| LC-MS | Liquid Chromatography–Mass Spectrometry |
| LC-TOFMS | Liquid Chromatography–Time-of-Flight Mass Spectrometry |
| MDA | Malondialdehyde |
| MPT | Mitochondrial Permeability Transition |
| MPTP | Mitochondrial Permeability Transition Pore |
| MTP | Mitochondrial Trifunctional Protein |
| PCA | Principal Component Analysis |
| ROS | Reactive Oxygen Species |
| TBARS | Thiobarbituric Acid Reactive Substances |
| TCA | Tricarboxylic Acid |
| TOF-MS | Time-of-Flight Mass Spectrometry |
References
- Hotchkiss, R.S.; Karl, I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003, 348, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Rittirsch, D.; Flierl, M.A.; Ward, P.A. Harmful molecular mechanisms in sepsis. Nat. Rev. Immunol. 2008, 8, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Aki, T.; Unuma, K.; Uemura, K. Emerging roles of mitochondria and autophagy in liver injury during sepsis. Cell Stress 2017, 1, 79–89. [Google Scholar] [CrossRef]
- Kobayashi, T.; Uchino, H.; Elmer, E.; Ogihara, Y.; Fujita, H.; Sekine, S.; Ishida, Y.; Saiki, I.; Shibata, S.; Kawachi, A. Disease Outcome and Brain Metabolomics of Cyclophilin-D Knockout Mice in Sepsis. Int. J. Mol. Sci. 2022, 23, 961. [Google Scholar] [CrossRef]
- Mohsin, M.; Zaki, A.; Tabassum, G.; Khan, S.; Ali, S.; Ahmad, T.; Syed, M.A. Urolithin-A supplementation alleviates sepsis-induced acute lung injury by reducing mitochondrial dysfunction and modulating macrophage polarization. Mitochondrion 2025, 84, 102047. [Google Scholar] [CrossRef]
- Bernardi, P.; Gerle, C.; Halestrap, A.P.; Jonas, E.A.; Karch, J.; Mnatsakanyan, N.; Pavlov, E.; Sheu, S.S.; Soukas, A.A. Identity, structure, and function of the mitochondrial permeability transition pore: Controversies, consensus, recent advances, and future directions. Cell Death Differ. 2023, 30, 1869–1885. [Google Scholar] [CrossRef]
- d’Avila, J.C.; Santiago, A.P.; Amancio, R.T.; Galina, A.; Oliveira, M.F.; Bozza, F.A. Sepsis induces brain mitochondrial dysfunction. Crit. Care Med. 2008, 36, 1925–1932. [Google Scholar] [CrossRef]
- Nakagawa, T.; Shimizu, S.; Watanabe, T.; Yamaguchi, O.; Otsu, K.; Yamagata, H.; Inohara, H.; Kubo, T.; Tsujimoto, Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 2005, 434, 652–658. [Google Scholar] [CrossRef]
- Mbye, L.H.; Singh, I.N.; Sullivan, P.G.; Springer, J.E.; Hall, E.D. Attenuation of acute mitochondrial dysfunction after traumatic brain injury in mice by NIM811, a non-immunosuppressive cyclosporin A analog. Exp. Neurol. 2008, 209, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Uchino, H.; Elmer, E.; Uchino, K.; Lindvall, O.; Siesjo, B.K. Cyclosporin A dramatically ameliorates CA1 hippocampal damage following transient forebrain ischaemia in the rat. Acta Physiol. Scand. 1995, 155, 469–471. [Google Scholar] [CrossRef]
- Uchino, H.; Elmer, E.; Uchino, K.; Li, P.A.; He, Q.P.; Smith, M.L.; Siesjo, B.K. Amelioration by cyclosporin A of brain damage in transient forebrain ischemia in the rat. Brain Res. 1998, 812, 216–226. [Google Scholar] [CrossRef]
- Uchino, H.; Minamikawa-Tachino, R.; Kristian, T.; Perkins, G.; Narazaki, M.; Siesjo, B.K.; Shibasaki, F. Differential neuroprotection by cyclosporin A and FK506 following ischemia corresponds with differing abilities to inhibit calcineurin and the mitochondrial permeability transition. Neurobiol. Dis. 2002, 10, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Demine, S.; Reddy, N.; Renard, P.; Raes, M.; Arnould, T. Unraveling biochemical pathways affected by mitochondrial dysfunctions using metabolomic approaches. Metabolites 2014, 4, 831–878. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, H.; Li, X.L.; Yang, Y.; Hairong, P. Rapid lipidomics analysis for sepsis-induced liver injury in rats and insights into lipid metabolic pathways using ultra-performance liquid chromatography/mass spectrometry. RSC Adv. 2019, 9, 35364–35371. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Gan, L.; Pan, J.; Zhong, L.; Wang, Q.; Luo, S.; Tian, J.; Liang, H. Transcriptomics combined with metabolomics analysis of the mechanism of agmatine in the treatment of septic liver injury. Ann. Transl. Med. 2022, 10, 578. [Google Scholar] [CrossRef]
- Hara, N.; Chijiiwa, M.; Yara, M.; Ishida, Y.; Ogiwara, Y.; Inazu, M.; Kuroda, M.; Karlsson, M.; Sjovall, F.; Elmer, E.; et al. Metabolomic Analyses of Brain Tissue in Sepsis Induced by Cecal Ligation Reveal Specific Redox Alterations—Protective Effects of the Oxygen Radical Scavenger Edaravone. Shock 2015, 44, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Scheff, S.W.; Sullivan, P.G. Cyclosporin A significantly ameliorates cortical damage following experimental traumatic brain injury in rodents. J. Neurotrauma 1999, 16, 783–792. [Google Scholar] [CrossRef]
- Dixon, C.E.; Bramlett, H.M.; Dietrich, W.D.; Shear, D.A.; Yan, H.Q.; Deng-Bryant, Y.; Mondello, S.; Wang, K.K.; Hayes, R.L.; Empey, P.E.; et al. Cyclosporine Treatment in Traumatic Brain Injury: Operation Brain Trauma Therapy. J. Neurotrauma 2016, 33, 553–566. [Google Scholar] [CrossRef]
- Crompton, M.; Virji, S.; Ward, J.M. Cyclophilin-D binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. Eur. J. Biochem. 1998, 258, 729–735. [Google Scholar] [CrossRef]
- Li, Y.; Johnson, N.; Capano, M.; Edwards, M.; Crompton, M. Cyclophilin-D promotes the mitochondrial permeability transition but has opposite effects on apoptosis and necrosis. Biochem. J. 2004, 383, 101–109. [Google Scholar] [CrossRef]
- Waldmeier, P.C.; Zimmermann, K.; Qian, T.; Tintelnot-Blomley, M.; Lemasters, J.J. Cyclophilin D as a drug target. Curr. Med. Chem. 2003, 10, 1485–1506. [Google Scholar] [CrossRef] [PubMed]
- Elrod, J.W.; Wong, R.; Mishra, S.; Vagnozzi, R.J.; Sakthievel, B.; Goonasekera, S.A.; Karch, J.; Gabel, S.; Farber, J.; Force, T.; et al. Cyclophilin D controls mitochondrial pore-dependent Ca2+ exchange, metabolic flexibility, and propensity for heart failure in mice. J. Clin. Investig. 2010, 120, 3680–3687. [Google Scholar] [CrossRef]
- Basso, E.; Fante, L.; Fowlkes, J.; Petronilli, V.; Forte, M.A.; Bernardi, P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J. Biol. Chem. 2005, 280, 18558–18561. [Google Scholar] [CrossRef] [PubMed]
- Chai, N.; Zhang, H.; Li, L.; Yu, X.; Liu, Y.; Lin, Y.; Wang, L.; Yan, J.; Nikolaevna, S.E.; Zhao, Y. Spermidine Prevents Heart Injury in Neonatal Rats Exposed to Intrauterine Hypoxia by Inhibiting Oxidative Stress and Mitochondrial Fragmentation. Oxid. Med. Cell. Longev. 2019, 2019, 5406468. [Google Scholar] [PubMed]
- Szabo, L.; Lejri, I.; Grimm, A.; Eckert, A. Spermidine Enhances Mitochondrial Bioenergetics in Young and Aged Human-Induced Pluripotent Stem Cell-Derived Neurons. Antioxidants 2024, 13, 1482. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, C.R.; Li, Z.X.; Gao, Y.H.; Jiang, L.; Zhang, J.; Wang, P.Y.; Liu, T. Spermine alleviates myocardial cell aging by inhibiting mitochondrial oxidative stress damage. Eur. J. Pharmacol. 2025, 997, 177477. [Google Scholar] [CrossRef]
- Al-Habsi, M.; Chamoto, K.; Matsumoto, K.; Nomura, N.; Zhang, B.; Sugiura, Y.; Sonomura, K.; Maharani, A.; Nakajima, Y.; Wu, Y.; et al. Spermidine activates mitochondrial trifunctional protein and improves antitumor immunity in mice. Science 2022, 378, eabj3510. [Google Scholar] [CrossRef]
- Rustenbeck, I.; Reiter, H.; Lenzen, S. Dissociation between effects of polyamines on mitochondrial calcium uptake and mitochondrial permeability transition by elongation of polyamine methylene backbone. Biochem. Mol. Biol. Int. 1996, 38, 1003–1011. [Google Scholar]
- Chen, H.Y.; Jia, X.L.; Zhao, S.Q.; Zheng, W.H.; Mei, Z.G.; Yang, H.W.; Zhang, S.Z. Dual role of polyamines in heart ischemia/reperfusion injury through regulation of mitochondrial permeability transition pore. Sheng Li Xue Bao 2019, 71, 681–688. [Google Scholar]
- Sims, N.R.; Anderson, M.F. Mitochondrial contributions to tissue damage in stroke. Neurochem. Int. 2002, 40, 511–526. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, N.; Shibata, S.; Sugimoto, M.; Elmer, E.; Uchino, H. Time-Resolved Metabolomics Reveals Mitochondrial Protection in Septic Liver Injury. Metabolites 2025, 15, 600. https://doi.org/10.3390/metabo15090600
Suzuki N, Shibata S, Sugimoto M, Elmer E, Uchino H. Time-Resolved Metabolomics Reveals Mitochondrial Protection in Septic Liver Injury. Metabolites. 2025; 15(9):600. https://doi.org/10.3390/metabo15090600
Chicago/Turabian StyleSuzuki, Naoki, Shoichiro Shibata, Masahiro Sugimoto, Eskil Elmer, and Hiroyuki Uchino. 2025. "Time-Resolved Metabolomics Reveals Mitochondrial Protection in Septic Liver Injury" Metabolites 15, no. 9: 600. https://doi.org/10.3390/metabo15090600
APA StyleSuzuki, N., Shibata, S., Sugimoto, M., Elmer, E., & Uchino, H. (2025). Time-Resolved Metabolomics Reveals Mitochondrial Protection in Septic Liver Injury. Metabolites, 15(9), 600. https://doi.org/10.3390/metabo15090600







