The Role of Visfatin/NAMPT in the Pathogenesis of Psoriasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Visfatin Analysis
2.2. Statistical Analysis
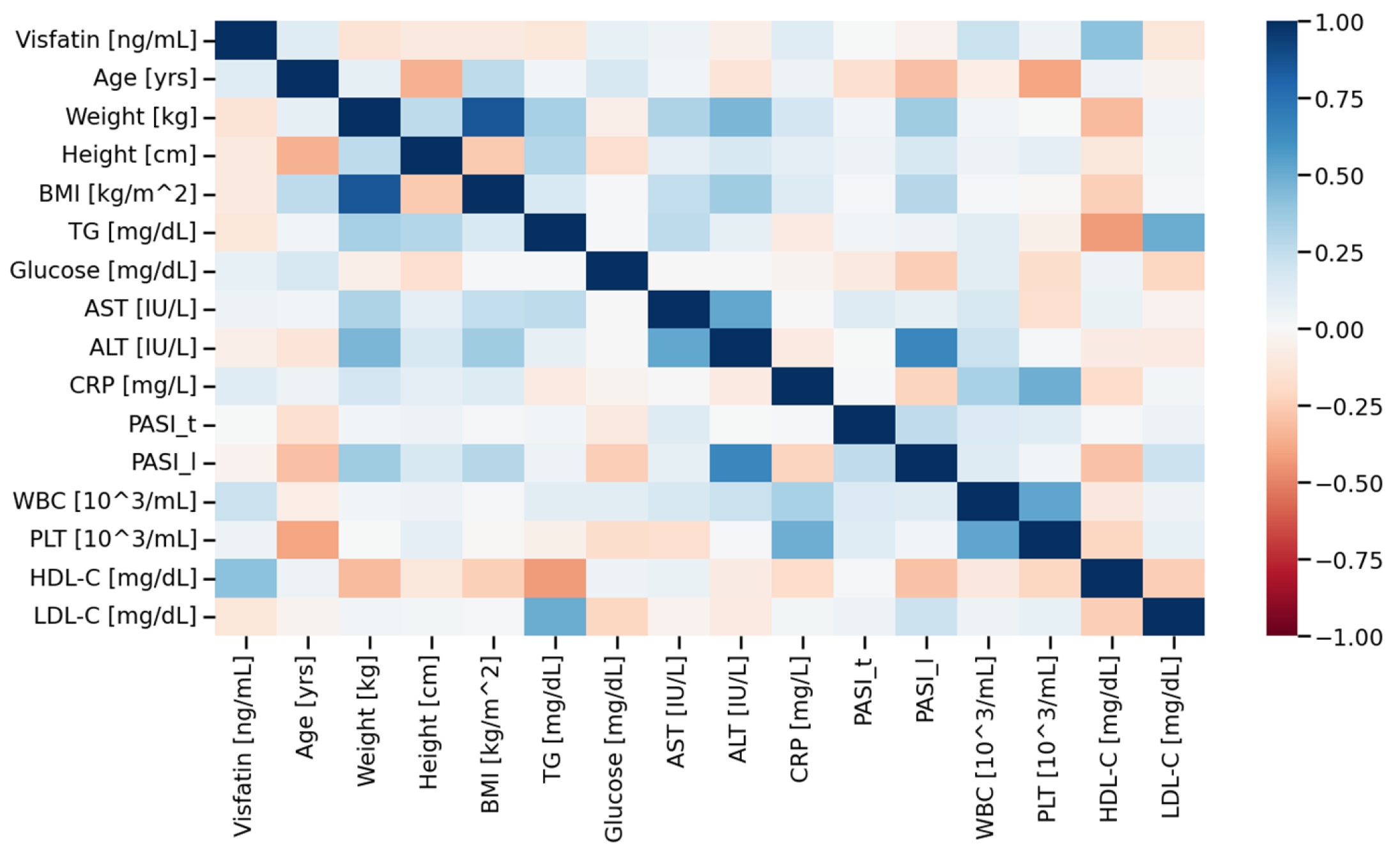
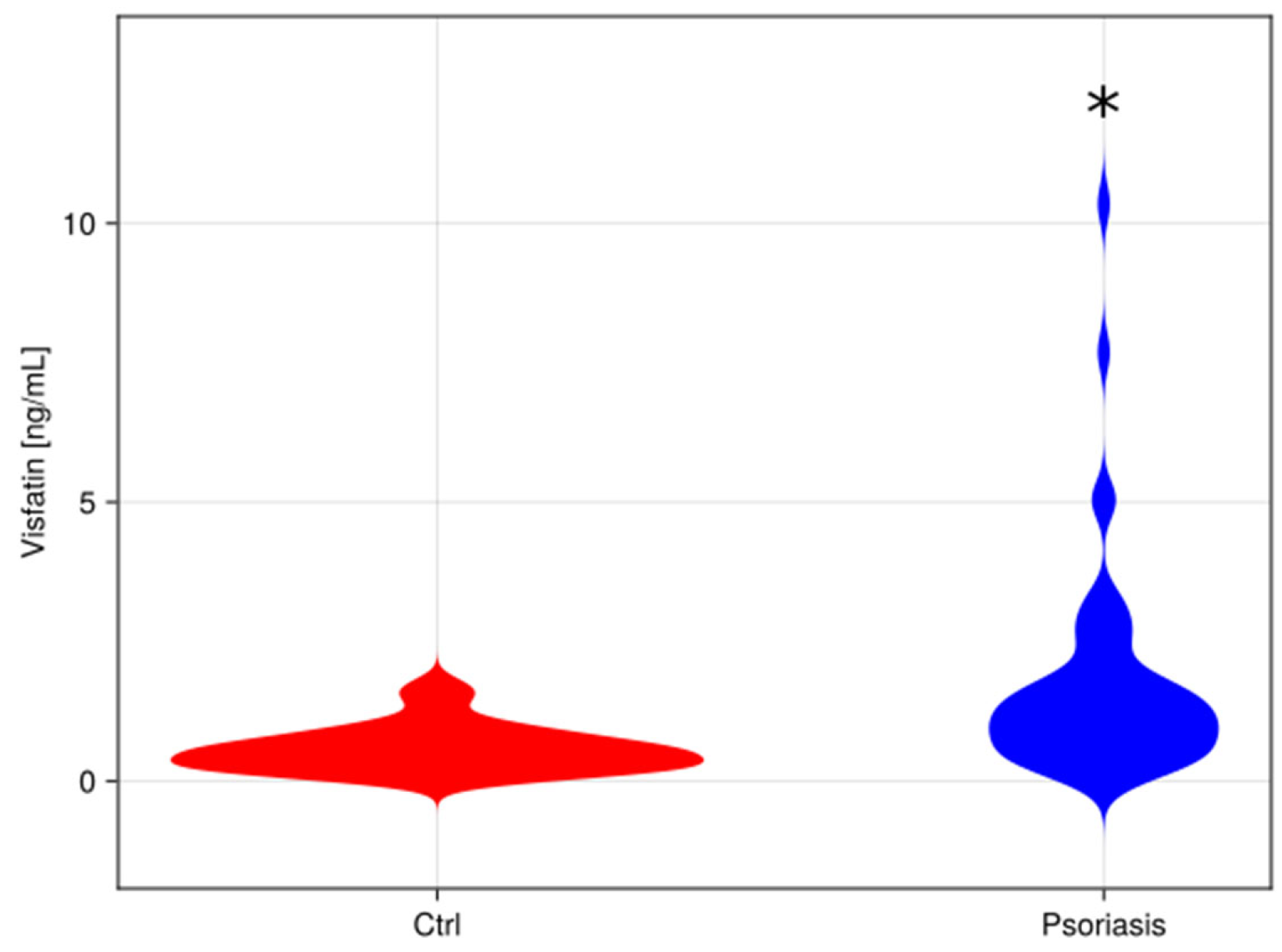
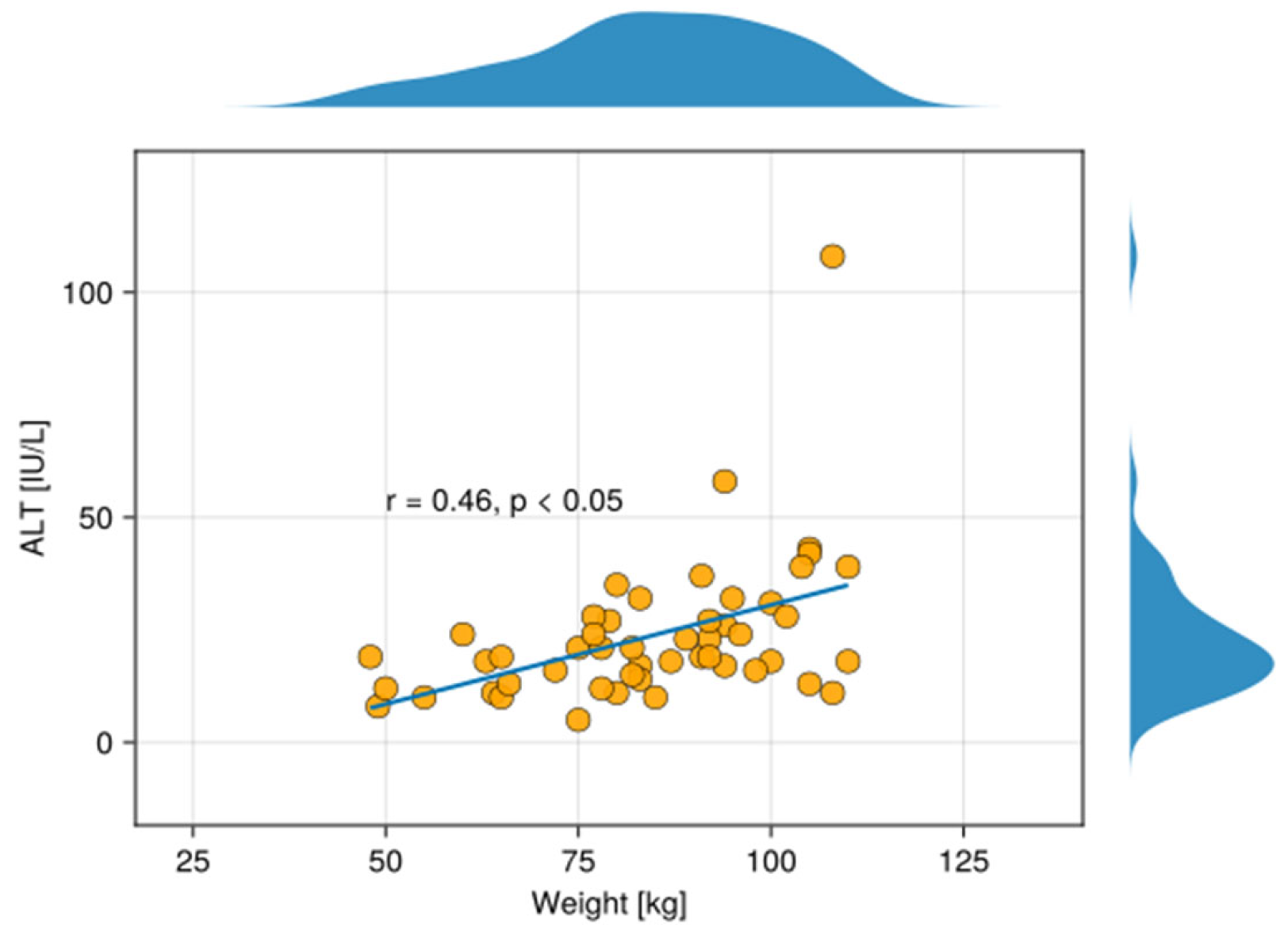
3. Results
3.1. Study Population
3.2. Visfatin Parameter
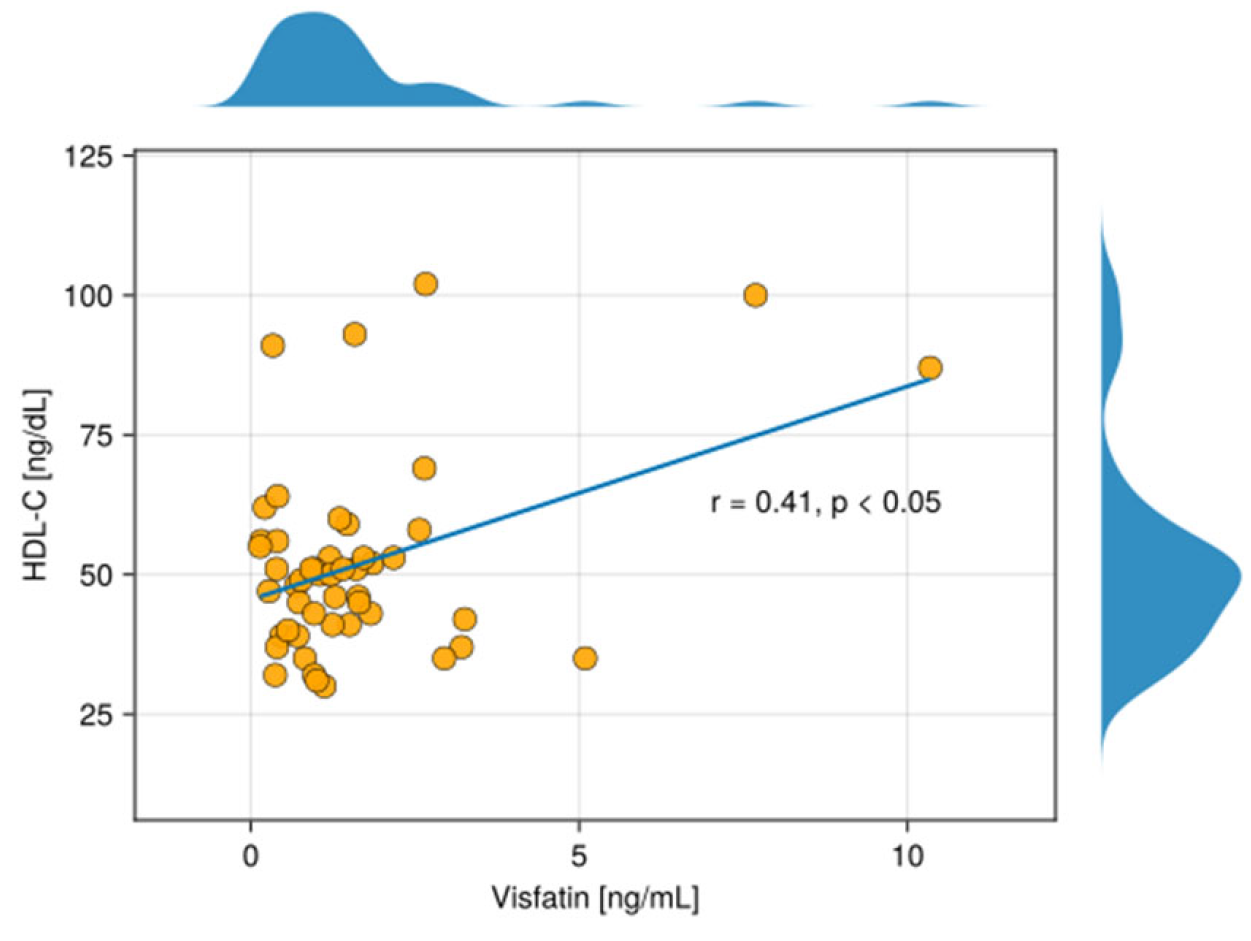
4. Discussion
The Role of Visfatin
The Link Between Visfatin and HDL-C
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MetS | metabolic syndrome |
| IFN-α | interferon α |
| TNF-α | tumour necrosis factor α |
| NAMPT | nicotinamide phosphoribosyltransferase |
| BMI | body mass index |
| CRP | C-reactive protein |
| TG | triacylglycerol |
| AST | Aspartate transaminase |
| ALT | Alanine transaminase |
References
- Sieminska, I.; Pieniawska, M.; Grzywa, T.M. The Immunology of Psoriasis—Current Concepts in Pathogenesis. Clin. Rev. Allergy Immunol. 2024, 66, 164–191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nussbaum, L.; Chen, Y.L.; Ogg, G.S. Role of regulatory T cells in psoriasis pathogenesis and treatment. Br. J. Dermatol. 2020, 184, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Report on Psoriasis; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Parisi, R.; Iskandar, I.Y.K.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.M.; Ashcroft, D.M.; Global Psoriasis Atlas. National, regional, and worldwide epidemiology of psoriasis: Systematic analysis and modelling study. BMJ 2020, 369, m1590. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iskandar, I.Y.K.; Parisi, R.; Griffiths, C.E.M.; Ashcroft, D.M.; The Global Psoriasis Atlas. Systematic review examining changes over time and variation in the incidence and prevalence of psoriasis by age and gender*. Br. J. Dermatol. 2020, 184, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Snekvik, I.; Smith, C.H.; Nilsen, T.I.L.; Langan, S.M.; Modalsli, E.H.; Romundstad, P.R.; Saunes, M. Obesity, Waist Circumference, Weight Change, and Risk of Incident Psoriasis: Prospective Data from the HUNT Study. J. Investig. Dermatol. 2017, 137, 2484–2490. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, A.; Yamagishi, K.; Imano, H.; Kiyama, M.; Cui, R.; Ohira, T.; Umesawa, M.; Muraki, I.; Sankai, T.; Saito, I.; et al. Impact of Hypertension and Subclinical Organ Damage on the Incidence of Cardiovascular Disease Among Japanese Residents at the Population and Individual Levels ― The Circulatory Risk in Communities Study (CIRCS). Circ. J. 2017, 81, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.T.; Shin, D.B.; Hubbard, R.A.; Noe, M.H.; Mehta, N.N.; Gelfand, J.M. Psoriasis and the risk of diabetes: A prospective population-based cohort study. J. Am. Acad. Dermatol. 2018, 78, 315–322.e1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gisondi, P.; Targher, G.; Zoppini, G.; Girolomoni, G. Non-alcoholic fatty liver disease in patients with chronic plaque psoriasis. J. Hepatol. 2009, 51, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, A.; Skov, L.; Joshi, A.A.; Mallbris, L.; Gislason, G.H.; Wu, J.J.; Rodante, J.; Lerman, J.B.; Ahlman, M.A.; Gelfand, J.M.; et al. The relationship between duration of psoriasis, vascular inflammation, and cardiovascular events. J. Am. Acad. Dermatol. 2017, 77, 650–656.e3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, L.; Cai, X.C.; Sun, X.Y.; Zhou, Y.Q.; Jin, M.Z.; Wang, J.; Ma, T.; Li, B.; Li, X. Global prevalence of metabolic syndrome in patients with psoriasis in the past two decades: Current evidence. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Suryavanshi, M.; Davidson, D.; Patel, V.; Jain, A.; Seigel, L. Economic Burden of Comorbidities in Patients with Psoriasis in the USA. Dermatol. Ther. 2022, 13, 207–219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raharja, A.; Mahil, S.K.; Barker, J.N. Psoriasis: A brief overview. Clin. Med. 2021, 21, 170–173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sobolev, V.V.; Soboleva, A.G.; Denisova, E.V.; Pechatnikova, E.A.; Dvoryankova, E.; Korsunskaya, I.M.; Mezentsev, A. Proteomic Studies of Psoriasis. Biomedicines 2022, 10, 619. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kromann, B.; Olsson, A.; Zhang, Y.M.; Løvendorf, M.B.; Skov, L.; Dyring-Andersen, B. Proteins in the Skin and Blood in Patients with Psoriasis: A Systematic Review of Proteomic Studies. Dermatology 2023, 240, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Protein adducts with lipid peroxidation products in patients with psoriasis. Redox Biol. 2023, 63, 102729. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zou, Q.; Si, J.; Guo, Y.; Yu, J.; Shi, H. Association between serum visfatin levels and psoriasis and their correlation with disease severity: A meta-analysis. J. Int. Med Res. 2021, 49, 3000605211002381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Minh, V.; Chau, N.; Trinh, H.; Xuan, H. Serum Visfatin Level in Psoriasis Patients: A Case-Control Study. Open Dermatol. J. 2024, 18, e18743722311288. [Google Scholar] [CrossRef]
- Tie, W.; Ma, T.; Liu, J.; Yi, Z.; Xiong, H.; Bai, J.; Li, Y.; Li, L.; Zhang, L. Visfatin promotes multiple myeloma cell proliferation and inhibits apoptosis by inducing IL-6 production via NF-κB pathways. Discov. Oncol. 2025, 16, 826. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jing, Z.; Xing, J.; Chen, X.; Stetler, R.A.; Weng, Z.; Gan, Y.; Zhang, F.; Gao, Y.; Chen, J.; Leak, R.K.; et al. Neuronal NAMPT is released after cerebral ischemia and protects against white matter injury. J. Cereb. Blood Flow Metab. 2014, 34, 1613–1621. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soncini, D.; Caffa, I.; Zoppoli, G.; Cea, M.; Cagnetta, A.; Passalacqua, M.; Mastracci, L.; Boero, S.; Montecucco, F.; Sociali, G.; et al. Nicotinamide phosphoribosyltransferase promotes epithelial-to-mesenchymal transition as a soluble factor independent of its enzymatic activity. J. Biol. Chem. 2014, 289, 34189–34204. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Audrito, V.; Serra, S.; Brusa, D.; Mazzola, F.; Arruga, F.; Vaisitti, T.; Coscia, M.; Maffei, R.; Rossi, D.; Wang, T.; et al. Extracellular nicotinamide phosphoribosyltransferase (NAMPT) promotes M2 macrophage polarization in chronic lymphocytic leukemia. Blood 2015, 125, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Schilling, E.; Hauschildt, S. Extracellular ATP induces P2X7-dependent nicotinamide phosphoribosyltransferase release in LPS-activated human monocytes. Innate Immun. 2012, 18, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Rajput, P.K.; Sharma, J.R.; Yadav, U.C.S. Cellular and molecular insights into the roles of visfatin in breast cancer cells plasticity programs. Life Sci. 2022, 304, 120706. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.C. Updated Functional Roles of NAMPT in Carcinogenesis and Therapeutic Niches. Cancers 2022, 14, 2059. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moschen, A.R.; Kaser, A.; Enrich, B.; Mosheimer, B.; Theurl, M.; Niederegger, H.; Tilg, H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J. Immunol. 2007, 178, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Stofkova, A. Resistin and visfatin: Regulators of insulin sensitivity, inflammation and immunity. Endocr. Regul. 2010, 44, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Hau, C.S.; Tada, Y.; Tatsuta, A.; Sato, S.; Watanabe, S. Visfatin enhances CXCL8, CXCL10, and CCL20 production in human keratinocytes. Endocrinology 2011, 152, 3155–3164. [Google Scholar] [CrossRef] [PubMed]
- Hau, C.S.; Kanda, N.; Noda, S.; Tatsuta, A.; Kamata, M.; Shibata, S.; Asano, Y.; Sato, S.; Watanabe, S.; Tada, Y. Visfatin enhances the production of cathelicidin antimicrobial peptide, human β-defensin-2, human β-defensin-3, and S100A7 in human keratinocytes and their orthologs in murine imiquimod-induced psoriatic skin. Am. J. Pathol. 2013, 182, 1705–1717. [Google Scholar] [CrossRef] [PubMed]
- Adya, R.; Tan, B.K.; Punn, A.; Chen, J.; Randeva, H.S. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: Novel insights into visfatin-induced angiogenesis. Cardiovasc. Res. 2008, 78, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lu, X.; Nakamura, M.; Sekhon, S.; Jeon, C.; Bhutani, T.; Liao, W. A Pilot Study to Assess the Reliability of Digital Image-Based PASI Scores Across Patient Skin Tones and Provider Training Levels. Dermatol. Ther. 2022, 12, 1685–1695. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ardiansyah, D.; Avianto, D. The Implementation of a Body Mass Index (BMI) Calculator in an Android-Based Ideal Body Check and Nutrition Consultation Application. Int. J. Eng. Technol. Nat. Sci. 2024, 6, 105–120. [Google Scholar] [CrossRef]
- Mercurio, L.; Morelli, M.; Scarponi, C.; Scaglione, G.L.; Pallotta, S.; Avitabile, D.; Albanesi, C.; Madonna, S. Enhanced NAMPT-Mediated NAD Salvage Pathway Contributes to Psoriasis Pathogenesis by Amplifying Epithelial Auto-Inflammatory Circuits. Int. J. Mol. Sci. 2021, 22, 6860. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martínez-Morcillo, F.J.; Cantón-Sandoval, J.; Martínez-Navarro, F.J.; Cabas, I.; Martínez-Vicente, I.; Armistead, J.; Hatzold, J.; López-Muñoz, A.; Martínez-Menchón, T.; Corbalán-Vélez, R.; et al. NAMPT-derived NAD+ fuels PARP1 to promote skin inflammation through parthanatos cell death. PLoS Biol. 2021, 19, e3001455. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ismail, S.A.; Mohamed, S.A. Serum levels of visfatin and omentin-1 in patients with psoriasis and their relation to disease severity. Br. J. Dermatol. 2012, 167, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Okan, G.; Baki, A.M.; Yorulmaz, E.; Doğru-Abbasoğlu, S.; Vural, P. Serum Visfatin, Fetuin-A, and Pentraxin 3 Levels in Patients with Psoriasis and Their Relation to Disease Severity. J. Clin. Lab. Anal. 2015, 30, 284–289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zou, A.; Chen, Y.; Liu, T.; Yang, T.; Zhou, B. Identification and verification of three autophagy-related genes as potential biomarkers for the diagnosis of psoriasis. Sci. Rep. 2023, 13, 22918. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bai, R.; Wu, S.; Liu, X.; Xing, Z.; Luo, R.; Zhang, W.; Liu, M.; Ma, X.; Lei, H.; Wang, N.; et al. Bioinformatic Analysis to Identify and Cellular Experiments to Validate Autophagy-related Genes in Psoriasis. Comb. Chem. High Throughput Screen. 2024, 27, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Dağdelen, D.; Karadag, A.S.; Kasapoğlu, E.; Wang, J.V.; Erman, H. Correlation of metabolic syndrome with serum omentin-1 and visfatin levels and disease severity in psoriasis and psoriatic arthritis. Dermatol. Ther. 2020, 33, e14378. [Google Scholar] [CrossRef] [PubMed]
- Brazzelli, V.; Maffioli, P.; Bolcato, V.; Ciolfi, C.; D’ANgelo, A.; Tinelli, C.; Derosa, G. Psoriasis and Diabetes, a Dangerous Association: Evaluation of Insulin Resistance, Lipid Abnormalities, and Cardiovascular Risk Biomarkers. Front. Med. 2021, 8, 605691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Surdacka, K.M.C.; Bartosińska, J.; Kowal, M.; Przepiórka-Kosińśka, J.; Krasowska, D.; Chodorowska, G. Assessment of visfatin concentrations in the serum of male psoriatic patients in relation to metabolic abnormalities. Adv. Clin. Exp. Med. 2020, 29, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Coban, M.; Tasli, L.; Turgut, S.; Özkan, S.; Ata, M.T.; Akın, F. Association of Adipokines, Insulin Resistance, Hypertension and Dyslipidemia in Patients with Psoriasis Vulgaris. Ann. Dermatol. 2016, 28, 74–79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Madaudo, C.; Bono, G.; Ortello, A.; Astuti, G.; Mingoia, G.; Galassi, A.R.; Sucato, V. Dysfunctional High-Density Lipoprotein Cholesterol and Coronary Artery Disease: A Narrative Review. J. Pers. Med. 2024, 14, 996. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balak, D.M.W.; Piaserico, S.; Kasujee, I. Non-Alcoholic Fatty Liver Disease (NAFLD) in Patients with Psoriasis: A Review of the Hepatic Effects of Systemic Therapies. Psoriasis 2021, 11, 151–168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bekkelund, S.I.; Jorde, R. Alanine Aminotransferase and Body Composition in Obese Men and Women. Dis. Markers 2019, 2019, 1695874. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruiz, J.R.; Lasa, A.; Simon, E.; Larrarte, E.; Labayen, I. Lower plasma NAMPT/visfatin levels are associated with impaired hepatic mitochondrial function in non-diabetic obese women: A potential link between obesity and non-alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2012, 22, e1–e2. [Google Scholar] [CrossRef] [PubMed]
| Control | Psoriasis | Clinical and Laboratory Features |
|---|---|---|
| 42.0 (37.5–47.2) | 51.0 (34.2–66.0) | Age [years] |
| 69.50 (63.8–79.2) | 84.0 (75.5–95.8) * | Weight [kg] |
| 165.0 (161.5–170.2) | 172.5 (164.2–176.0) * | Height [cm] |
| 25.1 (23.5–27.9) | 29.0 (23.9–31.8) * | BMI [kg/m2] |
| 1.0 (1.0–2.0) | 3.2 (1.5–6.9) * | CRP [mg/dL] |
| 86.5 (78.8–91.0) | 85.0 (80.0–93.0) | Glucose [mg/dL] |
| 73.0 (67.5–82.0) | 116.0 (86.2–134.5) | TG [mg/dL] |
| 17.5 (15.0–21.0) | 20.0 (16.2–27.0) * | AST [U/L] |
| 15.5 (11.5–18.2) | 19.0 (14.2–27.8) * | ALT [U/L] |
| 24/4 | 20/30 * | Sex [no. female/no. male] |
| Coef. | Lower 95% | Upper 95% | p-Value | |
|---|---|---|---|---|
| (Intercept) | −5.292 | −8.734 | −1.85 | <0.05 * |
| Weight [kg] | 0.059 | 0.016 | 0.103 | <0.05 * |
| Visfatin [ng/mL] | 1.555 | 0.41 | 2.701 | <0.05 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matwiejuk, M.; Kulczyńska-Przybik, A.; Łukaszuk, B.; Myśliwiec, H.; Myśliwiec, P.; Chabowski, A.; Mroczko, B.; Flisiak, I. The Role of Visfatin/NAMPT in the Pathogenesis of Psoriasis. Metabolites 2025, 15, 590. https://doi.org/10.3390/metabo15090590
Matwiejuk M, Kulczyńska-Przybik A, Łukaszuk B, Myśliwiec H, Myśliwiec P, Chabowski A, Mroczko B, Flisiak I. The Role of Visfatin/NAMPT in the Pathogenesis of Psoriasis. Metabolites. 2025; 15(9):590. https://doi.org/10.3390/metabo15090590
Chicago/Turabian StyleMatwiejuk, Mateusz, Agnieszka Kulczyńska-Przybik, Bartłomiej Łukaszuk, Hanna Myśliwiec, Piotr Myśliwiec, Adrian Chabowski, Barbara Mroczko, and Iwona Flisiak. 2025. "The Role of Visfatin/NAMPT in the Pathogenesis of Psoriasis" Metabolites 15, no. 9: 590. https://doi.org/10.3390/metabo15090590
APA StyleMatwiejuk, M., Kulczyńska-Przybik, A., Łukaszuk, B., Myśliwiec, H., Myśliwiec, P., Chabowski, A., Mroczko, B., & Flisiak, I. (2025). The Role of Visfatin/NAMPT in the Pathogenesis of Psoriasis. Metabolites, 15(9), 590. https://doi.org/10.3390/metabo15090590









