Plasma Metabolomic Profiling Reveals Systemic Alterations in a Mouse Model of Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Usage
2.2. Plasma Metabolomic Analysis Using UPLC-MS/MS
2.3. Sample Preparation
2.4. Chromatographic Conditions
2.5. Data Preprocessing and Metabolite Identification
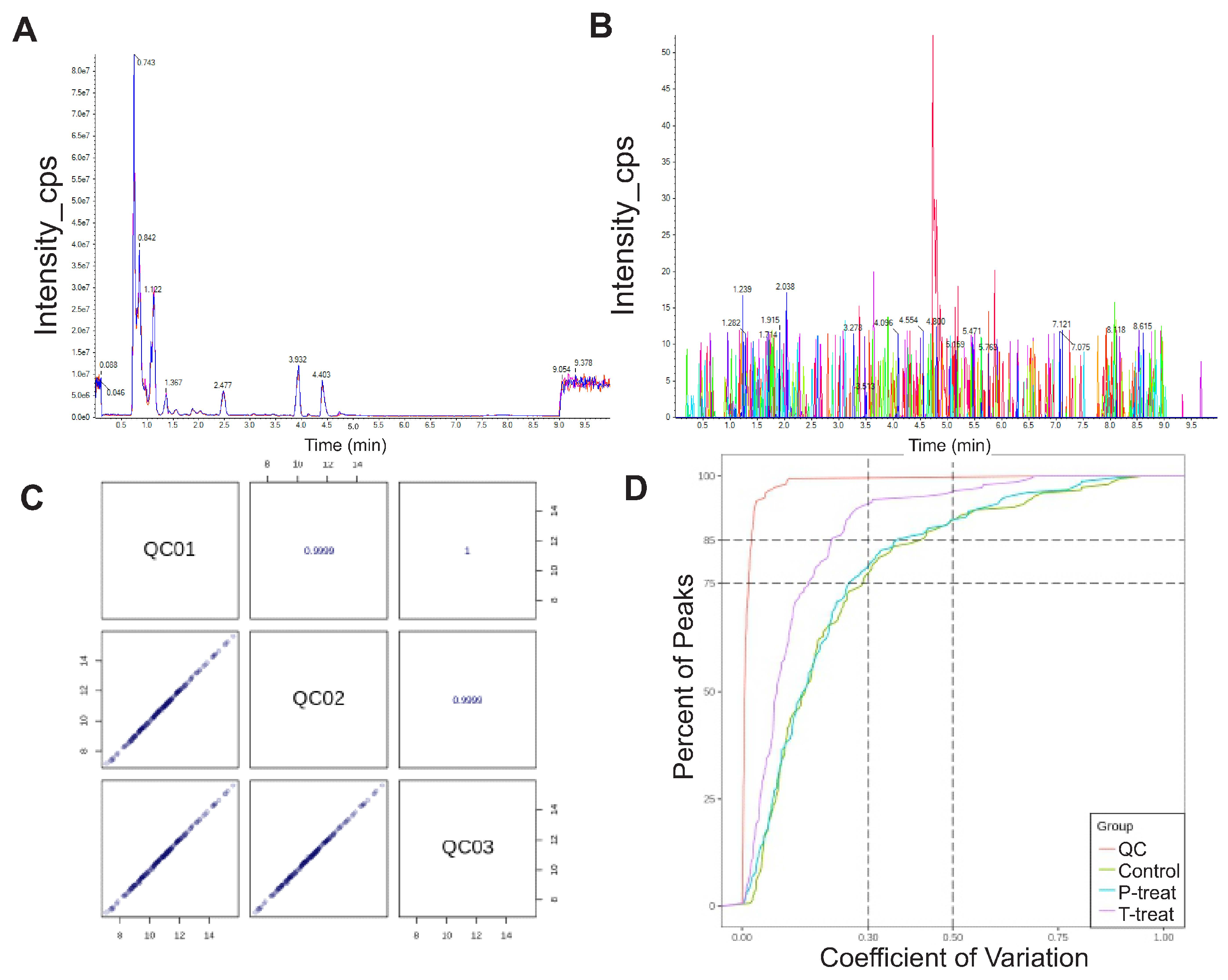
2.6. Data Quality Evaluation
2.7. Statistical Analysis
3. Results
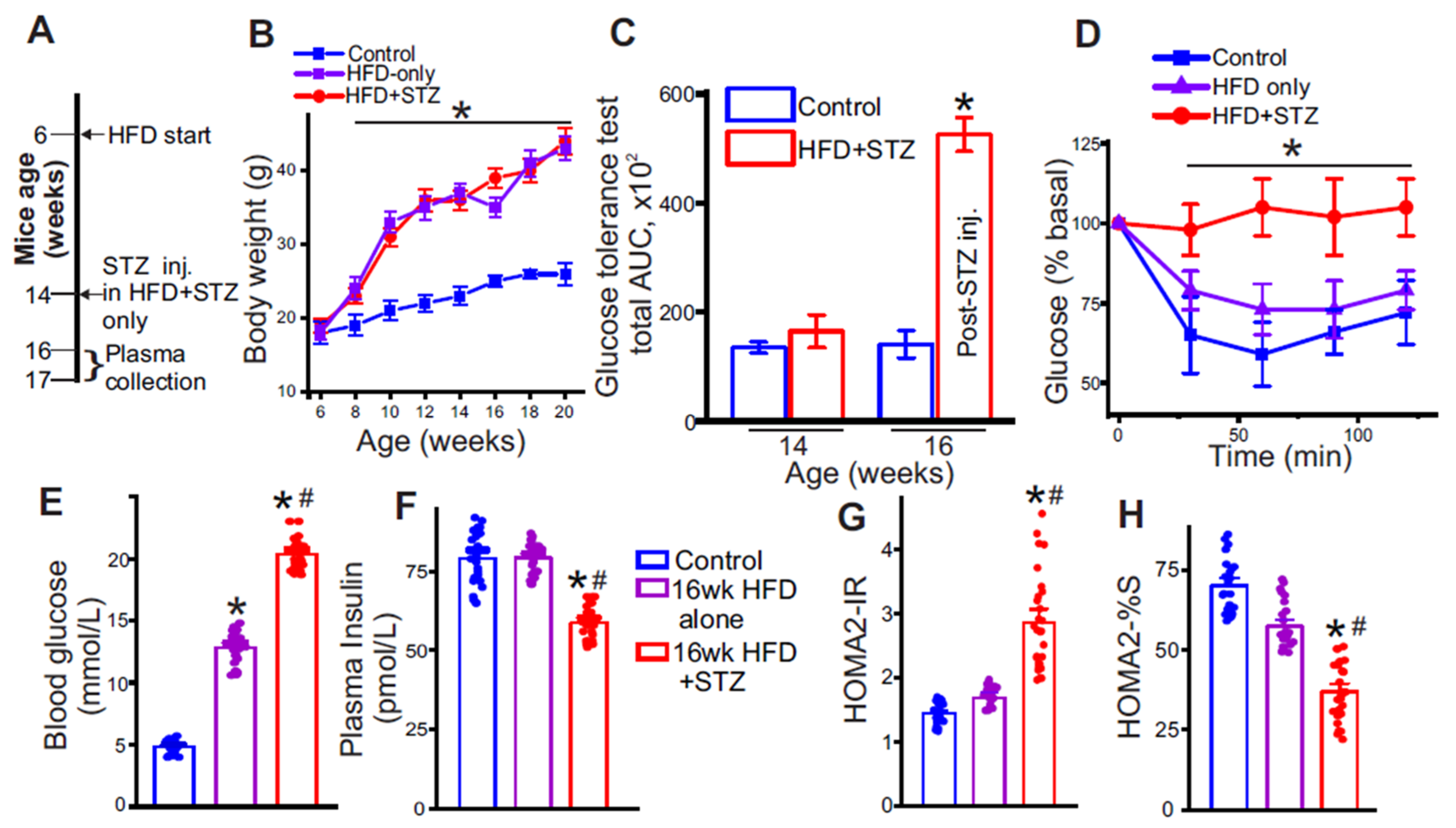
3.1. Low-Dose Streptozotocin Reliably Induces Insulin-Resistance in High-Fat Diet-Fed Mice
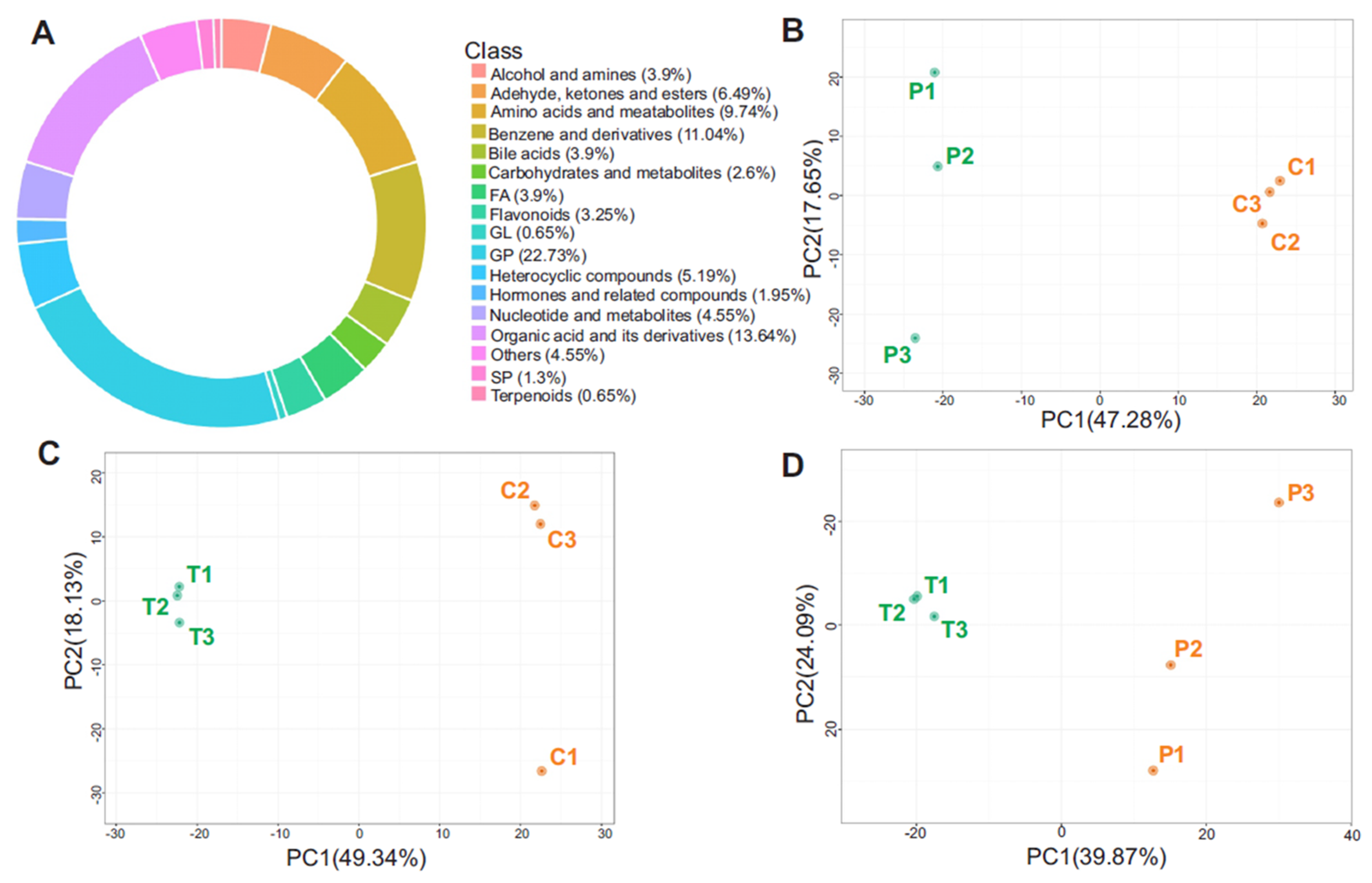
3.2. Metabolite Class Distribution and PCA Highlight Systemic Metabolic Remodeling
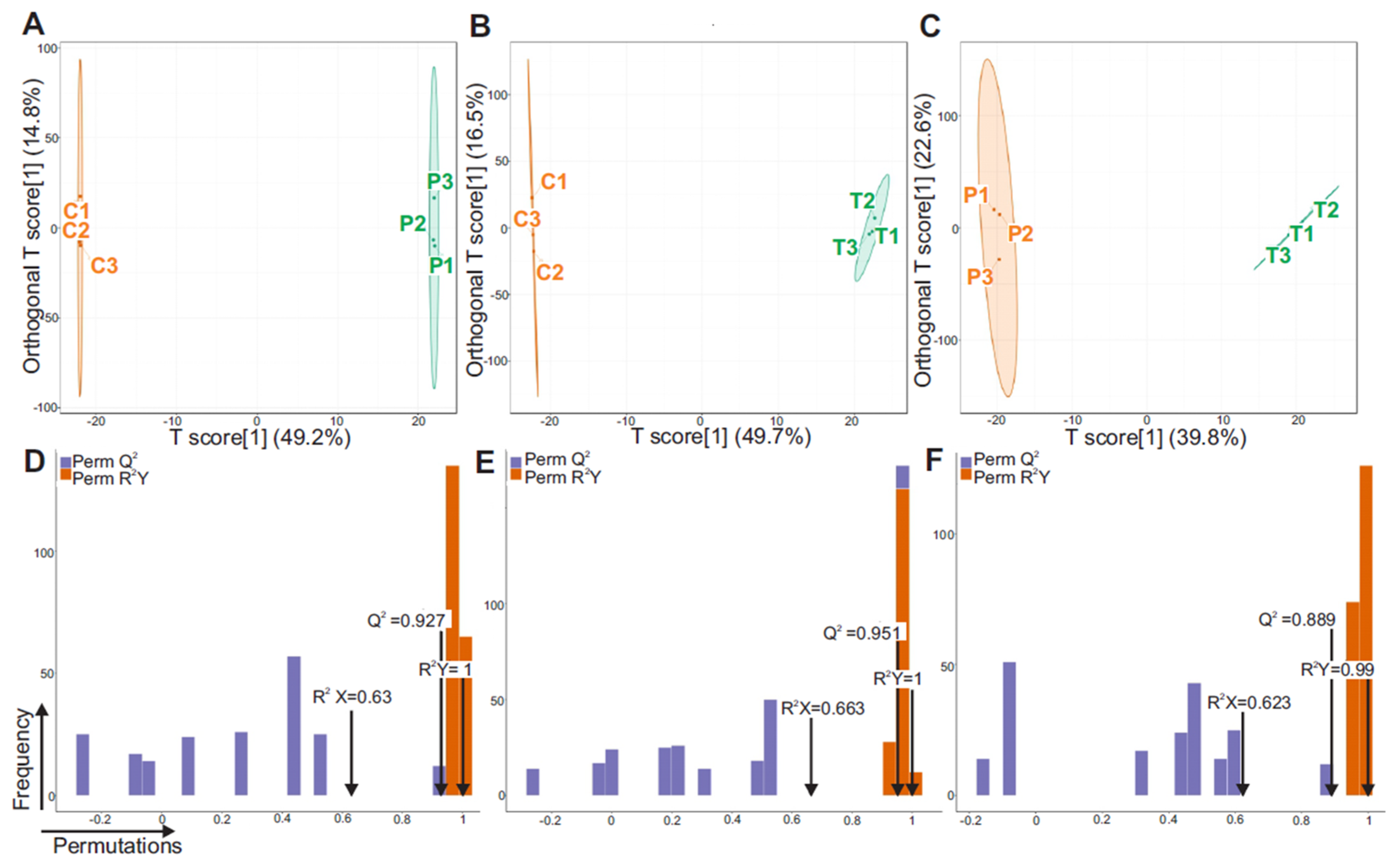
3.3. Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA)
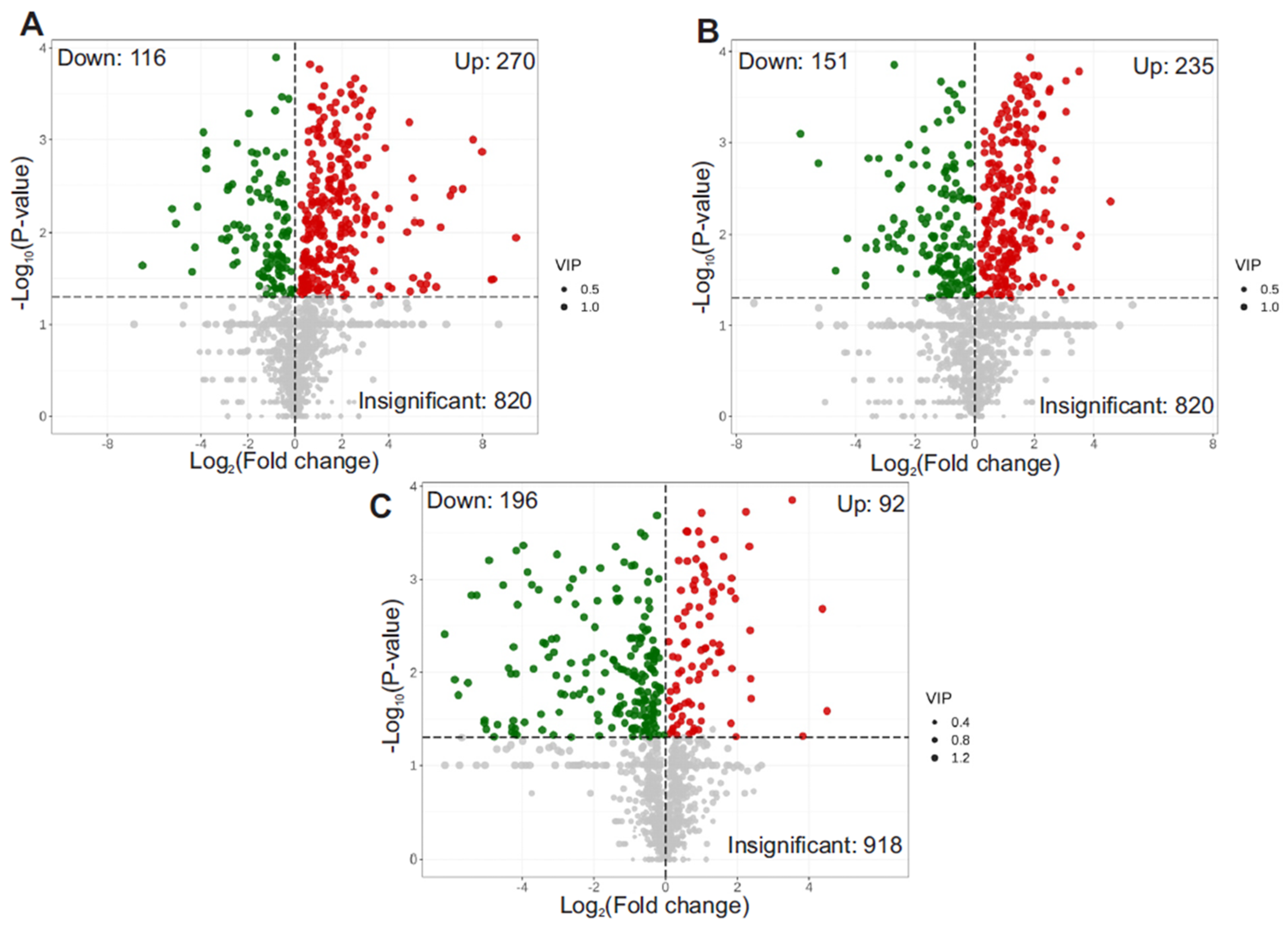
3.4. Volcano Plot of Differential Metabolites
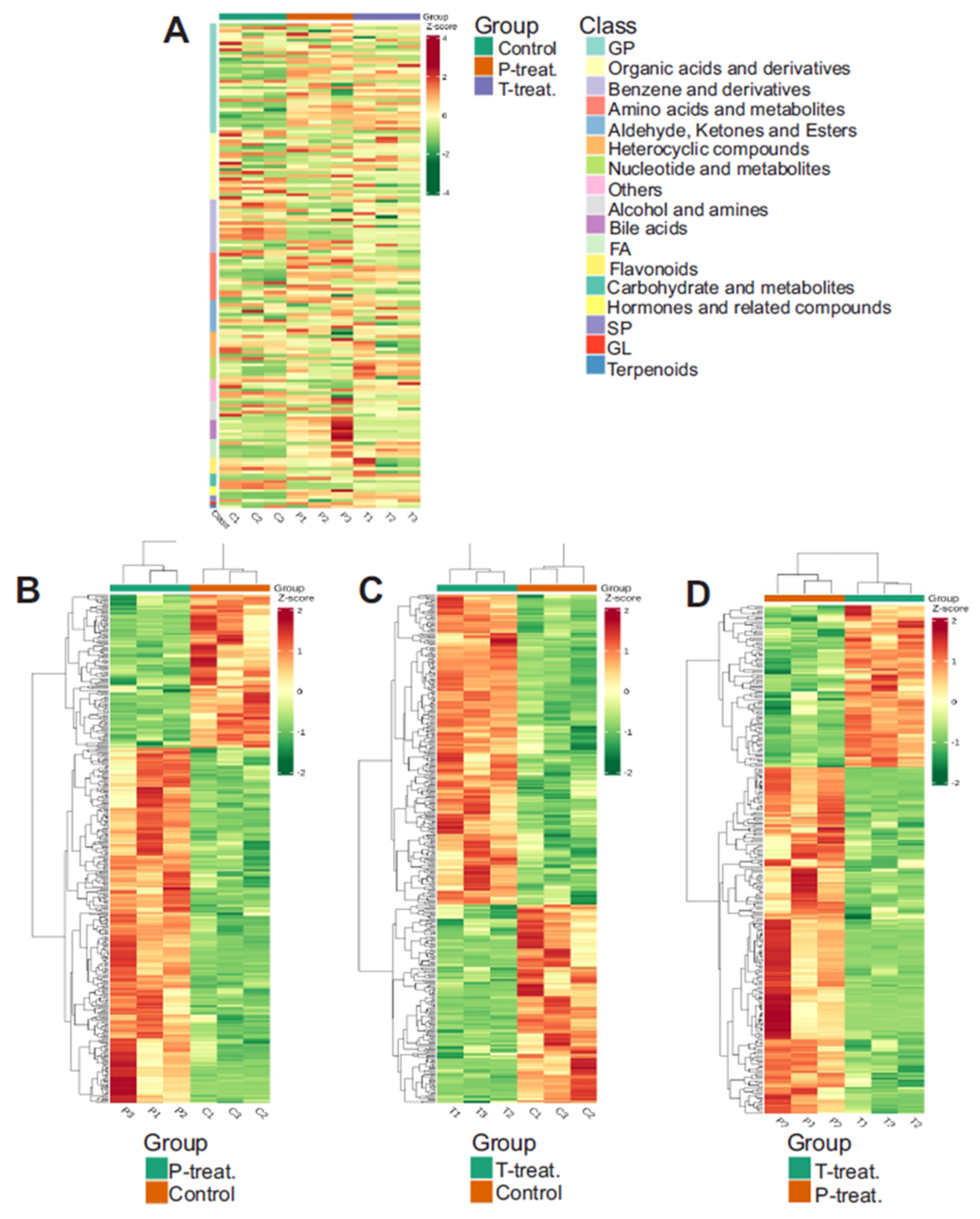
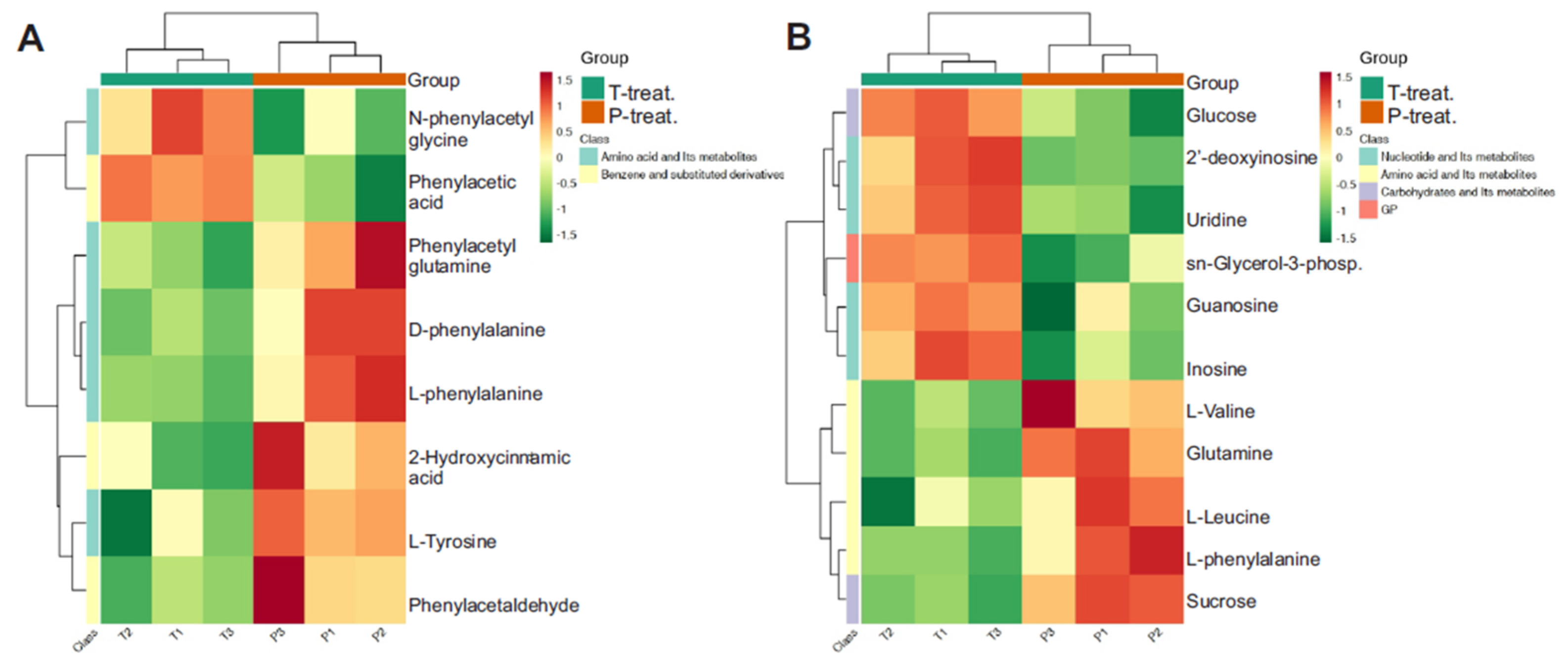
3.5. Heatmap of Differential Metabolite Abundance
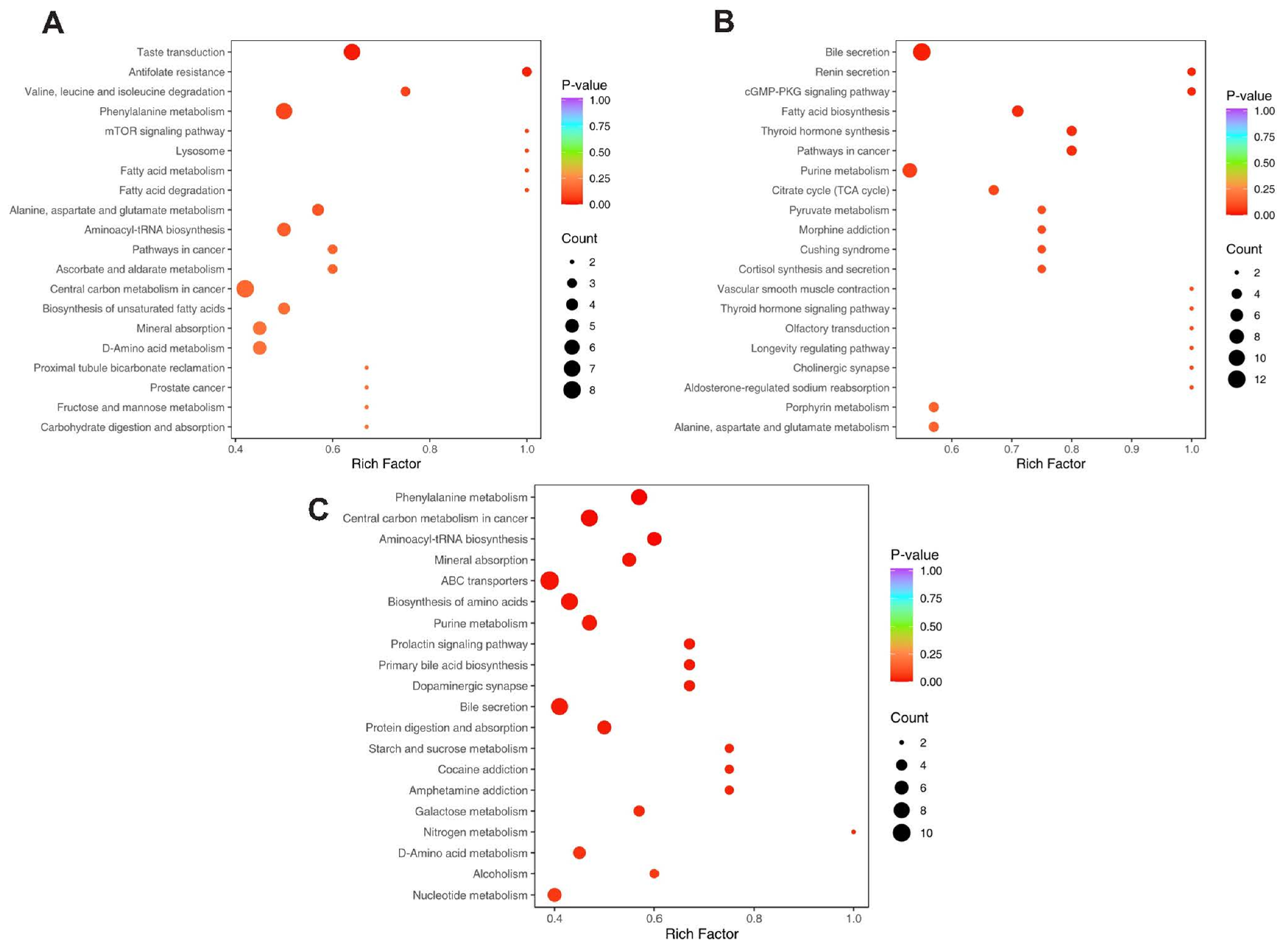
3.6. KEGG Pathway Enrichment
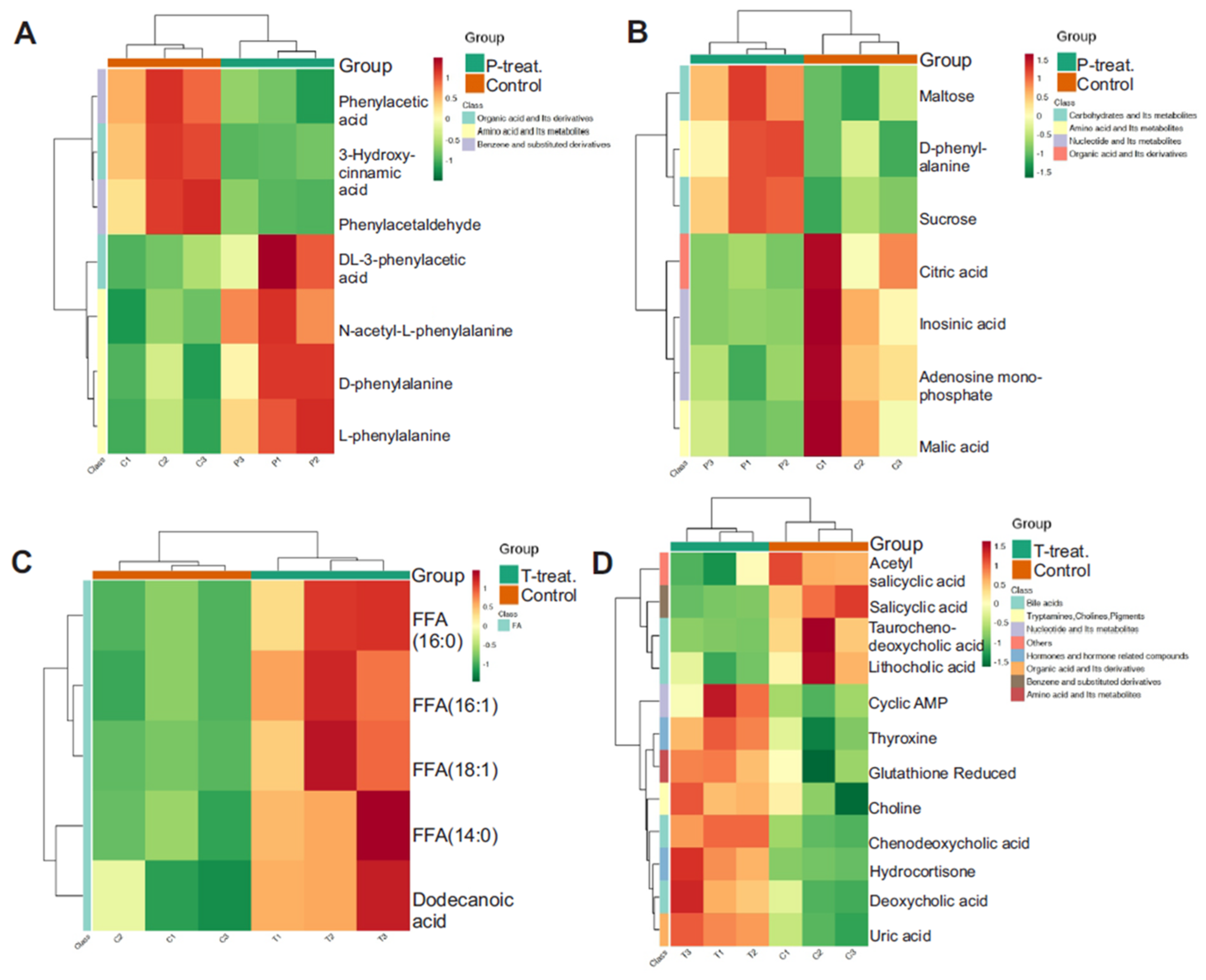
3.7. Pathway-Level Clustering Reveals Key Metabolic Disruptions in Diabetes
3.8. HMDB-Based Disease and Pathway Annotation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FA | Fatty Acid |
| T2D | Type 2 Diabetes |
| HFD | High-Fat Diet |
| STZ | Streptozotocin |
| IR | Insulin Resistance |
| LCMS | Liquid Chromatography Mass Spectrometry |
References
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. 15 May 2024. Available online: https://www.cdc.gov/diabetes/php/data-research/index.html (accessed on 17 August 2025).
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef]
- Green, J.B. Understanding the type 2 diabetes mellitus and cardiovascular disease risk paradox. Postgrad. Med. 2014, 126, 190–204. [Google Scholar] [CrossRef]
- Kalofoutis, C.; Piperi, C.; Kalofoutis, A.; Harris, F.; Phoenix, D.; Singh, J. Type II diabetes mellitus and cardiovascular risk factors: Current therapeutic approaches. Exp. Clin. Cardiol. 2007, 12, 17–28. [Google Scholar]
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Available online: https://pubmed.ncbi.nlm.nih.gov/12485966/ (accessed on 1 August 2025).
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Benabdelkamel, H.; Sebaa, R.; AlMalki, R.H.; Masood, A.; Alfadda, A.A.; Abdel Rahman, A.M. Untargeted metabolomics reveals the impact of Liraglutide treatment on metabolome profiling and metabolic pathways in type-2 diabetes mellitus. Saudi. Pharm. J. 2024, 32, 102172. [Google Scholar] [CrossRef] [PubMed]
- Galaviz, K.I.; Narayan, K.M.V.; Lobelo, F.; Weber, M.B. Lifestyle and the Prevention of Type 2 Diabetes: A Status Report. Am. J. Lifestyle Med. 2018, 12, 4–20. [Google Scholar] [CrossRef]
- Clee, S.M.; Attie, A.D. The genetic landscape of type 2 diabetes in mice. Endocr. Rev. 2007, 28, 48–83. [Google Scholar] [CrossRef] [PubMed]
- King, A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liao, J.K. A mouse model of diet-induced obesity and insulin resistance. Methods. Mol. Biol. 2012, 821, 421–433. [Google Scholar] [CrossRef]
- Leiter, E.H. Selecting the “right” mouse model for metabolic syndrome and type 2 diabetes research. Methods. Mol. Biol. 2009, 560, 1–17. [Google Scholar] [CrossRef]
- Brishti, M.A.; Raghavan, S.; Lamar, K.; Singh, U.P.; Collier, D.M.; Leo, M.D. Diabetic Endothelial Cell Glycogen Synthase Kinase 3β Activation Induces VCAM1 Ectodomain Shedding. Int. J. Mol. Sci. 2023, 24, 14105. [Google Scholar] [CrossRef]
- Raghavan, S.; Brishti, M.A.; Bernardelli, A.; Mata-Daboin, A.; Jaggar, J.H.; Leo, M.D. Extracellular glucose and dysfunctional insulin receptor signaling independently upregulate arterial smooth muscle TMEM16A expression. Am. J. Physiol. Cell. Physiol. 2024, 326, C1237–C1247. [Google Scholar] [CrossRef] [PubMed]
- Mosser, R.E.; Maulis, M.F.; Moullé, V.S.; Dunn, J.C.; Carboneau, B.A.; Arasi, K.; Pappan, K.; Poitout, V.; Gannon, M. High-fat diet-induced β-cell proliferation occurs prior to insulin resistance in C57Bl/6J male mice. Am. J. Physiol.-Endocrinol. Metab. 2015, 308, E573–E582. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Ghosh, S.K.; Choudhury, Y. A murine model of type 2 diabetes mellitus developed using a combination of high fat diet and multiple low doses of streptozotocin treatment mimics the metabolic characteristics of type 2 diabetes mellitus in humans. J. Pharmacol. Toxicol. Methods. 2017, 84, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Parilla, J.H.; Willard, J.R.; Barrow, B.M.; Zraika, S. A Mouse Model of Beta-Cell Dysfunction as Seen in Human Type 2 Diabetes. J. Diabetes. Res. 2018, 2018, 6106051. [Google Scholar] [CrossRef]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Innovation: Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef]
- Liu, X.; Locasale, J.W. Metabolomics: A Primer. Trends. Biochem. Sci. 2017, 42, 274–284. [Google Scholar] [CrossRef]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef]
- Heinken, A.; Thiele, I. Systems biology of host-microbe metabolomics. Wiley Interdiscip. Rev. Syst. Biol. Med. 2015, 7, 195–219. [Google Scholar] [CrossRef]
- Crestani, E.; Harb, H.; Charbonnier, L.M.; Leirer, J.; Motsinger-Reif, A.; Rachid, R.; Phipatanakul, W.; Kaddurah-Daouk, R.; Chatila, T.A. Untargeted metabolomic profiling identifies disease-specific signatures in food allergy and asthma. J. Allergy. Clin. Immunol. 2020, 145, 897–906. [Google Scholar] [CrossRef]
- Li, S.; Song, Z.; Fan, C.; Zhang, W.; Ma, T.; Li, X.; Zhang, Q.; Zhao, M.; Yu, T.; Li, S. Potential of FGF21 in type 2 diabetes mellitus treatment based on untargeted metabolomics. Biochem. Pharmacol. 2024, 225, 116306. [Google Scholar] [CrossRef]
- Banimfreg, B.H.; Shamayleh, A.; Alshraideh, H.; Semreen, M.H.; Soares, N.C. Untargeted approach to investigating the metabolomics profile of type 2 diabetes emiratis. J. Proteom. 2022, 269, 104718. [Google Scholar] [CrossRef]
- Jimenez-Luna, C.; Martin-Blazquez, A.; Dieguez-Castillo, C.; Diaz, C.; Martin-Ruiz, J.L.; Genilloud, O.; Vicente, F.; Del Palacio, J.P.; Prados, J.; Caba, O. Novel Biomarkers to Distinguish between Type 3c and Type 2 Diabetes Mellitus by Untargeted Metabolomics. Metabolites 2020, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Auguet, T.; Bertran, L.; Capellades, J.; Abelló, S.; Aguilar, C.; Sabench, F.; Del Castillo, D.; Correig, X.; Yanes, O.; Richart, C. LC/MS-Based Untargeted Metabolomics Analysis in Women with Morbid Obesity and Associated Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 7761. [Google Scholar] [CrossRef] [PubMed]
- Nagy, C.; Einwallner, E. Study of In Vivo Glucose Metabolism in High-fat Diet-fed Mice Using Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT). J. Vis. Exp. 2018, 131, 56672. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care. 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Leiter, E.H. Multiple low-dose streptozotocin-induced hyperglycemia and insulitis in C57BL mice: Influence of inbred background, sex, and thymus. Proc. Natl. Acad. Sci. USA 1982, 79, 630–634. [Google Scholar] [CrossRef]
- Gilbert, E.R.; Fu, Z.; Liu, D. Development of a nongenetic mouse model of type 2 diabetes. Exp. Diabetes Res. 2011, 2011, 416254. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef]
- Heydemann, A. An Overview of Murine High Fat Diet as a Model for Type 2 Diabetes Mellitus. J. Diabetes Res. 2016, 2016, 2902351. [Google Scholar] [CrossRef]
- Lai, M.; Chandrasekera, P.C.; Barnard, N.D. You are what you eat, or are you? The challenges of translating high-fat-fed rodents to human obesity and diabetes. Nutr. Diabetes 2014, 4, e135. [Google Scholar] [CrossRef]
- Austin, G.L.; Ogden, L.G.; Hill, J.O. Trends in carbohydrate, fat, and protein intakes and association with energy intake in normal-weight, overweight, and obese individuals: 1971–2006. Am. J. Clin. Nutr. 2011, 93, 836–843. [Google Scholar] [CrossRef]
- Floegel, A.; Stefan, N.; Yu, Z.; Mühlenbruch, K.; Drogan, D.; Joost, H.G.; Fritsche, A.; Häring, H.U.; Hrabě de Angelis, M.; Peters, A.; et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013, 62, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Rhee, E.P.; Larson, M.G.; Lewis, G.D.; McCabe, E.L.; Shen, D.; Palma, M.J.; Roberts, L.D.; Dejam, A.; Souza, A.L.; et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012, 125, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef]
- Yoon, M.S. The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients 2016, 8, 405. [Google Scholar] [CrossRef]
- Zhu, Y.; Dwidar, M.; Nemet, I.; Buffa, J.A.; Sangwan, N.; Li, X.S.; Anderson, J.T.; Romano, K.A.; Fu, X.; Funabashi, M.; et al. Two distinct gut microbial pathways contribute to meta-organismal production of phenylacetylglutamine with links to cardiovascular disease. Cell Host. Microbe. 2023, 31, 18–32.e19. [Google Scholar] [CrossRef]
- Rizo-Roca, D.; Henderson, J.D.; Zierath, J.R. Metabolomics in cardiometabolic diseases: Key biomarkers and therapeutic implications for insulin resistance and diabetes. J. Intern. Med. 2025, 297, 584–607. [Google Scholar] [CrossRef]
- Song, Y.; Wei, H.; Zhou, Z.; Wang, H.; Hang, W.; Wu, J.; Wang, D.W. Gut microbiota-dependent phenylacetylglutamine in cardiovascular disease: Current knowledge and new insights. Front. Med. 2024, 18, 31–45. [Google Scholar] [CrossRef]
- Shahisavandi, M.; Wang, K.; Ghanbari, M.; Ahmadizar, F. Exploring Metabolomic Patterns in Type 2 Diabetes Mellitus and Response to Glucose-Lowering Medications-Review. Genes 2023, 14, 1464. [Google Scholar] [CrossRef]
- Deng, K.; Gupta, D.K.; Shu, X.O.; Lipworth, L.; Zheng, W.; Cai, H.; Cai, Q.; Yu, D. Circulating Metabolite Profiles and Risk of Coronary Heart Disease Among Racially and Geographically Diverse Populations. Circ. Genom. Precis. Med. 2024, 17, e004437. [Google Scholar] [CrossRef] [PubMed]
- Delrue, C.; Speeckaert, R.; Moresco, R.N.; Speeckaert, M.M. Cyclic Adenosine Monophosphate Signaling in Chronic Kidney Disease: Molecular Targets and Therapeutic Potentials. Int. J. Mol. Sci. 2024, 25, 9441. [Google Scholar] [CrossRef] [PubMed]
- Yarwood, S.J. Special Issue on “New Advances in Cyclic AMP Signalling”—An Editorial Overview. Cells 2020, 9, 2274. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.H.; Sun, J.L.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.J.; Pieper, K.S.; Haynes, C.; Hauser, E.R.; Kraus, W.E.; Granger, C.B.; et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am. Heart J. 2012, 163, 844–850.e841. [Google Scholar] [CrossRef]
- Boden, G. Obesity and free fatty acids. Endocrinol. Metab. Clin. N. Am. 2008, 37, 635–646. [Google Scholar] [CrossRef]
- Itani, S.I.; Ruderman, N.B.; Schmieder, F.; Boden, G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 2002, 51, 2005–2011. [Google Scholar] [CrossRef]
- Khatiwada, S.; Sah, S.K.; Kc, R.; Baral, N.; Lamsal, M. Thyroid dysfunction in metabolic syndrome patients and its relationship with components of metabolic syndrome. Clin. Diabetes Endocrinol. 2016, 2, 3. [Google Scholar] [CrossRef]
- Kupczyk, D.; Bilski, R.; Kozakiewicz, M.; Studzińska, R.; Kędziora-Kornatowska, K.; Kosmalski, T.; Pedrycz-Wieczorska, A.; Głowacka, M. 11β-HSD as a New Target in Pharmacotherapy of Metabolic Diseases. Int. J. Mol. Sci. 2022, 23, 8984. [Google Scholar] [CrossRef]
- Lee, J.H.; Meyer, E.J.; Nenke, M.A.; Lightman, S.L.; Torpy, D.J. Cortisol, Stress, and Disease-Bidirectional Associations; Role for Corticosteroid-Binding Globulin? J. Clin. Endocrinol. Metab. 2024, 109, 2161–2172. [Google Scholar] [CrossRef]
- Gherghina, M.E.; Peride, I.; Tiglis, M.; Neagu, T.P.; Niculae, A.; Checherita, I.A. Uric Acid and Oxidative Stress-Relationship with Cardiovascular, Metabolic, and Renal Impairment. Int. J. Mol. Sci. 2022, 23, 3188. [Google Scholar] [CrossRef]
| Upregulated | Downregulated | |
|---|---|---|
| 1 | [(2R)-1-[(Z)-hexadec-9-enoyl]oxy-3-phosphonooxypropan-2-yl] tetracosanoate | N-[(3a,5b,7b)-7-hydroxy-24-oxo-3-(sulfooxy)cholan-24-yl]-Glycine |
| 2 | 14,15-Leukotriene C4(ExC4) | Gln-Leu-Glu-Lys |
| 3 | Inosinic acid | Phe-Leu-Gln-Lys |
| 4 | Trp-Ser-Ala | His-Val-Thr-Glu-Glu |
| 5 | PE-NMe(16:1(9Z)/20:1(11Z)) | Tyr-Gln-Thr-Lys |
| 6 | Linoleylethanolamide | Leu-Tyr-Asp-Lys |
| 7 | Aldehydo-D-Galactose | Leu-Ser-Ala-Leu-Glu |
| 8 | 3-Sulfocatechol | Val-Asp-Ile-Arg |
| 9 | Symmetric dimethylarginine | Lys-Gln-Ile-Glu |
| 10 | Glucose 1-phosphate | Thr-Val-Leu-Thr-Ser |
| 11 | D-Fructose-6-phosphate | Arg-Thr-Ile-Glu |
| 12 | Inosine | Ser-Phe-Val-Lys |
| 13 | 3-Hydroxybutyric acid | Asn-Lys-Arg-Asp |
| 14 | Glucose | Tyr-Gln-Asn-Glu |
| 15 | Isobutyrylglycine | Taurohyodeoxycholic acid |
| 16 | 2′-Deoxyinosine | Ile-Phe-Gln-Glu |
| 17 | 5-phospho-alpha-D-ribose cyclic-1,2-phosphate | Tauroursodeoxycholic acid |
| 18 | 1H-Indole-3-acetaldehyde, 5-methoxy- | Tyr-Glu-Val-Lys |
| 19 | (9Z)-N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]octadec-9-enamide | Glu-Ser-Val-Pro-Glu |
| 20 | Sedoheptulose | Leu-Ala-Gly-Glu-Phe |
| 21 | Aspartyl alanine | Ser-His-Glu-Ala-Glu |
| 22 | Allantoic acid | Glu-Pro-Gly-Tyr-Ser |
| 23 | Arabinose-5-phosphate | Val-Tyr-Ser-Lys |
| 24 | Val-Phe-Lys | Taurochenodeoxycholic acid |
| 25 | Arachidonoyl Serotonin | Arg-Gln-Ser-Lys |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brishti, M.A.; Vazhappully Francis, F.; Leo, M.D. Plasma Metabolomic Profiling Reveals Systemic Alterations in a Mouse Model of Type 2 Diabetes. Metabolites 2025, 15, 564. https://doi.org/10.3390/metabo15090564
Brishti MA, Vazhappully Francis F, Leo MD. Plasma Metabolomic Profiling Reveals Systemic Alterations in a Mouse Model of Type 2 Diabetes. Metabolites. 2025; 15(9):564. https://doi.org/10.3390/metabo15090564
Chicago/Turabian StyleBrishti, Masuma Akter, Fregi Vazhappully Francis, and M. Dennis Leo. 2025. "Plasma Metabolomic Profiling Reveals Systemic Alterations in a Mouse Model of Type 2 Diabetes" Metabolites 15, no. 9: 564. https://doi.org/10.3390/metabo15090564
APA StyleBrishti, M. A., Vazhappully Francis, F., & Leo, M. D. (2025). Plasma Metabolomic Profiling Reveals Systemic Alterations in a Mouse Model of Type 2 Diabetes. Metabolites, 15(9), 564. https://doi.org/10.3390/metabo15090564







