Modeling the Sensory Characteristics of Japanese Sake Using the Sake Metabolome Analysis Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Sensory Evaluations
2.3. General Properties and Aroma Components of Sake
2.4. Sake Metabolome Analysis
2.5. Statistical Analysis
3. Results
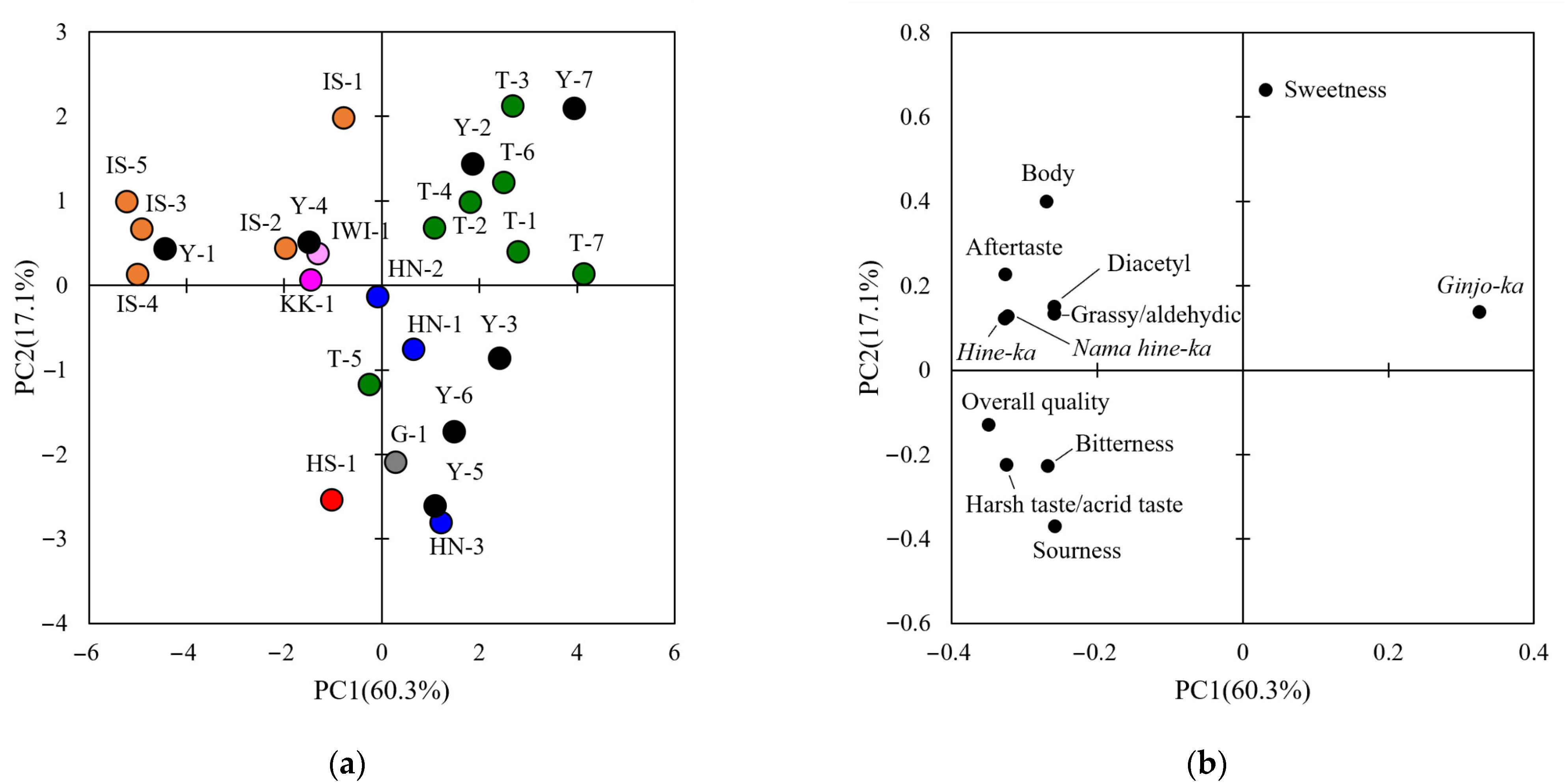
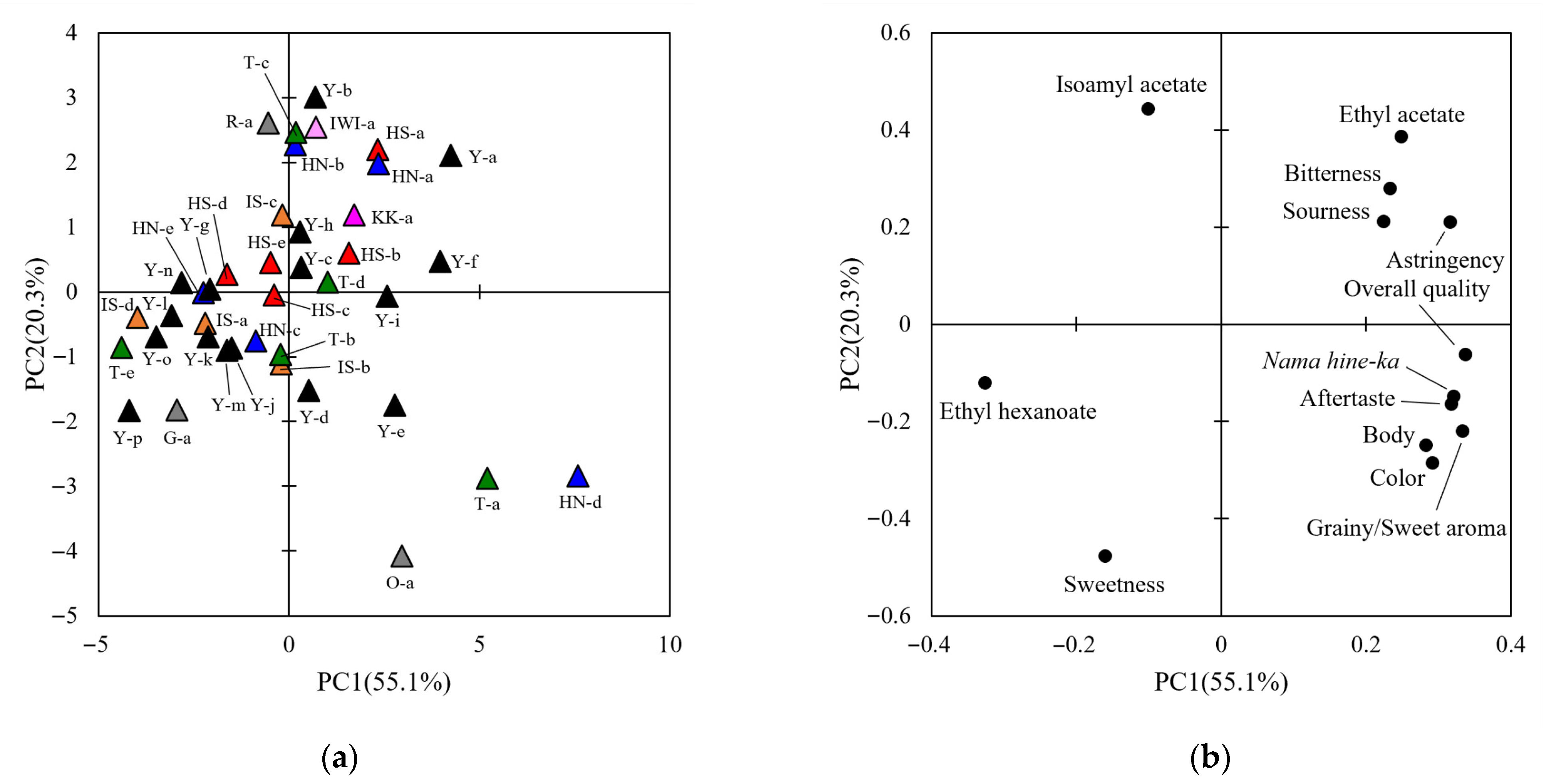
3.1. Sensory Evaluation of Sake Using QDA
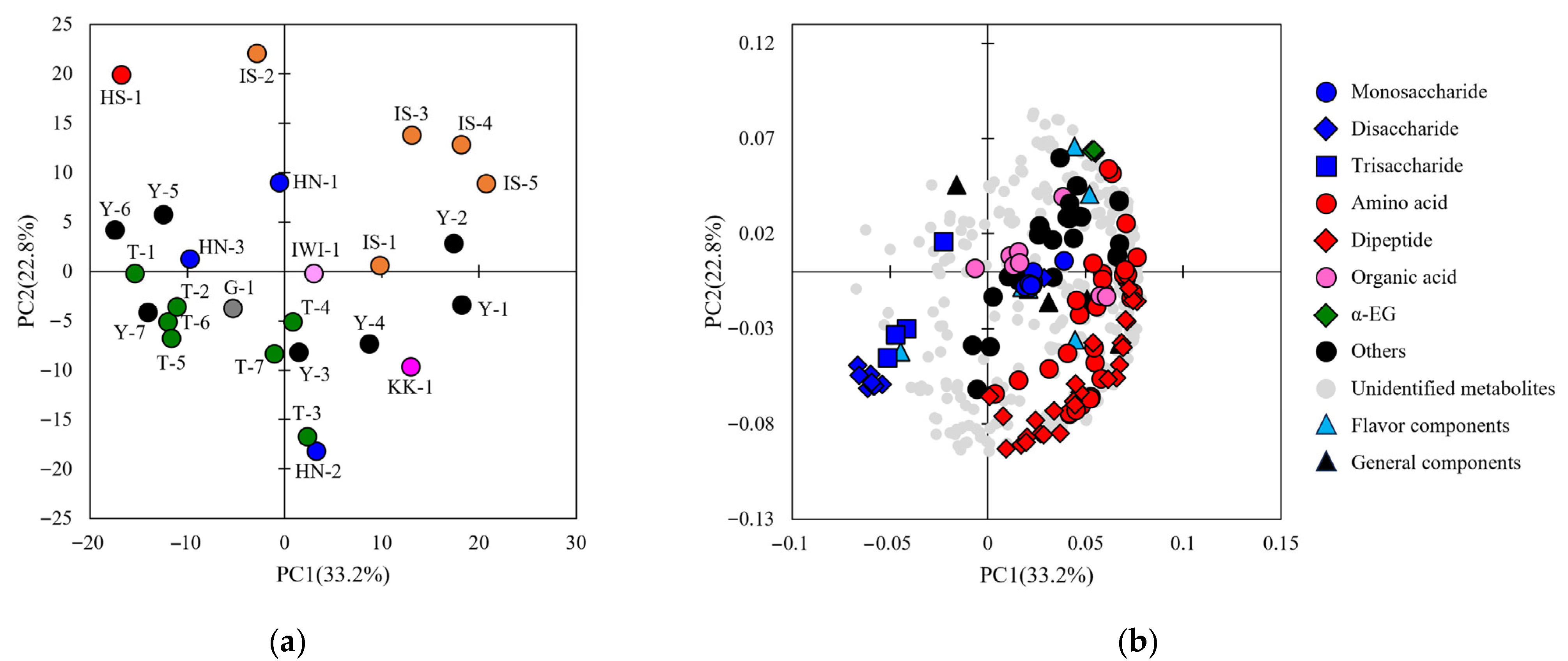
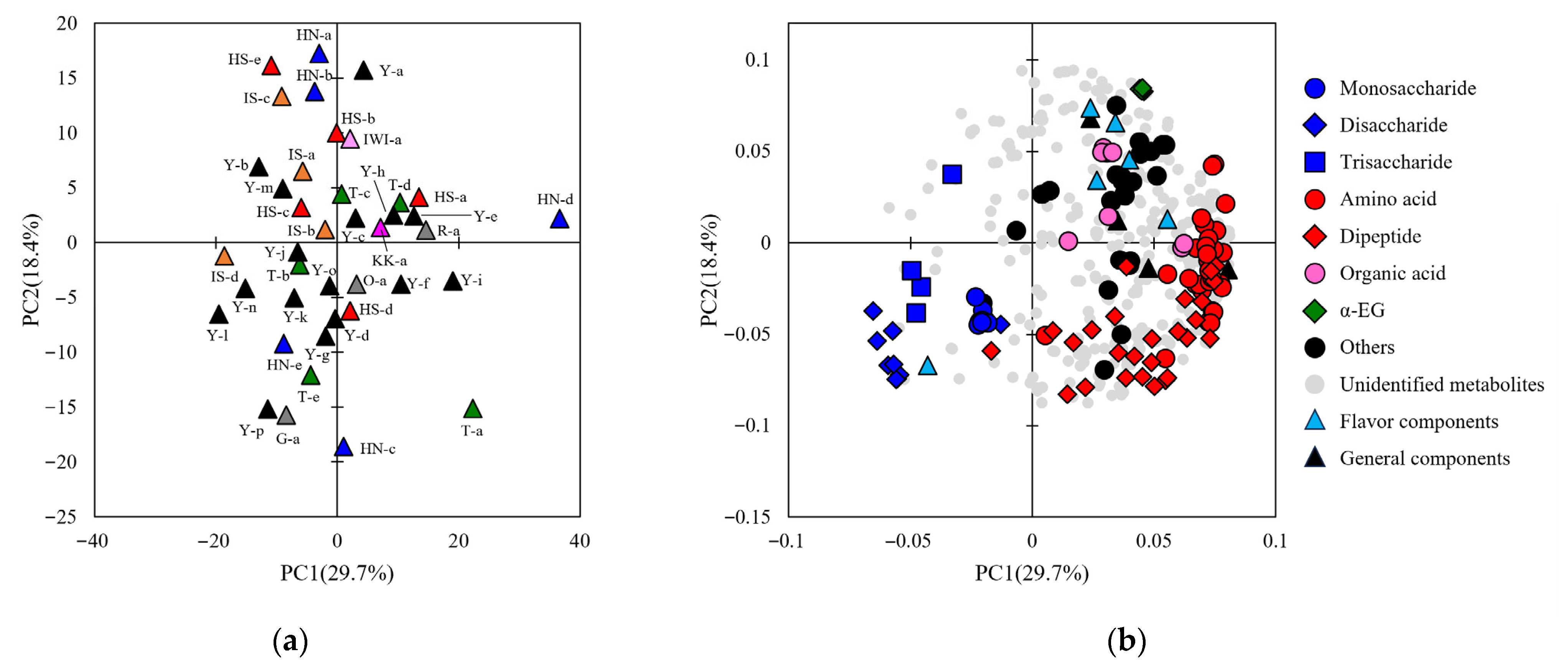
3.2. General and Comprehensive Analyses of Sake
3.3. Sake Metabolome Data Can Predict Sensory Evaluation Scores
3.4. Investigating the Correlation Between Sake Components and Sensory Evaluation Attributes
3.5. Comparison Between the Prediction Models of Different Sensory Evaluation Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| CE | Capillary electrophoresis |
| TOF-MS | Time-of-flight mass spectrometry |
| GC | Gas chromatography |
| QDA | Quantitative descriptive analysis |
| OPLS | Orthogonal projections to latent structures |
| UPLC-Q | Ultrahigh-performance liquid chromatography quadrupole |
| BY | Brewing year |
| RT | Retention time |
| PCA | Principal component analysis |
| VIPpred | Variable importance for prediction predictive |
References
- Available online: https://www.nta.go.jp/english/Report_pdf/2024.htm (accessed on 18 July 2025).
- Available online: https://www.nta.go.jp/taxes/sake/shiori-gaikyo/shiori/2025/index.htm (accessed on 18 July 2025).
- Yazawa, H.; Tokuoka, M.; Kozato, H.; Mori, Y.; Umeo, M.; Toyoura, R.; Oda, K.; Fukuda, H.; Iwashita, K. Investigation of Relationship between Sake-Making Parameters and Sake Metabolites Using a Newly Developed Sake Metabolome Analysis Method. J. Biosci. Bioeng. 2019, 128, 183–190. [Google Scholar] [CrossRef]
- Iwano, K.; Ito, T.; Nakazawa, N. Correlation Analysis of a Sensory Evaluation and the Chemical Components of Ginjyo-Shu. J. Brew. Soc. Jpn. 2005, 100, 639–649. [Google Scholar] [CrossRef]
- Yoshizawa, K.; Suzuki, D.; Shindo, H.; Kakuta, K.; Koizumi, T. Effects of Addition of Flavor Components in Sake on Sake Flavor and Taste. J. Brew. Soc. Jpn. 1997, 92, 217–223. [Google Scholar] [CrossRef]
- Ito, T.; Komatsu, Y.; Takato, A.; Takahashi, H.; Tamogami, S.; Koizumi, T.; Nakazawa, N.; Iwano, K. Tastes of the Aromatic Alcohols in Ginjyo-Syu. J. Brew. Soc. Jpn. 2008, 103, 562–569. [Google Scholar] [CrossRef]
- Ohba, T. Seisyu No Aji. J. Soc. Brew. Jpn. 1980, 75, 623–627. [Google Scholar]
- Available online: https://www.nrib.go.jp/English/kan/kaninfo.html (accessed on 1 May 2024).
- The Brewing Society of Japan. Jozo-Butsu-No-Seibun; The Brewing Society of Japan: Tokyo, Japan, 1999; pp. 2–108. [Google Scholar]
- Utsunomiya, H.; Isogai, A.; Iwata, H.; Nakano, S. Flavor Terminology and Reference Standards for Sensory Analysis of Sake. Rep. Res. Inst. Brew. 2006, 178, 45–52. [Google Scholar]
- Sato, S.; Kawashima, H.; Maruyama, Y. Studies on the Taste of Sake Part Iii. Application of Regression Models Relating Sweetness, Fullness and Chemical Date. J. Soc. Brew. Jpn. 1974, 69, 774–777. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Isogai, A.; Iwata, H. Amakara Categories for Type Designation. J. Brew. Soc. Jpn. 2004, 99, 882–889. [Google Scholar] [CrossRef]
- Putri, S.P.; Nakayama, Y.; Matsuda, F.; Uchikata, T.; Kobayashi, S.; Matsubara, A.; Fukusaki, E. Current Metabolomics: Practical Applications. J. Biosci. Bioeng. 2013, 115, 579–589. [Google Scholar] [CrossRef]
- Sugimoto, M.; Koseki, T.; Hirayama, A.; Abe, S.; Sano, T.; Tomita, M.; Soga, T. Correlation between Sensory Evaluation Scores of Japanese Sake and Metabolome Profiles. J. Agric. Food Chem. 2010, 58, 374–383. [Google Scholar] [CrossRef]
- Sugimoto, M.; Kaneko, M.; Onuma, H.; Sakaguchi, Y.; Mori, M.; Abe, S.; Soga, T.; Tomita, M. Changes in the Charged Metabolite and Sugar Profiles of Pasteurized and Unpasteurized Japanese Sake with Storage. J. Agric. Food Chem. 2012, 60, 2586–2593. [Google Scholar] [CrossRef] [PubMed]
- Mimura, N.; Isogai, A.; Iwashita, K.; Bamba, T.; Fukusaki, E. Gas Chromatography/Mass Spectrometry Based Component Profiling and Quality Prediction for Japanese Sake. J. Biosci. Bioeng. 2014, 118, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tsuchiya, F.; Isogai, A. Relationship between Medium-Chain Fatty Acid Contents and Organoleptic Properties of Japanese Sake. J. Agric. Food Chem. 2014, 62, 8478–8485. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Kabashima, F.; Tsuchiya, F. Comprehensive Two-Dimensional Gas Chromatography Coupled with Time-of-Flight Mass Spectrometry Reveals the Correlation between Chemical Compounds in Japanese Sake and Its Organoleptic Properties. J. Biosci. Bioeng. 2016, 121, 274–280. [Google Scholar] [CrossRef]
- Tatsukami, Y.; Morisaka, H.; Aburaya, S.; Aoki, W.; Kohsaka, C.; Tani, M.; Hirooka, K.; Yamamoto, Y.; Kitaoka, A.; Fujiwara, H.; et al. Metabolite Profiling of the Fermentation Process of “Yamahai-Ginjo-Shikomi” Japanese Sake. PLoS ONE 2018, 13, e0190040. [Google Scholar] [CrossRef]
- Shimofuji, S.; Matsui, M.; Muramoto, Y.; Moriyama, H.; Kato, R.; Hoki, Y.; Uehigashi, H. Machine Learning in Analyses of the Relationship between Japanese Sake Physicochemical Features and Comprehensive Evaluations. Jpn. J. Food Eng. 2020, 21, 37–50. [Google Scholar] [CrossRef]
- Tamada, Y.; Kabashima, F.; Sakurai, M.; Tokui, M.; Yamashita, N.; Kubodera, T.; Akashi, T. Modeling the Oshi-Aji Intensity of Sake Using the Component Profile Obtained from Gc/Ms-Based Non-Targeted Analysis. Seibutsu-Kogaku Kaishi 2018, 96, 234–239. [Google Scholar]
- Ichikawa, E.; Hirata, S.; Hata, Y.; Yazawa, H.; Tamura, H.; Kaneoke, M.; Iwashita, K.; Hirata, D. Analysis of Metabolites in Japanese Alcoholic Beverage Sake Made from the Sake Rice Koshitanrei. Biosci. Biotechnol. Biochem. 2019, 83, 1570–1582. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, K. Kasshoku gurasu wo mochiita kikizake ni tsuite. J. Soc. Brew. Jpn. 1965, 60, 636–637. [Google Scholar]
- Shiigi, M.; Kashiwagi, S.; Aritomi, K.; Moroki, Y. Studies on the Sensory Test of Sake Influence of Colors of Glass on the Results. J. Soc. Brew. Jpn. 1981, 76, 345–349. [Google Scholar] [CrossRef]
- The Brewing Society of Japan. Official Methods of Analysis of National Tax Administration Agency, 4th ed.; The Brewing Society of Japan: Tokyo, Japan, 1993; pp. 7–33. [Google Scholar]
- The Brewing Society of Japan. The Editorial Committee for the commentary for Brewing Standard Analysis Methods. In Commentary on Standard Analytical Methods of National Research Institute of Brewing, Japan; The Brewing Society of Japan: Tokyo, Japan, 2017; pp. 7–44. [Google Scholar]
- Mazzara, S.; Cerutti, S.; Iannaccone, S.; Conti, A.; Olivieri, S.; Alessio, M.; Pattini, L. Application of Multivariate Data Analysis for the Classification of Two Dimensional Gel Images in Neuroproteomics. J. Proteom. Bioinform. 2011, 4, 16–21. [Google Scholar] [CrossRef]
- Yamamoto, S.; Shiga, K.; Kodama, Y.; Imamura, M.; Uchida, R.; Obata, A.; Bamba, T.; Fukusaki, E. Analysis of the Correlation between Dipeptides and Taste Differences among Soy Sauces by Using Metabolomics-Based Component Profiling. J. Biosci. Bioeng. 2014, 118, 56–63. [Google Scholar] [CrossRef]
- Eriksson, L.; Kettaneh-Wold, N.; Trygg, J.; Wikström, C.; Wold, S. Multi-and Megavariate Data Analysis: Part I: Basic Principles and Applications; Umetrics Inc.: Umea, Sweden, 2006; p. 97. [Google Scholar]
- Oka, S.; Sato, S. Contribution of Ethyl & Alpha-D-Glucoside to Flavor Construction in Sake. Nippon. Nōgeikagaku Kaishi 1976, 50, 455–461. [Google Scholar]
- Stone, H.; Sidel, J.L. Sensory Evaluation Practices; Elsevier Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Kobayashi, T.; Kumazaki, T.; Morikawa, K.; Komatsu-Hata, Y.; Okuda, M.; Takahashi, M.; Saito, R.; Oda, K.; Yazawa, H.; Iwashita, K. Modeling the Sake Brewing Characteristics of Rice from Brown Rice Metabolites. J. Biosci. Bioeng. 2022, 134, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, K. Effects of Higher Fatty Acids on the Formation of Esters by Yeasts. Nippon. Nōgeikagaku Kaishi 1976, 50, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Inahashi, M.; Mutoh, T. The Questions from Kyokai Sake Yeast Users and Answers for Them Mainly on K-1801’s Questions. J. Brew. Soc. Jpn. 2008, 103, 824–835. [Google Scholar] [CrossRef]
- Okuda, M.; Joyo, M.; Bao, H.-B.; Takahashi, K.; Isogai, A.; Mukai, N.; Kishimoto, T.; Kanda, R.; Iizuka, S.; Izu, H.; et al. Relationship between Flavor Compounds in Sake Exhibited at the Annual Japan Sake Awards and Meteorological Conditions During Grain Filling and the Properties of Rice Starch. J. Brew. Soc. Jpn. 2021, 116, 839–852. [Google Scholar] [CrossRef]
- Shimofuji, S.; Matsui, M.; Muramoto, Y.; Moriyama, H.; Hoki, Y.; Uehigashi, H. Prediction of Sake Component Values Using E-Nose and E-Tongue Data by Machine Learning. Jpn. J. Food Eng. 2021, 22, 15–24. [Google Scholar] [CrossRef]
| No. | Sample ID | BY | Rice Cultivar | Rice Polishing Ratio (%) | Sake Brewery |
|---|---|---|---|---|---|
| 1 | Y-1 | H28/2016 | Yamadanishiki | 60 | Ishikawa-A |
| 2 | Y-2 | H28/2016 | Yamadanishiki | 60 | Hyogo-A |
| 3 | Y-3 | H28/2016 | Yamadanishiki | 50 | Hyogo-B |
| 4 | Y-4 | H28/2016 | Yamadanishiki | 60 | Kyoto-A |
| 5 | T-1 | H28/2016 | Yumesasara (Tochigisake-27) | Tochigi-A | |
| 6 | T-2 | H28/2016 | Yumesasara (Tochigisake-27) | Tochigi-B | |
| 7 | T-3 | H28/2016 | Yumesasara (Tochigisake-27) | Tochigi-C | |
| 8 | T-4 | H28/2016 | Yumesasara (Tochigisake-27) | 40 | Tochigi-D |
| 9 | T-5 | H28/2016 | Yumesasara (Tochigisake-27) | Tochigi-E | |
| 10 | T-6 | H28/2016 | Yumesasara (Tochigisake-27) | 40 | Tochigi-F |
| 11 | IS-1 | H28/2016 | Ishikawasake-68 | 50 | Ishikawa-A |
| 12 | IS-2 | H28/2016 | Ishikawasake-68 | 40 | Ishikawa-B |
| 13 | IS-3 | H28/2016 | Ishikawasake-68 | 50 | Ishikawa-B |
| 14 | IS-4 | H28/2016 | Ishikawasake-68 | 50 | Ishikawa-B |
| 15 | IS-5 | H28/2016 | Ishikawasake-68 | 50 | Ishikawa-B |
| 16 | IWI-1 | H28/2016 | Iwai | 60 | Kyoto-A |
| 17 | KK-1 | H28/2016 | Kyonokagayaki | 60 | Kyoto-A |
| 18 | HN-1 | H28/2016 | Hyogonishiki | 60 | Hyogo-A |
| 19 | HN-2 | H28/2016 | Hyogonishiki | 55 | Hyogo-B |
| 20 | Y-5 | H28/2016 | Yamadanishiki | 40 | Hiroshima-A |
| 21 | HN-3 | H28/2016 | Hyogonishiki | 40 | Hiroshima-A |
| 22 | HS-1 | H28/2016 | Hyogo Sake 85 | 40 | Hiroshima-A |
| 23 | G-1 | H28/2016 | Ginnosato | 40 | Hiroshima-A |
| 24 | Y-6 | H28/2016 | Yamadanishiki | 40 | Hiroshima-A |
| 25 | Y-7 | H28/2016 | Yamadanishiki | 40 | Hiroshima-A |
| 26 | T-7 | H28/2016 | Yumesasara (Tochigisake-27) | 40 | Hiroshima-A |
| 27 | HN-a | H29/2017 | Hyogonishiki | 70 | Hyogo-C |
| 28 | Y-a | H29/2017 | Yamadanishiki | 60 | Hyogo-C |
| 29 | IS-a | H29/2017 | Ishikawasake-68 | Ishikawa-C | |
| 30 | Y-b | H29/2017 | Yamadanishiki | 50 | Ishikawa-C |
| 31 | IS-b | H29/2017 | Ishikawasake-68 | 50 | Ishikawa-A |
| 32 | Y-c | H29/2017 | Yamadanishiki | 50 | Ishikawa-A |
| 33 | IS-c | H29/2017 | Ishikawasake-68 | 40 | Ishikawa-B |
| 34 | Y-d | H29/2017 | Yamadanishiki | 40 | Ishikawa-B |
| 35 | HN-b | H29/2017 | Hyogonishiki | 60 | Hyogo-A |
| 36 | Y-e | H29/2017 | Yamadanishiki | 60 | Hyogo-A |
| 37 | IWI-a | H29/2017 | Iwai | 60 | Kyoto-A |
| 38 | KK-a | H29/2017 | Kyonokagayaki | 60 | Kyoto-A |
| 39 | Y-f | H29/2017 | Yamadanishiki | 60 | Kyoto-A |
| 40 | HS-a | H29/2017 | Hyogo Sake 85 | 63 | Hyogo-D |
| 41 | R-a | H29/2017 | (undisclosed) | 63 | Hyogo-D |
| 42 | HN-c | H29/2017 | Hyogonishiki | 50 | Hyogo-B |
| 43 | Y-g | H29/2017 | Yamadanishiki | 50 | Hyogo-B |
| 44 | HS-b | H29/2017 | Hyogo Sake 85 | 60 | Hyogo-E |
| 45 | Y-h | H29/2017 | Yamadanishiki | 60 | Hyogo-E |
| 46 | HN-d | H29/2017 | Hyogonishiki | 55 | Hyogo-F |
| 47 | Y-i | H29/2017 | Yamadanishiki | 55 | Hyogo-F |
| 48 | HS-c | H29/2017 | Hyogo Sake 85 | 65 | Hyogo-G |
| 49 | Y-j | H29/2017 | Yamadanishiki | 55 | Hyogo-G |
| 50 | T-a | H29/2017 | Yumesasara (Tochigisake-27) | 55 | Tochigi-G |
| 51 | O-a | H29/2017 | Omachi | 50 | Tochigi-G |
| 52 | T-b | H29/2017 | Yumesasara (Tochigisake-27) | 40 | Tochigi-D |
| 53 | Y-k | H29/2017 | Yamadanishiki | 40 | Tochigi-D |
| 54 | T-c | H29/2017 | Yumesasara (Tochigisake-27) | 62 | Tochigi-E |
| 55 | Y-l | H29/2017 | Yamadanishiki | 40 | Tochigi-E |
| 56 | T-d | H29/2017 | Yumesasara (Tochigisake-27) | 55 | Tochigi-B |
| 57 | Y-m | H29/2017 | Yamadanishiki | 43 | Tochigi-B |
| 58 | Y-n | H29/2017 | Yamadanishiki | 40 | Hiroshima-A |
| 59 | Y-o | H29/2017 | Yamadanishiki | 60 | Hiroshima-A |
| 60 | HS-d | H29/2017 | Hyogo Sake 85 | 60 | Hiroshima-A |
| 61 | HS-e | H29/2017 | Hyogo Sake 85 | 85 | Hiroshima-A |
| 62 | IS-d | H29/2017 | Ishikawasake-68 | 40 | Hiroshima-A |
| 63 | Y-p | H29/2017 | Yamadanishiki | 40 | Hiroshima-A |
| 64 | G-a | H29/2017 | Ginnosato | 35 | Hiroshima-A |
| 65 | T-e | H29/2017 | Yumesasara (Tochigisake-27) | 40 | Hiroshima-A |
| 66 | HN-e | H29/2017 | Hyogonishiki | 40 | Hiroshima-A |
| Evaluation Attributes | Score | ||
|---|---|---|---|
| H28BY | H29BY | ||
| Appearance | |||
| Color | - | 0–5 | (colorless–deep) |
| Odor | |||
| Ginjo-ka | 0–5 | - | (none–strong) |
| Ethyl hexanoate | - | 0–5 | (none–strong) |
| Isoamyl acetate | - | 0–5 | (none–strong) |
| Hine-ka | 0–5 | - | (none–strong) |
| Nama hine-ka | 0–5 | 0–5 | (none–strong) |
| Ethyl acetate | - | 0–5 | (none–strong) |
| Fatty acid smell | 0–5 | - | (none–strong) |
| Grassy/aldehydic | 0–5 | - | (none–strong) |
| Diacetyl | 0–5 | - | (none–strong) |
| Grainy/sweet aroma | - | 0–5 | (none–strong) |
| Taste | |||
| Sweetness | 0–5 | 0–5 | (none–strong) |
| Sourness | 0–5 | 0–5 | (none–strong) |
| Body | 0–5 | 0–5 | (thin–thick) |
| Bitterness | 0–5 | 0–5 | (none–strong) |
| Astringency | - | 0–5 | (none–strong) |
| Harsh taste/acrid taste | 0–5 | - | (none–strong) |
| Aftertaste | 0–5 | 0–5 | (light–heavy) |
| Overall quality | 1–5 | 1–5 | (excellent–faulty) |
| Evaluation Attributes of Sensory Test | H28BY | H29BY | ||
|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | |
| Appearance | ||||
| Color | - | - | 24.5811 | <0.0001 |
| Odor | ||||
| Ginjo-ka | 5.9915 | <0.0001 | - | - |
| Ethyl hexanoate | - | - | 18.065 | <0.0001 |
| Isoamyl acetate | - | - | 2.4013 | <0.0001 |
| Hine-ka | 6.5311 | <0.0001 | - | - |
| Nama hine-ka | 16.6516 | <0.0001 | 9.1929 | <0.0001 |
| Ethyl acetate | - | - | 4.1869 | <0.0001 |
| Fatty acid smell | 1.0472 | 0.4016 | - | - |
| Grassy/aldehydic | 3.7718 | <0.0001 | - | - |
| Diacetyl | 2.6687 | <0.0001 | - | - |
| Grainy/sweet aroma | - | - | 7.653 | <0.0001 |
| Taste | ||||
| Sweetness | 7.2706 | <0.0001 | 9.4732 | <0.0001 |
| Sourness | 3.4983 | <0.0001 | 7.8414 | <0.0001 |
| Body | 6.3101 | <0.0001 | 6.519 | <0.0001 |
| Bitterness | 1.6625 | 0.0205 | 4.0764 | <0.0001 |
| Astringency | - | - | 2.8253 | <0.0001 |
| Harsh taste/acrid taste | 2.6811 | <0.0001 | - | - |
| Aftertaste | 4.2497 | <0.0001 | 5.6017 | <0.0001 |
| Overall quality | 9.5073 | <0.0001 | 16.7304 | <0.0001 |
| Evaluation Attributes of Sensory Test | H28BY | H29BY | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Latent Variable | R2 | RMSE | Q2 | No. of Selected Variable | CV-ANOVA p-Value | No. of Latent Variable | R2 | RMSE | Q2 | No. of Selected Variable | CV-ANOVA p-Value | |
| Color | 1 + 3 + 0 | 0.876 | 0.24 | 0.672 | 61 | 1 + 4 + 0 | 0.919 | 0.20 | 0.691 | 74 | 1.7 × 10−23 | |
| Ginjo-ka | 4.9 × 10−14 | |||||||||||
| Ethyl hexanoate | 1 + 2 + 0 | 0.834 | 0.33 | 0.712 | 60 | 2.4 × 10−28 | ||||||
| Isoamyl acetate | 1 + 3 + 0 | 0.768 | 0.15 | 0.371 | 60 | 1.2 × 10−8 | ||||||
| Hine-ka | 1 + 4 + 0 | 0.965 | 0.09 | 0.800 | 62 | 1.0 × 10−19 | ||||||
| Nama hine-ka | 1 + 5 + 0 | 0.990 | 0.09 | 0.834 | 73 | 9.5 × 10−21 | 1 + 2 + 0 | 0.826 | 0.27 | 0.646 | 50 | 2.4 × 10−23 |
| Ethyl acetate | 1 + 1 + 0 | 0.637 | 0.25 | 0.511 | 71 | 4.1 × 10−17 | ||||||
| Fatty acid smell | - | - | - | - | - | - | ||||||
| Grassy/aldehydic | 1 + 2 + 0 | 0.697 | 0.26 | 0.252 | 56 | 1.7 × 10−3 | ||||||
| Diacetyl | 1 + 0 + 0 | 0.508 | 0.19 | 0.389 | 70 | 9.7 × 10−9 | ||||||
| Grainy/sweet aroma | 1 + 3 + 0 | 0.866 | 0.22 | 0.624 | 68 | 2.2 × 10−20 | ||||||
| Sweetness | 1 + 4 + 0 | 0.950 | 0.14 | 0.682 | 52 | 3.3 × 10−13 | 1 + 1 + 0 | 0.747 | 0.26 | 0.659 | 80 | 5.4 × 10−26 |
| Sourness | 1 + 5 + 0 | 0.959 | 0.09 | 0.675 | 73 | 9.5 × 10−12 | 1 + 6 + 0 | 0.917 | 0.14 | 0.594 | 52 | 5.6 × 10−15 |
| Body | 1 + 0 + 0 | 0.690 | 0.34 | 0.615 | 40 | 2.9 × 10−16 | 1 + 1 + 0 | 0.758 | 0.22 | 0.514 | 64 | 2.8 × 10−17 |
| Bitterness | 1 + 0 + 0 | 0.450 | 0.20 | 0.258 | 49 | 1.4 × 10−5 | 1 + 0 + 0 | 0.535 | 0.24 | 0.421 | 50 | 1.3 × 10−14 |
| Astringency | 1 + 0 + 0 | 0.585 | 0.21 | 0.508 | 43 | 9.7 × 10−19 | ||||||
| Harsh taste/acrid taste | 1 + 5 + 0 | 0.950 | 0.10 | 0.610 | 65 | 2.3 × 10−9 | ||||||
| Aftertaste | 1 + 0 + 0 | 0.545 | 0.36 | 0.437 | 39 | 4.5 × 10−10 | 1 + 0 + 0 | 0.640 | 0.26 | 0.522 | 69 | 1.7 × 10−19 |
| Overall quality | 1 + 3 + 0 | 0.917 | 0.18 | 0.759 | 70 | 1.6 × 10−18 | 1 + 3 + 0 | 0.859 | 0.22 | 0.648 | 45 | 5.9 × 10−22 |
| Evaluation Attributes of Sensory Test | No. of Selected Variables in Both Years (Vippred > 1.5) | H28BY | H29BY | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| For Model Construction | For Model Validation | No. of Latent Variables | R2 of Calibration Set | RMSE of Calibration Set | Q2 of Calibration Set | R2 of Validation Set | RMSE of Validation Set | CV-ANOVA p-Value | No. of Latent Variables | R2 of Calibration Set | RMSE of Calibration Set | Q2 of Calibration Set | R2 of Validation Set | RMSE of Validation Set |
CV-ANOVA p-Value | |
| H28BY Ginjo-ka * | H29BY Ethyl hexanoate | 35 | 1 + 3 + 0 | 0.876 | 0.24 | 0.672 | 0.583 | 0.53 | 4.9 × 10−14 | 1 + 0 + 0 | 0.620 | 0.42 | 0.588 | 0.647 | 0.50 | 3.5 × 10−15 |
| H28BY Ginjo-ka * | H29BY Isoamyl acetate | 0 | 1 + 3 + 0 | 0.876 | 0.24 | 0.672 | 0.063 | 0.92 | 4.9 × 10−14 | - | - | - | - | - | - | - |
| H28BY Nama hine-ka | H29BY Nama hine-ka | 23 | 1 + 5 + 0 | 0.990 | 0.09 | 0.834 | 0.265 | 0.63 | 9.5 × 10−21 | 1 + 0 + 0 | 0.731 | 0.44 | 0.688 | 0.696 | 0.40 | 1.0 × 10−19 |
| H28BY Sweetness | H29BY Sweetness | 31 | 1 + 4 + 0 | 0.950 | 0.14 | 0.682 | 0.317 | 0.46 | 3.3 × 10−13 | 1 + 0 + 0 | 0.557 | 0.39 | 0.520 | 0.491 | 0.44 | 1.1 × 10−12 |
| H28BY Sourness | H29BY Sourness | 12 | 1 + 5 + 0 | 0.959 | 0.09 | 0.675 | 0.368 | 0.44 | 9.5 × 10−12 | 1 + 0 + 0 | 0.362 | 0.34 | 0.325 | 0.526 | 0.34 | 3.9 × 10−7 |
| H28BY Body | H29BY Body | 21 | 1 + 0 + 0 | 0.690 | 0.34 | 0.615 | 0.583 | 0.39 | 2.9 × 10−16 | 1 + 2 + 0 | 0.849 | 0.24 | 0.629 | 0.404 | 0.67 | 3.3 × 10−14 |
| H28BY Bitterness | H29BY Bitterness | 29 | 1 + 0 + 0 | 0.450 | 0.20 | 0.258 | 0.477 | 0.38 | 1.4 × 10−5 | 1 + 1 + 0 | 0.617 | 0.17 | 0.388 | 0.154 | 0.46 | 2.4 × 10−8 |
| H28BY Aftertaste | H29BY Aftertaste | 19 | 1 + 0 + 0 | 0.545 | 0.36 | 0.437 | 0.591 | 0.28 | 4.5 × 10−10 | 1 + 2 + 0 | 0.822 | 0.23 | 0.647 | 0.362 | 0.71 | 4.3 × 10−10 |
| H28BY Overall quality | H29BY Overall quality | 18 | 1 + 3 + 0 | 0.917 | 0.18 | 0.759 | 0.359 | 0.57 | 1.6 × 10−18 | 1 + 0 + 0 | 0.683 | 0.35 | 0.654 | 0.648 | 0.48 | 5.2 × 10−18 |
| H29BY Ethyl hexanoate | H28BY Ginjo-ka * | 35 | 1 + 2 + 0 | 0.834 | 0.33 | 0.712 | 0.539 | 0.50 | 2.4 × 10−28 | 1 + 1 + 0 | 0.748 | 0.41 | 0.700 | 0.592 | 0.47 | 3.3 × 10−29 |
| H29BY Isoamyl acetate | H28BY Ginjo-ka * | 0 | 1 + 3 + 0 | 0.768 | 0.15 | 0.371 | 0.001 | 0.77 | 1.2 × 10−8 | - | - | - | - | - | - | - |
| H29BY Nama hine-ka | H28BY Nama hine-ka | 23 | 1 + 2 + 0 | 0.826 | 0.27 | 0.646 | 0.558 | 0.56 | 2.4 × 10−23 | 1 + 0 + 0 | 0.702 | 0.34 | 0.688 | 0.731 | 0.47 | 2.5 × 10−30 |
| H29BY Sweetness | H28BY Sweetness | 31 | 1 + 1 + 0 | 0.747 | 0.26 | 0.659 | 0.387 | 0.53 | 5.4 × 10−26 | 1 + 0 + 0 | 0.505 | 0.36 | 0.474 | 0.558 | 0.46 | 4.8 × 10−17 |
| H29BY Sourness | H28BY Sourness | 12 | 1 + 6 + 0 | 0.917 | 0.14 | 0.594 | 0.450 | 0.31 | 5.6 × 10−15 | 1 + 0 + 0 | 0.525 | 0.33 | 0.467 | 0.348 | 0.36 | 1.0 × 10−16 |
| H29BY Body | H28BY Body | 21 | 1 + 1 + 0 | 0.758 | 0.22 | 0.514 | 0.578 | 0.42 | 2.8 × 10−17 | 1 + 0 + 0 | 0.600 | 0.28 | 0.573 | 0.597 | 0.45 | 2.5 × 10−22 |
| H29BY Bitterness | H28BY Bitterness | 29 | 1 + 0 + 0 | 0.535 | 0.24 | 0.421 | 0.317 | 0.34 | 1.3 × 10−14 | 1 + 0 + 0 | 0.471 | 0.25 | 0.438 | 0.431 | 0.36 | 2.3 × 10−15 |
| H29BY Aftertaste | H28BY Aftertaste | 19 | 1 + 0 + 0 | 0.640 | 0.26 | 0.522 | 0.429 | 0.41 | 1.7 × 10−19 | 1 + 0 + 0 | 0.611 | 0.27 | 0.599 | 0.454 | 0.40 | 6.1 × 10−24 |
| H29BY Overall quality | H28BY Overall quality | 18 | 1 + 3 + 0 | 0.859 | 0.22 | 0.648 | 0.496 | 0.58 | 5.9 × 10−22 | 1 + 1 + 0 | 0.703 | 0.32 | 0.659 | 0.650 | 0.50 | 8.7 × 10−26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, T.; Komatsu-Hata, Y.; Saito, R.; Yazawa, H.; Takahashi, M.; Oda, K.; Iwashita, K. Modeling the Sensory Characteristics of Japanese Sake Using the Sake Metabolome Analysis Method. Metabolites 2025, 15, 559. https://doi.org/10.3390/metabo15080559
Kobayashi T, Komatsu-Hata Y, Saito R, Yazawa H, Takahashi M, Oda K, Iwashita K. Modeling the Sensory Characteristics of Japanese Sake Using the Sake Metabolome Analysis Method. Metabolites. 2025; 15(8):559. https://doi.org/10.3390/metabo15080559
Chicago/Turabian StyleKobayashi, Takuji, Yuko Komatsu-Hata, Ryota Saito, Hisashi Yazawa, Masayuki Takahashi, Ken Oda, and Kazuhiro Iwashita. 2025. "Modeling the Sensory Characteristics of Japanese Sake Using the Sake Metabolome Analysis Method" Metabolites 15, no. 8: 559. https://doi.org/10.3390/metabo15080559
APA StyleKobayashi, T., Komatsu-Hata, Y., Saito, R., Yazawa, H., Takahashi, M., Oda, K., & Iwashita, K. (2025). Modeling the Sensory Characteristics of Japanese Sake Using the Sake Metabolome Analysis Method. Metabolites, 15(8), 559. https://doi.org/10.3390/metabo15080559







