Effects of IGF1 rs6214 Polymorphism and Milk Consumption on Serum Levels of IGF-1 and GH and Body Composition
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Methods
2.4. Body Composition Evaluation
2.5. Non-Dietary Covariates
2.6. Blood Sample Collection
2.7. Genotypification Assay
2.8. GH and IGF-1 Determination
2.9. Statistical Analysis
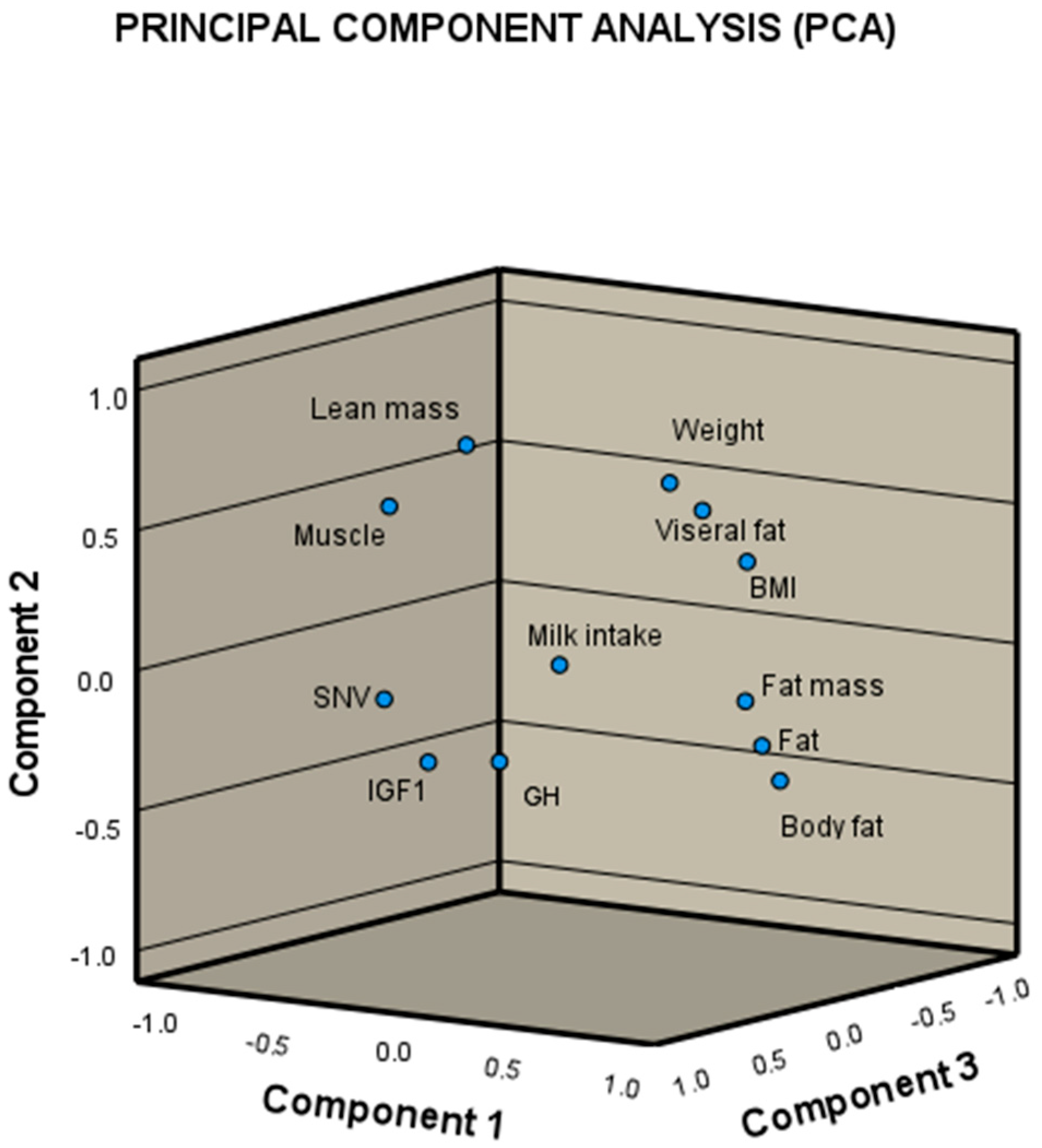
3. Results
3.1. Body Composition and GH and IGF-1 Levels
3.2. Genotyping
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | Protein Kinase B |
| BC | Body Composition |
| BIM | Body Index Mass |
| CI | Confidence Interval |
| CO | Codominant |
| CLA | Conjugated Linoleic Acid |
| CTAB | Cetyltrimethylammonium Bromide |
| dbSNP | Data Base Single Nucleotide Polymorphisms |
| DNA | Deoxyribonucleic Acid |
| DO | Dominant |
| DTAB | Dodecyltrimethylammonium Bromide |
| EDTA | Ethylenediaminetetraacetic Acid |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| ERK | Extracellular Signal-Regulated Kinases |
| GH | Growth Hormone |
| GHBP | Growth Hormone-Binding Protein |
| HDL | High-Density Lipoprotein |
| IGF1 | Insulin-Like Growth Factor Gene |
| IGF-1 | Insulin-Like Growth Factor, Type 1 Protein |
| LDL | Low-Density Lipoprotein |
| MEK | Mitogen-Activated Protein Kinase Kinase |
| mRNA | Messenger Ribonucleotide Acid |
| NFATC1 | Nuclear Factor of Activated T Cells, Cytoplasmic 1 Protein |
| OR | Odds Ratio |
| PCR | Polymerase Chain Reaction |
| PI3K | Phosphoinositide 3-Kinase |
| Raf-1 | Rapidly Accelerated Fibrosarcoma-1 |
| RE | Recessive |
| SNV | Single Nucleotide Variant |
| UTR | Untranslated Region |
References
- SSA I; GISAMAC; UNICEF. Guías Alimentarias Saludables y Sostenibles para la Población Mexicana 2023; Secretaría de Salud: México City, México, 2023.
- Grijalva-Avila, J.; Villanueva-Fierro, I.; Lares-Asseff, I.; Chairez-Hernández, I.; Rivera-Sanchez, G.; Martínez-Estrada, S.; Martínez-Rivera, I.; Quiñones, L.A.; Loera-Castañeda, V. Milk intake and IGF-1 rs6214 polymorphism as protective factors to obesity. Int. J. Food Sci. Nutr. 2020, 71, 388–393. [Google Scholar] [CrossRef]
- Wilkinson, K.R.; Tucker, L.A.; Davidson, L.E.; Bailey, B.W. Milk-Fat Intake and Differences in Abdominal Adiposity and BMI: Evidence Based on 13,544 Randomly-Selected Adults. Nutrients 2021, 13, 1832. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Xu, Y.; Yang, J.; Du, L.; Li, K.; Zhou, Y. Milk consumption and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses in humans. J. Nutr. Metab. 2021, 18, 7. [Google Scholar] [CrossRef]
- Kaplan, M.; Arslan, A.; Duman, H.; Karyelioğlu, M.; Baydemir, B.; Günar, B.B.; Alkan, M.; Bayraktar, A.; Tosun, H.I.; Ertürk, M.; et al. Production of bovine colostrum for human consumption to improve health. Front. Pharmacol. 2022, 12, 796824. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Aguirre, G.; De Ita, J.R.; De La Garza, R.; Castilla-Cortazar, I. Insulin-like growth factor-1 deficiency and metabolic syndrome. J. Transl. Med. 2016, 14, 3. [Google Scholar] [CrossRef]
- Kasprzak, A. Insulin-like growth factor 1 (IGF-1) signaling in glucose metabolism in colorectal cancer. Int. J. Mol. Sci. 2021, 22, 6434. [Google Scholar] [CrossRef]
- Akhtar, A.; Sah, S.P. Insulin signaling pathway and related molecules: Role in neurodegeneration and Alzheimer’s disease. Neurochem. Int. 2020, 135, 104707. [Google Scholar] [CrossRef]
- Rahmani, J.; Varkaneh, H.K.; Clark, C.; Zand, H.; Bawadi, H.; Ryan, P.M.; Fatahi, S.; Zhang, Y. The influence of fasting and energy restricting diets on IGF-1 levels in humans: A systematic review and meta-analysis. Ageing Res. Rev. 2019, 53, 100910. [Google Scholar] [CrossRef]
- AsghariHanjani, N.; Vafa, M. The role of IGF-1 in obesity, cardiovascular disease, and cancer. Med. J. Islam. Repub. Iran 2019, 33, 56. [Google Scholar] [CrossRef]
- Tahrani, A.A.; Morton, J. Benefits of weight loss of 10% or more in patients with overweight or obesity: A review. Obesity 2022, 30, 802–840. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Ahmad, K.; Lee, E.J.; Lee, Y.-H.; Choi, I. Implications of insulin-like growth factor-1 in skeletal muscle and various diseases. Cells 2020, 9, 1773. [Google Scholar] [CrossRef]
- Papa, S.; Choy, P.M.; Bubici, C. The ERK and JNK pathways in the regulation of metabolic reprogramming. Oncogene 2019, 38, 2223–2240. [Google Scholar] [CrossRef]
- Bailes, J.; Soloviev, M. Insulin-like growth factor-1 (IGF-1) and its monitoring in medical diagnostic and in sports. Biomolecules 2021, 11, 217. [Google Scholar] [CrossRef]
- Rzehak, P.; Grote, V.; Lattka, E.; Weber, M.; Gruszfeld, D.; Socha, P.; Closa-Monasterolo, R.; Escribano, J.; Giovannini, M.; Verduci, E.; et al. Associations of IGF-1 gene variants and milk protein intake with IGF-I concentrations in infants at age 6 months—Results from a randomized clinical trial. Growth Horm. IGF Res. 2013, 23, 149–158. [Google Scholar] [CrossRef]
- Khan, N.; Paterson, A.D.; Roshandel, D.; Raza, A.; Ajmal, M.; Waheed, N.K.; Azam, M.; Qamar, R. Association of IGF1 and VEGFA polymorphisms with diabetic retinopathy in Pakistani population. Acta Diabetol. 2020, 57, 237–245. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Geng, C.; Wang, C.; Gu, R.; Zhu, Z. A variant rs6214 within IGF-1 confers risk for ulcerative colitis in Chinese Han populations. Funct. Integr. Genom. 2022, 23, 1. [Google Scholar] [CrossRef]
- Short Genetic Variations. Reference SNP rs6214 Report [Internet]. 21 September 2022. Available online: https://www.ncbi.nlm.nih.gov/snp/rs6214 (accessed on 1 May 2025).
- Cronbach, L.J. Coefficient Alpha and the Internal Structure of Tests. Psychometrika 1951, 16, 297–334. [Google Scholar] [CrossRef]
- Pérez-Escamilla, R. The Mexican dietary and physical activity guidelines: Moving public nutrition forward in a globalized world. J. Nutr. 2020, 146, 1924S–1927S. [Google Scholar] [CrossRef]
- Rivera Dommarco, J.; Pérez Lizaur, A.B.; Batis Ruvalcaba, C.; Zendejas Vela, D. Consumir porciones recomendadas de alimentos según la edad. In Anabelle Bonvecchio Arenas ACF-G, Maite Plazas Belausteguigoitia; Kaufer-Horwitz, M., Lizaur, A.B.P., Dommarco, J.Á.R., Eds.; Guías alimentarias y de actividad física en contexto de sobrepeso y obesidad en la población mexicana; Academia Nacional de Medicina: Mexico City, México, 2020; pp. 63–75. [Google Scholar]
- Bowman, S.A.; Friday, J.E.; Moshfegh, A.J. MyPyramid Equivalents Database, 2.0 for USDA Survey Foods, 2003–2004: Documentation and User Guide; US Department of Agriculture: Beltsville, MD, USA, 2021.
- Gustincich, S.; Manfioletti, G.; Del Sal, G.; Schneider, C.; Carninci, P. A fast method for high-quality genomic DNA extraction from whole human blood. Biotechniques 1991, 11, 298–300. [Google Scholar]
- Rosenthal, R.; Rubin, D.B. A simple, general purpose display of magnitude of experimental effect. J. Educ. Psychol. 1982, 74, 166. [Google Scholar] [CrossRef]
- Rosenthal, R.; Rubin, D.B. The counternull value of an effect size: A new statistic. Psychol. Sci. 1994, 5, 329–334. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, Version 23.0; IBM Corp.: Armonk, NY, USA, 2015. [Google Scholar]
- Ohira, M.; Watanabe, Y.; Yamaguchi, T.; Onda, H.; Yamaoka, S.; Abe, K.; Nakamura, S.; Tanaka, S.; Kawagoe, N.; Nabekura, T.; et al. The relationship between serum insulin-like growth factor-1 levels and body composition changes after sleeve gastrectomy. J. Obes. Facts 2021, 14, 641–649. [Google Scholar] [CrossRef]
- Farag, A.G.A.; Allah, A.M.K.A.; El-Rebey, H.S.; Ibraheem, K.I.M.; Mohamed, A.S.E.D.; Labeeb, A.Z.; Elgazzar, A.E.; Haggag, M.M. Role of insulin-like growth factor-1 in skin tags: A clinical, genetic and immunohistochemical study in a sample of Egyptian patients. Clin. Cosmet. Investig. Dermatol. 2019, 12, 255–266. [Google Scholar] [CrossRef]
- Gilbert, J.-A.; Bendsen, N.; Tremblay, A.; Astrup, A. Effect of proteins from different sources on body composition. Nutr. Metab. Cardiovasc. Dis. 2011, 21, B16–B31. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.O.; Huggins, C.E.; Wattanapenpaiboon, N.; Nowson, C.A. Effect of increasing dietary calcium through supplements and dairy food on body weight and body composition: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 114, 1013–1025. [Google Scholar] [CrossRef]
- Yang, Y.; Kang, B.; Lee, E.Y.; Yang, H.K.; Kim, H.-S.; Lim, S.-Y.; Lee, J.-H.; Lee, S.-S.; Suh, B.-K.; Yoon, K.-H. Effect of an obesity prevention program focused on motivating environments in childhood: A school-based prospective study. Int. J. Obes. 2017, 41, 1027–1034. [Google Scholar] [CrossRef]
- Acosta Balcazar, I.C.; Granados Rivera, L.D.; Salinas Chavira, J.; Estrada Drouaillet, B.; Albarrán, M.R.; Bautista Martínez, Y. Relationship between the composition of lipids in forages and the concentration of conjugated linoleic acid in cow’s milk: A review. Animals 2022, 12, 1621. [Google Scholar] [CrossRef]
- Gómez-Cortés, P.; Juárez, M.; de la Fuente, M.A. Milk fatty acids and potential health benefits: An updated vision. Trends Food Sci. Technol. 2018, 81, 1–9. [Google Scholar] [CrossRef]
- Hoeflich, A.; Meyer, Z. Functional analysis of the IGF-system in milk. Best Pr. Res. Clin. Endocrinol. 2017, 31, 409–418. [Google Scholar] [CrossRef]
- Romo Ventura, E.; Konigorski, S.; Rohrmann, S.; Schneider, H.; Stalla, G.K.; Pischon, T.; Linseisen, J.; Nimptsch, K. Association of dietary intake of milk and dairy products with blood concentrations of insulin-like growth factor 1 (IGF-1) in bavarian adults. Eur. J. Nutr. 2020, 59, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Koca, T.T.; Berk, E.; Seyithanoğlu, M.; Koçyiğit, B.F.; Demirel, A. Relationship of leptin, growth hormone, and insulin-like growth factor levels with body mass index and disease severity in patients with fibromyalgia syndrome. Acta Neurol. Belg. 2020, 120, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Buroker, N.E. Computational EPAS1 rSNP analysis, transcriptional factor binding sites and high altitude sickness or adaptation. J. Proteom. Genom. Res. 2016, 1, 31–59. [Google Scholar] [CrossRef]
- Wu, C.; Chen, H.; Mei, Y.; Yang, B.; Zhao, J.; Stanton, C.; Chen, W. Advances in research on microbial conjugated linoleic acid bioconversion. Prog. Lipid Res. 2023, 93, 101257. [Google Scholar] [CrossRef]
- Sadee, W.; Wang, D.; Papp, A.C.; Pinsonneault, J.K.; Smith, R.M.; A Moyer, R.; Johnson, A.D. Pharmacogenomics of the RNA world: Structural RNA polymorphisms in drug therapy. Clin. Pharmacol. Ther. 2011, 9, 355–365. [Google Scholar] [CrossRef]
- Allen, M.D.; Dalton, B.H.; Gilmore, K.J.; McNeil, C.J.; Doherty, T.J.; Rice, C.L.; Power, G.A. Neuroprotective effects of exercise on the aging human neuromuscular system. Exp. Gerontol. 2021, 152, 111465. [Google Scholar] [CrossRef]
- Velloso, C.P. Regulation of muscle mass by growth hormone and IGF-I. Br. J. Pharmacol. 2008, 154, 557–568. [Google Scholar] [CrossRef]
- NCfBI. 1000 Genomes Browser, Phase 3. 2019. Available online: https://www.internationalgenome.org/data-portal/population/MXL (accessed on 6 May 2025).
- Metlapally, R.; Ki, C.-S.; Li, Y.-J.; Tran-Viet, K.-N.; Abbott, D.; Malecaze, F.; Calvas, P.; Mackey, D.A.; Rosenberg, T.; Paget, S.; et al. Genetic association of insulin-like growth factor-1 polymorphisms with high-grade myopia in an international family cohort. Invest. Ophthalmol. Vis. Sci. 2010, 51, 4476–4479. [Google Scholar] [CrossRef]
- Zhuang, W.; Yang, P.; Li, Z.; Sheng, X.; Zhao, J.; Li, S.; Yang, X.; Xiang, W.; Rong, W.; Liu, Y.; et al. Association of insulin-like growth factor-1 polymorphisms with high myopia in the Chinese population. Mol. Vis. 2012, 18, 634. [Google Scholar]
- Rydzanicz, M.; Nowak, D.M.; Karolak, J.A.; Frajdenberg, A.; Podfigurna-Musielak, M.; Mrugacz, M.; Gajecka, M. IGF-1 gene polymorphisms in Polish families with high-grade myopia. Mol. Vis. 2011, 17, 2428. [Google Scholar]
- Savaiano, D.A.; Hutkins, R.W. Yogurt, cultured fermented milk, and health: A systematic review. Nutr. Rev. 2021, 79, 599–614. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, Y.; Liu, J.; Huang, Z.; Yang, X.; Qin, P.; Chen, C.; Luo, X.; Li, Y.; Wu, Y.; et al. Consumption of Dairy Products and the Risk of Overweight or Obesity, Hypertension, and Type 2 Diabetes Mellitus: A Dose–Response Meta-Analysis and Systematic Review of Cohort Studies. Adv. Nutr. 2022, 13, 2165–2179. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Li, Y.; Wang, C.; Mao, Z.; Zhang, L.; Yang, X.; Cui, S.; Li, L. Consumption of dairy products in relation to type 2 diabetes mellitus in Chinese people: The Henan Rural Cohort study and an updated meta-analysis. Nutrients 2020, 12, 3827. [Google Scholar] [CrossRef] [PubMed]
- Marín-Quiroga, A.; Villanueva-Fierro, I.; Rodríguez-Pérez, M.A.; Lares-Asseff, I.A.; Cháirez-Hernández, I.; Proal-Nájera, J.B. Association of IGF-1 content with whole, reduced-fat, and low-fat milk in México. Agrociencia 2015, 49, 113–123. [Google Scholar]
- Grenov, B.; Larnkjær, A.; Ritz, C.; Michaelsen, K.F.; Damsgaard, C.T.; Mølgaard, C. The effect of milk and rapeseed protein on growth factors in 7–8 year-old healthy children–A randomized controlled trial. Growth Horm. IGF Res. 2021, 60, 101418. [Google Scholar] [CrossRef]
- Melnik, B.C.; Schmitz, G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp. Dermatol. 2009, 18, 833–841. [Google Scholar] [CrossRef]
- Grenov, B.; Larnkjær, A.; Mølgaard, C.; Michaelsen, K.F. Role of milk and dairy products in growth of the child. Nestle Nutr. Inst. Workshop Ser. 2020, 93, 77–90. [Google Scholar] [CrossRef]
- Stounbjerg, N.G.; Thams, L.; Hansen, M.; Larnkjær, A.; Clerico, J.W.; Cashman, K.D.; Mølgaard, C.; Damsgaard, C.T. Effects of vitamin D and high dairy protein intake on bone mineralization and linear growth in 6-to 8-year-old children: The D-pro randomized trial. Am. J. Clin. Nutr. 2021, 114, 1971–1985. [Google Scholar] [CrossRef]
- Wallace, T.C.; Bailey, R.L.; Lappe, J.; O’bRien, K.O.; Wang, D.D.; Sahni, S.; Weaver, C.M. Dairy intake and bone health across the lifespan: A systematic review and expert narrative. Crit. Rev. Food Sci. Nutr. 2021, 61, 3661–3707. [Google Scholar] [CrossRef]
- Asl, E.R.; Amini, M.; Najafi, S.; Mansoori, B.; Mokhtarzadeh, A.; Mohammadi, A.; Lotfinejad, P.; Bagheri, M.; Shirjang, S.; Lotfi, Z.; et al. Interplay between MAPK/ERK signaling pathway and MicroRNAs: A crucial mechanism regulating cancer cell metabolism and tumor progression. Life Sci. 2021, 278, 119499. [Google Scholar] [CrossRef]
| Variable | Milk Intake | Without Milk Intake | p-Value X2 |
|---|---|---|---|
| BMI (Kg/m2) > 25, N | 18 | 37 | 0.0003 |
| Fat percentage > 30, N | 22 | 34 | 0.021 |
| Lean mass > 35, N | 19 | 10 | 0.051 |
| Visceral fat > 5, N | 32 | 44 | 0.011 |
| GH (ng/mL), Mean ± SD | 0.698 ± 0.08 | 0.793 ± 0.16 | 0.0001 |
| IGF-1 (ng/mL) Mean ± SD | 224.5 ± 50.5 | 118.4 ± 32.8 | 0.0002 |
| Age (years) | |||
| 20–29 | 22 | 20 | 0.254 |
| 30–39 | 12 | 12 | 0.345 |
| 40–49 | 11 | 12 | 0.567 |
| 50–59 | 10 | 11 | 0.132 |
| Sex | |||
| Male | 25 | 20 | 0.063 |
| Female | 30 | 35 | 0.075 |
| GH | IGF-1 | |||||
|---|---|---|---|---|---|---|
| OR | CI95 | p-Value | OR | CI95 | p-Value | |
| Milk intake No | Ref. | Ref. | ||||
| Yes | 1.04 | 0.49–2.18 | 0.904 | 17.09 | 3.63–19.10 | 0.0003 |
| Codominant model CC | Ref. | Ref. | ||||
| CT | 2.85 | 0.7–11.40 | 0.13 | 2.5 | 0.9–6.68 | 0.065 |
| TT | 2.65 | 0.33–14.45 | 0.33 | 10.6 | 2.3–18.63 | 0.0021 |
| Dominant model CC | Ref. | Ref. | ||||
| TT + CT | 1.85 | 0.8–6.42 | 0.10 | 3.41 | 1.31–8.56 | 0.008 |
| Recessive model CC + CT | Ref. | Ref. | ||||
| TT | 1.89 | 0.22–16.05 | 0.55 | 6.75 | 1.57–28.9 | 0.009 |
| C Allele | Ref. | Ref. | ||||
| T Allele | 2.20 | 0.7–6.18 | 0.12 | 3.05 | 1.5–5.9 | 0.001 |
| Variables | Milk Intake | BMI | Fat % | Lean Mass | Visceral Fat | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| p-Value | r | p-Value | r | p-Value | r | p-Value | r | p-Value | ||
| Milk intake | 1 | −0.278 | 0.000 | −0.210 | 0.006 | 0.101 | 0.190 | −0.117 | 0.130 | |
| BMI | −0.278 | 0.000 | 1 | 0.480 | 0.000 | 0.195 | 0.011 | 0.648 | 0.000 | |
| GH | −0.042 | 0.588 | 0.101 | 0.193 | −0.018 | 0.817 | 0.101 | 0.193 | 0.053 | 0.494 |
| IGF-1 | 0.237 | 0.002 | −0.147 | 0.056 | −0.102 | 0.187 | 0.065 | 0.040 | 0.038 | 0.620 |
| Total Fat Mass | Lean Mass | Visceral Fat | Obesity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | CI95 | p-Value | OR | CI95 | p-Value | OR | CI95 | p-Value | OR | CI95 | p-Value | |
| GH | 2.18 | 0.60–7.82 | 0.22 | 0.68 | 0.19–2.34 | 0.54 | 0.6 | 0.11–3.02 | 0.022 | 0.66 | 0.15–2.80 | 0.57 |
| IGF-1 | 0.68 | 0.28–1.65 | 0.39 | 1.10 | 1.45–2.68 | 0.014 | 0.69 | 0.26–1.83 | 0.45 | 0.62 | 0.25–1.52 | 0.19 |
| BMI > 25 | % Fat | Lean Mas | Visceral Fat | |
|---|---|---|---|---|
| X2 | X2 | X2 | X2 | |
| p Value | p Value | p Value | p Value | |
| CC | Reference | Reference | Reference | Reference |
| CT | 0.154 | 0.90 | 0.025 | 0.48 |
| TT | 0.0087 | 0.033 | 0.12 | 0.17 |
| TT + CT | 0.029 | 0.41 | 0.016 | 0.28 |
| Model | Genotype | <Median BMI | >Median BMI | OR | IC95% | p Value |
|---|---|---|---|---|---|---|
| CO | IGF-1 | |||||
| rs6214 | ||||||
| CC | 27 (48) | 28 (51.02) | 1 | ------------ | ||
| CT | 18 (32) | 23 (44.89) | 1.32 | 0.55–3.12 | >0.05 | |
| TT | 10(20) | 3 (4.08) | 0.19 | 0.05–0.68 | 0.002 | |
| Allele C | 69 (62.7) | 78 (70.9) | 1 | ----------- | ||
| Allele T | 41 (37.3) | 32 (29.1) | 0.64 | 0.34–1.18 | >0.05 | |
| DO | CC | 25 (48) | 28 (51.02) | 1 | ||
| CT-TT | 30 (52) | 27 (48.97) | 0.88 | 0.37–2.08 | >0.05 | |
| RE | CC-CT | 43 (80) | 50 (95.91) | 1 | ||
| TT | 12 (20) | 5 (4.08) | 0.17 | 0.04–0.71 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grijalva-Avila, J.C.; Villanueva-Fierro, I.; Martínez-Estrada, S.C.; Grijalva-Avila, G.; Gándara-Mireles, A.; Rivera, G.; Loera-Castañeda, A.; Almanza-Reyes, H.; Patrón-Romero, L.; Loera-Castañeda, V. Effects of IGF1 rs6214 Polymorphism and Milk Consumption on Serum Levels of IGF-1 and GH and Body Composition. Metabolites 2025, 15, 556. https://doi.org/10.3390/metabo15080556
Grijalva-Avila JC, Villanueva-Fierro I, Martínez-Estrada SC, Grijalva-Avila G, Gándara-Mireles A, Rivera G, Loera-Castañeda A, Almanza-Reyes H, Patrón-Romero L, Loera-Castañeda V. Effects of IGF1 rs6214 Polymorphism and Milk Consumption on Serum Levels of IGF-1 and GH and Body Composition. Metabolites. 2025; 15(8):556. https://doi.org/10.3390/metabo15080556
Chicago/Turabian StyleGrijalva-Avila, Julio Cesar, Ignacio Villanueva-Fierro, Sandra Consuelo Martínez-Estrada, Gerardo Grijalva-Avila, Alonso Gándara-Mireles, Gildardo Rivera, Antonio Loera-Castañeda, Horacio Almanza-Reyes, Leslie Patrón-Romero, and Verónica Loera-Castañeda. 2025. "Effects of IGF1 rs6214 Polymorphism and Milk Consumption on Serum Levels of IGF-1 and GH and Body Composition" Metabolites 15, no. 8: 556. https://doi.org/10.3390/metabo15080556
APA StyleGrijalva-Avila, J. C., Villanueva-Fierro, I., Martínez-Estrada, S. C., Grijalva-Avila, G., Gándara-Mireles, A., Rivera, G., Loera-Castañeda, A., Almanza-Reyes, H., Patrón-Romero, L., & Loera-Castañeda, V. (2025). Effects of IGF1 rs6214 Polymorphism and Milk Consumption on Serum Levels of IGF-1 and GH and Body Composition. Metabolites, 15(8), 556. https://doi.org/10.3390/metabo15080556












