Non-Targeted Metabolomics Analysis of Metabolic Differences Between Different Concentrations of Protein Diets in the Longest Dorsal Muscle of Tibetan Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Animals and Husbandry Management
2.2. Longest Dorsal Muscle Sample Collection
2.3. Reagents and Instruments
2.4. Metabolite Extraction
2.5. LC-MS Analysis
2.5.1. Chromatograph
2.5.2. Mass Spectrometry Conditions
2.6. Data Processing and Statistical Analysis
3. Results
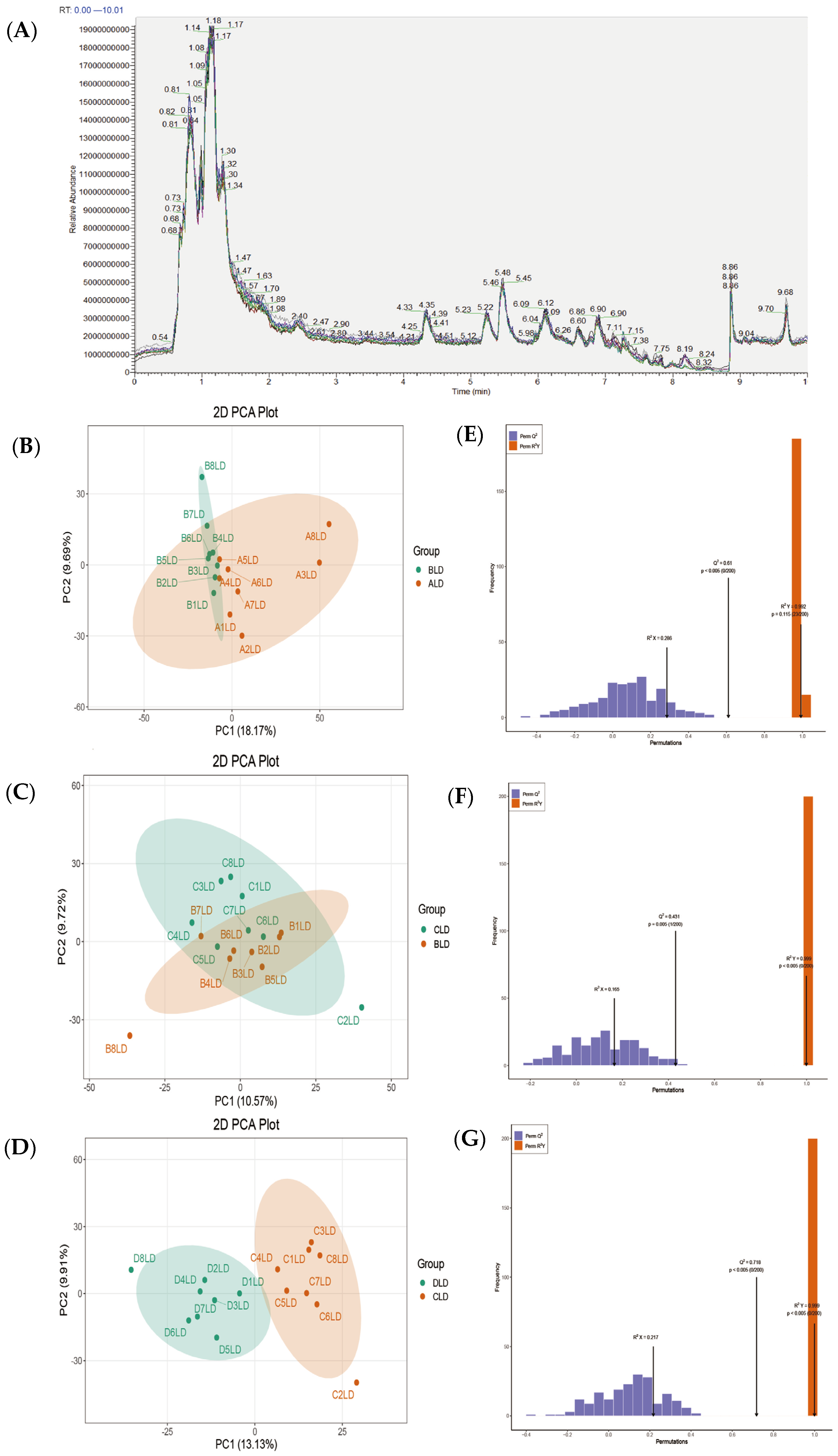
3.1. LC-MS Data QC and Total Spectrum Characterization
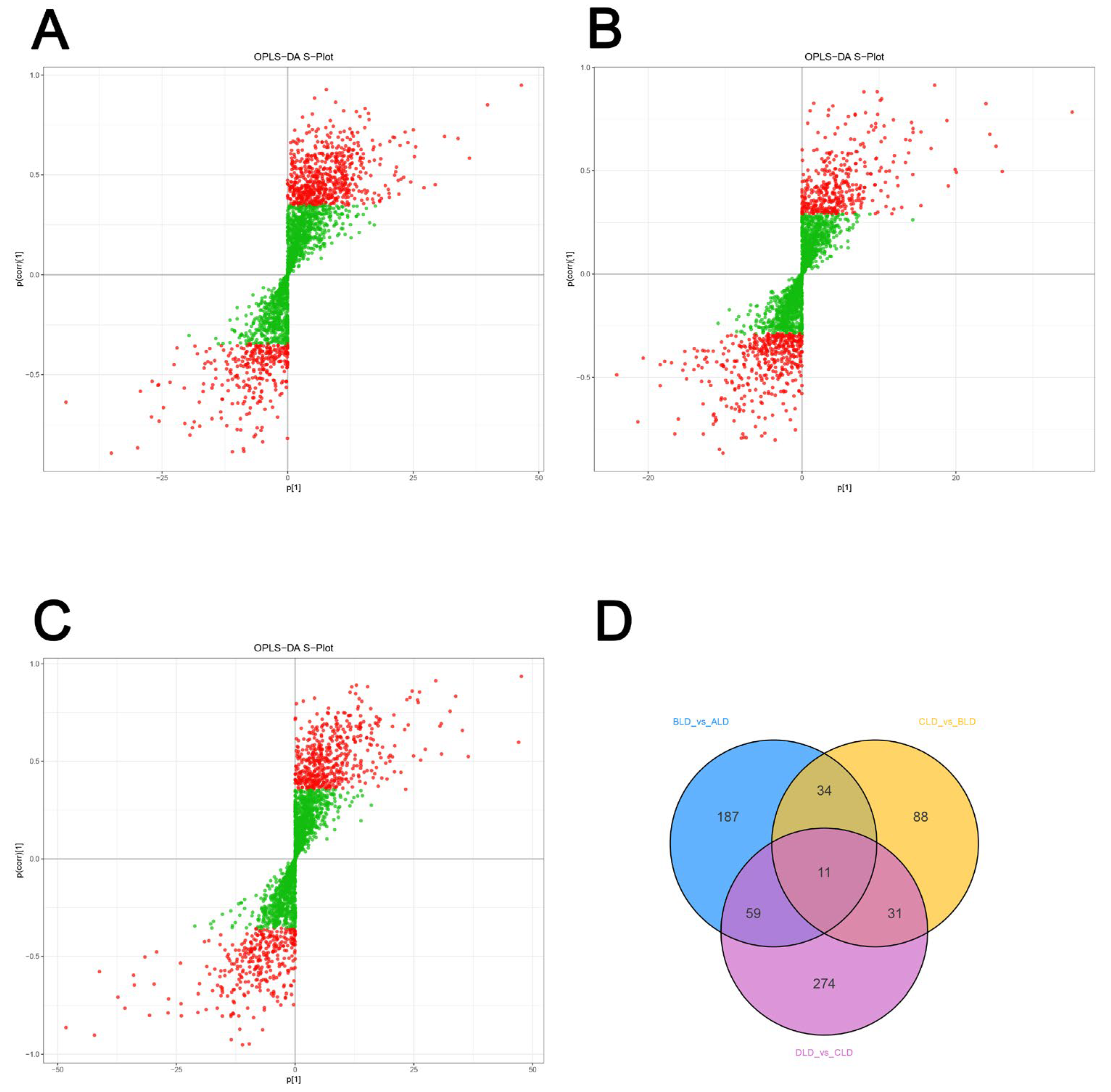
3.2. Differential Metabolite Identification
3.3. Analysis of Key Metabolic Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.W.; Gormley, A.; Jang, K.B.; Duarte, M.E. Invited Review—Current status of global pig production: An overview and research trends. Anim. Biosci. 2024, 37, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, Y.; Ren, Y.; Xu, L.; Liu, X.; Qi, X.; Jiao, T.; Sun, G.; Han, H.; Zhang, J.; et al. Genetic characterization of Tibetan pigs adapted to high altitude under natural selection based on a large whole-genome dataset. Sci. Rep. 2024, 14, 17062. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yuan, H.; Yao, B.; Zhao, S.; Wang, X.; Xu, L.; Zhang, L. Genetic Adaptations of the Tibetan Pig to High-Altitude Hypoxia on the Qinghai-Tibet Plateau. Int. J. Mol. Sci. 2024, 25, 11303. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liang, Y.; Li, L.; Lv, X.; He, Z.; Gu, Y. Identification of Selection Signatures and Candidate Genes Related to Environmental Adaptation and Economic Traits in Tibetan Pigs. Animals 2024, 14, 654. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, C.; Yang, T.; Sha, Y.; Cai, Y.; Wang, X.; Yang, Q.; Liu, C.; Wang, B.; Zhao, S. Characteristics of Tibetan pig lung tissue in response to a hypoxic environment on the Qinghai-Tibet Plateau. Arch. Anim. Breed. 2021, 64, 283–292. [Google Scholar] [CrossRef]
- Bai, X.; Huang, Z.; Tan, H.; Gu, Y.; Wang, X.; Jin, L.; Shang, P.; Long, K.; Li, D.; Li, M. Insights into high-altitude adaptation and meat quality regulation by gastrointestinal metabolites in Tibetan and black pigs. Front. Vet. Sci. 2025, 12, 1569196. [Google Scholar] [CrossRef]
- Shang, P.; Wei, M.; Duan, M.; Yan, F.; Chamba, Y. Healthy Gut Microbiome Composition Enhances Disease Resistance and Fat Deposition in Tibetan Pigs. Front. Microbiol. 2022, 13, 965292. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, X.; Cheng, Z.; Tian, M.; Qiangba, Y.; Fu, Q.; Ren, Z. Comparative proteomic analysis of Tibetan pig spermatozoa at high and low altitudes. BMC Genom. 2019, 20, 569. [Google Scholar] [CrossRef]
- Wang, S.; Tang, C.; Li, J.; Wang, Z.; Meng, F.; Luo, G.; Xin, H.; Zhong, J.; Wang, Y.; Li, B.; et al. The Effects of Dietary Inclusion of Mulberry Leaf Powder on Growth Performance, Carcass Traits and Meat Quality of Tibetan Pigs. Animals 2022, 12, 2743. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Xie, Y.; Qiao, S.; Yang, L.; Pan, H. Comparative Study on Jejunal Immunity and Microbial Composition of Growing-Period Tibetan Pigs and Duroc × (Landrace × Yorkshire) Pigs. Front. Vet. Sci. 2022, 9, 890585. [Google Scholar] [CrossRef]
- Wang, S.; Tian, J.; Jiang, X.; Li, C.; Ge, Y.; Hu, X.; Cheng, L.; Shi, X.; Shi, L.; Jia, Z. Effects of Different Dietary Protein Levels on the Growth Performance, Physicochemical Indexes, Quality, and Molecular Expression of Yellow River Carp (Cyprinus carpio haematopterus). Animals 2023, 13, 1237. [Google Scholar] [CrossRef]
- Beals, J.W.; Burd, N.A.; Moore, D.R.; van Vliet, S. Obesity Alters the Muscle Protein Synthetic Response to Nutrition and Exercise. Front. Nutr. 2019, 6, 87. [Google Scholar] [CrossRef] [PubMed]
- Hanai, M.; Esashi, T. Comparison of the effects of dietary protein on the sexual organ development of male mice and rats kept under constant darkness. J. Nutr. Sci. Vitaminol. 2013, 59, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Liufu, S.; Lan, Q.; Liu, X.; Chen, B.; Xu, X.; Ai, N.; Li, X.; Yu, Z.; Ma, H. Transcriptome Analysis Reveals the Age-Related Developmental Dynamics Pattern of the Longissimus Dorsi Muscle in Ningxiang Pigs. Genes 2023, 14, 1050. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, D.; Zhang, X.; Liu, X.; Niu, X.; Li, S.; Huang, S.; Ran, X.; Wang, J. Comparative transcriptome analysis of longissimus dorsi muscle reveal potential genes affecting meat trait in Chinese indigenous Xiang pig. Sci. Rep. 2024, 14, 8486. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Wu, L.; Wei, H.; Liu, Y.; Li, T.; Tan, B.; Kong, X.; Yao, K.; Chen, S.; et al. Effects of dietary protein restriction on muscle fiber characteristics and mTORC1 pathway in the skeletal muscle of growing-finishing pigs. J. Anim. Sci. Biotechnol. 2016, 7, 47. [Google Scholar] [CrossRef]
- Powell, D.J.; McFarland, D.C.; Cowieson, A.J.; Muir, W.I.; Velleman, S.G. The effect of nutritional status on myogenic satellite cell proliferation and differentiation. Poult. Sci. 2013, 92, 2163–2173. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Kong, X.; Tan, B.; Li, Y.; Duan, Y.; Blachier, F.; Hu, C.A.; Yin, Y. Signaling Pathways Related to Protein Synthesis and Amino Acid Concentration in Pig Skeletal Muscles Depend on the Dietary Protein Level, Genotype and Developmental Stages. PLoS ONE 2015, 10, e0138277. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, W.; Liu, T.; Mosenthin, R.; Bao, Y.; Chen, P.; Hao, W.; Zhao, L.; Zhang, J.; Ji, C.; et al. Improved Satellite Cell Proliferation Induced by L-Carnosine Benefits Muscle Growth of Pigs in Part through Activation of the Akt/mTOR/S6K Signaling Pathway. Agriculture 2022, 12, 988. [Google Scholar] [CrossRef]
- Kwak, M.; Kang, K.; Wang, Y. Methods of Metabolite Identification Using MS/MS Data. J. Comput. Inf. Syst. 2019, 62, 12–18. [Google Scholar] [CrossRef]
- Dervishi, E.; Bai, X.; Dyck, M.K.; Harding, J.C.S.; Fortin, F.; Dekkers, J.C.M.; Plastow, G. GWAS and genetic and phenotypic correlations of plasma metabolites with complete blood count traits in healthy young pigs reveal implications for pig immune response. Front. Mol. Biosci. 2023, 10, 1140375. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.T.; Faleiros, C.A.; Poleti, M.D.; Novais, F.J.; López-Hernández, Y.; Mandal, R.; Wishart, D.S.; Fukumasu, H. Unraveling Ruminant Feed Efficiency Through Metabolomics: A Systematic Review. Metabolites 2024, 14, 675. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yao, Y.; Wang, Y.; Yu, T.; Cai, W.; Zhou, D.; Yin, F.; Liu, W.; Liu, Y.; Xie, C.; et al. An end-to-end deep learning method for mass spectrometry data analysis to reveal disease-specific metabolic profiles. Nat. Commun. 2024, 15, 7136. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Hu, C.; Xu, C.; Zhang, Z.; Hu, G. Metabolomics reveals changes in soil metabolic profiles during vegetation succession in karst area. Front. Microbiol. 2024, 15, 1337672. [Google Scholar] [CrossRef]
- Lu, Y.; Pang, Z.; Xia, J. Comprehensive investigation of pathway enrichment methods for functional interpretation of LC-MS global metabolomics data. Brief. Bioinform. 2023, 24, bbac553. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, H.; Guo, J.; Chen, Y.; Li, W.; Wang, S.; Zhen, L. Non-targeted Metabolomics Analysis Based on LC-MS to Assess the Effects of Different Cold Exposure Times on Piglets. Front. Physiol. 2022, 13, 853995. [Google Scholar] [CrossRef]
- Dibble, C.C.; Barritt, S.A.; Perry, G.E.; Lien, E.C.; Geck, R.C.; DuBois-Coyne, S.E.; Bartee, D.; Zengeya, T.T.; Cohen, E.B.; Yuan, M.; et al. PI3K drives the de novo synthesis of coenzyme A from vitamin B5. Nature 2022, 608, 192–198. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Geerts, C.; Furtos, A.; Waters, P.; Cyr, D.; Wang, S.; Mitchell, G.A. The multiple facets of acetyl-CoA metabolism: Energetics, biosynthesis, regulation, acylation and inborn errors. Mol. Genet. Metab. 2023, 138, 106966. [Google Scholar] [CrossRef]
- Xi, C.; Pang, J.; Xue, W.; Cui, Y.; Jiang, N.; Zhi, W.; Shi, H.; Horuzsko, A.; Pace, B.S.; Zhu, X. Transsulfuration pathway activation attenuates oxidative stress and ferroptosis in sickle primary erythroblasts and transgenic mice. Commun. Biol. 2025, 8, 15. [Google Scholar] [CrossRef]
- Rasch, I.; Görs, S.; Tuchscherer, A.; Viergutz, T.; Metges, C.C.; Kuhla, B. Substitution of Dietary Sulfur Amino Acids by dl-2-Hydroxy-4-Methylthiobutyric Acid Reduces Fractional Glutathione Synthesis in Weaned Piglets. J. Nutr. 2020, 150, 722–729. [Google Scholar] [CrossRef]
- Wang, W.; Cui, J.; Ma, H.; Lu, W.; Huang, J. Targeting Pyrimidine Metabolism in the Era of Precision Cancer Medicine. Front. Oncol. 2021, 11, 684961. [Google Scholar] [CrossRef]
- Fraczek, P.M.; Duran, P.; Yang, B.A.; Ferre, V.; Alawieh, L.; Castor-Macias, J.A.; Wong, V.T.; Guzman, S.D.; Piotto, C.; Itsani, K.; et al. Vitamin A retinoic acid contributes to muscle stem cell and mitochondrial function loss in old age. JCI Insight 2025, 10, e183706. [Google Scholar] [CrossRef]
- Yan, H.F.; Zou, T.; Tuo, Q.Z.; Xu, S.; Li, H.; Belaidi, A.A.; Lei, P. Ferroptosis: Mechanisms and links with diseases. Signal Transduct. Target. Ther. 2021, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Chow, B.K. Gonadotropin-releasing hormone. FEBS J. 2008, 275, 5457. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Santos, T.; da Cruz-Filho, J.; Costa, D.M.; da Silva, R.P.; dos Anjos-Santos, H.C.; Dos Santos, J.R.; Reis, L.C.; do Carmo Kettelhut, Í.; Navegantes, L.C.; Camargo, E.A.; et al. Non-canonical Ca(2+)—Akt signaling pathway mediates the antiproteolytic effects induced by oxytocin receptor stimulation in skeletal muscle. Biochem. Pharmacol. 2023, 217, 115850. [Google Scholar] [CrossRef]
- Sun, J.; Xie, F.; Wang, J.; Luo, J.; Chen, T.; Jiang, Q.; Xi, Q.; Liu, G.E.; Zhang, Y. Integrated meta-omics reveals the regulatory landscape involved in lipid metabolism between pig breeds. Microbiome 2024, 12, 33. [Google Scholar] [CrossRef]
- Cruz-Topete, D.; Cidlowski, J.A. One hormone, two actions: Anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation 2015, 22, 20–32. [Google Scholar] [CrossRef]
- Glezer, I.; Scavone, C.; Avellar, M.C.W. Editorial: Updates and new concepts in regulation of pro-inflammatory gene expression by steroid hormones, volume II. Front. Endocrinol. 2023, 14, 1337386. [Google Scholar] [CrossRef]
- Kido, K.; Watanabe, S.; Kusano, M.; Ito, A.; Sakai, K.; Kosugi, M.; Gotoh, Y.; Suzuki, T.; Kawanaka, K.; Higaki, Y. Additive impact of soy protein dietary intake and exercise on visceral fat mass reduction and mitochondrial complex I activation in skeletal muscle. Am. J. Physiol.-Endocrinol. Metab. 2024, 326, E124–E133. [Google Scholar] [CrossRef]
- Gao, J.; Yang, P.; Cui, Y.; Meng, Q.; Feng, Y.; Hao, Y.; Liu, J.; Piao, X.; Gu, X. Identification of Metabonomics Changes in Longissimus Dorsi Muscle of Finishing Pigs Following Heat Stress through LC-MS/MS-Based Metabonomics Method. Animals 2020, 10, 129. [Google Scholar] [CrossRef]
- Huang, N.J.; Lin, Y.C.; Lin, C.Y.; Pishesha, N.; Lewis, C.A.; Freinkman, E.; Farquharson, C.; Millán, J.L.; Lodish, H. Enhanced phosphocholine metabolism is essential for terminal erythropoiesis. Blood 2018, 131, 2955–2966. [Google Scholar] [CrossRef]
- Siudeja, K.; Grzeschik, N.A.; Rana, A.; de Jong, J.; Sibon, O.C. Cofilin/Twinstar phosphorylation levels increase in response to impaired coenzyme a metabolism. PLoS ONE 2012, 7, e43145. [Google Scholar] [CrossRef]
- Sommakia, S.; Baker, O.J. Regulation of inflammation by lipid mediators in oral diseases. Oral. Dis. 2017, 23, 576–597. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, Y.; Nakamura, N.; Ikeda, N.; Sugiyama, R.; Ishii, C.; Maki, M.; Shibata, H.; Takahara, T. Amino Acid-Mediated Intracellular Ca(2+) Rise Modulates mTORC1 by Regulating the TSC2-Rheb Axis through Ca(2+)/Calmodulin. Int. J. Mol. Sci. 2021, 22, 6897. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Deng, S.; Lian, Z.; Yu, K. Skeletal muscle oxidative stress and inflammation in aging: Focus on antioxidant and anti-inflammatory therapy. Front. Cell Dev. Biol. 2022, 10, 964130. [Google Scholar] [CrossRef]

| Raw Materials | 10% (ALD) | 12% (BLD) | 14% (CLD) | 16% (DLD) |
|---|---|---|---|---|
| corn | 257.4 | 232.93 | 208.46 | 184 |
| wheat bran | 120.6 | 125.33 | 130.07 | 134.8 |
| soybean (oil) meal | 5.2 | 26.67 | 48.13 | 69.6 |
| talc | 3.48 | 3.56 | 3.64 | 3.72 |
| calcium biphosphate | 1.12 | 0.75 | 0.37 | 0 |
| Lys-HCl | 3.68 | 2.92 | 2.16 | 1.4 |
| DL-Met | 0.84 | 0.63 | 0.41 | 0.2 |
| DL-Thr | 1.6 | 1.25 | 0.91 | 0.56 |
| Trp | 0.36 | 0.24 | 0.12 | 0 |
| edible salt | 1.2 | 1.20 | 1.20 | 1.2 |
| choline | 0.4 | 0.40 | 0.40 | 0.4 |
| multidimensional | 0.12 | 0.12 | 0.12 | 0.12 |
| limestone | 0.8 | 0.80 | 0.80 | 0.8 |
| silica earth | 3.2 | 3.20 | 3.20 | 3.2 |
| add up the total | 400 | 400 | 400 | 400 |
| Metabolites | Class I | Formula |
|---|---|---|
| Dihydro-3-methyl-2(3H)-furanone | Aldehyde, Ketones, Esters | C5H8O2 |
| Phosphocholine | Organic acid and its derivatives | C5H15NO4P+ |
| 4,4’-Sulfonyldiphenol | Benzene and substituted derivatives | C12H10O4S |
| 4-(Aminomethyl)benzoic acid | Organic acid and its derivatives | C8H9NO2 |
| Benzyln-[(2 s)-4-methyl-1-[[(2 r)-4-methyl-1-[[(2 s)-4-methyl-1-oxopentan-2-yl]amino]-1-oxopentan-2-yl]amino]-1-oxopentan-2-yl]carbamate | Amino acid and its metabolites | C26H41N3O5 |
| 4-Chlorobenzaldehyde | Benzene and substituted derivatives | C7H5ClO |
| Val-Ile-Pro-Lys-Ser | Amino acid and its metabolites | C25H46N6O7 |
| Erythrose | Carbohydrates and their metabolites | C4H8O4 |
| Deoxypeganine | Heterocyclic compounds | C11H12N2 |
| (10-Methoxy-1,4,14,19,19-pentamethyl-8,17-dioxo-2,7,18-trioxapentacyclo [11.9.0.03,11.05,9.014,20]docosa-3(11),4,9-trien-21-yl) acetate | Heterocyclic compounds | C27H34O8 |
| 2-Cyclohexen-1-one | Aldehyde, Ketones, Esters | C6H8O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Liang, J.; Zhang, H.; Duan, M.; Yang, D.; Yangzom, C.; Shang, P. Non-Targeted Metabolomics Analysis of Metabolic Differences Between Different Concentrations of Protein Diets in the Longest Dorsal Muscle of Tibetan Pigs. Metabolites 2025, 15, 555. https://doi.org/10.3390/metabo15080555
Zhang F, Liang J, Zhang H, Duan M, Yang D, Yangzom C, Shang P. Non-Targeted Metabolomics Analysis of Metabolic Differences Between Different Concentrations of Protein Diets in the Longest Dorsal Muscle of Tibetan Pigs. Metabolites. 2025; 15(8):555. https://doi.org/10.3390/metabo15080555
Chicago/Turabian StyleZhang, Feifan, Jinhui Liang, Hongliang Zhang, Mengqi Duan, Dong Yang, Chamba Yangzom, and Peng Shang. 2025. "Non-Targeted Metabolomics Analysis of Metabolic Differences Between Different Concentrations of Protein Diets in the Longest Dorsal Muscle of Tibetan Pigs" Metabolites 15, no. 8: 555. https://doi.org/10.3390/metabo15080555
APA StyleZhang, F., Liang, J., Zhang, H., Duan, M., Yang, D., Yangzom, C., & Shang, P. (2025). Non-Targeted Metabolomics Analysis of Metabolic Differences Between Different Concentrations of Protein Diets in the Longest Dorsal Muscle of Tibetan Pigs. Metabolites, 15(8), 555. https://doi.org/10.3390/metabo15080555






