Integration of Pseudotargeted Metabolomics and Microbiomics Reveals That Hugan Tablets Ameliorate NASH with Liver Fibrosis in Mice by Modulating Bile Acid Metabolism via the Gut Microbiome
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals
2.3. Experimental Design
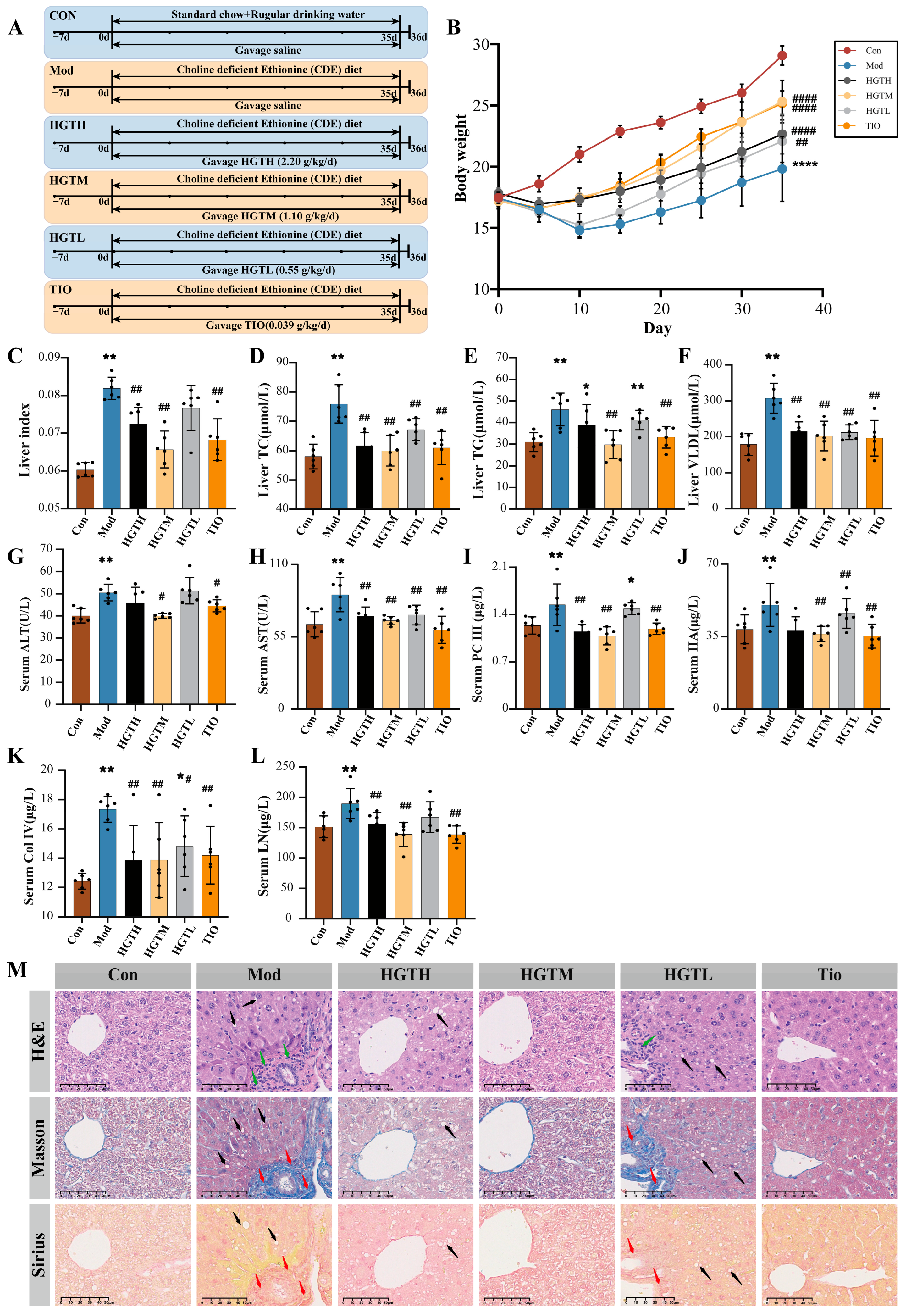
2.3.1. The Pharmacological Effects of HGTs on Hepatic Fibrosis in Mice with NASH
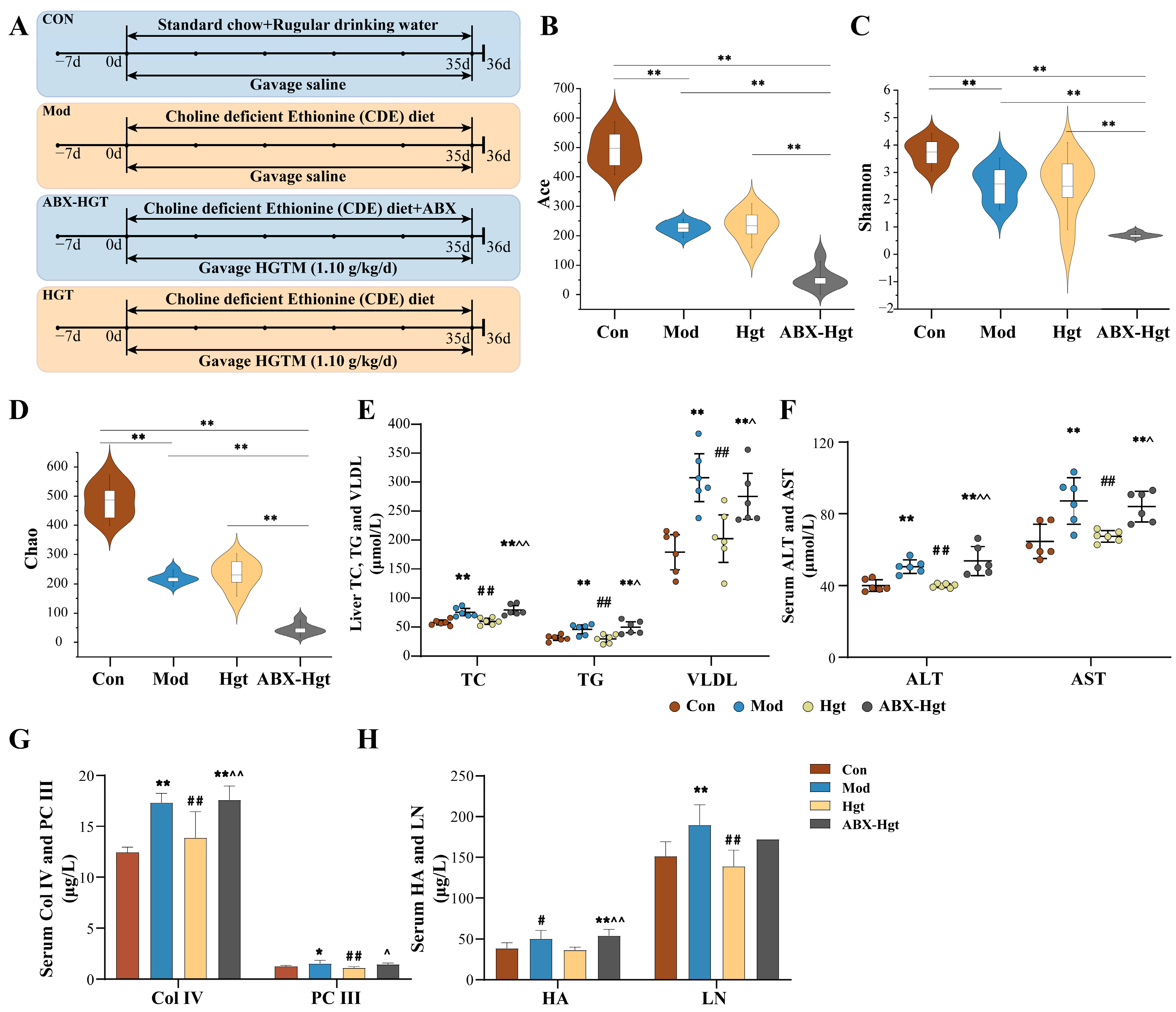
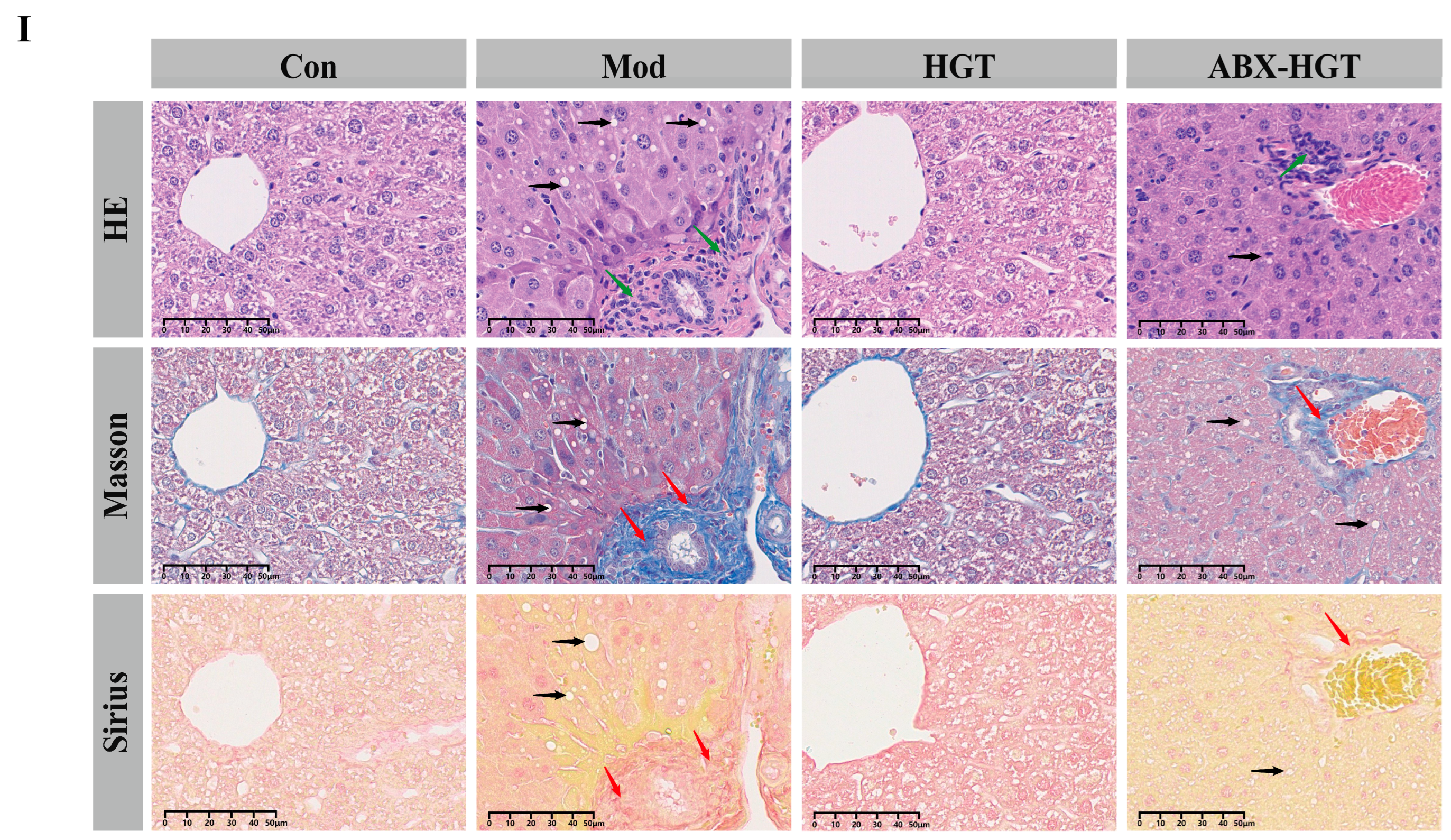
2.3.2. Antibiotic Cocktail Animal Experiment
2.3.3. General Observations and Sample Collection
2.4. Enzyme-Linked Immunosorbent Assay
2.5. Histopathological Analysis
2.6. 16S rDNA Gene Sequencing Analysis of Mouse Colonic Contents Samples
2.7. Untargeted Metabolomics Assay
2.7.1. Sample Preparation
2.7.2. UPLC-Q-Exactive-MS
2.7.3. Data Analysis
2.8. Pseudotargeted Metabolomics Assay
2.9. Literature Mining and Database Searching
2.10. Molecular Docking
2.11. Statistical Analysis
3. Results
3.1. HGTs Alleviate NASH-Related Hepatic Fibrosis in CDE Mice
3.2. Effects of HGTs on the Intestinal Flora of Mice with NASH Fibrosis
3.2.1. Diversity Analysis
3.2.2. Structural Composition Analysis
3.2.3. Linear Discriminant Analysis Effect Quantity (LEfSe) and Differential Bacteria Genus Analysis
3.2.4. PICRUSt Functional Prediction
3.2.5. Correlation Analysis Between Gut Microbiota and Hepatic Fibrosis
3.3. ABX Attenuated the Anti-Hepatic Fibrosis Effect of HGTs in NASH Mice
3.4. HGTs Regulate the Bile Acid Metabolism in the Liver and Colonic Contents of Mice with NASH Fibrosis
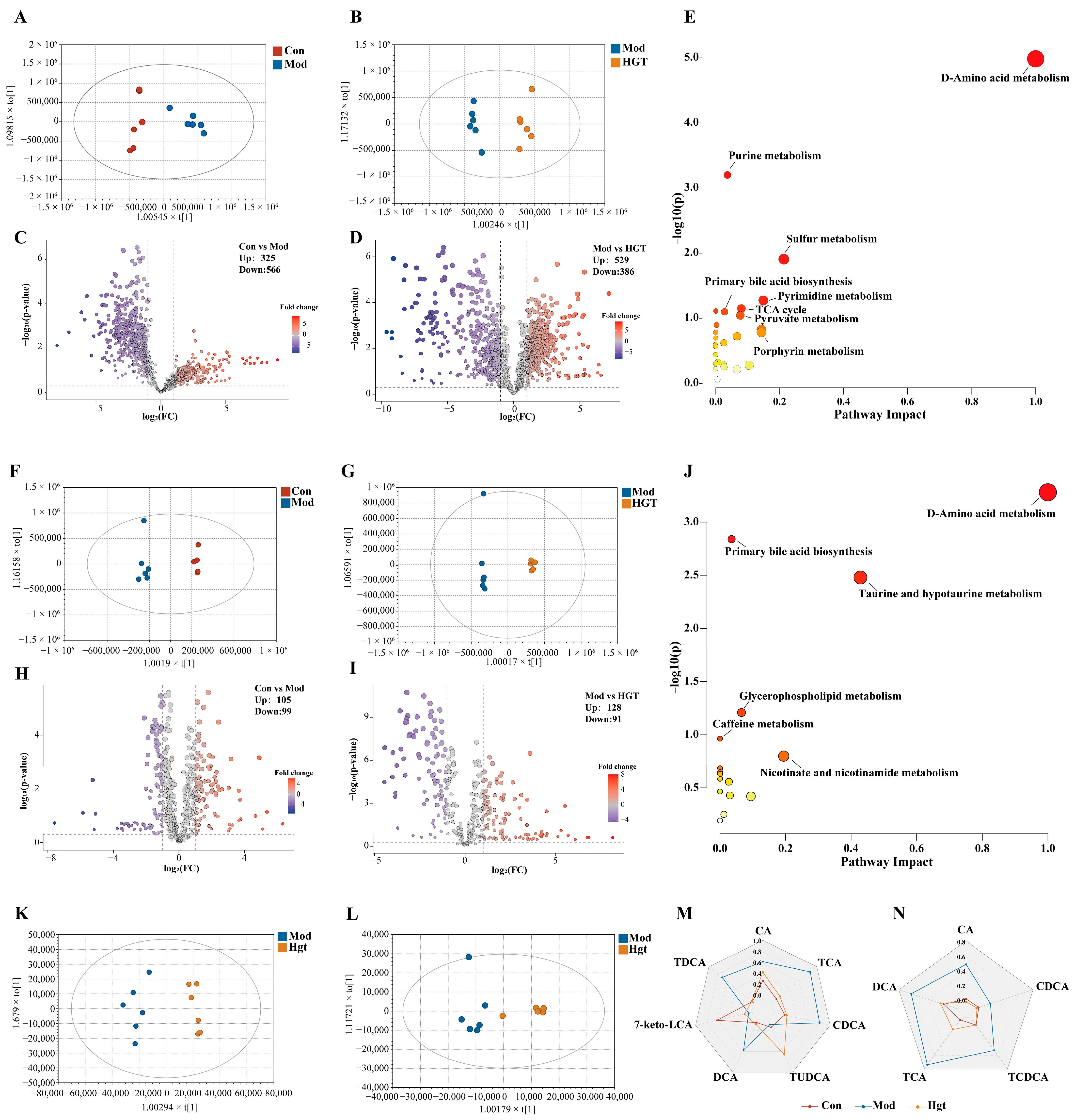
3.4.1. Untargeted Metabolomics
Metabolic Profiles of Colonic Contents
Liver Metabolic Profiles
3.4.2. Pseudotargeted Metabolomics
Fecal TBA Levels and Colonic Content BA Composition
Hepatic TBA Levels and BAs Composition
3.5. The Multidimensional Mechanism of HGTs Targeting BA-Metabolizing Enzymes to Regulate BA Profiles via Gut Microbiome in Improving Hepatic Fibrosis
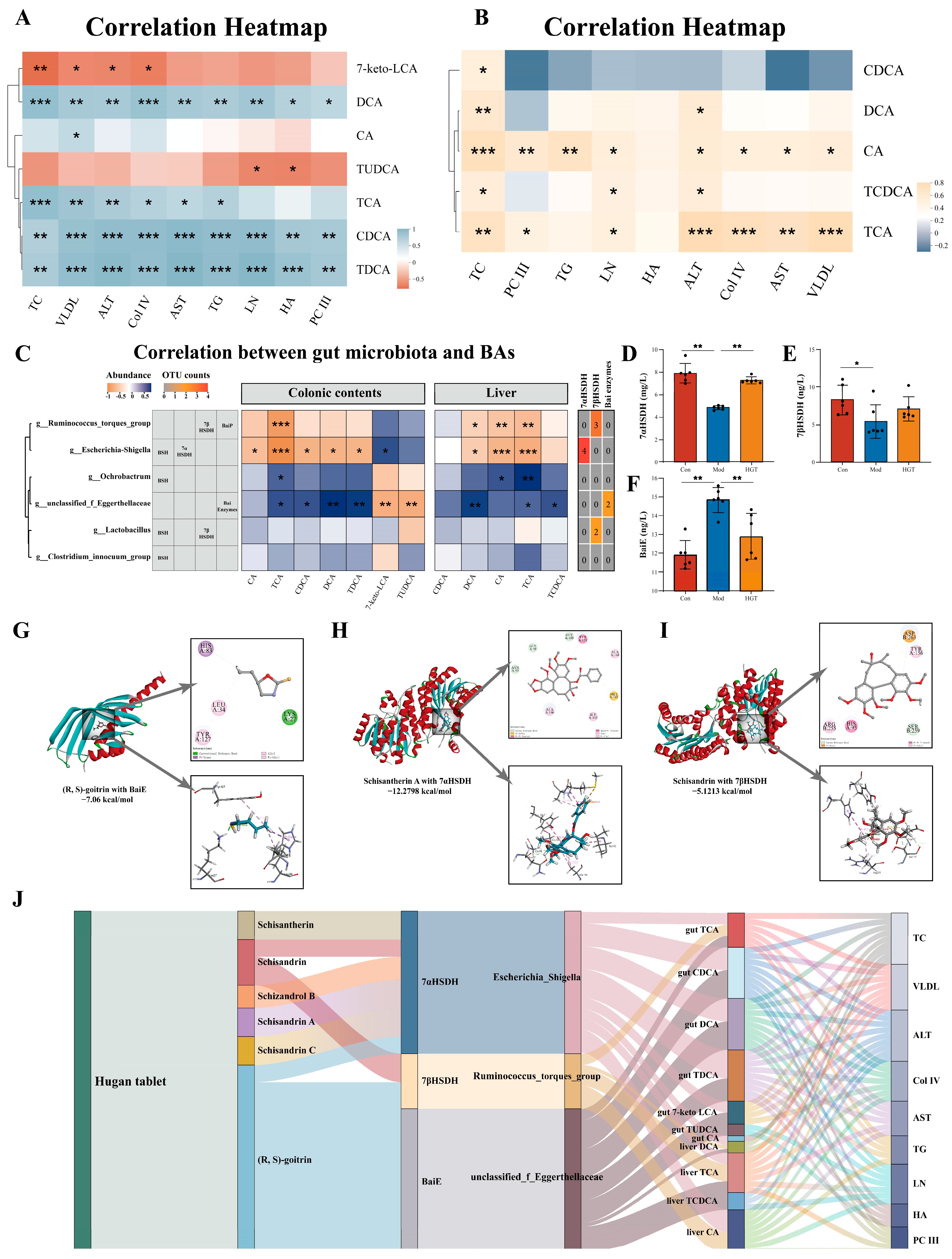
3.5.1. Correlation Analysis Between BAs and Hepatic Fibrosis
3.5.2. Identification of Potential Targets of HGTs in Regulating Bile Acid Metabolism
3.5.3. Molecular Docking of Bile-Acid-Metabolizing Enzymes and Active Ingredients of HGTs
3.5.4. Multidimensional TCM-Enzyme-Intestinal Genera-Bile Acids-Disease Interaction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parola, M.; Pinzani, M. Liver fibrosis in NAFLD/NASH: From pathophysiology towards diagnostic and therapeutic strategies. Mol. Aspects Med. 2024, 95, 101231. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Man, S.; Deng, Y.; Ma, Y.; Fu, J.; Bao, H.; Yu, C.; Lv, J.; Liu, H.; Wang, B.; Li, L. Prevalence of Liver Steatosis and Fibrosis in the General Population and Various High-Risk Populations: A Nationwide Study With 5.7 Million Adults in China. Gastroenterology 2023, 165, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Le, P.; Payne, J.Y.; Zhang, L.; Deshpande, A.; Rothberg, M.B.; Alkhouri, N.; Herman, W.; Hernandez, A.V.; Schleicher, M.; Ye, W.; et al. Disease State Transition Probabilities Across the Spectrum of NAFLD: A Systematic Review and Meta-Analysis of Paired Biopsy or Imaging Studies. Clin. Gastroenterol. Hepatol. 2023, 21, 1154–1168. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.A.; Kendall, B.J.; El-Serag, H.B.; Thrift, A.P.; Macdonald, G.A. Hepatocellular and extrahepatic cancer risk in people with non-alcoholic fatty liver disease. Lancet Gastroenterol. Hepatol. 2024, 9, 159–169. [Google Scholar] [CrossRef]
- Hammerich, L.; Tacke, F. Hepatic inflammatory responses in liver fibrosis. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 633–646. [Google Scholar] [CrossRef]
- Nie, Q.; Luo, X.; Wang, K.; Ding, Y.; Jia, S.; Zhao, Q.; Li, M.; Zhang, J.; Zhuo, Y.; Lin, J.; et al. Gut symbionts alleviate MASH through a secondary bile acid biosynthetic pathway. Cell 2024, 187, 2717–2734.e2733. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Gaskins, H.R. Another renaissance for bile acid gastrointestinal microbiology. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 348–364. [Google Scholar] [CrossRef]
- Chow, M.D.; Lee, Y.H.; Guo, G.L. The role of bile acids in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mol. Aspects Med. 2017, 56, 34–44. [Google Scholar] [CrossRef]
- Smirnova, E.; Muthiah, M.D.; Narayan, N.; Siddiqui, M.S.; Puri, P.; Luketic, V.A.; Contos, M.J.; Idowu, M.; Chuang, J.C.; Billin, A.N.; et al. Metabolic reprogramming of the intestinal microbiome with functional bile acid changes underlie the development of NAFLD. Hepatology 2022, 76, 1811–1824. [Google Scholar] [CrossRef]
- Puri, P.; Daita, K.; Joyce, A.; Mirshahi, F.; Santhekadur, P.K.; Cazanave, S.; Luketic, V.A.; Siddiqui, M.S.; Boyett, S.; Min, H.K.; et al. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology 2018, 67, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Meibom, K.L.; Marion, S.; Volet, C.; Nass, T.; Vico-Oton, E.; Menin, L.; Bernier-Latmani, R. BaiJ and BaiB are key enzymes in the chenodeoxycholic acid 7α-dehydroxylation pathway in the gut microbe Clostridium scindens ATCC 35704. Gut Microbes 2024, 16, 2323233. [Google Scholar] [CrossRef] [PubMed]
- Devlin, A.S.; Fischbach, M.A. A biosynthetic pathway for a prominent class of microbiota-derived bile acids. Nat. Chem. Biol. 2015, 11, 685–690. [Google Scholar] [CrossRef]
- Guzior, D.V.; Quinn, R.A. Review: Microbial transformations of human bile acids. Microbiome 2021, 9, 140. [Google Scholar] [CrossRef]
- Heinken, A.; Ravcheev, D.A.; Baldini, F.; Heirendt, L.; Fleming, R.M.T.; Thiele, I. Systematic assessment of secondary bile acid metabolism in gut microbes reveals distinct metabolic capabilities in inflammatory bowel disease. Microbiome 2019, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Wirbel, J.; Pyl, P.T.; Kartal, E.; Zych, K.; Kashani, A.; Milanese, A.; Fleck, J.S.; Voigt, A.Y.; Palleja, A.; Ponnudurai, R.; et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med. 2019, 25, 679–689. [Google Scholar] [CrossRef]
- Liu, H.; Wang, R.B.; Xie, Y.M.; Li, Y.Y.; Liao, X.; Liu, S.N.; Ye, Y.A.; Gou, C.Y.; Zhan, S.Y.; Wang, Y.Y. Expert consensus statement on Hugan Tablets in clinical practice. China J. Chin. Mater. Medica 2019, 44, 2943–2946. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, M.; Gang, H.; Li, X.; Zhai, C.; Feng, Z.; Luo, G.; Gao, X. A funnel-type stepwise filtering strategy for identification of potential Q-markers of traditional Chinese medicine formulas. Front. Pharmacol. 2023, 14, 1143768. [Google Scholar] [CrossRef]
- Lv, S.; Yu, H.; Liu, X.; Gao, X. The Study on the Mechanism of Hugan Tablets in Treating Drug-Induced Liver Injury Induced by Atorvastatin. Front. Pharmacol. 2021, 12, 683707. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, M.; Zhao, X.; Li, X. Effects of garlic polysaccharide on alcoholic liver fibrosis and intestinal microflora in mice. Pharm. Biol. 2018, 56, 325–332. [Google Scholar] [CrossRef]
- Tsurusaki, S.; Tsuchiya, Y.; Koumura, T.; Nakasone, M.; Sakamoto, T.; Matsuoka, M.; Imai, H.; Yuet-Yin Kok, C.; Okochi, H.; Nakano, H.; et al. Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis. Cell Death Dis. 2019, 10, 449. [Google Scholar] [CrossRef] [PubMed]
- Knight, B.; Akhurst, B.; Matthews, V.B.; Ruddell, R.G.; Ramm, G.A.; Abraham, L.J.; Olynyk, J.K.; Yeoh, G.C. Attenuated liver progenitor (oval) cell and fibrogenic responses to the choline deficient, ethionine supplemented diet in the BALB/c inbred strain of mice. J. Hepatol. 2007, 46, 134–141. [Google Scholar] [CrossRef]
- Van Hul, N.K.; Abarca-Quinones, J.; Sempoux, C.; Horsmans, Y.; Leclercq, I.A. Relation between liver progenitor cell expansion and extracellular matrix deposition in a CDE-induced murine model of chronic liver injury. Hepatology 2009, 49, 1625–1635. [Google Scholar] [CrossRef]
- Quan, L.H.; Zhang, C.; Dong, M.; Jiang, J.; Xu, H.; Yan, C.; Liu, X.; Zhou, H.; Zhang, H.; Chen, L.; et al. Myristoleic acid produced by enterococci reduces obesity through brown adipose tissue activation. Gut 2020, 69, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Guo, H.; Liu, S.; Jin, W.; Zhang, Z.W.; Zhang, Y.; Liu, K.; Mao, S.; Zhou, Z.; Xie, L.; et al. Aberrant branched-chain amino acid accumulation along the microbiota-gut-brain axis: Crucial targets affecting the occurrence and treatment of ischaemic stroke. Br. J. Pharmacol. 2023, 180, 347–368. [Google Scholar] [CrossRef]
- Agus, A.; Clément, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef]
- Koh, A.; Bäckhed, F. From Association to Causality: The Role of the Gut Microbiota and Its Functional Products on Host Metabolism. Mol. Cell 2020, 78, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Xu, X.; Fang, W.; Qu, S.; Jin, F.; Zhou, J.; Han, X.; Raza, H.K.; Li, X.; et al. Hugan tablets for the treatment of RUCAM based drug-induced liver injury: A propensity score matching analysis using a nationwide database. Expert. Rev. Clin. Pharmacol. 2021, 14, 1543–1550. [Google Scholar] [CrossRef]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef]
- Patel, R.; DuPont, H.L. New approaches for bacteriotherapy: Prebiotics, new-generation probiotics, and synbiotics. Clin. Infect. Dis. 2015, 60 (Suppl. 2), S108–S121. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.; Stringer, A.M.; Prestidge, C.A.; Joyce, P. Targeting the gut microbiome to control drug pharmacomicrobiomics: The next frontier in oral drug delivery. Expert. Opin. Drug Deliv. 2023, 20, 1315–1331. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Yaskolka Meir, A.; Rinott, E.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Rosen, P.; Shelef, I.; Youngster, I.; Shalev, A.; Blüher, M.; et al. Effect of green-Mediterranean diet on intrahepatic fat: The DIRECT PLUS randomised controlled trial. Gut 2021, 70, 2085–2095. [Google Scholar] [CrossRef]
- Yueh, M.F.; He, F.; Chen, C.; Vu, C.; Tripathi, A.; Knight, R.; Karin, M.; Chen, S.; Tukey, R.H. Triclosan leads to dysregulation of the metabolic regulator FGF21 exacerbating high fat diet-induced nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA 2020, 117, 31259–31266. [Google Scholar] [CrossRef] [PubMed]
- Astbury, S.; Atallah, E.; Vijay, A.; Aithal, G.P.; Grove, J.I.; Valdes, A.M. Lower gut microbiome diversity and higher abundance of proinflammatory genus Collinsella are associated with biopsy-proven nonalcoholic steatohepatitis. Gut Microbes 2020, 11, 569–580. [Google Scholar] [CrossRef]
- Li, C.; Stražar, M.; Mohamed, A.M.T.; Pacheco, J.A.; Walker, R.L.; Lebar, T.; Zhao, S.; Lockart, J.; Dame, A.; Thurimella, K.; et al. Gut microbiome and metabolome profiling in Framingham heart study reveals cholesterol-metabolizing bacteria. Cell 2024, 187, 1834–1852.e1819. [Google Scholar] [CrossRef]
- de Aguiar Vallim, T.Q.; Tarling, E.J.; Edwards, P.A. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013, 17, 657–669. [Google Scholar] [CrossRef]
- Arab, J.P.; Karpen, S.J.; Dawson, P.A.; Arrese, M.; Trauner, M. Bile acids and nonalcoholic fatty liver disease: Molecular insights and therapeutic perspectives. Hepatology 2017, 65, 350–362. [Google Scholar] [CrossRef]
- Pizarro, M.; Balasubramaniyan, N.; Solís, N.; Solar, A.; Duarte, I.; Miquel, J.F.; Suchy, F.J.; Trauner, M.; Accatino, L.; Ananthanarayanan, M.; et al. Bile secretory function in the obese Zucker rat: Evidence of cholestasis and altered canalicular transport function. Gut 2004, 53, 1837–1843. [Google Scholar] [CrossRef]
- Segovia-Miranda, F.; Morales-Navarrete, H.; Kücken, M.; Moser, V.; Seifert, S.; Repnik, U.; Rost, F.; Brosch, M.; Hendricks, A.; Hinz, S.; et al. Three-dimensional spatially resolved geometrical and functional models of human liver tissue reveal new aspects of NAFLD progression. Nat. Med. 2019, 25, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Arab, J.P.; Arrese, M.; Trauner, M. Recent Insights into the Pathogenesis of Nonalcoholic Fatty Liver Disease. Annu. Rev. Pathol. 2018, 13, 321–350. [Google Scholar] [CrossRef] [PubMed]
- Jansen, P.L.; Ghallab, A.; Vartak, N.; Reif, R.; Schaap, F.G.; Hampe, J.; Hengstler, J.G. The ascending pathophysiology of cholestatic liver disease. Hepatology 2017, 65, 722–738. [Google Scholar] [CrossRef]
- Kjærgaard, K.; Frisch, K.; Sørensen, M.; Munk, O.L.; Hofmann, A.F.; Horsager, J.; Schacht, A.C.; Erickson, M.; Shapiro, D.; Keiding, S. Obeticholic acid improves hepatic bile acid excretion in patients with primary biliary cholangitis. J. Hepatol. 2021, 74, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Jiao, N.; Baker, S.S.; Chapa-Rodriguez, A.; Liu, W.; Nugent, C.A.; Tsompana, M.; Mastrandrea, L.; Buck, M.J.; Baker, R.D.; Genco, R.J.; et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 2018, 67, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.; Charles, E.D.; Neuschwander-Tetri, B.A.; Loomba, R.; Harrison, S.A.; Abdelmalek, M.F.; Lawitz, E.J.; Halegoua-DeMarzio, D.; Kundu, S.; Noviello, S.; et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: A randomised, double-blind, placebo-controlled, phase 2a trial. Lancet 2019, 392, 2705–2717. [Google Scholar] [CrossRef]
- Luo, Y.; Decato, B.E.; Charles, E.D.; Shevell, D.E.; McNaney, C.; Shipkova, P.; Apfel, A.; Tirucherai, G.S.; Sanyal, A.J. Pegbelfermin selectively reduces secondary bile acid concentrations in patients with non-alcoholic steatohepatitis. JHEP Rep. 2022, 4, 100392. [Google Scholar] [CrossRef]
- Larabi, A.B.; Masson, H.L.P.; Bäumler, A.J. Bile acids as modulators of gut microbiota composition and function. Gut Microbes 2023, 15, 2172671. [Google Scholar] [CrossRef]
- Yoshimoto, T.; Higashi, H.; Kanatani, A.; Lin, X.S.; Nagai, H.; Oyama, H.; Kurazono, K.; Tsuru, D. Cloning and sequencing of the 7 alpha-hydroxysteroid dehydrogenase gene from Escherichia coli HB101 and characterization of the expressed enzyme. J. Bacteriol. 1991, 173, 2173–2179. [Google Scholar] [CrossRef]
- Zheng, M.M.; Wang, R.F.; Li, C.X.; Xu, J.H. Two-step enzymatic synthesis of ursodeoxycholic acid with a new 7β-hydroxysteroid dehydrogenase from Ruminococcus torques. Process Biochem. 2015, 50, 598–604. [Google Scholar] [CrossRef]


| No. | Bile Acid | Molecular Formula | [M − H]− (m/z) | NCE (eV) | |
|---|---|---|---|---|---|
| Abbreviation | Full Name | ||||
| 1 | 3-oxoLCA | 3-Oxolithocholic acid | C24H38O3 | 373.2737 | −50 |
| 2 | NorDCA | Nordeoxycholic acid | C23H38O4 | 377.2686 | −22 |
| 3 | 12-ketoLCA | 12-Ketolithocholic acid | C24H38O4 | 389.2686 | −15 |
| 4 | 7-ketoLCA | 7-Ketolithocholic acid | C24H38O4 | 389.2686 | −15 |
| 5 | 12-oxoCDCA | 12-Oxochenodeoxycholic acid | C24H38O5 | 405.2636 | −15 |
| 6 | 7-keto DCA | 7-Ketodeoxycholic acid | C24H38O5 | 405.2719 | −15 |
| 7 | alloLCA | Allolithocholic acid | C24H40O3 | 375.2894 | −22 |
| 8 | iso-alloLCA | Isoallolithocholic acid | C24H40O3 | 375.2894 | −50 |
| 9 | isoLCA | Isolithocholic acid | C24H40O3 | 375.2894 | −50 |
| 10 | CDCA | Chenodeoxycholic acid | C24H40O4 | 391.2843 | −15 |
| 11 | DCA | Deoxycholic acid | C24H40O4 | 391.2843 | −15 |
| 12 | HDCA | Hyodeoxycholic acid | C24H40O4 | 391.2843 | −15 |
| 13 | MDCA | Murideoxycholic acid | C24H40O4 | 391.2843 | −15 |
| 14 | UDCA | Ursodesoxycholic acid | C24H40O4 | 391.2843 | −15 |
| 15 | isoDCA | Isodeoxycholic acid | C24H40O4 | 391.2927 | −15 |
| 16 | alloCA | Allocholic acid | C24H40O5 | 407.2792 | −10 |
| 17 | CA | Cholic acid | C24H40O5 | 407.2792 | −10 |
| 18 | LCA | Lithocholic acid | C24H40O3 | 407.2792 | −10 |
| 19 | HCA | Hyocholic acid | C24H40O5 | 407.2792 | −10 |
| 20 | UCA | Ursocholic acid | C24H40O5 | 407.2792 | −10 |
| 21 | αMCA | alpha-Muricholic acid | C24H40O5 | 407.2792 | −10 |
| 22 | βMCA | beta-Muricholic acid | C24H40O5 | 407.2792 | −10 |
| 23 | TLCA | Taurolithocholic Acid | C26H45NO5S | 482.2935 | −80 |
| 24 | TCDCA | Taurochenodeoxycholic acid | C26H45NO6S | 498.2884 | −72 |
| 25 | TDCA | Taurodesoxycholic acid | C26H45NO6S | 498.2884 | −72 |
| 26 | THDCA | Taurohyodeoxycholic acid | C26H45NO6S | 498.2884 | −72 |
| 27 | TUDCA | Tauroursodeoxycholic acid | C26H45NO6S | 498.2884 | −72 |
| 28 | TCA | Taurocholic acid | C26H45NO7S | 514.2833 | −80 |
| 29 | THCA | Taurohyocholic acid | C26H45NO7S | 514.2833 | −80 |
| 30 | TαMCA | Tauro-alpha-muricholic acid | C26H45NO7S | 514.2833 | −80 |
| 31 | TβMCA | Tauro-beta-muricholic acid | C26H45NO7S | 514.2833 | −80 |
| Position | Class | BAs | VIP | Trend |
|---|---|---|---|---|
| Colonic contents | Primary BAs | CA | 1.072 | ↓ * |
| Colonic contents | Primary BAs | CDCA | 1.534 | ↓ ** |
| Colonic contents | Primary BAs | TCA | 1.954 | ↓ ** |
| Colonic contents | Primary BAs | 7-keto LCA | 1.971 | ↑ ** |
| Colonic contents | Primary BAs | TUDCA | 1.473 | ↑ ** |
| Colonic contents | Secondary BAs | DCA | 1.982 | ↓ ** |
| Colonic contents | Secondary BAs | TDCA | 1.914 | ↓ ** |
| Liver | Primary BAs | CA | 1.550 | ↓ ** |
| Liver | Primary BAs | CDCA | 2.370 | ↓ ** |
| Liver | Primary BAs | TCA | 3.684 | ↓ ** |
| Liver | Primary BAs | TCDCA | 2.050 | ↓ * |
| Liver | Secondary BAs | DCA | 1.299 | ↓ * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, W.; Wang, Y.; Li, H.; Ma, H.; Gong, Y.; Luo, G.; Gao, X. Integration of Pseudotargeted Metabolomics and Microbiomics Reveals That Hugan Tablets Ameliorate NASH with Liver Fibrosis in Mice by Modulating Bile Acid Metabolism via the Gut Microbiome. Metabolites 2025, 15, 433. https://doi.org/10.3390/metabo15070433
Dong W, Wang Y, Li H, Ma H, Gong Y, Luo G, Gao X. Integration of Pseudotargeted Metabolomics and Microbiomics Reveals That Hugan Tablets Ameliorate NASH with Liver Fibrosis in Mice by Modulating Bile Acid Metabolism via the Gut Microbiome. Metabolites. 2025; 15(7):433. https://doi.org/10.3390/metabo15070433
Chicago/Turabian StyleDong, Wenran, Ying Wang, Huajinzi Li, Huilin Ma, Yingxi Gong, Gan Luo, and Xiaoyan Gao. 2025. "Integration of Pseudotargeted Metabolomics and Microbiomics Reveals That Hugan Tablets Ameliorate NASH with Liver Fibrosis in Mice by Modulating Bile Acid Metabolism via the Gut Microbiome" Metabolites 15, no. 7: 433. https://doi.org/10.3390/metabo15070433
APA StyleDong, W., Wang, Y., Li, H., Ma, H., Gong, Y., Luo, G., & Gao, X. (2025). Integration of Pseudotargeted Metabolomics and Microbiomics Reveals That Hugan Tablets Ameliorate NASH with Liver Fibrosis in Mice by Modulating Bile Acid Metabolism via the Gut Microbiome. Metabolites, 15(7), 433. https://doi.org/10.3390/metabo15070433





