Insomnia, Cognitive Impairment, or a Combination of Both, Alter Lipid Metabolism Due to Changes in Acylcarnitine Concentration in Older Persons
Abstract
1. Introduction
2. Materials and Methods
2.1. Cohort Description
2.2. Sample Collection
2.3. Insomnia Evaluation Symptoms
2.4. Cognition Status Evaluation
2.5. Targeted Metabolomic Determinations
2.6. Sample Processing
2.7. Assessment of the Acylcarnitine (AC)/L-Carnitine (LC) Ratio
2.8. Statistical Analysis
2.9. Ethical Statement
3. Results
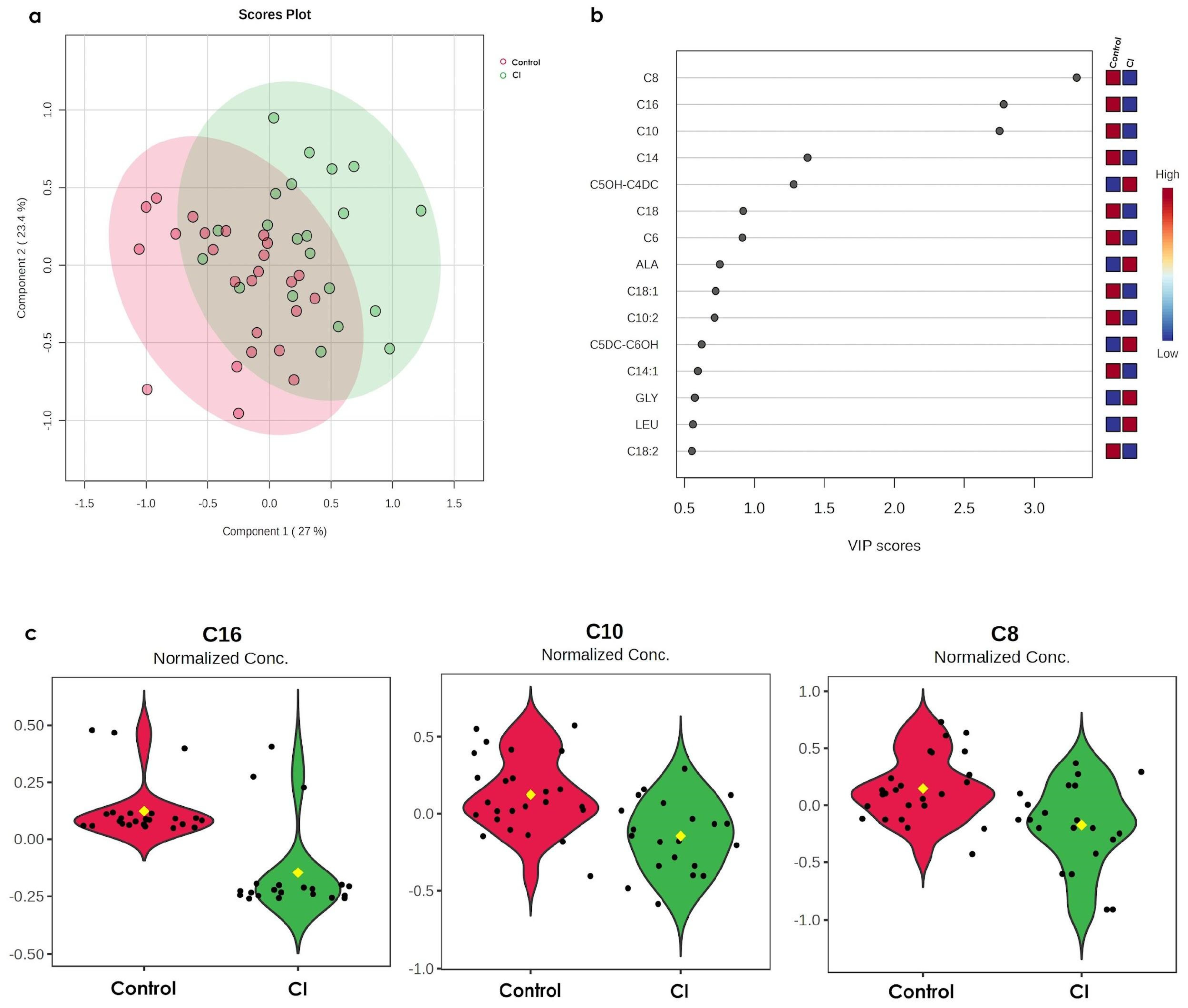
3.1. CI Induces Alterations of C16, C10, and C8 in Older Persons
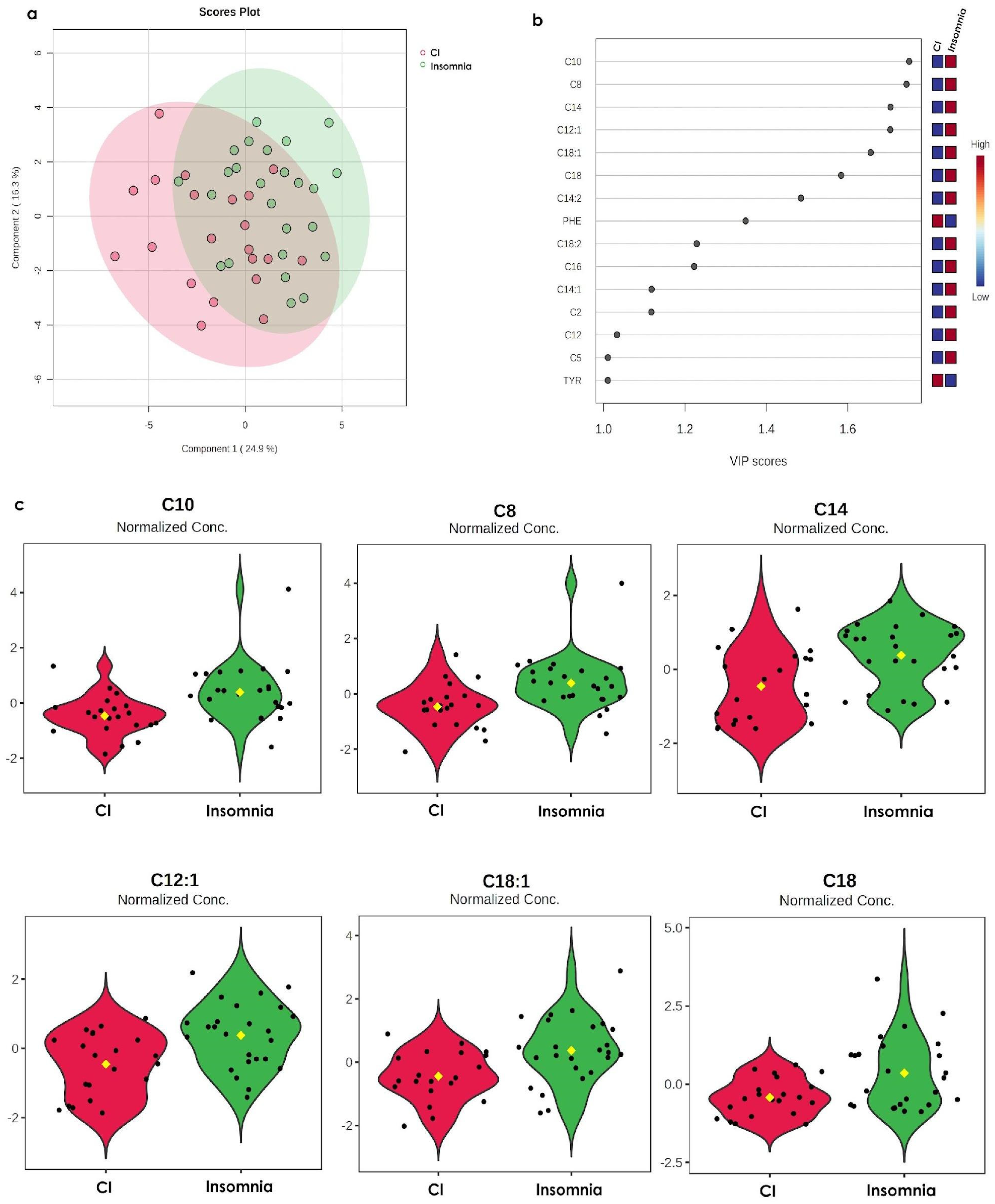
3.2. The Metabolomic Profile of Insomnia Is Opposite to the CI Profile
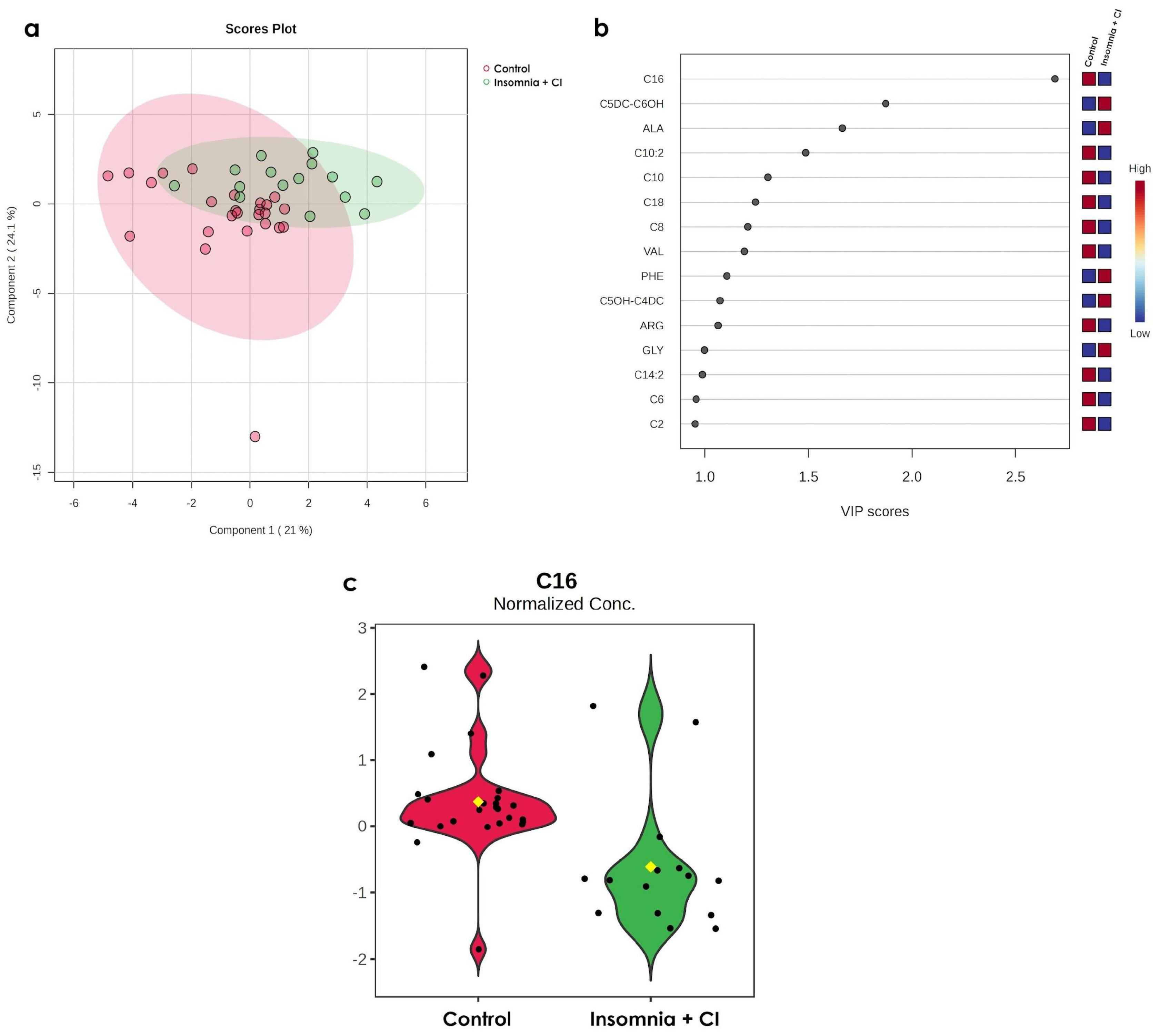
3.3. Only C16 Increases in Older Persons with Both Conditions (Insomnia + CI)
3.4. Impairments in C16OH/C16 Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | directory of open access journals |
| TLA | three letter acronym |
| LD | linear dichroism |
| CI | cognitive impairment |
| CAAs | branched-chain amino acids |
| COSFOMA | Cohort of Obesity, Sarcopenia, and Frailty of Older Mexican Adults |
| AA | amino acid |
| ORN | ornithine |
| TYR | tyrosine |
| PHE | l-phenylalanine |
| ALA | alanine |
| C5:1 | tiglylcarnitine |
| C0 | free carnitine |
| C12:1 | dodecenoylcarnitine |
| C10 | decanoylcarnitine |
| T2D | Type 2 diabetes |
| MCI | mild cognitive impairment |
| AD | Alzheimer’s disease |
| IMSS | Mexican Social Security Institute |
| AIS | Athens Insomnia Scale |
| MMSE | Mini-Mental State Examination |
| LC-ESI-MS/MS | liquid chromatography-electrospray ionization positive-tandem mass spectrometry |
| MRM | multiple reaction monitoring |
| GLY | glycine |
| CIT | citrulline |
| ARG | arginine |
| LEU | leucine |
| MET | L-methionine |
| VAL | valine |
| PRO | proline |
| SA | succinylacetone |
| C2 | acetylcarnitine |
| C3 | propionyl carnitine |
| C6DC | methylglutarylcarnitine |
| C4 | isobutyric-L-carnitine |
| C5 | isovalerylcarnitine |
| C6 | hexanoylcarnitine |
| C8 | octanoyl carnitine |
| C16 | palmitoyl-L-carnitine |
| C14 | myristoylcarnitine |
| C18 | sterearoylcarnitine |
| PLS-DA | partial least squares discriminant analysis |
| VIP | variable importance in the projection |
| Tukey’s HSD | Tukey’s honestly significant difference |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| SMPDB | small molecule pathway database |
| SMC | subjective memory complaint |
References
- Palmer, A.K.; Jensen, M.D. Metabolic Changes in Aging Humans: Current Evidence and Therapeutic Strategies. J. Clin. Investig. 2022, 132, e158451. [Google Scholar] [CrossRef] [PubMed]
- Bartke, A.; Brannan, S.; Hascup, E.; Hascup, K.; Darcy, J. Energy Metabolism and Aging. World J. Men’s Health 2021, 39, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ma, Y.; Luo, Y.; Song, Y.; Xiong, G.; Ma, Y.; Sun, X.; Kan, C. Metabolic Diseases and Healthy Aging: Identifying Environmental and Behavioral Risk Factors and Promoting Public Health. Front. Public Health 2023, 11, 1253506. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, J.; Sun, X.; Qu, Z.; Zhang, J.; Zhang, H. The Association between Insomnia and Cognitive Decline: A Scoping Review. Sleep Med. 2024, 124, 540–550. [Google Scholar] [CrossRef]
- Shaha, D.P. Insomnia Management: A Review and Update. J Fam. Pract. 2023, 72, S31–S36. [Google Scholar] [CrossRef]
- Patel, D.; Steinberg, J.; Patel, P. Insomnia in the Elderly: A Review. J. Clin. Sleep Med. 2018, 14, 1017–1024. [Google Scholar] [CrossRef]
- Wennberg, A.M.V.; Wu, M.N.; Rosenberg, P.B.; Spira, A.P. Sleep Disturbance, Cognitive Decline, and Dementia: A Review. Semin. Neurol. 2017, 37, 395–406. [Google Scholar]
- Dalamaga, M. Clinical Metabolomics: Useful Insights, Perspectives and Challenges. Metab. Open 2024, 22, 100290. [Google Scholar] [CrossRef] [PubMed]
- Steuer, A.E.; Brockbals, L.; Kraemer, T. Metabolomic Strategies in Biomarker Research–New Approach for Indirect Identification of Drug Consumption and Sample Manipulation in Clinical and Forensic Toxicology? Front. Chem. 2019, 7, 449452. [Google Scholar] [CrossRef]
- Gehrman, P.; Sengupta, A.; Harders, E.; Ubeydullah, E.; Pack, A.I.; Weljie, A. Altered Diurnal States in Insomnia Reflect Peripheral Hyperarousal and Metabolic Desynchrony: A Preliminary Study. Sleep 2018, 41, zsy043. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, B.; Sheng, D.; Yang, J.; Fu, S.; Wang, J.; Zhao, C.; Wang, Y.; Gai, X.; Wang, J.; et al. Multiomics Analysis Reveals Aberrant Metabolism and Immunity Linked Gut Microbiota with Insomnia. Microbiol. Spectr. 2022, 10, e0099822. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, X.; Li, Z.; Zou, Z.; Dou, S.; Li, G.; Yan, F.; Chen, B.; Li, Y. Alterations in Gut Microbiota Are Correlated with Serum Metabolites in Patients with Insomnia Disorder. Front. Cell. Infect. Microbiol. 2022, 12, 722662. [Google Scholar] [CrossRef] [PubMed]
- Even, C.; Magzal, F.; Shochat, T.; Haimov, I.; Agmon, M.; Tamir, S. Microbiota Metabolite Profiles and Dietary Intake in Older Individuals with Insomnia of Short vs. Normal Sleep Duration. Biomolecules 2024, 14, 419. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association Mild Cognitive Impairment (MCI). Available online: https://www.alz.org/alzheimers-dementia/what-is-dementia/related_conditions/mild-cognitive-impairment (accessed on 26 February 2025).
- Zhao, Y.; Song, P.; Zhang, H.; Chen, X.; Han, P.; Yu, X.; Fang, C.; Xie, F.; Guo, Q. Alteration of Plasma Metabolic Profile and Physical Performance Combined with Metabolites Is More Sensitive to Early Screening for Mild Cognitive Impairment. Front. Aging Neurosci. 2022, 14, 951146. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.; Chen, J.; Luo, M.; Xie, Q.; Rong, Y.; Wu, Y.; Cao, Z.; Liu, Y. High-Resolution NMR Metabolomics of Patients with Subjective Cognitive Decline plus: Perturbations in the Metabolism of Glucose and Branched-Chain Amino Acids. Neurobiol. Dis. 2022, 171, 105782. [Google Scholar] [CrossRef]
- Wang, J.; Wei, R.; Xie, G.; Arnold, M.; Kueider-Paisley, A.; Louie, G.; Mahmoudian, D.S.; Blach, C.; Baillie, R.; Han, X.; et al. Peripheral Serum Metabolomic Profiles Inform Central Cognitive Impairment. Sci. Rep. 2020, 10, 14059. [Google Scholar] [CrossRef]
- Sánchez-García, S.; García-Peña, C.; Salvà, A.; Sánchez-Arenas, R.; Granados-García, V.; Cuadros-Moreno, J.; Velázquez-Olmedo, L.B.; Cárdenas-Bahena, Á. Frailty in Community-Dwelling Older Adults: Association with Adverse Outcomes. Clin. Interv. Aging 2017, 12, 1003–1011. [Google Scholar] [CrossRef]
- Jahrami, H.; Trabelsi, K.; Saif, Z.; Manzar, M.D.; BaHammam, A.S.; Vitiello, M.V. Reliability Generalization Meta-Analysis of the Athens Insomnia Scale and Its Translations: Examining Internal Consistency and Test-Retest Validity. Sleep Med. 2023, 111, 133–145. [Google Scholar] [CrossRef]
- Shahid, A.; Wilkinson, K.; Marcu, S.; Shapiro, C.M. Athens Insomnia Scale (AIS). In STOP, THAT and One Hundred Other Sleep Scales; Springer: New York, NY, USA, 2011; pp. 53–54. ISBN 9781441998927. [Google Scholar]
- Nenclares Portocarrero, A.; Jiménez-Genchi, A. Estudio de validación de la traducción al español de la Escala Atenas de Insomnio. Salud Ment. 2005, 28, 34–39. [Google Scholar]
- Sánchez-García, S.; Moreno-Tamayo, K.; Ramírez-Aldana, R.; García-Peña, C.; Medina-Campos, R.H.; García Dela Torre, P.; Rivero-Segura, N.A. Insomnia Impairs Both the Pro-BDNF and the BDNF Levels Similarly to Older Adults with Cognitive Decline: An Exploratory Study. Int. J. Mol. Sci. 2023, 24, 7387. [Google Scholar] [CrossRef]
- Franco-Marina, F.; García-González, J.J.; Wagner-Echeagaray, F.; Gallo, J.; Ugalde, O.; Sánchez-García, S.; Espinel-Bermúdez, C.; Juárez-Cedillo, T.; Rodríguez, M.Á.V.; García-Peña, C. The Mini-Mental State Examination Revisited: Ceiling and Floor Effects after Score Adjustment for Educational Level in an Aging Mexican Population. Int. Psychogeriatr. 2010, 22, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Pons, R.; De Vivo, D.C. Primary and Secondary Carnitine Deficiency Syndromes. J. Child Neurol. 1995, 10, 2S8–2S24. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a Unified Platform for Metabolomics Data Processing, Analysis and Interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef] [PubMed]
- Baydakova, G.V.; Tsygankova, P.G.; Pechatnikova, N.L.; Bazhanova, O.A.; Nazarenko, Y.D.; Zakharova, E.Y. New Acylcarnitine Ratio as a Reliable Indicator of Long-Chain 3-Hydroxyacyl-CoA Dehydrogenase Deficiency. Int. J. Neonatal Screen. 2023, 9, 48. [Google Scholar] [CrossRef]
- Al-Thihli, K.; Sinclair, G.; Sirrs, S.; Mezei, M.; Nelson, J.; Vallance, H. Performance of Serum and Dried Blood Spot Acylcarnitine Profiles for Detection of Fatty Acid β-Oxidation Disorders in Adult Patients with Rhabdomyolysis. J. Inherit. Metab. Dis. 2014, 37, 207–213. [Google Scholar] [CrossRef]
- Panyard, D.J.; Yu, B.; Snyder, M.P. The Metabolomics of Human Aging: Advances, Challenges, and Opportunities. Sci. Adv. 2022, 8, eadd6155. [Google Scholar] [CrossRef]
- Spinelli, R.; Parrillo, L.; Longo, M.; Florese, P.; Desiderio, A.; Zatterale, F.; Miele, C.; Raciti, G.A.; Beguinot, F. Molecular Basis of Ageing in Chronic Metabolic Diseases. J. Endocrinol. Investig. 2020, 43, 1373–1389. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, H.; Xiong, W. Metabolism and Metabolomics in Senescence, Aging, and Age-Related Diseases: A Multiscale Perspective. Front. Med. 2025, 19, 200–225. [Google Scholar] [CrossRef]
- Lee, L.C.; Liong, C.-Y.; Jemain, A.A. Partial Least Squares-Discriminant Analysis (PLS-DA) for Classification of High-Dimensional (HD) Data: A Review of Contemporary Practice Strategies and Knowledge Gaps. Analyst 2018, 143, 3526–3539. [Google Scholar] [CrossRef]
- Xu, S.; Bai, C.; Chen, Y.; Yu, L.; Wu, W.; Hu, K. Comparing Univariate Filtration Preceding and Succeeding PLS-DA Analysis on the Differential Variables/metabolites Identified from Untargeted LC-MS Metabolomics Data. Anal. Chim. Acta 2024, 1287, 342103. [Google Scholar] [CrossRef]
- Miccheli, A.; Marini, F.; Capuani, G.; Miccheli, A.T.; Delfini, M.; Di Cocco, M.E.; Puccetti, C.; Paci, M.; Rizzo, M.; Spataro, A. The Influence of a Sports Drink on the Postexercise Metabolism of Elite Athletes as Investigated by NMR-Based Metabolomics. J. Am. Coll. Nutr. 2009, 28, 553–564. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, Z.R.; Ryan Smith, M.; Hu, X.; Orr, M.; Liu, K.H.; Quyyumi, A.A.; Jones, D.P.; Go, Y.-M. Plasma Acylcarnitine Levels Increase with Healthy Aging. Aging 2020, 12, 13555. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.K.; Ang, J.E.; Revell, V.L.; Holmes, B.; Mann, A.; Robertson, F.P.; Cui, N.; Middleton, B.; Ackermann, K.; Kayser, M.; et al. Effect of Sleep Deprivation on the Human Metabolome. Proc. Natl. Acad. Sci. USA 2014, 111, 10761. [Google Scholar] [CrossRef]
- Kadyrov, M.; Whiley, L.; Brown, B.; Erickson, K.I.; Holmes, E. Associations of the Lipidome with Ageing, Cognitive Decline and Exercise Behaviours. Metabolites 2022, 12, 822. [Google Scholar] [CrossRef]
- González-Domínguez, R.; García, A.; García-Barrera, T.; Barbas, C.; Gómez-Ariza, J.L. Metabolomic Profiling of Serum in the Progression of Alzheimer’s Disease by Capillary Electrophoresis-Mass Spectrometry. Electrophoresis 2014, 35, 3321–3330. [Google Scholar] [CrossRef]
- Huo, Z.; Yu, L.; Yang, J.; Zhu, Y.; Bennett, D.A.; Zhao, J. Corrigendum to Brain and Blood Metabolome for Alzheimer’s Dementia: Findings from a Targeted Metabolomics Analysis [Neurobiology of Aging Volume 86, February 2020, Pages 123–133]. Neurobiol. Aging 2020, 91, 169. [Google Scholar] [CrossRef]
- Olazarán, J.; Gil-de-Gómez, L.; Rodríguez-Martín, A.; Valentí-Soler, M.; Frades-Payo, B.; Marín-Muñoz, J.; Antúnez, C.; Frank-García, A.; Acedo-Jiménez, C.; Morlán-Gracia, L.; et al. A Blood-Based, 7-Metabolite Signature for the Early Diagnosis of Alzheimer’s Disease. J. Alzheimers Dis. 2015, 45, 1157–1173. [Google Scholar] [CrossRef]
- Cristofano, A.; Sapere, N.; La Marca, G.; Angiolillo, A.; Vitale, M.; Corbi, G.; Scapagnini, G.; Intrieri, M.; Russo, C.; Corso, G.; et al. Serum Levels of Acyl-Carnitines along the Continuum from Normal to Alzheimer’s Dementia. PLoS ONE 2016, 11, e0155694. [Google Scholar] [CrossRef]
- Jia, L.; Yang, J.; Zhu, M.; Pang, Y.; Wang, Q.; Wei, Q.; Li, Y.; Li, T.; Li, F.; Wang, Q.; et al. A Metabolite Panel That Differentiates Alzheimer’s Disease from Other Dementia Types. Alzheimers Dement. 2022, 18, 1345–1356. [Google Scholar] [CrossRef]
- Ciavardelli, D.; Piras, F.; Consalvo, A.; Rossi, C.; Zucchelli, M.; Di Ilio, C.; Frazzini, V.; Caltagirone, C.; Spalletta, G.; Sensi, S.L. Medium-Chain Plasma Acylcarnitines, Ketone Levels, Cognition, and Gray Matter Volumes in Healthy Elderly, Mildly Cognitively Impaired, or Alzheimer’s Disease Subjects. Neurobiol. Aging 2016, 43, 1–12. [Google Scholar] [CrossRef]
- Mai, M.; Tönjes, A.; Kovacs, P.; Stumvoll, M.; Fiedler, G.M.; Leichtle, A.B. Serum Levels of Acylcarnitines Are Altered in Prediabetic Conditions. PLoS ONE 2013, 8, e82459. [Google Scholar] [CrossRef]
- Adams, S.H.; Hoppel, C.L.; Lok, K.H.; Zhao, L.; Wong, S.W.; Minkler, P.E.; Hwang, D.H.; Newman, J.W.; Garvey, W.T. Plasma Acylcarnitine Profiles Suggest Incomplete Long-Chain Fatty Acid β-Oxidation and Altered Tricarboxylic Acid Cycle Activity in Type 2 Diabetic African-American Women. J. Nutr. 2009, 139, 1073–1081. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, X.-F.; Huang, T.; Luo, H.-H.; Chen, J.-X.; Zeng, J.; Gu, M.; Li, J.; Sun, X.-Y.; Sun, D.; et al. The Association Between Acylcarnitine Metabolites and Cardiovascular Disease in Chinese Patients With Type 2 Diabetes Mellitus. Front. Endocrinol. 2020, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- El-Gharbawy, A.; Vockley, J. Inborn Errors of Metabolism with Myopathy: Defects of Fatty Acid Oxidation and the Carnitine Shuttle System. Pediatr. Clin. N. Am. 2018, 65, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Dambrova, M.; Makrecka-Kuka, M.; Kuka, J.; Vilskersts, R.; Nordberg, D.; Attwood, M.M.; Smesny, S.; Sen, Z.D.; Guo, A.C.; Oler, E.; et al. Acylcarnitines: Nomenclature, Biomarkers, Therapeutic Potential, Drug Targets, and Clinical Trials. Pharmacol. Rev. 2022, 74, 506–551. [Google Scholar] [CrossRef] [PubMed]
- Kalim, S.; Clish, C.B.; Wenger, J.; Elmariah, S.; Yeh, R.W.; Deferio, J.J.; Pierce, K.; Deik, A.; Gerszten, R.E.; Thadhani, R.; et al. A Plasma Long-Chain Acylcarnitine Predicts Cardiovascular Mortality in Incident Dialysis Patients. J. Am. Heart Assoc. 2013, 2, e000542. [Google Scholar] [CrossRef]
- Cho, K.; Yoon, D.W.; Lee, M.; So, D.; Hong, I.-H.; Rhee, C.-S.; Park, J.-W.; Cho, J.-Y.; Shin, H.-W. Urinary Metabolomic Signatures in Obstructive Sleep Apnea through Targeted Metabolomic Analysis: A Pilot Study. Metabolomics 2017, 13, 88. [Google Scholar] [CrossRef]
- Jaspan, V.N.; Greenberg, G.S.; Parihar, S.; Park, C.M.; Somers, V.K.; Shapiro, M.D.; Lavie, C.J.; Virani, S.S.; Slipczuk, L. The Role of Sleep in Cardiovascular Disease. Curr. Atheroscler. Rep. 2024, 26, 249–262. [Google Scholar] [CrossRef]
- Yoon, G.; Kam, M.K.; Koh, Y.H.; Jo, C. Palmitoyl-L-Carnitine Induces Tau Phosphorylation and Mitochondrial Dysfunction in Neuronal Cells. PLoS ONE 2024, 19, e0313507. [Google Scholar] [CrossRef]
- Ahmed, A.T.; MahmoudianDehkordi, S.; Bhattacharyya, S.; Arnold, M.; Liu, D.; Neavin, D.; Arthur Moseley, M.; Will Thompson, J.; St John Williams, L.; Louie, G.; et al. Acylcarnitine Metabolomic Profiles Inform Clinically-Defined Major Depressive Phenotypes. J. Affect. Disord. 2019, 264, 90. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, R.; Mook-Kanamori, D.O.; Donga, E.; van Dijk, M.; van Dijk, J.G.; Lammers, G.J.; van Kralingen, K.W.; Prehn, C.; Adamski, J.; Romijn, J.A.; et al. A Single Night of Sleep Curtailment Increases Plasma Acylcarnitines: Novel Insights in the Relationship between Sleep and Insulin Resistance. Arch. Biochem. Biophys. 2016, 589, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Nassir, F.; Arndt, J.J.; Johnson, S.A.; Ibdah, J.A. Regulation of Mitochondrial Trifunctional Protein Modulates Nonalcoholic Fatty Liver Disease in Mice. J. Lipid Res. 2018, 59, 967–973. [Google Scholar] [CrossRef]
- Tonin, A.M.; Grings, M.; Busanello, E.N.B.; Moura, A.P.; Ferreira, G.C.; Viegas, C.M.; Fernandes, C.G.; Schuck, P.F.; Wajner, M. Long-Chain 3-Hydroxy Fatty Acids Accumulating in LCHAD and MTP Deficiencies Induce Oxidative Stress in Rat Brain. Neurochem. Int. 2010, 56, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.A.; Mejia, E.M.; Mitchell, R.W.; Choy, P.C.; Sparagna, G.C.; Hatch, G.M. Human Trifunctional Protein Alpha Links Cardiolipin Remodeling to Beta-Oxidation. PLoS ONE 2012, 7, e48628. [Google Scholar] [CrossRef]
- Vianey-Saban, C.; Guffon, N.; Fouilhoux, A.; Acquaviva, C. Fifty Years of Research on Mitochondrial Fatty Acid Oxidation Disorders: The Remaining Challenges. J. Inherit. Metab. Dis. 2023, 46, 848–873. [Google Scholar] [CrossRef]
- Angdisen, J.; Moore, V.D.G.; Cline, J.M.; Payne, R.M.; Ibdah, J.A. Mitochondrial Trifunctional Protein Defects: Molecular Basis and Novel Therapeutic Approaches. Curr. Drug Targets Immune Endocr. Metab. Disord. 2005, 5, 27–40. [Google Scholar] [CrossRef]
- Borroni, E.; Frigerio, G.; Polledri, E.; Mercadante, R.; Maggioni, C.; Fedrizzi, L.; Pesatori, A.C.; Fustinoni, S.; Carugno, M. Metabolomic Profiles in Night Shift Workers: A Cross-Sectional Study on Hospital Female Nurses. Front. Public Health 2023, 11, 1082074. [Google Scholar] [CrossRef]
| Variables | Data |
|---|---|
| Age y.o. (mean ± SD) | 70.6 ± 6.3 |
| Sex | |
| Female (n, %) | 57 (71.2) |
| BMI (kg/m2) (mean ± SD) | |
| Controls | 27.93 ± 4.09 |
| Cognitive impairment (CI) | 27.40 ± 4.27 |
| Insomnia | 29.09 ± 3.81 |
| Insomnia + CI | 29.7 7± 4.47 |
| Body composition | |
| Controls | |
| Fat mass (%) (mean ± SD) | 33.03 ± 6.33 |
| Weight (kg) (mean ± SD) | 67.93 ± 11.38 |
| CI | |
| Fat mass (%) (mean ± SD) | 27.41 ± 4.27 |
| Weight (kg) (mean ± SD) | 64.18 ± 13.70 |
| Insomnia | |
| Fat mass (%) (mean ± SD) | 33.82 ± 7.79 |
| Weight (kg) (mean ± SD) | 71.00 ± 10.66 |
| Insomnia + CI | |
| Fat mass (%) (mean ± SD) | 36.6 ± 9.02 |
| Weight (kg) (mean ± SD) | 63.55 ± 11.41 |
| Education in years (n, %) | |
| None (Illiterate) | 3 (3.75) |
| <6 | 11 (13.75) |
| From 6 to 9 | 22 (27.5) |
| From 10 to 11 | 12 (15) |
| 12 and more | 32 (40) |
| Living arrangement | |
| Accompanied (n, %) | 75 (93.75) |
| Multimorbidity (n, %) | 20 (25) |
| Medication | |
| Number of medicines ± SD (n, %) | 3.8 ± 2.9 |
| Antidepressant consumption (n, %) | 17 (21.3) |
| Anxiety (n, %) | 17 (21.3) |
| Current smoking (n, %) | 8 (10) |
| Current alcohol consumption (n, %) | 14 (17.5) |
| Groups (n, %) | |
| Controls | 22 (27.5) |
| CI | 21 (26.3) |
| Insomnia | 23 (46.3) |
| Insomnia + CI | 14 (17.5) |
| Total of participants (n, %) | 80 (100) |
| C16OH/C16 | C16/C2 | C18/C2 | |
|---|---|---|---|
| Control | 0.6842 ± 0.1514 | 0.0255 ± 0.0115 | 0.0146 ± 0.0069 |
| CI | 0.7575 ± 0.2057 | 0.0263 ± 0.0128 | 0.0144 ± 0.0076 |
| Insomnia | 0.6550 ± 0.1318 | 0.0257 ± 0.0126 | 0.0144 ± 0.0088 |
| Insomnia + CI | 0.6727 ± 0.1429 | 0.0284 ± 0.0123 | 0.0159 ± 0.0067 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Vazquez, S.K.; Palacios-González, B.; Vela-Amieva, M.; Ibarra-González, I.; Morales, R.; García-delaTorre, P.; Sánchez-García, S.; García-Peña, C.; Reyes-Chilpa, R.; Medina-Campos, R.H.; et al. Insomnia, Cognitive Impairment, or a Combination of Both, Alter Lipid Metabolism Due to Changes in Acylcarnitine Concentration in Older Persons. Metabolites 2025, 15, 417. https://doi.org/10.3390/metabo15060417
Castillo-Vazquez SK, Palacios-González B, Vela-Amieva M, Ibarra-González I, Morales R, García-delaTorre P, Sánchez-García S, García-Peña C, Reyes-Chilpa R, Medina-Campos RH, et al. Insomnia, Cognitive Impairment, or a Combination of Both, Alter Lipid Metabolism Due to Changes in Acylcarnitine Concentration in Older Persons. Metabolites. 2025; 15(6):417. https://doi.org/10.3390/metabo15060417
Chicago/Turabian StyleCastillo-Vazquez, Selma Karime, Berenice Palacios-González, Marcela Vela-Amieva, Isabel Ibarra-González, Ricardo Morales, Paola García-delaTorre, Sergio Sánchez-García, Carmen García-Peña, Ricardo Reyes-Chilpa, Raúl Hernán Medina-Campos, and et al. 2025. "Insomnia, Cognitive Impairment, or a Combination of Both, Alter Lipid Metabolism Due to Changes in Acylcarnitine Concentration in Older Persons" Metabolites 15, no. 6: 417. https://doi.org/10.3390/metabo15060417
APA StyleCastillo-Vazquez, S. K., Palacios-González, B., Vela-Amieva, M., Ibarra-González, I., Morales, R., García-delaTorre, P., Sánchez-García, S., García-Peña, C., Reyes-Chilpa, R., Medina-Campos, R. H., Hernández-Pineda, J., Gomez-Verjan, J. C., & Rivero-Segura, N. A. (2025). Insomnia, Cognitive Impairment, or a Combination of Both, Alter Lipid Metabolism Due to Changes in Acylcarnitine Concentration in Older Persons. Metabolites, 15(6), 417. https://doi.org/10.3390/metabo15060417








