Mitochondrial Translation Inhibition Uncovers a Critical Metabolic–Epigenetic Interface in Renal Cell Carcinoma
Abstract
1. Introduction
2. Material and Methods
2.1. Immunoblotting
2.2. Immunofluorescence and Lipid Droplet Labeling
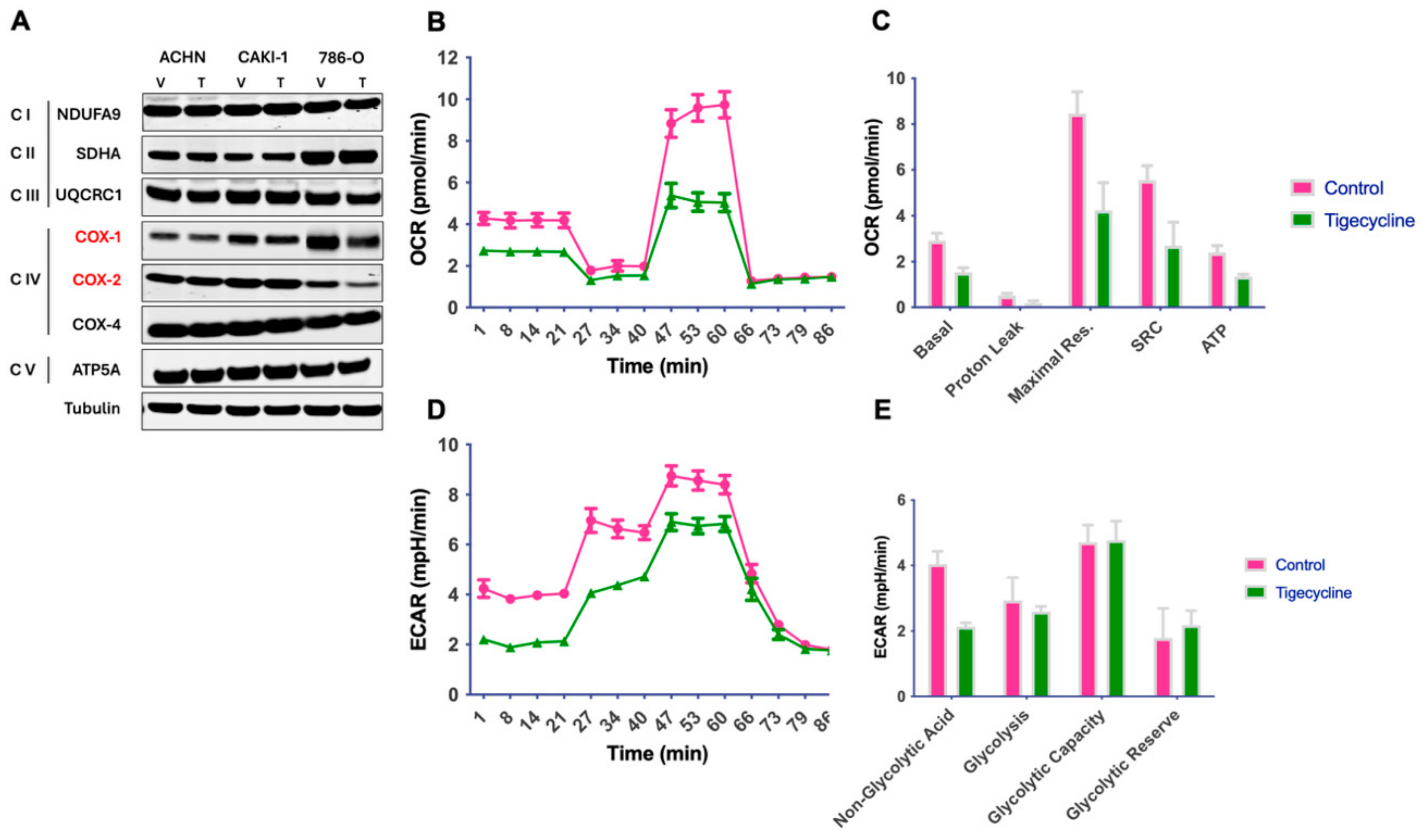
2.3. Oxygen Consumption Rate Assay
2.4. Cell Culture
2.5. In Vivo Xenograft Studies
2.6. Organoid Sourcing and Culture
2.7. Cell and Organoid Viability Assays
2.8. Whole Cell and Mitochondrial Isolation Prep for Proteomics
2.9. Metabolomics
2.10. Mass Spectrometry
3. Results
3.1. Tigecycline Inhibits Mitochondrial Translation and Impairs Cellular Bioenergetics in RCC
3.2. Tigecycline Treatment Induces Global Metabolic Reprogramming in RCC Cells
3.3. Tigecycline Profoundly Alters Lipid Metabolism in RCC Cells
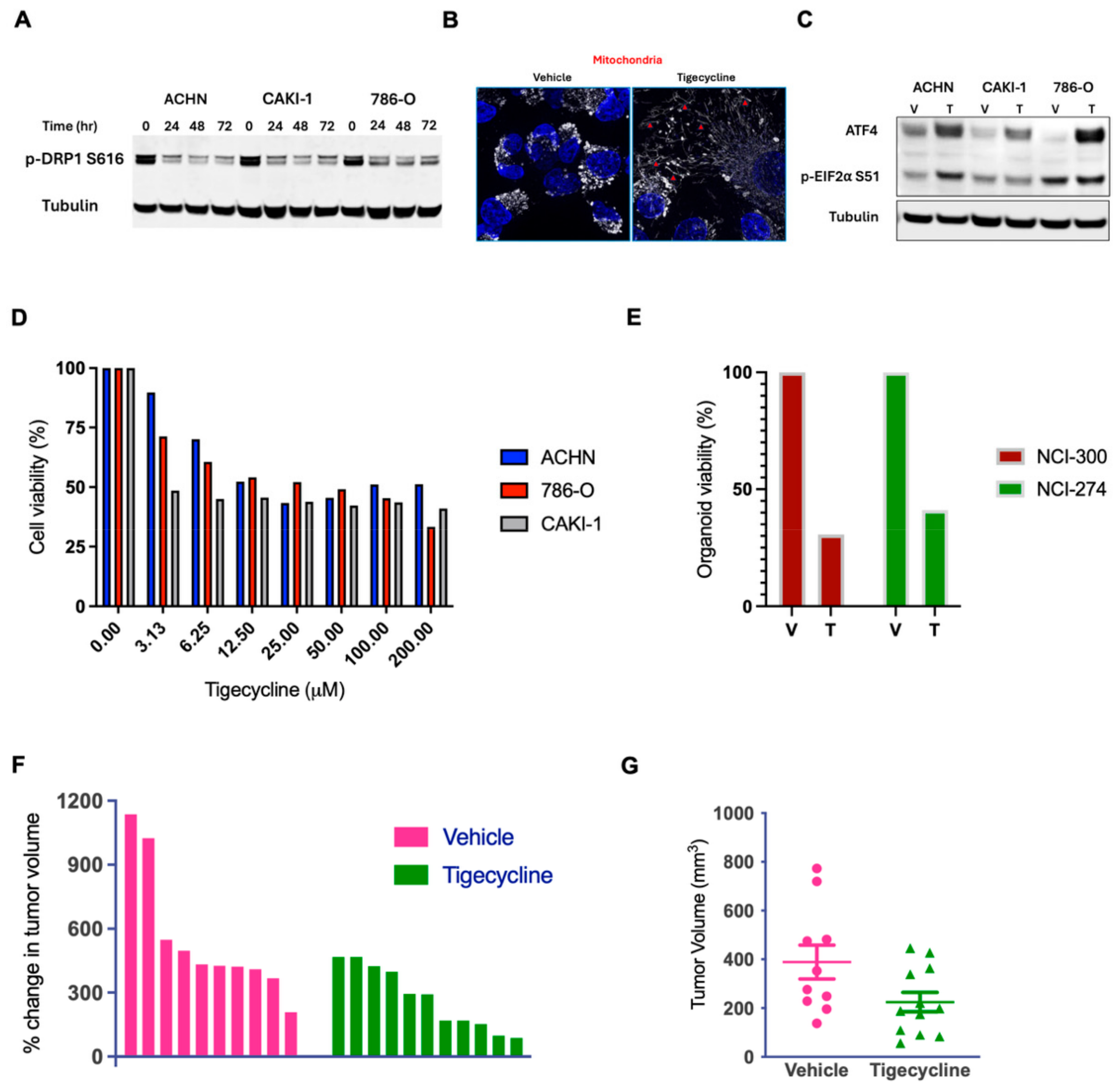
3.4. Tigecycline Modulates Mitochondrial Dynamics and Integrated Stress Response
3.5. Tigecycline Demonstrates Robust Anticancer Activity in RCC Cell Lines, Patient-Derived Organoids, and Human RCC Xenografts
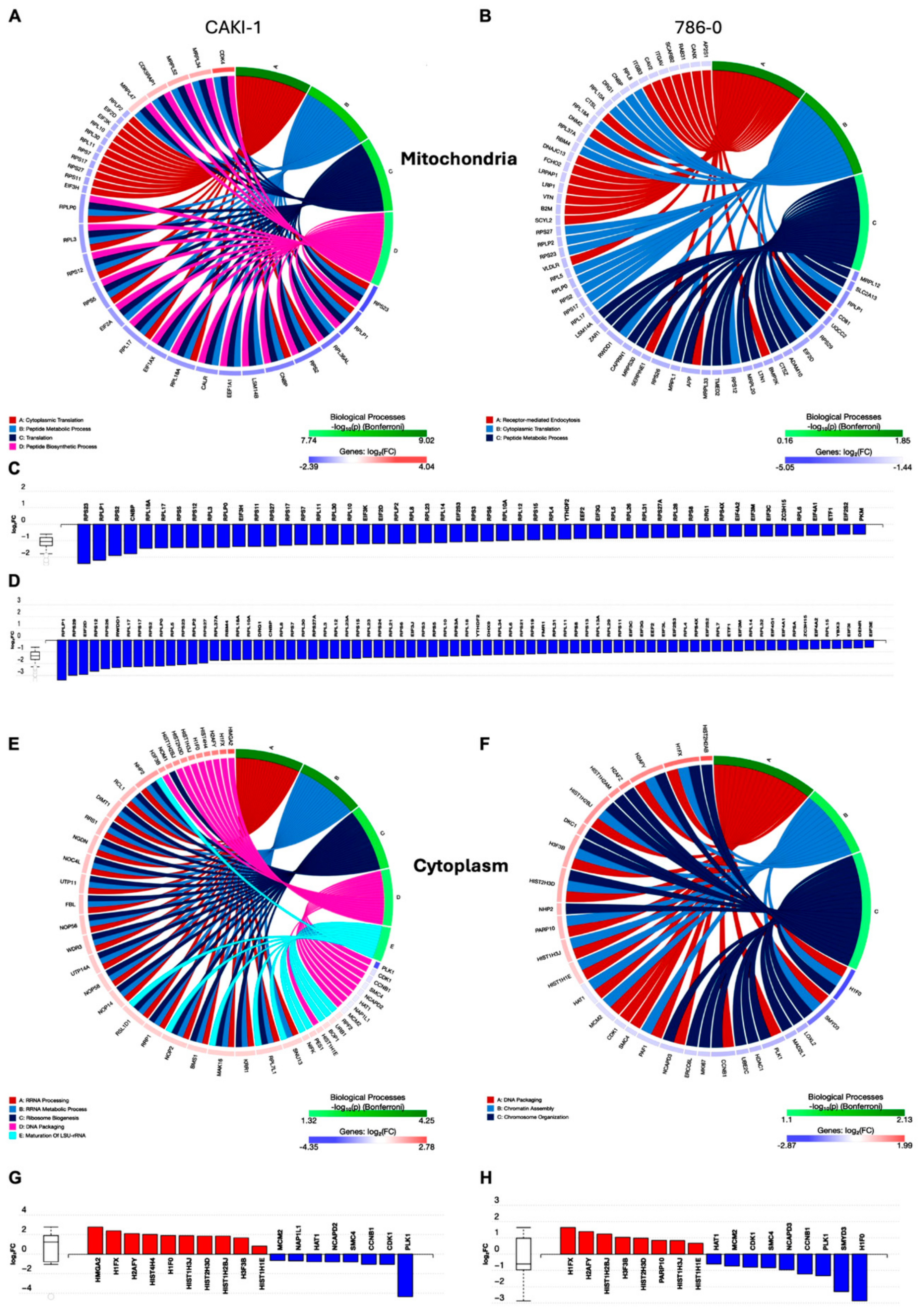
3.6. Compartment-Specific Proteomic Analysis Reveals a Novel Metabolic–Epigenetic Link
3.7. Tigecycline Treatment Drives Dramatic Remodeling of the Cytoplasmic Proteome with Histone Accumulation and Cell Cycle Perturbation in RCC Cells
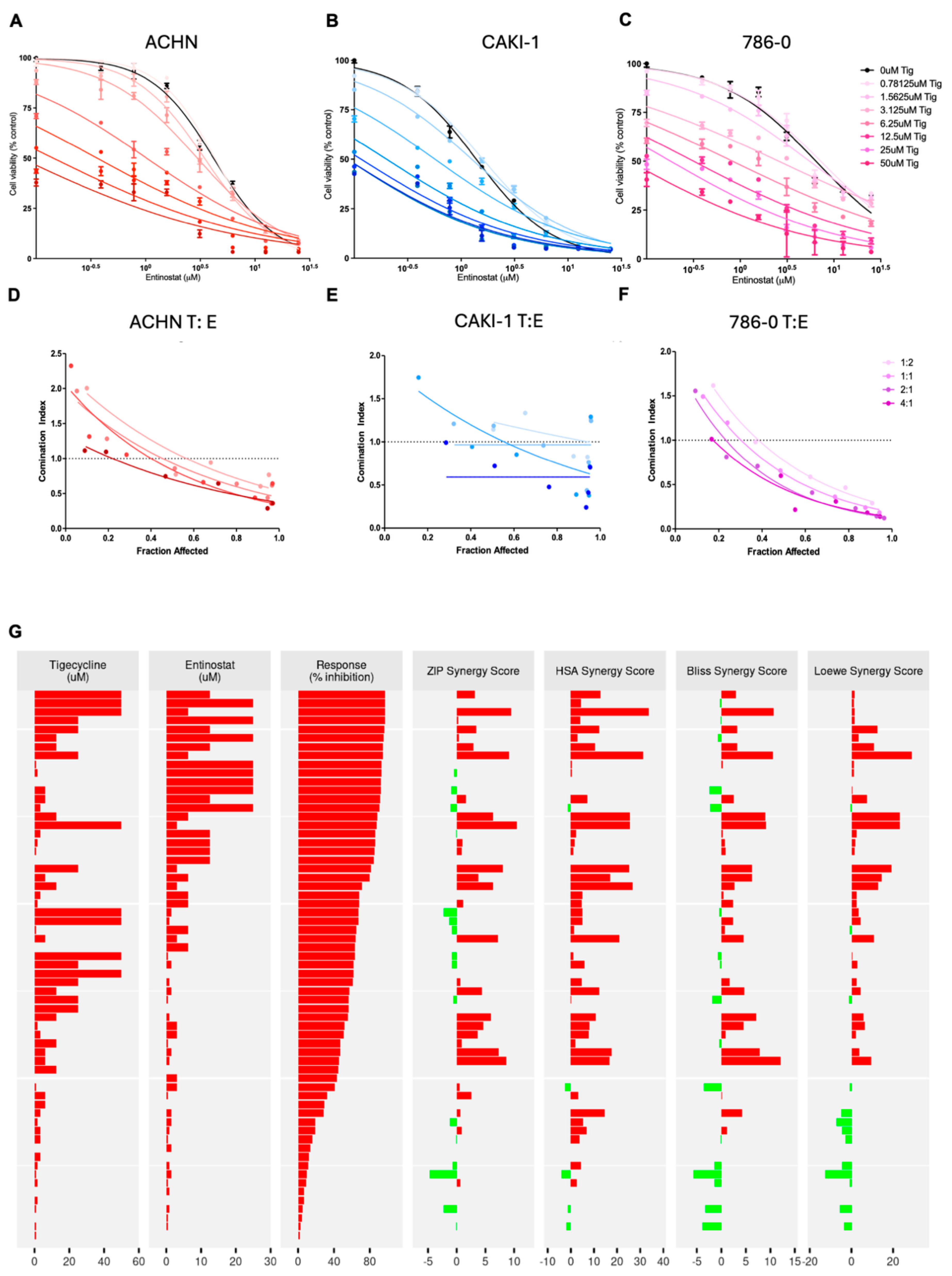
3.8. Combined Tigecycline and Entinostat Treatment Demonstrates Enhanced Efficacy in Human RCC Cells and Patient-Derived RCC Organoids
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ricketts, C.J.; Crooks, D.R.; Sourbier, C.; Schmidt, L.S.; Srinivasan, R.; Linehan, W.M. SnapShot: Renal Cell Carcinoma. Cancer Cell 2016, 29, 610–610.e1. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.X.; Rabinowitz, J.D.; White, E. Mitochondria and Cancer. Mol. Cell 2016, 61, 667–676. [Google Scholar] [CrossRef]
- Anderson, R.G.; Ghiraldeli, L.P.; Pardee, T.S. Mitochondria in cancer metabolism, an organelle whose time has come? Biochim. Biophys. Acta Rev. Cancer 2018, 1870, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.; Schmidt, S.; Ma, B.; Schiefelbein, L.; Rand, K.H.; Burkhardt, O.; Derendorf, H. Clinical pharmacokinetics and pharmacodynamics of tigecycline. Clin. Pharmacokinet. 2009, 48, 575–584. [Google Scholar] [CrossRef]
- Baron, J.; Cai, S.; Klein, N.; Cunha, B.A. Once Daily High Dose Tigecycline Is Optimal: Tigecycline PK/PD Parameters Predict Clinical Effectiveness. J. Clin. Med. 2018, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Bucaneve, G.; Micozzi, A.; Picardi, M.; Ballanti, S.; Cascavilla, N.; Salutari, P.; Specchia, G.; Fanci, R.; Luppi, M.; Cudillo, L.; et al. Results of a multicenter, controlled, randomized clinical trial evaluating the combination of piperacillin/tazobactam and tigecycline in high-risk hematologic patients with cancer with febrile neutropenia. J. Clin. Oncol. 2014, 32, 1463–1471. [Google Scholar] [CrossRef]
- Cunha, B.A.; Baron, J.; Cunha, C.B. Once daily high dose tigecycline-pharmacokinetic/pharmacodynamic based dosing for optimal clinical effectiveness: Dosing matters, revisited. Expert. Rev. Anti Infect. Ther. 2017, 15, 257–267. [Google Scholar] [CrossRef]
- Maseda, E.; Denis, S.E.; Riquelme, A.; Gilsanz, F. Use of tigecycline in critically ill patients with serious nosocomial intra-abdominal infections. Rev. Esp. Quimioter. 2013, 26, 56–63. [Google Scholar]
- Fu, X.; Liu, W.; Huang, Q.; Wang, Y.; Li, H.; Xiong, Y. Targeting mitochondrial respiration selectively sensitizes pediatric acute lymphoblastic leukemia cell lines and patient samples to standard chemotherapy. Am. J. Cancer Res. 2017, 7, 2395–2405. [Google Scholar]
- Jones, R.A.; Robinson, T.J.; Liu, J.C.; Shrestha, M.; Voisin, V.; Ju, Y.; Chung, P.E.; Pellecchia, G.; Fell, V.L.; Bae, S.; et al. RB1 deficiency in triple-negative breast cancer induces mitochondrial protein translation. J. Clin. Investig. 2016, 126, 3739–3757. [Google Scholar] [CrossRef]
- Rava, M.; D’Andrea, A.; Nicoli, P.; Gritti, I.; Donati, G.; Doni, M.; Giorgio, M.; Olivero, D.; Amati, B. Therapeutic synergy between tigecycline and venetoclax in a preclinical model of MYC/BCL2 double-hit B cell lymphoma. Sci. Transl. Med. 2018, 10, eaan8723. [Google Scholar] [CrossRef] [PubMed]
- Skrtic, M.; Sriskanthadevan, S.; Jhas, B.; Gebbia, M.; Wang, X.; Wang, Z.; Hurren, R.; Jitkova, Y.; Gronda, M.; Maclean, N.; et al. Inhibition of mitochondrial translation as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell 2011, 20, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Koch, D.T.; Yu, H.; Beirith, I.; Schirren, M.; Drefs, M.; Liu, Y.; Knoblauch, M.; Koliogiannis, D.; Sheng, W.; De Toni, E.N.; et al. Tigecycline causes loss of cell viability mediated by mitochondrial OXPHOS and RAC1 in hepatocellular carcinoma cells. J. Transl. Med. 2023, 21, 876. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, E.M.; Baquero, P.; Michie, A.M.; Dunn, K.; Tardito, S.; Holyoake, T.L.; Helgason, G.V.; Gottlieb, E. Targeting mitochondrial oxidative phosphorylation eradicates therapy-resistant chronic myeloid leukemia stem cells. Nat. Med. 2017, 23, 1234–1240. [Google Scholar] [CrossRef]
- Khalaf, A.; de Beauchamp, L.; Kalkman, E.; Rattigan, K.; Himonas, E.; Jones, J.; James, D.; Shokry, E.S.A.; Scott, M.T.; Dunn, K.; et al. Nutrient-sensitizing drug repurposing screen identifies lomerizine as a mitochondrial metabolism inhibitor of chronic myeloid leukemia. Sci. Transl. Med. 2024, 16, eadi5336. [Google Scholar] [CrossRef]
- Reed, G.A.; Schiller, G.J.; Kambhampati, S.; Tallman, M.S.; Douer, D.; Minden, M.D.; Yee, K.W.; Gupta, V.; Brandwein, J.; Jitkova, Y.; et al. A Phase 1 study of intravenous infusions of tigecycline in patients with acute myeloid leukemia. Cancer Med. 2016, 5, 3031–3040. [Google Scholar] [CrossRef]
- Ahsan, S.; Draghici, S. Identifying Significantly Impacted Pathways and Putative Mechanisms with iPathwayGuide. Curr. Protoc. Bioinform. 2017, 57, 7.15.11–7.15.30. [Google Scholar] [CrossRef]
- Lue, H.W.; Podolak, J.; Kolahi, K.; Cheng, L.; Rao, S.; Garg, D.; Xue, C.H.; Rantala, J.K.; Tyner, J.W.; Thornburg, K.L.; et al. Metabolic reprogramming ensures cancer cell survival despite oncogenic signaling blockade. Genes Dev. 2017, 31, 2067–2084. [Google Scholar] [CrossRef]
- Hall, A.R.; Burke, N.; Dongworth, R.K.; Hausenloy, D.J. Mitochondrial fusion and fission proteins: Novel therapeutic targets for combating cardiovascular disease. Br. J. Pharmacol. 2014, 171, 1890–1906. [Google Scholar] [CrossRef]
- McConkey, D.J. The integrated stress response and proteotoxicity in cancer therapy. Biochem. Biophys. Res. Commun. 2017, 482, 450–453. [Google Scholar] [CrossRef]
- Lue, H.W.; Cole, B.; Rao, S.A.; Podolak, J.; Van Gaest, A.; King, C.; Eide, C.A.; Wilmot, B.; Xue, C.; Spellman, P.T.; et al. Src and STAT3 inhibitors synergize to promote tumor inhibition in renal cell carcinoma. Oncotarget 2015, 6, 44675–44687. [Google Scholar] [CrossRef][Green Version]
- Suwaki, N.; Vanhecke, E.; Atkins, K.M.; Graf, M.; Swabey, K.; Huang, P.; Schraml, P.; Moch, H.; Cassidy, A.M.; Brewer, D.; et al. A HIF-regulated VHL-PTP1B-Src signaling axis identifies a therapeutic target in renal cell carcinoma. Sci Transl Med 2011, 3, 85ra47. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.V.; Tran, C.; Mellinghoff, I.K.; Welsbie, D.S.; Chan, E.; Fueger, B.; Czernin, J.; Sawyers, C.L. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med 2006, 12, 122–127. [Google Scholar] [CrossRef]
- Rath, S.; Sharma, R.; Gupta, R.; Ast, T.; Chan, C.; Durham, T.J.; Goodman, R.P.; Grabarek, Z.; Haas, M.E.; Hung, W.H.W.; et al. MitoCarta3.0: An updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 2021, 49, D1541–D1547. [Google Scholar] [CrossRef]
- Tsunaka, Y.; Furukawa, A.; Nishimura, Y. Histone tail network and modulation in a nucleosome. Curr. Opin. Struct. Biol. 2022, 75, 102436. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Gaullier, G.; Luger, K. Nucleosome structure and dynamics are coming of age. Nat. Struct. Mol. Biol. 2019, 26, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Kastan, M.B.; Bartek, J. Cell-cycle checkpoints and cancer. Nature 2004, 432, 316–323. [Google Scholar] [CrossRef]
- Talbert, P.B.; Henikoff, S. Histone variants at a glance. J. Cell Sci. 2021, 134, jcs244749. [Google Scholar] [CrossRef]
- Trapani, D.; Esposito, A.; Criscitiello, C.; Mazzarella, L.; Locatelli, M.; Minchella, I.; Minucci, S.; Curigliano, G. Entinostat for the treatment of breast cancer. Expert. Opin. Investig. Drugs 2017, 26, 965–971. [Google Scholar] [CrossRef]
- de Gooijer, M.C.; van den Top, A.; Bockaj, I.; Beijnen, J.H.; Wurdinger, T.; van Tellingen, O. The G2 checkpoint-a node-based molecular switch. FEBS Open Bio 2017, 7, 439–455. [Google Scholar] [CrossRef]
- Preuss, U.; Landsberg, G.; Scheidtmann, K.H. Novel mitosis-specific phosphorylation of histone H3 at Thr11 mediated by Dlk/ZIP kinase. Nucleic Acids Res. 2003, 31, 878–885. [Google Scholar] [CrossRef]
- Redon, C.E.; Nakamura, A.J.; Martin, O.A.; Parekh, P.R.; Weyemi, U.S.; Bonner, W.M. Recent developments in the use of gamma-H2AX as a quantitative DNA double-strand break biomarker. Aging 2011, 3, 168–174. [Google Scholar] [CrossRef]
- Islam, S.; Moinuddin; Mir, A.R.; Raghav, A.; Habib, S.; Alam, K.; Ali, A. Glycation, oxidation and glycoxidation of IgG: A biophysical, biochemical, immunological and hematological study. J Biomol Struct Dyn 2018, 36, 2637–2653. [Google Scholar] [CrossRef]
- Matilainen, O.; Quiros, P.M.; Auwerx, J. Mitochondria and Epigenetics—Crosstalk in Homeostasis and Stress. Trends Cell Biol. 2017, 27, 453–463. [Google Scholar] [CrossRef]
- Santos, J.H. Mitochondria signaling to the epigenome: A novel role for an old organelle. Free Radic. Biol. Med. 2021, 170, 59–69. [Google Scholar] [CrossRef]
- Tian, Y.; Garcia, G.; Bian, Q.; Steffen, K.K.; Joe, L.; Wolff, S.; Meyer, B.J.; Dillin, A. Mitochondrial Stress Induces Chromatin Reorganization to Promote Longevity and UPR(mt). Cell 2016, 165, 1197–1208. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Hiltunen, M.; Kauppinen, A. Krebs cycle dysfunction shapes epigenetic landscape of chromatin: Novel insights into mitochondrial regulation of aging process. Cell. Signal. 2014, 26, 1598–1603. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckenstein, K.; Cengiz, B.; Chang, M.E.K.; Cartier, J.M.; Flory, M.R.; Thomas, G.V. Mitochondrial Translation Inhibition Uncovers a Critical Metabolic–Epigenetic Interface in Renal Cell Carcinoma. Metabolites 2025, 15, 393. https://doi.org/10.3390/metabo15060393
Eckenstein K, Cengiz B, Chang MEK, Cartier JM, Flory MR, Thomas GV. Mitochondrial Translation Inhibition Uncovers a Critical Metabolic–Epigenetic Interface in Renal Cell Carcinoma. Metabolites. 2025; 15(6):393. https://doi.org/10.3390/metabo15060393
Chicago/Turabian StyleEckenstein, Kazumi, Beyza Cengiz, Matthew E. K. Chang, Jessie May Cartier, Mark R. Flory, and George V. Thomas. 2025. "Mitochondrial Translation Inhibition Uncovers a Critical Metabolic–Epigenetic Interface in Renal Cell Carcinoma" Metabolites 15, no. 6: 393. https://doi.org/10.3390/metabo15060393
APA StyleEckenstein, K., Cengiz, B., Chang, M. E. K., Cartier, J. M., Flory, M. R., & Thomas, G. V. (2025). Mitochondrial Translation Inhibition Uncovers a Critical Metabolic–Epigenetic Interface in Renal Cell Carcinoma. Metabolites, 15(6), 393. https://doi.org/10.3390/metabo15060393





