Salivaomics: New Frontiers in Studying the Relationship Between Periodontal Disease and Alzheimer’s Disease
Abstract
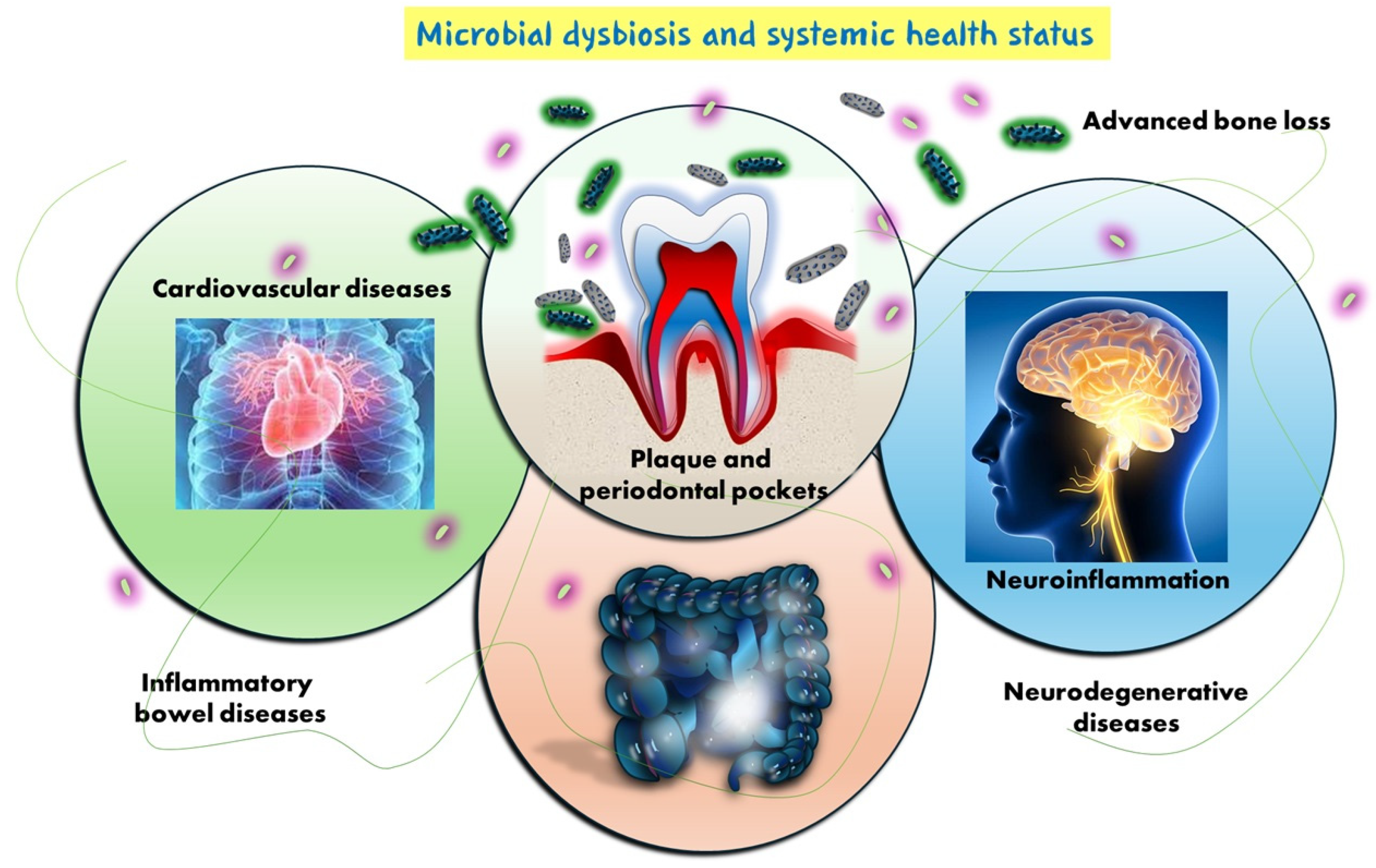
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria and PICO Framework and Research Question
- Population: Human subjects of any age diagnosed with Alzheimer’s disease (AD) or mild cognitive impairment (MCI), or cognitively healthy controls, with or without a diagnosis of periodontal disease (PD).
- Intervention/Exposure: Assessment of salivary metabolomic or microbiome profiles, including techniques such as LC-MS/MS, GC-MS, NMR, and 16S rRNA sequencing. Studies analyzing gingival crevicular fluid (GCF) were also considered if saliva was included.
- Comparison: Studies comparing subjects with AD versus healthy controls, or patients with periodontitis versus those without, or studies examining within-group correlations (e.g., between salivary markers and cognitive scores).
- Outcomes: Diagnostic or prognostic relevance of salivary biomarkers, correlation with clinical measures (e.g., MMSE, MoCA, periodontal indices), or characterization of oral microbial/metabolomic signatures in systemic disease contexts.
- Study Design: Observational studies including cross-sectional, case–control, and cohort designs, as well as randomized controlled trials (RCTs) where applicable.
- Language and Access: Published in English and available in full text.
- Were conducted using in vitro or animal models;
- Were narrative or systematic reviews, editorials, case reports, or conference abstracts lacking sufficient methodological detail;
- Did not include salivary (or GCF) analysis relevant to AD or PD;
- Focused exclusively on unrelated conditions without addressing oral–systemic interactions.
2.3. Data Extraction and Analysis
3. Results
3.1. Study Selection and Characteristics
3.2. Synthesis of Findings Across Studies
3.3. Risk of Bias Assessment
3.4. Summary of Findings, Evidence Certainty (GRADE), Publication Bias, Heterogeneity, and Meta-Analysis Considerations
3.4.1. GRADE Assessment
3.4.2. Publication Bias
3.4.3. Meta-Analysis
3.4.4. Heterogeneity
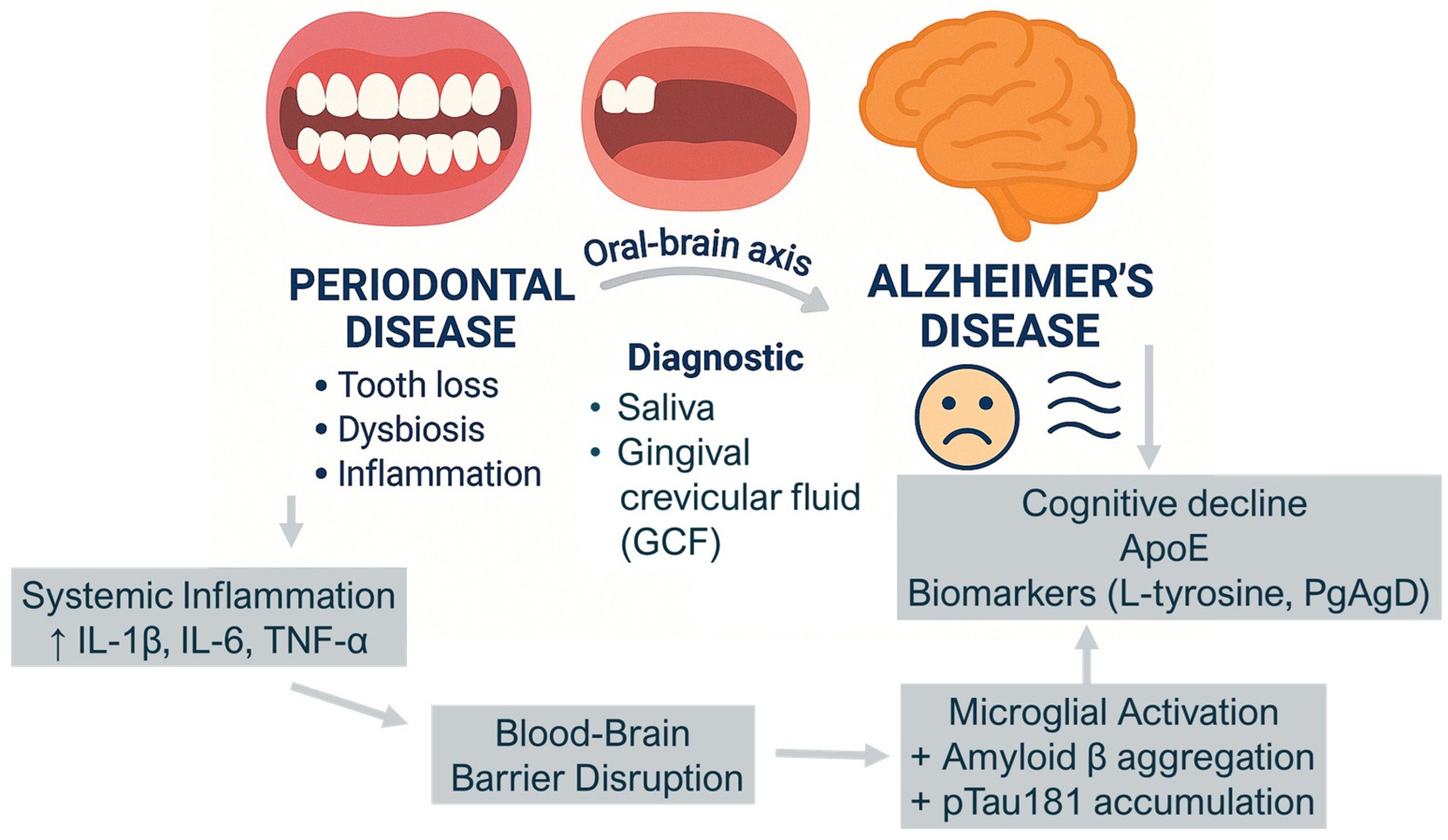
4. Discussion
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aβ | Amyloid beta |
| AD | Alzheimer’s disease |
| AUC | Area Under the Curve |
| aMCI | Amnestic mild cognitive impairment |
| BOP | Bleeding on probing |
| CAL CHC | Clinical attachment level Cognitively healthy control |
| DIABLO | Data Integration Analysis for Biomarker discovery using Latent cOmponents |
| GCF | Gingival crevicular fluid |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| LC-MS/MS | Liquid chromatography coupled to tandem mass spectrometry |
| MoCA | Montreal Cognitive Assessment |
| PD | Periodontal disease |
| PgAgD | Agmatine deiminase from Porphyromonas gingivalis |
| PI | Plaque index |
| pTau181 | Phosphorylated Tau protein at threonine 181 |
| rRNA | Ribosomal ribonucleic acid |
| TNF-α | Tumor necrosis factor-alpha |
References
- Miebach, L.; Wolfsgruber, S.; Polcher, A.; Peters, O.; Menne, F.; Luther, K.; Incesoy, E.; Priller, J.; Spruth, E.; Altenstein, S.; et al. Which features of subjective cognitive decline are related to amyloid pathology? Findings from the DELCODE study. Alzheimer’s Res. Ther. 2019, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Cognat, E.; Mouton Liger, F.; Troussière, A.-C.; Wallon, D.; Dumurgier, J.; Magnin, E.; Duron, E.; Gabelle, A.; Croisile, B.; de la Sayette, V.; et al. What is the clinical impact of cerebrospinal fluid biomarkers on final diagnosis and management in patients with mild cognitive impairment in clinical practice? Results from a nation-wide prospective survey in France. BMJ Open 2019, 9, e026380. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lv, J.; Bai, H.; Ren, L.; Yang, J.; Ding, Y.; Liu, C.; Chen, X. Periodontal Status and Saliva Metabolic Signature in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 95, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Figueira, J.; Jonsson, P.; Nordin Adolfsson, A.; Adolfsson, R.; Nyberg, L.; Öhman, A. NMR analysis of the human saliva metabolome distinguishes dementia patients from matched controls. Mol. Biosyst. 2016, 12, 2562–2571. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Qian, X.; Song, X.; Liu, X.; Chen, S.; Tang, H. Inflammatory pathways in Alzheimer’s disease mediated by gut microbiota. Ageing Res. Rev. 2021, 68, 101317. [Google Scholar] [CrossRef]
- Xi, J.; Ding, D.; Zhu, H.; Wang, R.; Su, F.; Wu, W.; Xiao, Z.; Liang, X.; Zhao, Q.; Hong, Z.; et al. Disturbed microbial ecology in Alzheimer’s disease: Evidence from the gut microbiota and fecal metabolome. BMC Microbiol. 2021, 21, 226. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Zamora, C.; Ceron, J.J.; Bravo-Cantero, A.F.; Pardo-Marin, L.; Valverde, S.; Lopez-Jornet, P. Salivary biomarkers in Alzheimer’s disease. Clin. Oral Investig. 2020, 24, 3437–3444. [Google Scholar] [CrossRef]
- Fujii, Y.; Khasnobish, A.; Morita, H. Relationship between Alzheimer’s Disease and the Human Microbiome. In Alzheimer’s Disease; Wisniewski, T., Ed.; Codon Publications: Brisbane, Australia, 2019; ISBN 978-0-646-80968-7. [Google Scholar]
- Periodontitis—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/periodontitis (accessed on 5 October 2024).
- Woods, L.T.; Ajit, D.; Camden, J.M.; Erb, L.; Weisman, G.A. Purinergic receptors as potential therapeutic targets in Alzheimer’s disease. Neuropharmacology 2016, 104, 169–179. [Google Scholar] [CrossRef]
- Toots, A.; Littbrand, H.; Holmberg, H.; Nordström, P.; Lundin-Olsson, L.; Gustafson, Y.; Rosendahl, E. Walking Aids Moderate Exercise Effects on Gait Speed in People with Dementia: A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2017, 18, 227–233. [Google Scholar] [CrossRef]
- Robinson, M.D.; Mishra, I.; Deodhar, S.; Patel, V.; Gordon, K.V.; Vintimilla, R.; Brown, K.; Johnson, L.; O’Bryant, S.; Cistola, D.P. Water T(2) as an early, global and practical biomarker for metabolic syndrome: An observational cross-sectional study. J. Transl. Med. 2017, 15, 258. [Google Scholar] [CrossRef] [PubMed]
- Borda, M.G.; Barreto, G.E.; Baldera, J.P.; de Lucia, C.; Khalifa, K.; Bergland, A.K.; Pola, I.; Botero-Rodríguez, F.; Siow, R.C.; Kivipelto, M.; et al. A randomized, placebo-controlled trial of purified anthocyanins on cognitive function in individuals at elevated risk for dementia: Analysis of inflammatory biomarkers toward personalized interventions. Exp. Gerontol. 2024, 196, 112569. [Google Scholar] [CrossRef] [PubMed]
- Ueba, Y.; Murakami, T.; Yamamoto, T.; Kuroe, A.; Yamasaki, M.; Kaneda, D.; Otani, D.; Kiyobayashi, S.; Ikeda, K.; Yabe, D.; et al. Voxel-based specific regional analysis system for Alzheimer’s disease utility as a screening tool for unrecognized cognitive dysfunction of elderly patients in diabetes outpatient clinics: Multicenter retrospective exploratory study. J. Diabetes Investig. 2022, 13, 177–184. [Google Scholar] [CrossRef]
- Shohei, I.; Daita, K.; Keita, S.; Yuto, U.; Osamu, A.; Yoshio, H. Voxel-based Morphometry of Alzheimer’s Disease Using a Localizer Image: A Comparative Study with Magnetization Prepared Rapid Acquisition with Gradient Echo. Magn. Reson. Med. Sci. MRMS Off. J. Jpn. Soc. Magn. Reson. Med. 2025, 24, 103–111. [Google Scholar] [CrossRef]
- Oltra-Cucarella, J.; Sánchez-SanSegundo, M.; Lipnicki, D.M.; Sachdev, P.S.; Crawford, J.D.; Pérez-Vicente, J.A.; Cabello-Rodríguez, L.; Ferrer-Cascales, R. Using Base Rate of Low Scores to Identify Progression from Amnestic Mild Cognitive Impairment to Alzheimer’s Disease. J. Am. Geriatr. Soc. 2018, 66, 1360–1366. [Google Scholar] [CrossRef]
- Malcangi, G.; Inchingolo, A.M.; Casamassima, L.; Trilli, I.; Ferrante, L.; Inchingolo, F.; Palermo, A.; Inchingolo, A.D.; Dipalma, G. Effectiveness of Herbal Medicines with Anti-Inflammatory, Antimicrobial, and Antioxidant Properties in Improving Oral Health and Treating Gingivitis and Periodontitis: A Systematic Review. Nutrients 2025, 17, 762. [Google Scholar] [CrossRef]
- Törmälehto, S.; Martikainen, J.; Bell, J.S.; Hallikainen, I.; Koivisto, A.M. Use of psychotropic medications in relation to neuropsychiatric symptoms, cognition and functional performance in Alzheimer’s disease over a three-year period: Kuopio ALSOVA study. Int. Psychogeriatr. 2017, 29, 1723–1733. [Google Scholar] [CrossRef]
- Lacombe, V.; Lacout, C.; Lozac’h, P.; Ghali, A.; Gury, A.; Lavigne, C.; Urbanski, G. Unstimulated whole saliva flow for diagnosis of primary Sjögren’s syndrome: Time to revisit the threshold? Arthritis Res. Ther. 2020, 22, 38. [Google Scholar] [CrossRef]
- Sperling, R.A.; Donohue, M.C.; Rissman, R.A.; Johnson, K.A.; Rentz, D.M.; Grill, J.D.; Heidebrink, J.L.; Jenkins, C.; Jimenez-Maggiora, G.; Langford, O.; et al. Amyloid and Tau Prediction of Cognitive and Functional Decline in Unimpaired Older Individuals: Longitudinal Data from the A4 and LEARN Studies. J. Prev. Alzheimer’s Dis. 2024, 11, 802–813. [Google Scholar] [CrossRef]
- Loo, J.A.; Yan, W.; Ramachandran, P.; Wong, D.T. Comparative Human Salivary and Plasma Proteomes. J. Dent. Res. 2010, 89, 1016–1023. [Google Scholar] [CrossRef]
- Barnes, L.L.; Dhana, K.; Liu, X.; Carey, V.J.; Ventrelle, J.; Johnson, K.; Hollings, C.S.; Bishop, L.; Laranjo, N.; Stubbs, B.J.; et al. Trial of the MIND Diet for Prevention of Cognitive Decline in Older Persons. N. Engl. J. Med. 2023, 389, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Honig, L.S.; Vellas, B.; Woodward, M.; Boada, M.; Bullock, R.; Borrie, M.; Hager, K.; Andreasen, N.; Scarpini, E.; Liu-Seifert, H.; et al. Trial of Solanezumab for Mild Dementia Due to Alzheimer’s Disease. N. Engl. J. Med. 2018, 378, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Stelmokas, J.; Yassay, L.; Giordani, B.; Dodge, H.H.; Dinov, I.D.; Bhaumik, A.; Sathian, K.; Hampstead, B.M. Translational MRI Volumetry with NeuroQuant: Effects of Version and Normative Data on Relationships with Memory Performance in Healthy Older Adults and Patients with Mild Cognitive Impairment. J. Alzheimer’s Dis. 2017, 60, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Sforzini, L.; Marizzoni, M.; Bottanelli, C.; Kunšteková, V.; Zonca, V.; Saleri, S.; Kose, M.; Lombardo, G.; Mariani, N.; Nettis, M.A.; et al. Transcriptomic profiles in major depressive disorder: The role of immunometabolic and cell-cycle-related pathways in depression with different levels of inflammation. Mol. Psychiatry 2025, 30, 1308–1318. [Google Scholar] [CrossRef]
- Narita, Z.; Yokoi, Y. Transcranial direct current stimulation for depression in Alzheimer’s disease: Study protocol for a randomized controlled trial. Trials 2017, 18, 285. [Google Scholar] [CrossRef]
- Guo, H.; Li, B.; Yao, H.; Liu, D.; Chen, R.; Zhou, S.; Ji, Y.; Zeng, L.; Du, M. Profiling the oral microbiomes in patients with Alzheimer’s disease. Oral Dis. 2023, 29, 1341–1355. [Google Scholar] [CrossRef]
- Bystad, M.; Grønli, O.; Rasmussen, I.D.; Gundersen, N.; Nordvang, L.; Wang-Iversen, H.; Aslaksen, P.M. Transcranial direct current stimulation as a memory enhancer in patients with Alzheimer’s disease: A randomized, placebo-controlled trial. Alzheimer’s Res. Ther. 2016, 8, 13. [Google Scholar] [CrossRef]
- Tang, Y.; Xing, Y.; Sun, L.; Wang, Z.; Wang, C.; Yang, K.; Zhu, W.; Shi, X.; Xie, B.; Yin, Y.; et al. TRanscranial AlterNating current stimulation for patients with mild Alzheimer’s Disease (TRANSFORM-AD): A randomized controlled clinical trial. Alzheimer’s Res. Ther. 2024, 16, 203. [Google Scholar] [CrossRef]
- David, N.D.; Lin, F.; Porsteinsson, A.P. Trajectories of Neuropsychiatric Symptoms and Cognitive Decline in Mild Cognitive Impairment. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2016, 24, 70–80. [Google Scholar] [CrossRef]
- Collado, M.C.; Engen, P.A.; Bandín, C.; Cabrera-Rubio, R.; Voigt, R.M.; Green, S.J.; Naqib, A.; Keshavarzian, A.; Scheer, F.A.J.L.; Garaulet, M. Timing of food intake impacts daily rhythms of human salivary microbiota: A randomized, crossover study. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 2060–2072. [Google Scholar] [CrossRef]
- Qiu, C.; Zhou, W.; Shen, H.; Wang, J.; Tang, R.; Wang, T.; Xie, X.; Hong, B.; Ren, R.; Wang, G.; et al. Profiles of subgingival microbiomes and gingival crevicular metabolic signatures in patients with amnestic mild cognitive impairment and Alzheimer’s disease. Alzheimer’s Res. Ther. 2024, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.B.; Saito, M.T.; Matheus, F.C.; Prediger, R.D.; Yamada, E.S.; Maia, C.S.F.; Lima, R.R. Periodontitis and Alzheimer’s Disease: A Possible Comorbidity between Oral Chronic Inflammatory Condition and Neuroinflammation. Front. Aging Neurosci. 2017, 9, 327. [Google Scholar] [CrossRef]
- Zeng, F.; Liu, Y.; Huang, W.; Qing, H.; Kadowaki, T.; Kashiwazaki, H.; Ni, J.; Wu, Z. Receptor for advanced glycation end products up-regulation in cerebral endothelial cells mediates cerebrovascular-related amyloid β accumulation after Porphyromonas gingivalis infection. J. Neurochem. 2021, 158, 724–736. [Google Scholar] [CrossRef]
- Schaap, T.; Thropp, P.; Tosun, D. Timing of Alzheimer’s disease biomarker progressions: A two-decade observational study from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Alzheimer’s Dement. J. Alzheimer’s Assoc. 2024, 20, 9060–9067. [Google Scholar] [CrossRef]
- Dalrymple, W.A.; Pusso, A.; Sperling, S.A.; Flanigan, J.L.; Huss, D.S.; Harrison, M.B.; Elias, W.J.; Shah, B.B.; Barrett, M.J. Comparison of Parkinson’s Disease Patients’ Characteristics by Indication for Deep Brain Stimulation: Men Are More Likely to Have DBS for Tremor. Tremor Other Hyperkinet. Mov. 2019, 9, 10-7916. [Google Scholar] [CrossRef]
- Florian, H.; Wang, D.; Arnold, S.E.; Boada, M.; Guo, Q.; Jin, Z.; Zheng, H.; Fisseha, N.; Kalluri, H.V.; Rendenbach-Mueller, B.; et al. Tilavonemab in early Alzheimer’s disease: Results from a phase 2, randomized, double-blind study. Brain J. Neurol. 2023, 146, 2275–2284. [Google Scholar] [CrossRef]
- Sang, S.; Pan, X.; Chen, Z.; Zeng, F.; Pan, S.; Liu, H.; Jin, L.; Fei, G.; Wang, C.; Ren, S.; et al. Thiamine diphosphate reduction strongly correlates with brain glucose hypometabolism in Alzheimer’s disease, whereas amyloid deposition does not. Alzheimer’s Res. Ther. 2018, 10, 26. [Google Scholar] [CrossRef]
- Lanchec, E.; Désilets, A.; Béliveau, F.; Flamier, A.; Mahmoud, S.; Bernier, G.; Gris, D.; Leduc, R.; Lavoie, C. The type II transmembrane serine protease matriptase cleaves the amyloid precursor protein and reduces its processing to β-amyloid peptide. J. Biol. Chem. 2017, 292, 20669–20682. [Google Scholar] [CrossRef]
- Albahri, J.; Allison, H.; Whitehead, K.A.; Muhamadali, H. The role of salivary metabolomics in chronic periodontitis: Bridging oral and systemic diseases. Metabolomics 2025, 21, 24. [Google Scholar] [CrossRef]
- Lim, Y.; Totsika, M.; Morrison, M.; Punyadeera, C. The saliva microbiome profiles are minimally affected by collection method or DNA extraction protocols. Sci. Rep. 2017, 7, 8523. [Google Scholar] [CrossRef]
- Clemmensen, F.K.; Hoffmann, K.; Siersma, V.; Sobol, N.; Beyer, N.; Andersen, B.B.; Vogel, A.; Lolk, A.; Gottrup, H.; Høgh, P.; et al. The role of physical and cognitive function in performance of activities of daily living in patients with mild-to-moderate Alzheimer’s disease—A cross-sectional study. BMC Geriatr. 2020, 20, 513. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.J.A.; Wallace, R.B. The Role of Incarceration as a Risk Factor for Cognitive Impairment. J. Gerontol. B. Psychol. Sci. Soc. Sci. 2022, 77, e247–e262. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiao, X.; Yang, Y.; Yao, R.; Yang, Q.; Zhu, Y.; Yang, X.; Zhang, S.; Shen, L.; Jiao, B. The risk of Alzheimer’s disease and cognitive impairment characteristics in eight mental disorders: A UK Biobank observational study and Mendelian randomization analysis. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2024, 20, 4841–4853. [Google Scholar] [CrossRef] [PubMed]
- Hancock, S.E.; Friedrich, M.G.; Mitchell, T.W.; Truscott, R.J.W.; Else, P.L. The phospholipid composition of the human entorhinal cortex remains relatively stable over 80 years of adult aging. GeroScience 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Hooghiemstra, A.M.; Bertens, A.S.; Leeuwis, A.E.; Bron, E.E.; Bots, M.L.; Brunner-La Rocca, H.-P.; de Craen, A.J.M.; van der Geest, R.J.; Greving, J.P.; Kappelle, L.J.; et al. The Missing Link in the Pathophysiology of Vascular Cognitive Impairment: Design of the Heart-Brain Study. Cerebrovasc. Dis. Extra 2017, 7, 140–152. [Google Scholar] [CrossRef]
- Akhgar, C.K.; Ebner, J.; Alcaraz, M.R.; Kopp, J.; Goicoechea, H.; Spadiut, O.; Schwaighofer, A.; Lendl, B. Application of Quantum Cascade Laser-Infrared Spectroscopy and Chemometrics for In-Line Discrimination of Coeluting Proteins from Preparative Size Exclusion Chromatography. Anal. Chem. 2022, 94, 11192–11200. [Google Scholar] [CrossRef]
- O’Donnell, L.E.; Robertson, D.; Nile, C.J.; Cross, L.J.; Riggio, M.; Sherriff, A.; Bradshaw, D.; Lambert, M.; Malcolm, J.; Buijs, M.J.; et al. The Oral Microbiome of Denture Wearers Is Influenced by Levels of Natural Dentition. PLoS ONE 2015, 10, e0137717. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, F.; Wang, C.; Chen, Z.; Zhao, B.; Li, K. The nitric oxide synthase 3 G894T polymorphism associated with Alzheimer’s disease risk: A meta-analysis. Sci. Rep. 2015, 5, 13598. [Google Scholar] [CrossRef]
- Hsieh, S.-W.; Kim, S.-Y.; Shim, Y.-S.; Huang, L.-C.; Yang, Y.-H. A comparison of sociobehavioral impact on cognitive preservation in Alzheimer’s disease between Taiwan and Korea: A cross-national study. Medicine 2020, 99, e19690. [Google Scholar] [CrossRef]
- Rasmussen, P.M.; Aamand, R.; Weitzberg, E.; Christiansen, M.; Østergaard, L.; Lund, T.E. APOE gene-dependent BOLD responses to a breath-hold across the adult lifespan. NeuroImage Clin. 2019, 24, 101955. [Google Scholar] [CrossRef]
- Toscani, F.; Finetti, S.; Giunco, F.; Basso, I.; Rosa, D.; Pettenati, F.; Bussotti, A.; Villani, D.; Gentile, S.; Boncinelli, L.; et al. The last week of life of nursing home residents with advanced dementia: A retrospective study. BMC Palliat. Care 2019, 18, 117. [Google Scholar] [CrossRef] [PubMed]
- Kapila, Y.L. Oral health’s inextricable connection to systemic health: Special populations bring to bear multimodal relationships and factors connecting periodontal disease to systemic diseases and conditions. Periodontology 2000 2021, 87, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Parra-Torres, V.; Melgar-Rodríguez, S.; Muñoz-Manríquez, C.; Sanhueza, B.; Cafferata, E.A.; Paula-Lima, A.C.; Díaz-Zúñiga, J. Periodontal bacteria in the brain—Implication for Alzheimer’s disease: A systematic review. Oral Dis. 2023, 29, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Louzada, L.L.; Machado, F.V.; Quintas, J.L.; Ribeiro, G.A.; Silva, M.V.; Mendonça-Silva, D.L.; Gonçalves, B.S.B.; Nóbrega, O.T.; Camargos, E.F. The efficacy and safety of zolpidem and zopiclone to treat insomnia in Alzheimer’s disease: A randomized, triple-blind, placebo-controlled trial. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2022, 47, 570–579. [Google Scholar] [CrossRef]
- Tian, J.; Shi, J.; Wei, M.; Qin, R.; Ni, J.; Zhang, X.; Li, T.; Wang, Y. The efficacy and safety of Fufangdanshen tablets (Radix Salviae miltiorrhizae formula tablets) for mild to moderate vascular dementia: A study protocol for a randomized controlled trial. Trials 2016, 17, 281. [Google Scholar] [CrossRef]
- Schlafer, S.; Johnsen, K.K.; Kjærbølling, I.; Schramm, A.; Meyer, R.L.; Jørgensen, M.R. The efficacy and safety of an enzyme-containing lozenge for dental biofilm control-a randomized controlled pilot trial. J. Dent. 2024, 147, 105107. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef]
- Hu, Y.; Li, H.; Zhang, J.; Zhang, X.; Xia, X.; Qiu, C.; Liao, Y.; Chen, H.; Song, Z.; Zhou, W. Periodontitis Induced by P. gingivalis-LPS Is Associated with Neuroinflammation and Learning and Memory Impairment in Sprague-Dawley Rats. Front. Neurosci. 2020, 14, 658. [Google Scholar] [CrossRef]
- Choi, W.-Y.; Lee, W.-K.; Kim, T.-H.; Ryu, Y.-K.; Park, A.; Lee, Y.-J.; Heo, S.-J.; Oh, C.; Chung, Y.-C.; Kang, D.-H. The Effects of Spirulina maxima Extract on Memory Improvement in Those with Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2022, 14, 3714. [Google Scholar] [CrossRef]
- Blanc, F.; Colloby, S.J.; Philippi, N.; de Pétigny, X.; Jung, B.; Demuynck, C.; Phillipps, C.; Anthony, P.; Thomas, A.; Bing, F.; et al. Cortical Thickness in Dementia with Lewy Bodies and Alzheimer’s Disease: A Comparison of Prodromal and Dementia Stages. PLoS ONE 2015, 10, e0127396. [Google Scholar] [CrossRef]
- Yuan, Y.; Leung, A.W.S.; Duan, H.; Zhang, L.; Zhang, K.; Wu, J.; Qin, S. The effects of long-term stress on neural dynamics of working memory processing: An investigation using ERP. Sci. Rep. 2016, 6, 23217. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B.; Luo, T.; Srinivasan, U.; Ramadugu, K.; Wen, A.; Goldberg, D.; Shedden, K.; Crout, R.; McNeil, D.W.; Weyant, R.; et al. The effects of family, dentition, and dental caries on the salivary microbiome. Ann. Epidemiol. 2016, 26, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Howshigan, J.; Perera, K.; Samita, S.; Rajapakse, P.S. The effects of an Ayurvedic medicinal toothpaste on clinical, microbiological and oral hygiene parameters in patients with chronic gingivitis: A double-blind, randomised, placebo-controlled, parallel allocation clinical trial. Ceylon Med. J. 2015, 60, 126–132. [Google Scholar] [CrossRef]
- Hives, B.A.; Buckler, E.J.; Weiss, J.; Schilf, S.; Johansen, K.L.; Epel, E.S.; Puterman, E. The Effects of Aerobic Exercise on Psychological Functioning in Family Caregivers: Secondary Analyses of a Randomized Controlled Trial. Ann. Behav. Med. Publ. Soc. Behav. Med. 2021, 55, 65–76. [Google Scholar] [CrossRef]
- Dioguardi, M.; Di Gioia, G.; Caloro, G.A.; Capocasale, G.; Zhurakivska, K.; Troiano, G.; Lo Russo, L.; Lo Muzio, L. The Association between Tooth Loss and Alzheimer’s Disease: A Systematic Review with Meta-Analysis of Case Control Studies. Dent. J. 2019, 7, 49. [Google Scholar] [CrossRef]
- Betz, M.E.; Portz, J.; Knoepke, C.; Ranney, M.L.; Fischer, S.M.; Peterson, R.A.; Johnson, R.L.; Omeragic, F.; Castaneda, M.; Greenway, E.; et al. The Effect of the “Safety in Dementia” Online Tool to Assist Decision Making for Caregivers of Persons with Dementia and Access to Firearms: A Randomized Trial. Ann. Intern. Med. 2024, 177, 1630–1640. [Google Scholar] [CrossRef]
- Konečná, B.; Chobodová, P.; Janko, J.; Baňasová, L.; Bábíčková, J.; Celec, P.; Tóthová, Ľ. The Effect of Melatonin on Periodontitis. Int. J. Mol. Sci. 2021, 22, 2390. [Google Scholar] [CrossRef]
- Peng, W.; Zhou, J.; Xu, M.; Feng, Q.; Bin, L.; Liu, Z. The effect of electroacupuncture combined with donepezil on cognitive function in Alzheimer’s disease patients: Study protocol for a randomized controlled trial. Trials 2017, 18, 301. [Google Scholar] [CrossRef]
- Karssemeijer, E.G.A.; Aaronson, J.A.; Bossers, W.J.R.; Donders, R.; Olde Rikkert, M.G.M.; Kessels, R.P.C. The quest for synergy between physical exercise and cognitive stimulation via exergaming in people with dementia: A randomized controlled trial. Alzheimer’s Res. Ther. 2019, 11, 3. [Google Scholar] [CrossRef]
- Falahati, F.; Ferreira, D.; Soininen, H.; Mecocci, P.; Vellas, B.; Tsolaki, M.; Kłoszewska, I.; Lovestone, S.; Eriksdotter, M.; Wahlund, L.-O.; et al. The Effect of Age Correction on Multivariate Classification in Alzheimer’s Disease, with a Focus on the Characteristics of Incorrectly and Correctly Classified Subjects. Brain Topogr. 2016, 29, 296–307. [Google Scholar] [CrossRef]
- Papp, K.V.; Rentz, D.M.; Maruff, P.; Sun, C.-K.; Raman, R.; Donohue, M.C.; Schembri, A.; Stark, C.; Yassa, M.A.; Wessels, A.M.; et al. The Computerized Cognitive Composite (C3) in an Alzheimer’s Disease Secondary Prevention Trial. J. Prev. Alzheimer’s Dis. 2021, 8, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, X.; Liu, J.; Hou, Z.; Wei, Y.; Chen, M.; Wang, B.; Cao, H.; Qiu, R.; Zhang, Y.; et al. Distinctive subgingival microbial signatures in older adults with different levels of cognitive function. J. Clin. Periodontol. 2024, 51, 1066–1080. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-F.; Lee, W.-F.; Salamanca, E.; Yao, W.-L.; Su, J.-N.; Wang, S.-Y.; Hu, C.-J.; Chang, W.-J. Oral Microbiota Changes in Elderly Patients, an Indicator of Alzheimer’s Disease. Int. J. Environ. Res. Public Health 2021, 18, 4211. [Google Scholar] [CrossRef]
- Libowitz, M.R.; Wei, K.; Tran, T.; Chu, K.; Moncrieffe, K.; Harrington, M.G.; King, K. Regional brain volumes relate to Alzheimer’s disease cerebrospinal fluid biomarkers and neuropsychometry: A cross-sectional, observational study. PLoS ONE 2021, 16, e0254332. [Google Scholar] [CrossRef]
- Chan, P.-C.; Wei, C.-Y.; Hung, G.-U.; Chiu, P.-Y. Reduced vascular risk factors in Parkinson’s disease dementia and dementia with Lewy bodies compared to Alzheimer’s disease. Brain Behav. 2018, 8, e00916. [Google Scholar] [CrossRef]
- Cavedo, E.; Grothe, M.J.; Colliot, O.; Lista, S.; Chupin, M.; Dormont, D.; Houot, M.; Lehéricy, S.; Teipel, S.; Dubois, B.; et al. Reduced basal forebrain atrophy progression in a randomized Donepezil trial in prodromal Alzheimer’s disease. Sci. Rep. 2017, 7, 11706. [Google Scholar] [CrossRef]
- Aigbogun, M.S.; Cloutier, M.; Gauthier-Loiselle, M.; Guerin, A.; Ladouceur, M.; Baker, R.A.; Grundman, M.; Duffy, R.A.; Hartry, A.; Gwin, K.; et al. Real-World Treatment Patterns and Characteristics Among Patients with Agitation and Dementia in the United States: Findings from a Large, Observational, Retrospective Chart Review. J. Alzheimer’s Dis. 2020, 77, 1181–1194. [Google Scholar] [CrossRef]
- Cohen, M.L.; Kim, C.; Haldiman, T.; ElHag, M.; Mehndiratta, P.; Pichet, T.; Lissemore, F.; Shea, M.; Cohen, Y.; Chen, W.; et al. Rapidly progressive Alzheimer’s disease features distinct structures of amyloid-β. Brain J. Neurol. 2015, 138, 1009–1022. [Google Scholar] [CrossRef]
- Li, J.; Xu, H.; Pan, W.; Wu, B. Association between tooth loss and cognitive decline: A 13-year longitudinal study of Chinese older adults. PLoS ONE 2017, 12, e0171404. [Google Scholar] [CrossRef]
- Lu, H.; Ma, S.L.; Wong, S.W.H.; Tam, C.W.C.; Cheng, S.-T.; Chan, S.S.M.; Lam, L.C.W. Aberrant interhemispheric functional connectivity within default mode network and its relationships with neurocognitive features in cognitively normal APOE ε 4 elderly carriers. Int. Psychogeriatr. 2017, 29, 805–814. [Google Scholar] [CrossRef]
- Krueger, K.R.; Dhana, K.; Aggarwal, N.T.; Arfanakis, K.; Carey, V.J.; Sacks, F.M.; Barnes, L.L. Properties of the Cognitive Function Battery for the MIND Diet Intervention to Prevent Alzheimer’s Disease. J. Int. Neuropsychol. Soc. 2022, 28, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Wetterö, J.; von Löhneysen, S.; Cobar, F.; Kristenson, M.; Garvin, P.; Sjöwall, C. Pronounced Diurnal Pattern of Salivary C-Reactive Protein (CRP) with Modest Associations to Circulating CRP Levels. Front. Immunol. 2020, 11, 607166. [Google Scholar] [CrossRef] [PubMed]
- Street, D.; Jabbari, E.; Costantini, A.; Jones, P.S.; Holland, N.; Rittman, T.; Jensen, M.T.; Chelban, V.; Goh, Y.Y.; Guo, T.; et al. Progression of atypical parkinsonian syndromes: PROSPECT-M-UK study implications for clinical trials. Brain J. Neurol. 2023, 146, 3232–3242. [Google Scholar] [CrossRef] [PubMed]
- Baser, U.; Gamsiz-Isik, H.; Cifcibasi, E.; Ademoglu, E.; Yalcin, F. Plasma and salivary total antioxidant capacity in healthy controls compared with aggressive and chronic periodontitis patients. Saudi Med. J. 2015, 36, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Fereshtehnejad, S.; Garcia-Ptacek, S.; Religa, D.; Holmer, J.; Buhlin, K.; Eriksdotter, M.; Sandborgh-Englund, G. Dental care utilization in patients with different types of dementia: A longitudinal nationwide study of 58,037 individuals. Alzheimer’s Dement. 2018, 14, 10–19. [Google Scholar] [CrossRef]
- Tiisanoja, A.; Syrjälä, A.; Tertsonen, M.; Komulainen, K.; Pesonen, P.; Knuuttila, M.; Hartikainen, S.; Ylöstalo, P. Oral diseases and inflammatory burden and Alzheimer’s disease among subjects aged 75 years or older. Spec. Care Dent. 2019, 39, 158–165. [Google Scholar] [CrossRef]
- Yoshida, K.; Roberts, R.; Suzuki, T.; Lebowitz, B.; Reeves, S.; Howard, R.; Abe, T.; Mimura, M.; Uchida, H. Lack of Early Improvement with Antipsychotics is a Marker for Subsequent Nonresponse in Behavioral and Psychological Symptoms of Dementia: Analysis of CATIE-AD Data. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2017, 25, 708–716. [Google Scholar] [CrossRef]
- Spichiger, F.; Koppitz, A.L.; Riese, F.; Kipfer, S.; Nagl-Cupal, M.; Büscher, A.; Volken, T.; Larkin, P.; Meichtry, A. Person Profile Dementia Intervention in Long-Term Care: A Stepped-Wedge Cluster-Randomized Trial. J. Am. Med. Dir. Assoc. 2025, 26, 105351. [Google Scholar] [CrossRef]
- Ide, M.; Harris, M.; Stevens, A.; Sussams, R.; Hopkins, V.; Culliford, D.; Fuller, J.; Ibbett, P.; Raybould, R.; Thomas, R.; et al. Periodontitis and Cognitive Decline in Alzheimer’s Disease. PLoS ONE 2016, 11, e0151081. [Google Scholar] [CrossRef]
- Fowler, M.E.; Triebel, K.L.; Cutter, G.R.; Schneider, L.S.; Kennedy, R.E. Progression of Alzheimer’s Disease by Self-Reported Cancer History in the Alzheimer’s Disease Neuroimaging Initiative. J. Alzheimer’s Dis. 2020, 76, 691–701. [Google Scholar] [CrossRef]
- Makiura, T.; Ikeda, Y.; Hirai, T.; Terasawa, H.; Hamaue, N.; Minami, M. Influence of diet and occlusal support on learning memory in rats behavioral and biochemical studies. Res. Commun. Mol. Pathol. Pharmacol. 2000, 107, 269–277. [Google Scholar] [PubMed]
- Jones, J.A.; Moss, K.; Finlayson, T.L.; Preisser, J.S.; Weintraub, J.A. Edentulism Predicts Cognitive Decline in the US Health and Retirement Cohort Study. J. Dent. Res. 2023, 102, 863–870. [Google Scholar] [CrossRef]
- François, M.; Pascovici, D.; Wang, Y.; Vu, T.; Liu, J.-W.; Beale, D.; Hor, M.; Hecker, J.; Faunt, J.; Maddison, J.; et al. Saliva Proteome, Metabolome and Microbiome Signatures for Detection of Alzheimer’s Disease. Metabolites 2024, 14, 714. [Google Scholar] [CrossRef]
- François, M.; Karpe, A.; Liu, J.-W.; Beale, D.; Hor, M.; Hecker, J.; Faunt, J.; Maddison, J.; Johns, S.; Doecke, J.; et al. Salivaomics as a Potential Tool for Predicting Alzheimer’s Disease During the Early Stages of Neurodegeneration. J. Alzheimer’s Dis. 2021, 82, 1301–1313. [Google Scholar] [CrossRef]
- Issilbayeva, A.; Kaiyrlykyzy, A.; Vinogradova, E.; Jarmukhanov, Z.; Kozhakhmetov, S.; Kassenova, A.; Nurgaziyev, M.; Mukhanbetzhanov, N.; Alzhanova, D.; Zholdasbekova, G.; et al. Oral Microbiome Stamp in Alzheimer’s Disease. Pathogen 2024, 13, 195. [Google Scholar] [CrossRef]
- Sansores-España, L.D.; Morales, F.; Arriola-Pacheco, F.; Astorga, J.; Paula-Lima, A.; Carrillo-Ávila, A.; Melgar-Rodríguez, S.; Martínez-Aguilar, V.; Díaz-Zúñiga, J.; Sansores-España, L.D.; et al. Gingival crevicular fluid as Biomarker’s source for alzheimer’s disease. Odovtos Int. J. Dent. Sci. 2022, 24, 156–176. [Google Scholar] [CrossRef]
- Na, H.S.; Jung, N.-Y.; Song, Y.; Kim, S.Y.; Kim, H.-J.; Lee, J.Y.; Chung, J. A distinctive subgingival microbiome in patients with periodontitis and Alzheimer’s disease compared with cognitively unimpaired periodontitis patients. J. Clin. Periodontol. 2024, 51, 43–53. [Google Scholar] [CrossRef]
- Hamdi, A.; Baroudi, S.; Gharbi, A.; Babay, W.; Laaribi, A.B.; Kacem, I.; Mrabet, S.; Zidi, I.; Klibi, N.; Gouider, R.; et al. Dysregulation of Porphyromonas gingivalis Agmatine Deiminase Expression in Alzheimer’s Disease. Curr. Alzheimer Res. 2024, 21, 232–241. [Google Scholar] [CrossRef]
- Belstrøm, D.; Sembler-Møller, M.L.; Grande, M.A.; Kirkby, N.; Cotton, S.L.; Paster, B.J.; Holmstrup, P. Microbial profile comparisons of saliva, pooled and site-specific subgingival samples in periodontitis patients. PLoS ONE 2017, 12, e0182992. [Google Scholar] [CrossRef]
- Sutter, C.; Marti, Y.; Haas, C.; Neubauer, J. Methylation-based forensic age estimation in blood, buccal cells, saliva and semen: A comparison of two technologies. Forensic Sci. Int. 2025, 367, 112325. [Google Scholar] [CrossRef]
- Taga, M.; Minett, T.; Classey, J.; Matthews, F.E.; Brayne, C.; Ince, P.G.; Nicoll, J.A.; Hugon, J.; Boche, D. Metaflammasome components in the human brain: A role in dementia with Alzheimer’s pathology? Brain Pathol. 2017, 27, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, Y.; Jastrzab, L.; Dutt, S.; Miller, B.L.; Seeley, W.W.; Kramer, J.H. Memory profiles in pathology or biomarker confirmed Alzheimer disease and frontotemporal dementia. Alzheimer Dis. Assoc. Disord. 2015, 29, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, G.T.; Alva, G.; Hendrix, S.; Ellison, N.; Kane, M.C.; Edwards, J. Memantine ER Maintains Patient Response in Moderate to Severe Alzheimer’s Disease: Post Hoc Analyses from a Randomized, Controlled, Clinical Trial of Patients Treated with Cholinesterase Inhibitors. Alzheimer Dis. Assoc. Disord. 2018, 32, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Lanctôt, K.L.; Scherer, R.W.; Li, A.; Vieira, D.; Coulibaly, H.; Rosenberg, P.B.; Herrmann, N.; Lerner, A.J.; Padala, P.R.; Brawman-Mintzer, O.; et al. Measuring Apathy in Alzheimer’s Disease in the Apathy in Dementia Methylphenidate Trial 2 (ADMET 2): A Comparison of Instruments. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2021, 29, 81–89. [Google Scholar] [CrossRef]
- Mimmack, K.J.; Sprague, E.H.; Amariglio, R.E.; Vannini, P.; Marshall, G.A. Longitudinal Evolution of Financial Capacity and Cerebral Tau and Amyloid Burden in Older Adults with Normal Cognition or Mild Cognitive Impairment. J. Prev. Alzheimer’s Dis. 2024, 11, 966–974. [Google Scholar] [CrossRef]
- Jimenez-Maggiora, G.A.; Schulz, A.P.; Donohue, M.C.; Qiu, H.; Jaiswal, S.N.; Adegoke, O.; Gallardo, R.; Baryshnikava, O.; Rissman, R.A.; Abdel-Latif, S.; et al. Maximizing the Utility of Alzheimer’s Disease Trial Data: Sharing of Baseline A4 and LEARN Data. J. Prev. Alzheimer’s Dis. 2024, 11, 889–894. [Google Scholar] [CrossRef]
- Chera, B.S.; Amdur, R.J.; Tepper, J.E.; Tan, X.; Weiss, J.; Grilley-Olson, J.E.; Hayes, D.N.; Zanation, A.; Hackman, T.G.; Patel, S.; et al. Mature results of a prospective study of deintensified chemoradiotherapy for low-risk human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cancer 2018, 124, 2347–2354. [Google Scholar] [CrossRef]
- Amariglio, R.E.; Grill, J.D.; Rentz, D.M.; Marshall, G.A.; Donohue, M.C.; Liu, A.; Aisen, P.S.; Sperling, R.A. Longitudinal Trajectories of the Cognitive Function Index in the A4 Study. J. Prev. Alzheimer’s Dis. 2024, 11, 838–845. [Google Scholar] [CrossRef]
- Soler, A.; Amer, G.; Leiva, A.; Ripoll, J.; Llorente, M.A.; Leiva, A.; Taltavull, J.M.; Molina, R.; Llobera, J. Continuation versus discontinuation of treatment for severe dementia: Randomized, pragmatic, open-label, clinical trial to evaluate the efficacy of continuing drug treatment in patients with severe dementia (STOP-DEM). BMC Geriatr. 2019, 19, 101. [Google Scholar] [CrossRef]
- Besser, L.M.; Litvan, I.; Monsell, S.E.; Mock, C.; Weintraub, S.; Zhou, X.-H.; Kukull, W. Mild cognitive impairment in Parkinson’s disease versus Alzheimer’s disease. Parkinsonism Relat. Disord. 2016, 27, 54–60. [Google Scholar] [CrossRef]
- Huang, S.; Li, Z.; He, T.; Bo, C.; Chang, J.; Li, L.; He, Y.; Liu, J.; Charbonneau, D.; Li, R.; et al. Microbiota-based Signature of Gingivitis Treatments: A Randomized Study. Sci. Rep. 2016, 6, 24705. [Google Scholar] [CrossRef] [PubMed]
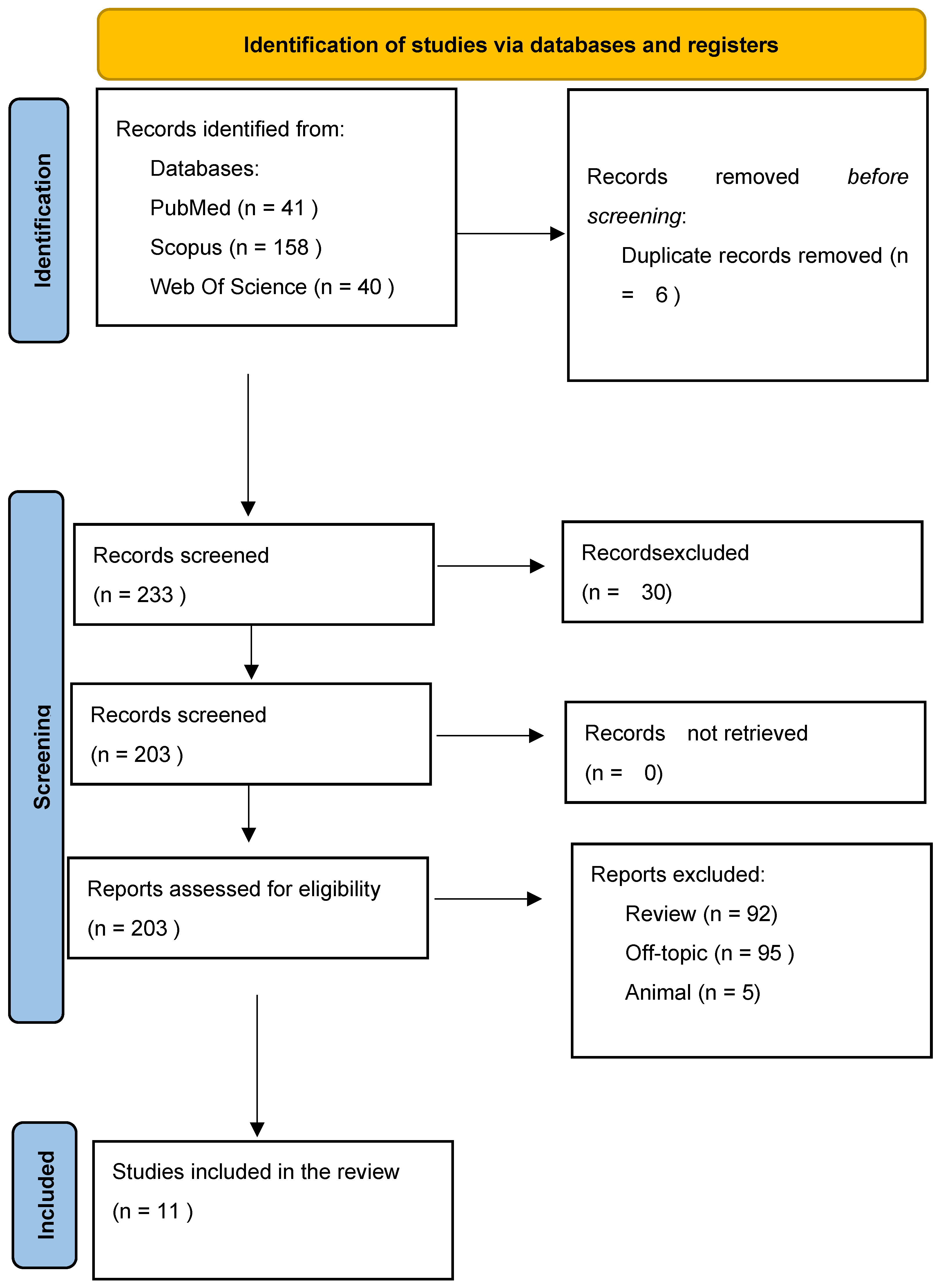
| Database | Search String | Number of Record Retrieved |
|---|---|---|
| Pubmed | (metabolome OR metabolite OR saliva OR salivome) AND (“Alzheimer disease” OR “periodontal disease”) | 41 |
| Web of science | (metabolome OR metabolite OR saliva OR salivome) AND (“Alzheimer disease” OR “periodontal disease”) | 40 |
| Scopus | (metabolome OR metabolite OR saliva OR salivome) AND (“Alzheimer disease” OR “periodontal disease”) | 158 |
| References | Type of Study | N. of Patients | Aim of the Study | Outcomes |
|---|---|---|---|---|
| François et al. (2024) [95] | Cross-sectional, observational | 80 participants: 40 CHC, 20 MCI, 20 AD |
|
|
| François et al. (2021) [96] | Observational, cross-sectional pilot study | 80 participants: 40 CHC, 20 with MCI, 20 with AD |
|
|
| Issilbayeva et al. (2024) [97] | Case–control study | 135 participants: 64 with AD, 71 CHC |
|
|
| Sansores-España et al. (2022) [98] | Clinical observational pilot study | 30 participants: CHC, periodontitis without AD, and periodontitis with AD |
|
|
| Qiu et al. (2024) [33] | Cross-sectional, observational study | 96 participants: 32 CHC, 32 MCI, and 32 AD |
|
|
| Guo et al., 2023 [28] | Observational (cross-sectional) study | 60 participants (33 with AD, 27 CHC) | To investigate oral microbiomes of Alzheimer’s disease patients and controls. | AD patients had an altered oral microbiome composition, with increased pathogenic bacteria (P. gingivalis, F. alocis) and reduced beneficial bacteria (R. mucilaginosa, C. matruchotii). Changes were more pronounced in subgingival plaque and seemed independent of oral hygiene status. |
| Ide et al., 2016 [91] | Observational cohort (6 months) | 60 participants | To investigate the correlation between periodontitis and AD. | Periodontitis is associated with significantly faster cognitive decline and heightened systemic inflammation in AD patients. |
| Na et al., 2024 [99] | Cross-sectional observational study | 43 participants (21 with AD + PD; 22 with PD only) | To investigate the influence between AD and periodontitis. | AD influences oral microbiota and may worsen periodontal condition. |
| Hamdi et al., 2024 [100] | Observational, cross-sectional | 54 participants (27 AD patients, 27 CHC) | To investigate the dysregulation of Porphyromonas gingivalis agmatine deiminase (PgAgD) expression in Alzheimer’s disease and its correlation with periodontitis and cognitive decline. | Correlation between decreased PgAgD expression and lower Mini-Mental State Examination (MMSE) scores. |
| Chen et al. (2024) [74] | Cross-sectional study | 165 older adults | To explore differences in subgingival microbiota composition and function across cognitive states. | Decreased microbial richness with cognitive decline; specific bacterial signatures associated with cognitive level. |
| Yang et al. (2023) [3] | Observational case–control study | 79 participants (60 for saliva analysis) | To investigate the correlation between periodontal status and identify salivary metabolic biomarkers in AD. | AD linked with worse periodontal status; four salivary biomarkers identified. |
| Study | Confounding | Selection of Participants | Classification of Interventions | Deviations from Intended Interventions | Missing Data | Measurement of Outcomes | Selection of Reported Results | Overall Risk |
|---|---|---|---|---|---|---|---|---|
| François et al. (2024) [95] | Moderate risk | Low risk | Low risk | Low risk | Low risk | Moderate risk | Low risk | Moderate risk |
| François et al. (2021) [96] | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk | Low risk | Low risk |
| Issilbayeva et al. (2024) [97] | Moderate risk | Moderate risk | Low risk | Low risk | Low risk | Moderate risk | Low risk | Moderate risk |
| Sansores-España et al. (2022) [98] | Serious risk | Moderate risk | Low risk | Low risk | Low risk | Moderate risk | Low risk | High risk |
| Qiu et al. (2024) [33] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Guo et al. (2023) [28] | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk | Low risk | Low risk |
| Ide et al. (2016) [91] | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk | Low risk | Moderate risk |
| Na et al. (2024) [99] | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk | Low risk | Moderate risk |
| Hamdi et al. (2024) [100] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Chen et al. (2024) [74] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Yang et al. (2024) [3] | Moderate risk | Low risk | Low risk | Low risk | Low risk | Moderate risk | Low risk | Moderate risk |
| Key Finding | Inference/Implication |
|---|---|
| ↓ Oral microbial diversity in AD ↑ P. gingivalis, F. alocis, ↑ Firmicutes | Oral dysbiosis is associated with cognitive decline and may precede clinical signs of Alzheimer’s Disease (AD) |
| ↑ Salivary metabolites (e.g., L-tyrosine, 3-chlorotyrosine, taurine, 3-hydroxybutyric acid) | Oral metabolic changes reflect oxidative stress and inflammation, potentially linked to neurodegenerative processes |
| ↑ Inflammatory cytokines (IL-1β, IL-6, TNF-α) in saliva and GCF | Local oral inflammation may contribute to systemic neuroinflammation, particularly in genetically susceptible individuals (e.g., ApoE-ε4 carriers) |
| ↓ Expression of beneficial microbial genes (e.g., PgAgD) | Microbial functional changes suggest an active role of the oral microbiome in AD pathophysiology |
| Coexistence of AD and periodontitis = ↑ Inflammatory profile + ↑ Cognitive decline | Periodontal disease may be a modifiable risk factor for the progression of Alzheimer’s Disease |
| Gradual microbial and metabolic changes from healthy aging → MCI → AD | Oral biofluids could serve as early biomarkers for the diagnosis and monitoring of cognitive decline |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malcangi, G.; Marinelli, G.; Inchingolo, A.D.; Trilli, I.; Ferrante, L.; Casamassima, L.; Nardelli, P.; Inchingolo, F.; Palermo, A.; Inchingolo, A.M.; et al. Salivaomics: New Frontiers in Studying the Relationship Between Periodontal Disease and Alzheimer’s Disease. Metabolites 2025, 15, 389. https://doi.org/10.3390/metabo15060389
Malcangi G, Marinelli G, Inchingolo AD, Trilli I, Ferrante L, Casamassima L, Nardelli P, Inchingolo F, Palermo A, Inchingolo AM, et al. Salivaomics: New Frontiers in Studying the Relationship Between Periodontal Disease and Alzheimer’s Disease. Metabolites. 2025; 15(6):389. https://doi.org/10.3390/metabo15060389
Chicago/Turabian StyleMalcangi, Giuseppina, Grazia Marinelli, Alessio Danilo Inchingolo, Irma Trilli, Laura Ferrante, Lucia Casamassima, Paola Nardelli, Francesco Inchingolo, Andrea Palermo, Angelo Michele Inchingolo, and et al. 2025. "Salivaomics: New Frontiers in Studying the Relationship Between Periodontal Disease and Alzheimer’s Disease" Metabolites 15, no. 6: 389. https://doi.org/10.3390/metabo15060389
APA StyleMalcangi, G., Marinelli, G., Inchingolo, A. D., Trilli, I., Ferrante, L., Casamassima, L., Nardelli, P., Inchingolo, F., Palermo, A., Inchingolo, A. M., & Dipalma, G. (2025). Salivaomics: New Frontiers in Studying the Relationship Between Periodontal Disease and Alzheimer’s Disease. Metabolites, 15(6), 389. https://doi.org/10.3390/metabo15060389













