Comparison of the Metabolic Profiles Associated with Protonitazene and Protonitazepyne in Two Severe Poisonings
Abstract
1. Introduction
2. Case Reports
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Qualitative and Quantitative Untargeted Toxicological Screening Using LC-HRMS/MS
3.2.1. Sample Preparation
3.2.2. Analytical Conditions
3.2.3. Data Processing for Untargeted Analysis
3.2.4. Quantitative Analysis
4. Results
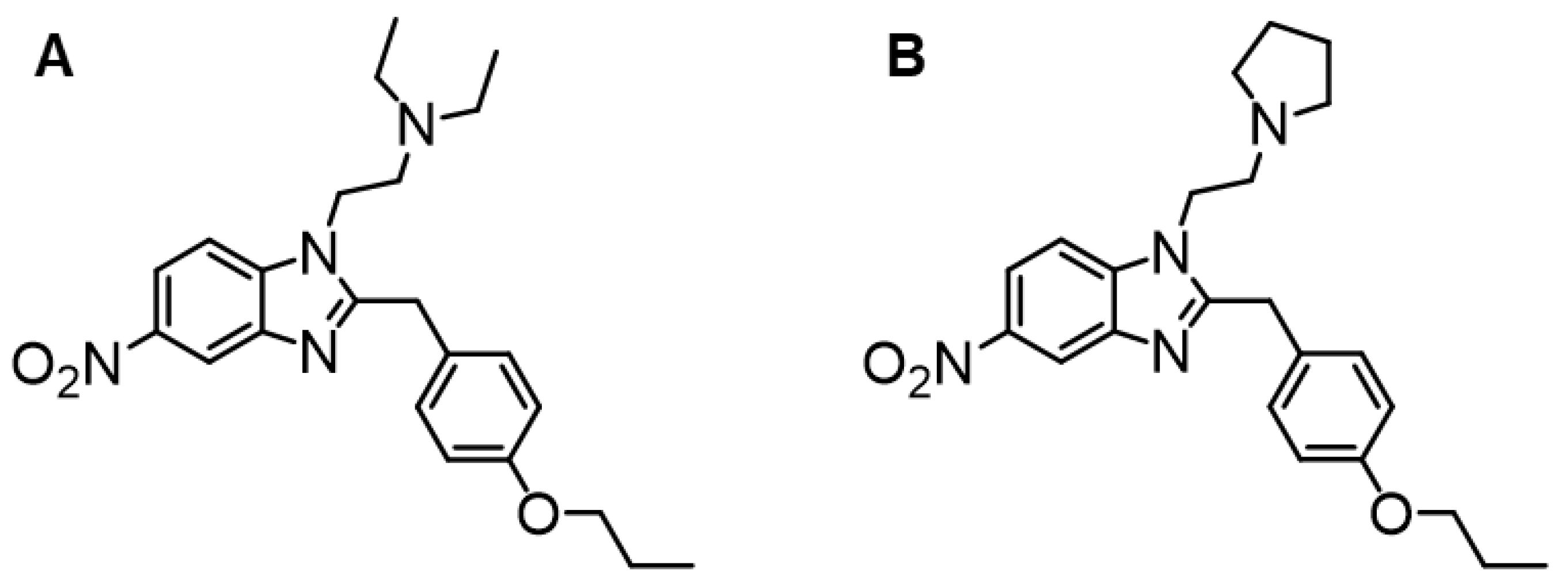
4.1. Analytical Features of Protonitazene and Protonitazepyne
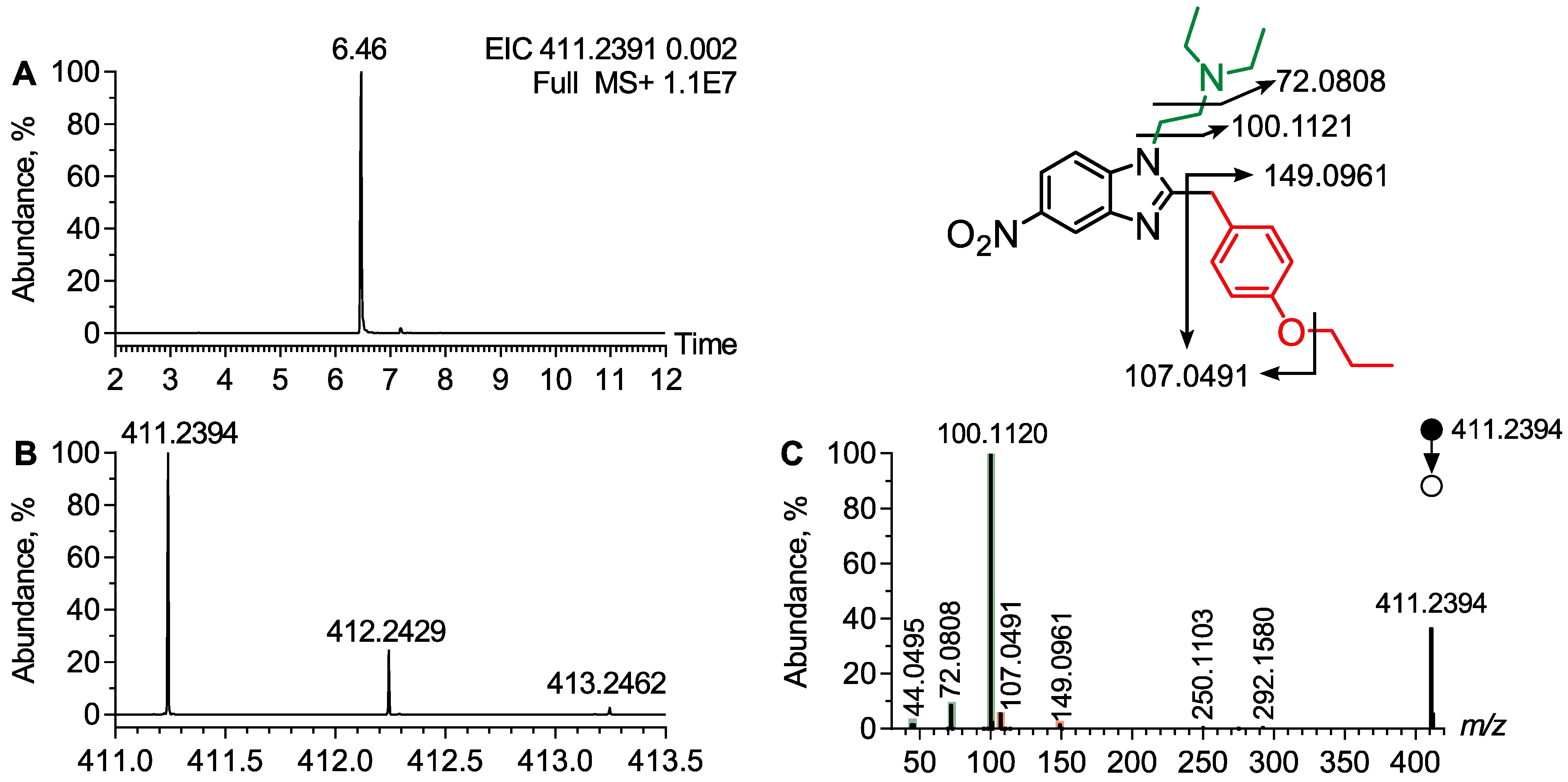
4.1.1. Analytical Features of Protonitazene
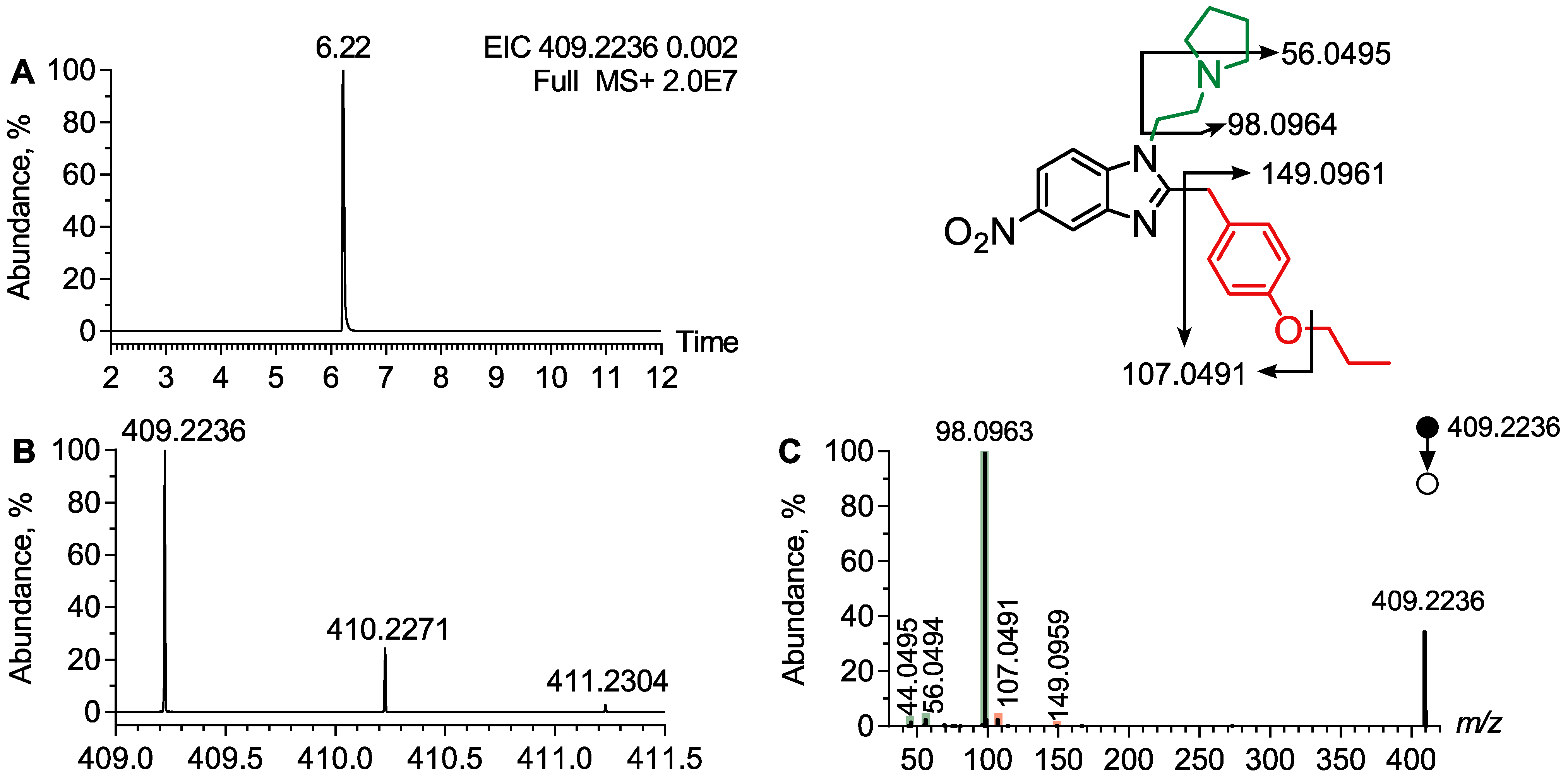
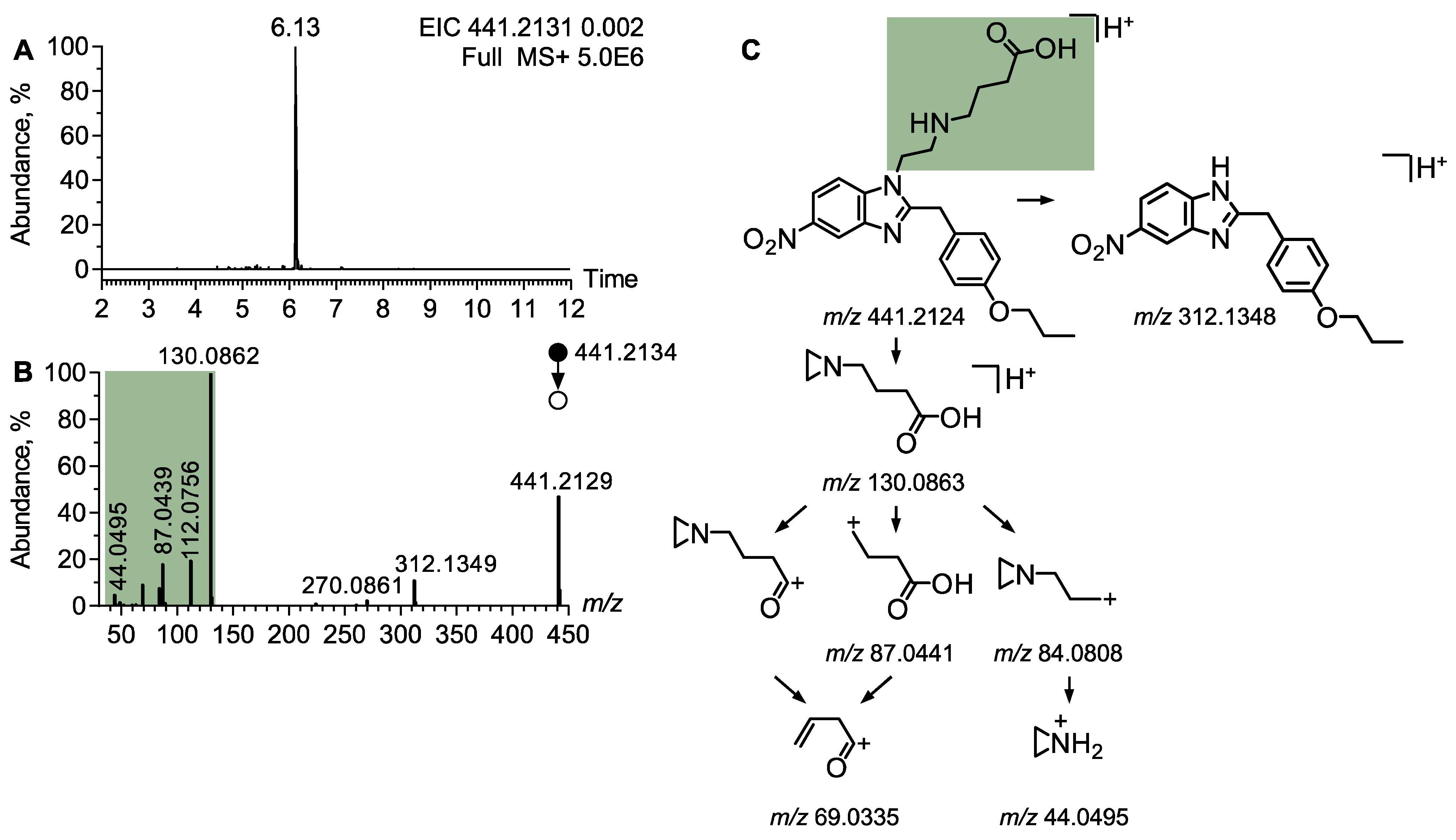
4.1.2. Analytical Features of Protonitazepyne
4.2. Qualitative Metabolite Profiling
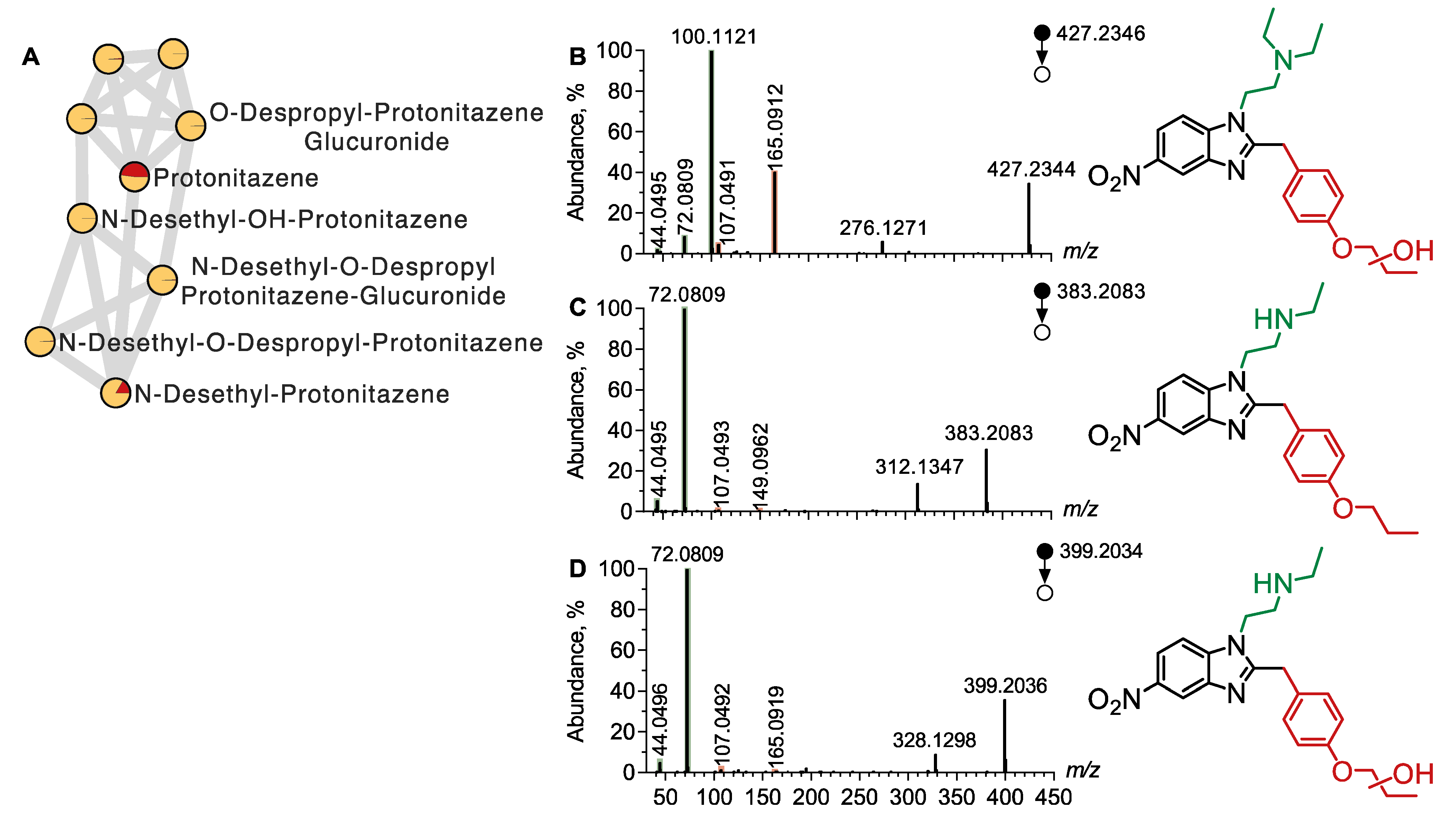
4.2.1. Metabolite Profiling of Protonitazene
4.2.2. Metabolite Profiling of Protonitazepyne
4.3. Relative Metabolite Profiling
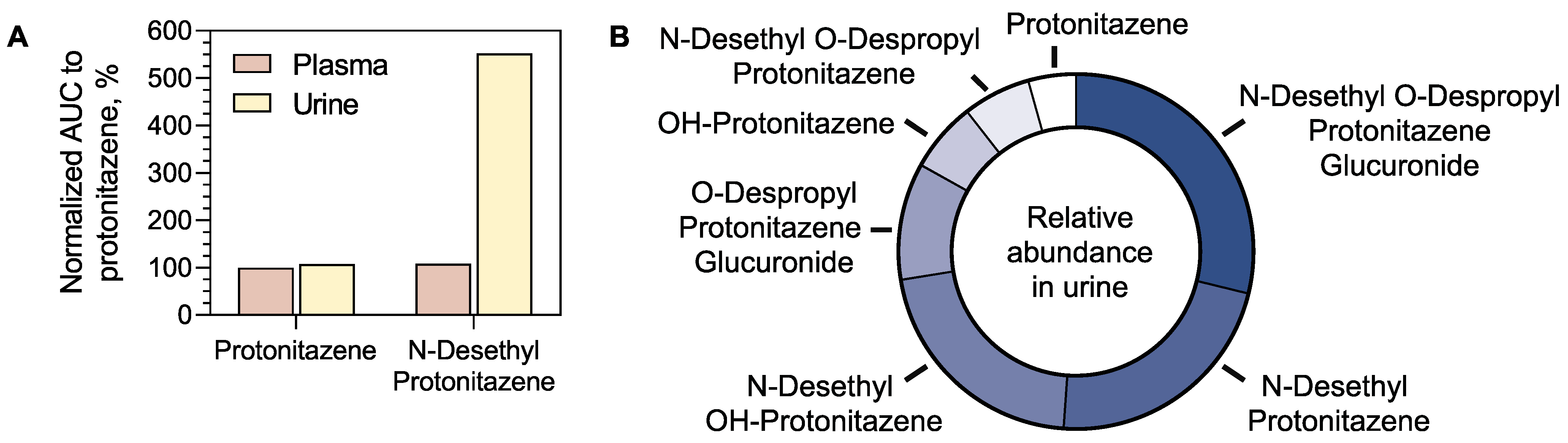
4.3.1. Relative Metabolite Profiling of Protonitazene
4.3.2. Relative Metabolite Profiling of Protonitazepyne
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edinoff, A.N.; Martinez Garza, D.M.; Vining, S.P.; Vasterling, M.E.; Jackson, E.D.; Murnane, K.S.; Kaye, A.M.; Fair, R.N.; Torres, Y.J.L.; Badr, A.E.; et al. New Synthetic Opioids: Clinical Considerations and Dangers. Pain Ther. 2023, 12, 399–421. [Google Scholar] [CrossRef] [PubMed]
- Jr, J.P.; Raffa, R.; LeQuang, J.A.K.; Breve, F.; Varrassi, G. Old Drugs and New Challenges: A Narrative Review of Nitazenes. Cureus 2023, 15, e40736. [Google Scholar] [CrossRef]
- Walton, S.E.; Krotulski, A.J.; Logan, B.K. A Forward-Thinking Approach to Addressing the New Synthetic Opioid 2-Benzylbenzimidazole Nitazene Analogs by Liquid Chromatography-Tandem Quadrupole Mass Spectrometry (LC-QQQ-MS). J. Anal. Toxicol. 2022, 46, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, N.J.; Palkovic, B.; Sprague, D.J.; Calkins, M.M.; Lanham, J.K.; Halberstadt, A.L.; Stucke, A.G.; McCorvy, J.D. Mu-opioid receptor selective superagonists produce prolonged respiratory depression. Iscience 2023, 26, 107121. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Adamowicz, P.; Kurpeta, M.; Wojcieszak, J. Non-fentanyl new synthetic opioids—An update. Forensic Sci. Int. 2023, 349, 111775. [Google Scholar] [CrossRef]
- Krotulski, A.J.; Papsun, D.M.; Kacinko, S.L.; Logan, B.K. Isotonitazene Quantitation and Metabolite Discovery in Authentic Forensic Casework. J. Anal. Toxicol. 2021, 44, 521–530. [Google Scholar] [CrossRef]
- Krotulski, A.J.; Papsun, D.M.; Walton, S.E.; Logan, B.K. Metonitazene in the United States—Forensic toxicology assessment of a potent new synthetic opioid using liquid chromatography mass spectrometry. Drug Test. Anal. 2021, 13, 1697–1711. [Google Scholar] [CrossRef]
- Vandeputte, M.M.; Krotulski, A.J.; Papsun, D.M.; Logan, B.K.; Stove, C.P. The Rise and Fall of Isotonitazene and Brorphine: Two Recent Stars in the Synthetic Opioid Firmament. J. Anal. Toxicol. 2022, 46, 115–121. [Google Scholar] [CrossRef]
- Vandeputte, M.M.; Van Uytfanghe, K.; Layle, N.K.; St. Germaine, D.M.; Iula, D.M.; Stove, C.P. Stove, Synthesis, Chemical Characterization, and μ-Opioid Receptor Activity Assessment of the Emerging Group of “nitazene” 2-Benzylbenzimidazole Synthetic Opioids. ACS Chem. Neurosci. 2021, 12, 1241–1251. [Google Scholar] [CrossRef]
- Monti, M.C.; De Vrieze, L.M.; Vandeputte, M.M.; Persson, M.; Gréen, H.; Stove, C.P.; Schlotterbeck, G. Detection of N-desethyl etonitazene in a drug checking sample: Chemical analysis and pharmacological characterization of a recent member of the 2-benzylbenzimidazole “nitazene” class. J. Pharm. Biomed. Anal. 2024, 251, 116453. [Google Scholar] [CrossRef]
- Glatfelter, G.C.; Vandeputte, M.M.; Chen, L.; Walther, D.; Tsai, M.H.M.; Shi, L.; Stove, C.P.; Baumann, M.H. Alkoxy chain length governs the potency of 2-benzylbenzimidazole ‘nitazene’ opioids associated with human overdose. Psychopharmacology 2023, 240, 2573–2584. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, L.M.; Walton, S.E.; Pottie, E.; Papsun, D.; Logan, B.K.; Krotulski, A.J.; Stove, C.P.; Vandeputte, M.M. In vitro structure–activity relationships and forensic case series of emerging 2-benzylbenzimidazole ‘nitazene’ opioids. Arch. Toxicol. 2024, 98, 2999–3018. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, M.M.; Krotulski, A.J.; Walther, D.; Glatfelter, G.C.; Papsun, D.; Walton, S.E.; Logan, B.K.; Baumann, M.H.; Stove, C.P. Pharmacological evaluation and forensic case series of N-pyrrolidino etonitazene (etonitazepyne), a newly emerging 2-benzylbenzimidazole “nitazene” synthetic opioid. Arch. Toxicol. 2022, 96, 1845–1863. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, M.M.; Glatfelter, G.C.; Walther, D.; Layle, N.K.; Germaine, D.M.S.; Ujváry, I.; Iula, D.M.; Baumann, M.H.; Stove, C.P. Characterization of novel nitazene recreational drugs: Insights into their risk potential from in vitro µ-opioid receptor assays and in vivo behavioral studies in mice. Pharmacol. Res. 2024, 210, 107503. [Google Scholar] [CrossRef]
- Kanamori, T.; Okada, Y.; Segawa, H.; Yamamuro, T.; Kuwayama, K.; Tsujikawa, K.; Iwata, Y.T. Analysis of highly potent synthetic opioid nitazene analogs and their positional isomers. Drug Test. Anal. 2023, 15, 449–457. [Google Scholar] [CrossRef]
- Pardi, J.; Ford, S.; Cooper, G. Validation of an analytical method for quantitation of metonitazene and isotonitazene in plasma, blood, urine, liver and brain and application to authentic postmortem casework in New York City. J. Anal. Toxicol. 2023, 47, 648–655. [Google Scholar] [CrossRef]
- Schüller, M.; Lucic, I.; Øiestad, Å.M.L.; Pedersen-Bjergaard, S.; Øiestad, E.L. High-Throughput quantification of emerging “nitazene” benzimidazole opioid analogs by microextraction and UHPLC-MS-MS. J. Anal. Toxicol. 2023, 47, 787–796. [Google Scholar] [CrossRef]
- Ververi, C.; Galletto, M.; Massano, M.; Alladio, E.; Vincenti, M.; Salomone, A. Method development for the quantification of nine nitazene analogs and brorphine in Dried Blood Spots utilizing liquid chromatography—Tandem mass spectrometry. J. Pharm. Biomed. Anal. 2024, 241, 115975. [Google Scholar] [CrossRef]
- Taoussi, O.; Berardinelli, D.; Zaami, S.; Tavoletta, F.; Basile, G.; Kronstrand, R.; Auwärter, V.; Busardò, F.P.; Carlier, J. Human metabolism of four synthetic benzimidazole opioids: Isotonitazene, metonitazene, etodesnitazene, and metodesnitazene. Arch. Toxicol. 2024, 98, 2101–2116. [Google Scholar] [CrossRef]
- Berardinelli, D.; Taoussi, O.; Carlier, J.; Tini, A.; Zaami, S.; Sundermann, T.; Busardò, F.P.; Auwärter, V. In vitro, in vivo metabolism and quantification of the novel synthetic opioid N-piperidinyl etonitazene (etonitazepipne). Clin. Chem. Lab. Med. 2024, 62, 1580–1590. [Google Scholar] [CrossRef]
- Huang, B.-Y.; Hua, Z.-D.; Liu, C.-M.; Li, J.; Shu, J.-Z.; Li, Z. Metabolism of Six Novel Nitazenes in Human Liver Microsomes Based on Ultra-High-Performance Liquid Chromatography Coupled with High-Resolution Mass Spectrometry. Drug Test. Anal. 2024. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, T.; Okada, Y.; Segawa, H.; Yamamuro, T.; Kuwayama, K.; Tsujikawa, K.; Iwata, Y.T. Metabolism of highly potent synthetic opioid nitazene analogs: N-ethyl-N-(1-glucuronyloxyethyl) metabolite formation and degradation to N-desethyl metabolites during enzymatic hydrolysis. Drug Test. Anal. 2024, 17, 238–249. [Google Scholar] [CrossRef]
- Magny, R.; Mégarbane, B.; Chevillard, L.; Roulland, E.; Bardèche-Trystram, B.; Dumestre-Toulet, V.; Labat, L.; Houzé, P. A combined toxicokinetic and metabolic approach to investigate deschloro-N-ethylketamine exposure in a multidrug user. J. Pharm. Biomed. Anal. 2024, 243, 116086. [Google Scholar] [CrossRef]
- Magny, R.; Lefrère, B.; Roulland, E.; Auzeil, N.; Farah, S.; Richeval, C.; Gish, A.; Vodovar, D.; Labat, L.; Houzé, P. Feature-Based Molecular Network for New Psychoactive Substance Identification: The Case of Synthetic Cannabinoids in a Seized e-Liquid and Biological Samples. J. Am. Soc. Mass. Spectrom. 2024, 35, 2276–2287. [Google Scholar] [CrossRef]
- Pelletier, R.; Le Daré, B.; Grandin, L.; Couette, A.; Ferron, P.J.; Morel, I.; Gicquel, T. New psychoactive substance cocktail in an intensive care intoxication case elucidated by molecular networking. Clin. Toxicol. 2022, 60, 122–125. [Google Scholar] [CrossRef]
- Pelletier, R.; Le Daré, B.; Le Bouëdec, D.; Bourdais, A.; Ferron, P.J.; Morel, I.; Porée, F.H.; Gicquel, T. Identification, synthesis and quantification of eutylone consumption markers in a chemsex context. Arch. Toxicol. 2024, 98, 151–158. [Google Scholar] [CrossRef]
- Gicquel, T.; Pelletier, R.; Richeval, C.; Gish, A.; Hakim, F.; Ferron, P.J.; Mesli, V.; Allorge, D.; Morel, I.; Gaulier, J.M. Metabolite elucidation of 2-fluoro-deschloroketamine (2F-DCK) using molecular networking across three complementary in vitro and in vivo models. Drug Test. Anal. 2022, 14, 144–153. [Google Scholar] [CrossRef]
- Vincenti, F.; Montesano, C.; Di Ottavio, F.; Gregori, A.; Compagnone, D.; Sergi, M.; Dorrestein, P. Molecular Networking: A Useful Tool for the Identification of New Psychoactive Substances in Seizures by LC–HRMS. Front. Chem. 2020, 8, 1039. [Google Scholar] [CrossRef]
- Magny, R.; Mégarbane, B.; Guillaud, P.; Chevillard, L.; Auzeil, N.; Thiebot, P.; Voicu, S.; Malissin, I.; Deye, N.; Labat, L.; et al. Life-Threatening Cardiogenic Shock Related to Venlafaxine Poisoning—A Case Report with Metabolomic Approach. Metabolites 2023, 13, 353. [Google Scholar] [CrossRef]
- Schmid, R.; Heuckeroth, S.; Korf, A.; Smirnov, A.; Myers, O.; Dyrlund, T.S.; Bushuiev, R.; Murray, K.J.; Hoffmann, N.; Lu, M.; et al. Integrative analysis of multimodal mass spectrometry data in MZmine 3. Nat. Biotechnol. 2023, 41, 447–449. [Google Scholar] [CrossRef]
- Heuckeroth, S.; Damiani, T.; Smirnov, A.; Mokshyna, O.; Brungs, C.; Korf, A.; Smith, J.D.; Stincone, P.; Dreolin, N.; Nothias, L.F.; et al. Reproducible mass spectrometry data processing and compound annotation in MZmine 3. Nat. Protoc. 2024, 19, 2597–2641. [Google Scholar] [CrossRef] [PubMed]
- Nothias, L.F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-based molecular networking in the GNPS analysis environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Olivon, F.; Elie, N.; Grelier, G.; Roussi, F.; Litaudon, M.; Touboul, D. MetGem Software for the Generation of Molecular Networks Based on the t-SNE Algorithm. Anal. Chem. 2018, 90, 13900–13908. [Google Scholar] [CrossRef] [PubMed]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef]
- Ludwig, M.; Fleischauer, M.; Dührkop, K.; Hoffmann, M.A.; Böcker, S. De Novo Molecular Formula Annotation and Structure Elucidation Using SIRIUS 4. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2020; pp. 185–207. [Google Scholar] [CrossRef]
- Syrjanen, R.; Schumann, J.L.; Castle, J.W.; Sharp, L.; Griffiths, A.; Blakey, K.; Dutch, M.; Maplesden, J.; Greene, S.L. Protonitazene detection in two cases of opioid toxicity following the use of tetrahydrocannabinol vape products in Australia. Clin. Toxicol. 2024, 62, 539–541. [Google Scholar] [CrossRef]
- Ujváry, I.; Christie, R.; Evans-Brown, M.; Gallegos, A.; Jorge, R.; De Morais, J.; Sedefov, R. DARK Classics in Chemical Neuroscience: Etonitazene and Related Benzimidazoles. ACS Chem. Neurosci. 2021, 12, 1072–1092. [Google Scholar] [CrossRef]
- Ameline, A.; Gheddar, L.; Pichini, S.; Stove, C.; Aknouche, F.; Maruejouls, C.; Raul, J.S.; Kintz, P. In vitro characterization of protonitazene metabolites, using human liver microsomes, and first application to two urines collected from death cases. Clin. Chim. Acta 2024, 561, 119764. [Google Scholar] [CrossRef]
- Blanckaert, P.; Balcaen, M.; Vanhee, C.; Risseeuw, M.; Canfyn, M.; Desmedt, B.; Van Calenbergh, S.; Deconinck, E. Analytical characterization of “etonitazepyne” a new pyrrolidinyl-containing 2-benzylbenzimidazole opioid sold online. Drug Test. Anal. 2021, 13, 1627–1634. [Google Scholar] [CrossRef]
- Sauer, C.; Peters, F.T.; Haas, C.; Meyer, M.R.; Fritschi, G.; Maurera, H.H. New designer drug α-pyrrolidinovalerophenone (PVP): Studies on itsmetabolism and toxicological detection in rat urine using gas chromatographic/mass spectrometric techniques. J. Mass. Spectrom. 2009, 44, 952–964. [Google Scholar] [CrossRef]
- Magny, R.; Bardèche-Trystram, B.; Oppon, C.; Thiebot, P.; Labat, L.; Houzé, P. Entactogen chemicals and drug facilitating sexual assault: A case report in a clinical toxicology context. Toxicol. Anal. Clin. 2024, 36, 62–69. [Google Scholar] [CrossRef]
| Name | [M + H]+ | tR | Substitution | Diagnostic Product Ions (m/z) |
|---|---|---|---|---|
| Protonitazene | 411.2391 | 6.46 | N,N-Diethylamine | 100.1120, 72.0808, 44.0495 |
| O-Propyl | 149.0961, 107.0491 | |||
| OH-Protonitazene | 427.2347 | 5.26 | N,N-Diethylamine | 100.1121, 72.0809, 44.0495 |
| O-Hydroxy propyl | 165.0912, 107.0491 | |||
| N-Desethyl Protonitazene | 383.2083 | 6.34 | N-Ethylamine | 72.0808, 44.0495 |
| O-Propyl | 149.0956, 107.0491 | |||
| N-Desethyl OH- Protonitazene | 399.2023 | 5.08 | N-Ethylamine | 72.0809, 44.0496 |
| O-Hydroxy propyl | 165.0919, 107.0492 | |||
| N-Desethyl O-Despropyl Protonitazene | 341.1613 | 4.54 | N-Ethylamine | 72.0809, 44.0495 |
| OH | 107.0492 | |||
| O-Despropyl Protonitazene Glucuronide | 545.2249 | 4.05 | N,N-Diethylamine | 100.1121, 72.0809, 44.0496 |
| O-Glucuronide | 107.0492 | |||
| N-Desethyl O-Despropyl Protonitazene Glucuronide | 517.1936 | 3.84 | N-Ethylamine | 72.0808, 44.0495 |
| O-Glucuronide | 107.0492 | |||
| Protonitazepyne | 409.2236 | 6.22 | N-Pyrrolidine | 98.0963, 56.0494, 44.0495 |
| O-Propyl | 149.0959, 107.0492 | |||
| Protonitazepyne acid | 441.2131 | 6.22 | N-butanoic acid | 130.0862, 112,0757, 87.0441 |
| O-Propyl | 149.0958, 107.0492 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magny, R.; Schiestel, T.; M’Rad, A.; Lefrère, B.; Raphalen, J.-H.; Ledochowski, S.; Labat, L.; Mégarbane, B.; Houzé, P. Comparison of the Metabolic Profiles Associated with Protonitazene and Protonitazepyne in Two Severe Poisonings. Metabolites 2025, 15, 371. https://doi.org/10.3390/metabo15060371
Magny R, Schiestel T, M’Rad A, Lefrère B, Raphalen J-H, Ledochowski S, Labat L, Mégarbane B, Houzé P. Comparison of the Metabolic Profiles Associated with Protonitazene and Protonitazepyne in Two Severe Poisonings. Metabolites. 2025; 15(6):371. https://doi.org/10.3390/metabo15060371
Chicago/Turabian StyleMagny, Romain, Thomas Schiestel, Aymen M’Rad, Bertrand Lefrère, Jean-Herlé Raphalen, Stanislas Ledochowski, Laurence Labat, Bruno Mégarbane, and Pascal Houzé. 2025. "Comparison of the Metabolic Profiles Associated with Protonitazene and Protonitazepyne in Two Severe Poisonings" Metabolites 15, no. 6: 371. https://doi.org/10.3390/metabo15060371
APA StyleMagny, R., Schiestel, T., M’Rad, A., Lefrère, B., Raphalen, J.-H., Ledochowski, S., Labat, L., Mégarbane, B., & Houzé, P. (2025). Comparison of the Metabolic Profiles Associated with Protonitazene and Protonitazepyne in Two Severe Poisonings. Metabolites, 15(6), 371. https://doi.org/10.3390/metabo15060371










