Secreted Phosphoprotein 1 in Lung Diseases
Abstract
1. Introduction
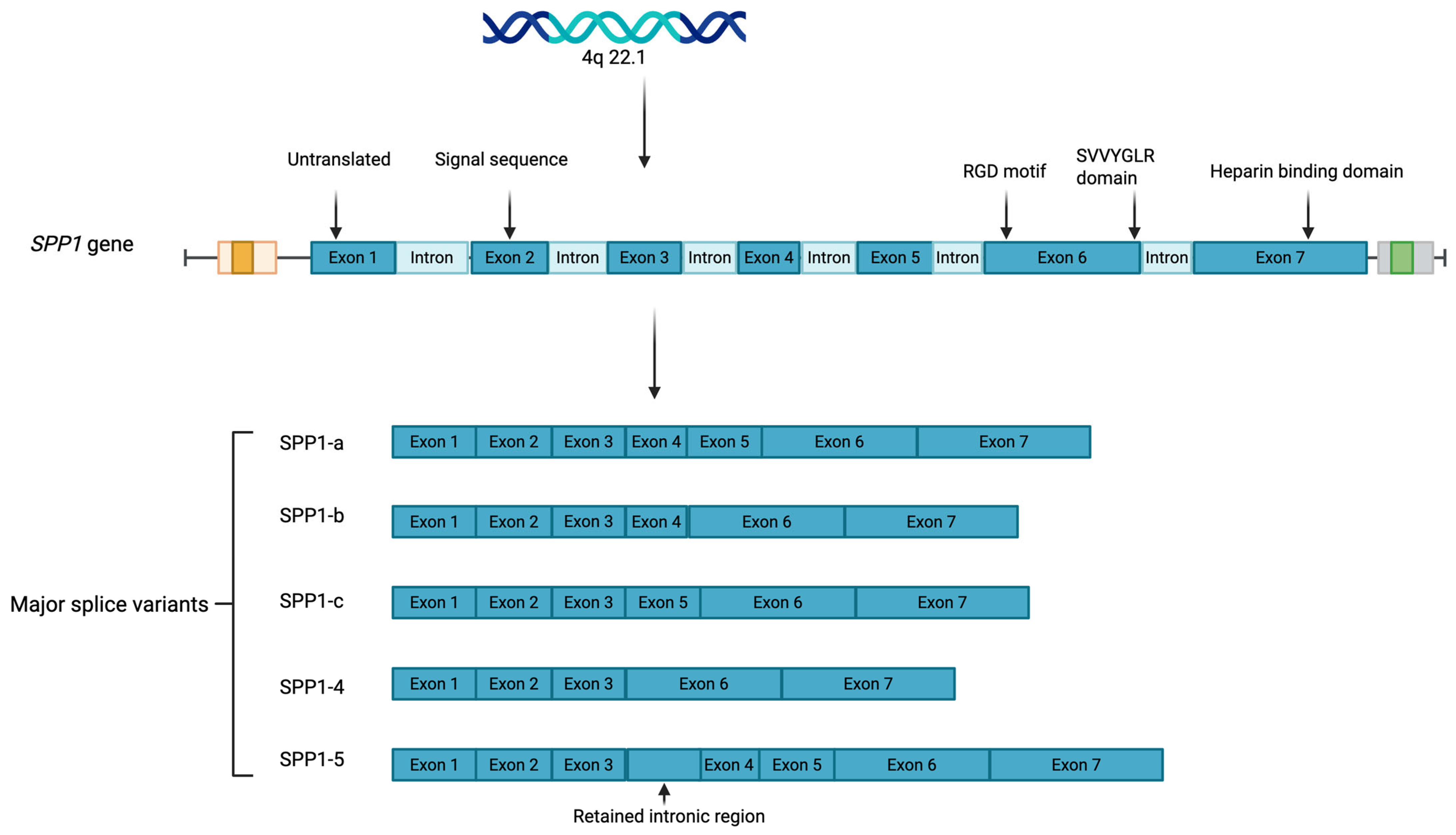
- SPP1-a, the full-length transcript containing all seven exons;
- SPP1-b, lacking exon 5;
- SPP1-c, lacking exon 4;
- SPP1-4, which lacks both exons 4 and 5;
- SPP1-5, which includes a retained intronic region between exons 3 and 4 that adds a novel exon and alters the translation start site. Subvariants of SPP1-5 (e.g., SPP1-5b through SPP1-5e) contain specific insertions or deletions in the novel exon. These splice variants differ in motifs for phosphorylation and transglutaminase crosslinking, contributing to isoform-specific functions in disease. Moreover, SPP1 exists in both intracellular and secreted forms and undergoes extensive post-translational modifications, including serine/threonine phosphorylation and glycosylation [2], which differentially influence its function. Exon 2 encodes the signal peptide necessary for secretion, while exons 3 through 7 contribute to its phosphorylation sites, calcium-binding domains, and receptor-binding motifs. The extracellular form of SPP1 primarily exerts its effects through interactions with integrins and CD44, regulating cellular functions such as migration, adhesion, and proliferation [3,4,5]. SPP1 contains a conserved Arg-Gly-Asp (RGD) motif, which facilitates integrin binding and is regulated by active thrombin cleavage [6]. The integrin-binding Arg-Gly-Asp (RGD) motif, located in exon 6, is essential for binding to αvβ3, αvβ5, and α8β1 integrins, and the SVVYGLR domain enables interaction with α4β1, α9β1, and α4β7 integrins. In addition to thrombin, matrix metalloproteinases (MMPs)—specifically MMP-3 and MMP-7—can also cleave SPP1 [7]. While most research has focused on the extracellular form, intracellular SPP1, located in the cytoplasm and nucleus, can act independently of receptor-mediated pathways, influencing processes such as calcium signaling, cytoskeletal organization, and apoptosis [8]. This dual localization raises important questions about how each form of SPP1 contributes to disease progression, either through extracellular signaling or intracellular regulation. The context of SPP1 expression—whether as part of a physiological adaptation or a pathological response—is also critical. In some instances, SPP1 upregulation may reflect an adaptive response to injury or inflammation, promoting tissue repair and cellular survival. Indeed, SPP1 has been shown to be a critical factor for lung development in mice [9]. However, SPP1 may contribute to disease processes such as fibrosis, immune dysregulation, or cancer metastasis. This multifaceted nature—being both protective and pathogenic—underscores the need for a nuanced understanding of SPP1’s isoform- and cell-specific functions, subcellular localization, and disease context.
2. SPP1 in Interstitial Lung Diseases
3. SPP1 in Infectious Granulomatous Lung Diseases
4. SPP1 in Lung and Pleural Malignancies
5. SPP1 in Obstructive Lung Diseases and Reactive Airway Diseases
6. SPP1 in COVID-19-Associated Lung Disease
7. Future Research
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denhardt, D.T.; Noda, M.; O’Regan, A.W.; Pavlin, D.; Berman, J.S. Osteopontin as a means to cope with environmental insults: Regulation of inflammation, tissue remodeling, and cell survival. J. Clin. Investig. 2001, 107, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Yim, A.; Smith, C.; Brown, A.M. Osteopontin/secreted phosphoprotein-1 harnesses glial-, immune-, and neuronal cell ligand-receptor interactions to sense and regulate acute and chronic neuroinflammation. Immunol. Rev. 2022, 311, 224–233. [Google Scholar] [CrossRef]
- Hu, D.D.; Lin, E.C.; Kovach, N.L.; Hoyer, J.R.; Smith, J.W. A biochemical characterization of the binding of osteopontin to integrins αvβ1 and αvβ5. J. Biol. Chem. 1995, 270, 26232–26238. [Google Scholar] [CrossRef]
- Weber, G.F.; Ashkar, S.; Glimcher, M.J.; Cantor, H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1). Science 1996, 271, 509–512. [Google Scholar] [CrossRef]
- Lee, J.L.; Wang, M.J.; Sudhir, P.R.; Chen, G.D.; Chi, C.W.; Chen, J.Y. Osteopontin promotes integrin activation through outside-in and inside-out mechanisms: OPN-CD44V interaction enhances survival in gastrointestinal cancer cells. Cancer Res. 2007, 67, 2089–2097. [Google Scholar] [CrossRef]
- Liaw, L.; Birk, D.E.; Ballas, C.B.; Whitsitt, J.S.; Davidson, J.M.; Hogan, B.L. Altered wound healing in mice lacking a functional osteopontin gene (spp1). J. Clin. Investig. 1998, 101, 1468–1478. [Google Scholar] [CrossRef]
- Agnihotri, R.; Crawford, H.C.; Haro, H.; Matrisian, L.M.; Havrda, M.C.; Liaw, L. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin). J. Biol. Chem. 2001, 276, 28261–28267. [Google Scholar] [CrossRef]
- Reggio, A.; Fuoco, C.; Deodati, R.; Palma, A. SPP1 macrophages across diseases: A call for reclassification? FASEB J. 2025, 39, e70448. [Google Scholar] [CrossRef]
- Ganguly, K.; Martin, T.M.; Concel, V.J.; Upadhyay, S.; Bein, K.; Brant, K.A.; George, L.; Mitra, A.; Thimraj, T.A.; Fabisiak, J.P.; et al. Secreted phosphoprotein 1 is a determinant of lung function development in mice. Am. J. Respir. Cell Mol. Biol. 2014, 51, 637–651. [Google Scholar] [CrossRef]
- White, E.S.; Xia, M.; Murray, S.; Dyal, R.; Flaherty, C.M.; Flaherty, K.R.; Moore, B.B.; Cheng, L.; Doyle, T.J.; Villalba, J.; et al. Plasma Surfactant Protein-D, Matrix Metalloproteinase-7, and Osteopontin Index Distinguishes Idiopathic Pulmonary Fibrosis from Other Idiopathic Interstitial Pneumonias. Am. J. Respir. Crit. Care Med. 2016, 194, 1242–1251. [Google Scholar] [CrossRef]
- Carlson, I.; Tognazzi, K.; Manseau, E.J.; Dvorak, H.F.; Brown, L.F. Osteopontin is strongly expressed by histiocytes in granulomas of diverse etiology. Lab. Investig. J. Tech. Methods Pathol. 1997, 77, 103–108. [Google Scholar]
- Bharat, A.; Querrey, M.; Markov, N.S.; Kim, S.; Kurihara, C.; Garza-Castillon, R.; Manerikar, A.; Shilatifard, A.; Tomic, R.; Politanska, Y.; et al. Lung transplantation for patients with severe COVID-19. Sci. Transl. Med. 2020, 12, eabe4282. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Li, M.; Frid, M.G.; Kumar, B.; Gerasimovskaya, E.V.; Riddle, S.R.; McKeon, B.A.; Thukaram, R.; Meyrick, B.O.; Fini, M.A.; et al. Osteopontin is an endogenous modulator of the constitutively activated phenotype of pulmonary adventitial fibroblasts in hypoxic pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L1–L11. [Google Scholar] [CrossRef]
- Shan, M.; Yuan, X.; Song, L.-Z.; Roberts, L.; Zarinkamar, N.; Seryshev, A.; Zhang, Y.; Hilsenbeck, S.; Chang, S.-H.; Dong, C.; et al. Cigarette smoke induction of osteopontin (SPP1) mediates TH17 inflammation in human and experimental emphysema. Sci. Transl. Med. 2012, 4, 117ra9. [Google Scholar] [CrossRef]
- Zheng, Q.; Cox, I.A.; Campbell, J.A.; Xia, Q.; Otahal, P.; de Graaff, B.; Corte, T.J.; Teoh, A.K.; Walters, E.H.; Palmer, A.J. Mortality and survival in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. ERJ Open Res. 2022, 8, 00591–2021. [Google Scholar] [CrossRef]
- Maher, T.M.; Bendstrup, E.; Dron, L.; Langley, J.; Smith, G.; Khalid, J.M.; Patel, H.; Kreuter, M. Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir. Res. 2021, 22, 197. [Google Scholar] [CrossRef]
- Podolanczuk, A.J.; Wong, A.W.; Saito, S.; Lasky, J.A.; Ryerson, C.J.; Eickelberg, O. Update in Interstitial Lung Disease 2020. Am. J. Respir. Crit. Care Med. 2021, 203, 1343–1352. [Google Scholar] [CrossRef]
- Oldham, J.M.; Huang, Y.; Bose, S.; Ma, S.-F.; Kim, J.S.; Schwab, A.; Ting, C.; Mou, K.; Lee, C.T.; Adegunsoye, A.; et al. Proteomic Biomarkers of Survival in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2024, 209, 1111–1120. [Google Scholar] [CrossRef]
- Pardo, A.; Gibson, K.; Cisneros, J.; Richards, T.J.; Yang, Y.; Becerril, C.; Yousem, S.; Herrera, I.; Ruiz, V.; Selman, M.; et al. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2005, 2, e251. [Google Scholar] [CrossRef]
- Foster, M.W.; Morrison, L.D.; Todd, J.L.; Snyder, L.D.; Thompson, J.W.; Soderblom, E.J.; Plonk, K.; Weinhold, K.J.; Townsend, R.; Minnich, A.; et al. Quantitative proteomics of bronchoalveolar lavage fluid in idiopathic pulmonary fibrosis. J. Proteome Res. 2015, 14, 1238–1249. [Google Scholar] [CrossRef]
- Kaminski, N.; Allard, J.D.; Pittet, J.F.; Zuo, F.; Griffiths, M.J.D.; Morris, D.; Huang, X.; Sheppard, D.; Heller, R.A. Global analysis of gene expression in pulmonary fibrosis reveals distinct programs regulating lung inflammation and fibrosis. Proc. Natl. Acad. Sci. USA 2000, 97, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Takahashi, K.; Okazaki, T.; Maeda, K.; Ienaga, H.; Maeda, M.; Kon, S.; Uede, T.; Fukuchi, Y. Role of osteopontin in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2001, 24, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.F.; Berse, B.; Van de Water, L.; Papadopoulos-Sergiou, A.; Perruzzi, C.A.; Manseau, E.J.; Dvorak, H.F.; Senger, D.R. Expression and distribution of osteopontin in human tissues: Widespread association with luminal epithelial surfaces. Mol. Biol. Cell 1992, 3, 1169–1180. [Google Scholar] [CrossRef]
- Mould, K.J.; Moore, C.M.; McManus, S.A.; McCubbrey, A.L.; McClendon, J.D.; Griesmer, C.L.; Henson, P.M.; Janssen, W.J. Airspace Macrophages and Monocytes Exist in Transcriptionally Distinct Subsets in Healthy Adults. Am. J. Respir. Crit. Care Med. 2021, 203, 946–956. [Google Scholar] [CrossRef]
- Morse, C.; Tabib, T.; Sembrat, J.; Buschur, K.L.; Bittar, H.T.; Valenzi, E.; Jiang, Y.; Kass, D.J.; Gibson, K.; Chen, W.; et al. Proliferating SPP1/MERTK-expressing macrophages in idiopathic pulmonary fibrosis. Eur. Respir. J. 2019, 54, 1802441. [Google Scholar] [CrossRef]
- Reyfman, P.A.; Walter, J.M.; Joshi, N.; Anekalla, K.R.; McQuattie-Pimentel, A.C.; Chiu, S.; Fernandez, R.; Akbarpour, M.; Chen, C.-I.; Ren, Z.; et al. Single-Cell Transcriptomic Analysis of Human Lung Provides Insights into the Pathobiology of Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 199, 1517–1536. [Google Scholar] [CrossRef]
- Yue, B.; Xiong, D.; Chen, J.; Yang, X.; Zhao, J.; Shao, J.; Wei, D.; Gao, F.; Huang, M.; Chen, J. SPP1 induces idiopathic pulmonary fibrosis and NSCLC progression via the PI3K/Akt/mTOR pathway. Respir. Res. 2024, 25, 362. [Google Scholar] [CrossRef]
- Berman, J.S.; Serlin, D.; Li, X.; Whitley, G.; Hayes, J.; Rishikof, D.C.; Ricupero, D.A.; Liaw, L.; Goetschkes, M.; O’Regan, A.W. Altered bleomycin-induced lung fibrosis in osteopontin-deficient mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L1311–L1318. [Google Scholar] [CrossRef]
- Prasse, A.; Stahl, M.; Schulz, G.; Kayser, G.; Wang, L.; Ask, K.; Yalcintepe, J.; Kirschbaum, A.; Bargagli, E.; Zissel, G.; et al. Essential role of osteopontin in smoking-related interstitial lung diseases. Am. J. Pathol. 2009, 174, 1683–1691. [Google Scholar] [CrossRef]
- Hou, J.; Ji, J.; Chen, X.; Cao, H.; Tan, Y.; Cui, Y.; Xiang, Z.; Han, X. Alveolar epithelial cell-derived Sonic hedgehog promotes pulmonary fibrosis through OPN-dependent alternative macrophage activation. FEBS J. 2021, 288, 3530–3546. [Google Scholar] [CrossRef]
- Hatipoglu, O.F.; Uctepe, E.; Opoku, G.; Wake, H.; Ikemura, K.; Ohtsuki, T.; Inagaki, J.; Gunduz, M.; Gunduz, E.; Watanabe, S.; et al. Osteopontin silencing attenuates bleomycin-induced murine pulmonary fibrosis by regulating epithelial-mesenchymal transition. Biomed. Pharmacother. 2021, 139, 111633. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.; Seo, M.W.; Kim, Y.W.; Lee, D.S. Osteopontin Potentiates Pulmonary Inflammation and Fibrosis by Modulating IL-17/IFN-γ-secreting T-cell Ratios in Bleomycin-treated Mice. Immune Netw. 2015, 15, 142–149. [Google Scholar] [CrossRef]
- Bastos, A.C.S.d.F.; Gomes, A.V.P.; Silva, G.R.; Emerenciano, M.; Ferreira, L.B.; Gimba, E.R.P. The Intracellular and Secreted Sides of Osteopontin and Their Putative Physiopathological Roles. Int. J. Mol. Sci. 2023, 24, 2942. [Google Scholar] [CrossRef] [PubMed]
- Lederer, D.J.; Martinez, F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018, 378, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jia, G.; Guttman, A.; DePianto, D.J.; Morshead, K.B.; Sun, K.-H.; Ramamoorthi, N.; Heiden, J.A.V.; Modrusan, Z.; Wolters, P.J.; et al. Osteopontin Links Myeloid Activation and Disease Progression in Systemic Sclerosis. Cell Rep. Med. 2020, 1, 100140. [Google Scholar] [CrossRef]
- MacDonald, L.; Alivernini, S.; Tolusso, B.; Elmesmari, A.; Somma, D.; Perniola, S.; Paglionico, A.; Petricca, L.; Bosello, S.L.; Carfì, A.; et al. COVID-19 and RA share an SPP1 myeloid pathway that drives PD-L1+ neutrophils and CD14+ monocytes. JCI Insight 2021, 6, e147413. [Google Scholar] [CrossRef]
- Qiu, Y.; Feng, X.; Liu, C.; Shi, Y.; Xu, L.; You, H.; Wang, L.; Lv, C.; Wang, F.; Tan, W. Proteomic profiling identifies SPP1 associated with rapidly progressive interstitial lung disease in anti-MDA5-positive dermatomyositis. Arthritis Res. Ther. 2024, 26, 9. [Google Scholar] [CrossRef]
- Wu, M.; Schneider, D.J.; Mayes, M.D.; Assassi, S.; Arnett, F.C.; Tan, F.K.; Blackburn, M.R.; Agarwal, S.K. Osteopontin in systemic sclerosis and its role in dermal fibrosis. J. Investig. Dermatol. 2012, 132, 1605–1614. [Google Scholar] [CrossRef]
- Papazoglou, A.; Huang, M.; Bulik, M.; Lafyatis, A.; Tabib, T.; Morse, C.; Sembrat, J.; Rojas, M.; Valenzi, E.; Lafyatis, R. Epigenetic Regulation of Profibrotic Macrophages in Systemic Sclerosis-Associated Interstitial Lung Disease. Arthritis Rheumatol. 2022, 74, 2003–2014. [Google Scholar] [CrossRef]
- Valenzi, E.; Tabib, T.; Papazoglou, A.; Sembrat, J.; Bittar, H.E.T.; Rojas, M.; Lafyatis, R. Disparate Interferon Signaling and Shared Aberrant Basaloid Cells in Single-Cell Profiling of Idiopathic Pulmonary Fibrosis and Systemic Sclerosis-Associated Interstitial Lung Disease. Front. Immunol. 2021, 12, 595811. [Google Scholar] [CrossRef]
- Clemente, N.; Raineri, D.; Cappellano, G.; Boggio, E.; Favero, F.; Soluri, M.F.; Dianzani, C.; Comi, C.; Dianzani, U.; Chiocchetti, A. Osteopontin Bridging Innate and Adaptive Immunity in Autoimmune Diseases. J. Immunol. Res. 2016, 2016, 7675437. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, S.N.; Moore, J.G.; Kirou, K.A.; Crow, M.K.; Utset, T.O.; Niewold, T.B. Age- and gender-specific modulation of serum osteopontin and interferon-α by osteopontin genotype in systemic lupus erythematosus. Genes Immun. 2009, 10, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Sabo-Attwood, T.; Ramos-Nino, M.E.; Eugenia-Ariza, M.; Macpherson, M.B.; Butnor, K.J.; Vacek, P.C.; McGee, S.P.; Clark, J.C.; Steele, C.; Mossman, B.T. Osteopontin modulates inflammation, mucin production, and gene expression signatures after inhalation of asbestos in a murine model of fibrosis. Am. J. Pathol. 2011, 178, 1975–1985. [Google Scholar] [CrossRef]
- Pass, H.I.; Lott, D.; Lonardo, F.; Harbut, M.; Liu, Z.; Tang, N.; Carbone, M.; Webb, C.; Wali, A. Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N. Engl. J. Med. 2005, 353, 1564–1573. [Google Scholar] [CrossRef]
- Kotov, D.I.; Lee, O.V.; Fattinger, S.A.; Langner, C.A.; Guillen, J.V.; Peters, J.M.; Moon, A.; Burd, E.M.; Witt, K.C.; Stetson, D.B.; et al. Early cellular mechanisms of type I interferon-driven susceptibility to tuberculosis. Cell 2023, 186, 5536–5553.e22. [Google Scholar] [CrossRef]
- Latoche, J.D.; Ufelle, A.C.; Fazzi, F.; Ganguly, K.; Leikauf, G.D.; Fattman, C.L. Secreted Phosphoprotein 1 and Sex-Specific Differences in Silica-Induced Pulmonary Fibrosis in Mice. Environ. Health Perspect. 2016, 124, 1199–1207. [Google Scholar] [CrossRef]
- Feng, C.; Xu, Z.; Tang, X.; Cao, H.; Zhang, G.; Tan, J. Estrogen-Related Receptor α: A Significant Regulator and Promising Target in Bone Homeostasis and Bone Metastasis. Molecules 2022, 27, 3976. [Google Scholar] [CrossRef]
- Zirngibl, R.A.; Chan, J.S.; Aubin, J.E. Divergent regulation of the Osteopontin promoter by the estrogen receptor-related receptors is isoform- and cell context dependent. J. Cell. Biochem. 2013, 114, 2356–2362. [Google Scholar] [CrossRef]
- Craig, A.M.; Denhardt, D.T. The murine gene encoding secreted phosphoprotein 1 (osteopontin): Promoter structure, activity, and induction in vivo by estrogen and progesterone. Gene 1991, 100, 163–171. [Google Scholar] [CrossRef]
- Huang, R.; Hao, C.; Wang, D.; Zhao, Q.; Li, C.; Wang, C.; Yao, W. SPP1 derived from silica-exposed macrophage exosomes triggers fibroblast transdifferentiation. Toxicol. Appl. Pharmacol. 2021, 422, 115559. [Google Scholar] [CrossRef]
- Dong, J.; Ma, Q. Osteopontin enhances multi-walled carbon nanotube-triggered lung fibrosis by promoting TGF-β1 activation and myofibroblast differentiation. Part. Fibre Toxicol. 2017, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Khaliullin, T.O.; Kisin, E.R.; Murray, A.R.; Yanamala, N.; Shurin, M.R.; Gutkin, D.W.; Fatkhutdinova, L.M.; Kagan, V.E.; Shvedova, A.A. Mediation of the single-walled carbon nanotubes induced pulmonary fibrogenic response by osteopontin and TGF-β1. Exp. Lung Res. 2017, 43, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Lavi, H.; Assayag, M.; Schwartz, A.; Arish, N.; Fridlender, Z.G.; Berkman, N. The association between osteopontin gene polymorphisms, osteopontin expression and sarcoidosis. PLoS ONE 2017, 12, e0171945. [Google Scholar] [CrossRef]
- Maeda, K.; Takahashi, K.; Takahashi, F.; Tamura, N.; Maeda, M.; Kon, S.; Uede, T.; Fukuchi, Y. Distinct roles of osteopontin fragments in the development of the pulmonary involvement in sarcoidosis. Lung 2001, 179, 279–291. [Google Scholar] [CrossRef]
- Kadota, J.; Mizunoe, S.; Mito, K.; Mukae, H.; Yoshioka, S.; Kawakami, K.; Koguchi, Y.; Fukushima, K.; Kon, S.; Kohno, S.; et al. High plasma concentrations of osteopontin in patients with interstitial pneumonia. Respir. Med. 2005, 99, 111–117. [Google Scholar] [CrossRef][Green Version]
- Maver, A.; Medica, I.; Salobir, B.; Tercelj, M.; Peterlin, B. Genetic variation in osteopontin gene is associated with susceptibility to sarcoidosis in Slovenian population. Dis. Markers 2009, 27, 295–302. [Google Scholar] [CrossRef]
- Zhao, A.Y.; Unterman, A.; Abu Hussein, N.S.; Abu Hussein, N.S.; Prapti Sharma, P.; Nikola, F.; Flint, J.; Yan, X.; Adams, T.S.; Justet, A.; et al. Single-Cell Analysis Reveals Novel Immune Perturbations in Fibrotic Hypersensitivity Pneumonitis. Am. J. Respir. Crit. Care Med. 2024, 210, 1252–1266. [Google Scholar] [CrossRef]
- Wang, D.; Tong, X.; Wang, L.; Zhang, S.; Huang, J.; Zhang, L.; Fan, H. The association between osteopontin and tuberculosis: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0242702. [Google Scholar] [CrossRef]
- Yang, Q.; Qi, F.; Ye, T.; Li, J.; Xu, G.; Xiaomeng He, X.; Guofang Deng, G.; Peize Zhang, P.; Mingfeng Liao, M.; Qiao, K.; et al. The interaction of macrophages and CD8 T cells in bronchoalveolar lavage fluid is associated with latent tuberculosis infection. Emerg. Microbes Infect. 2023, 12, 2239940. [Google Scholar] [CrossRef] [PubMed]
- Hasibuan, F.M.; Shiratori, B.; Senoputra, M.A.; Chagan-Yasutan, H.; Koesoemadinata, R.C.; Apriani, L.; Takahashi, Y.; Niki, T.; Alisjahbana, B.; Hattori, T. Evaluation of matricellular proteins in systemic and local immune response to Mycobacterium tuberculosis infection. Microbiol. Immunol. 2015, 59, 623–632. [Google Scholar] [CrossRef]
- Koguchi, Y.; Kawakami, K.; Uezu, K.; Fukushima, K.; Kon, S.; Maeda, M.; Nakamoto, A.; Owan, I.; Kuba, M.; Kudeken, N.; et al. High plasma osteopontin level and its relationship with interleukin-12-mediated type 1 T helper cell response in tuberculosis. Am. J. Respir. Crit. Care Med. 2003, 167, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Medawar, L.; Tukiman, H.M.; Mbayo, G.; Donkor, S.; Owolabi, O.; Sutherland, J.S. Analysis of cellular and soluble profiles in QuantiFERON nonconverters, converters, and reverters in the Gambia. Immun. Inflamm. Dis. 2019, 7, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Shete, A.; Bichare, S.; Pujari, V.; Virkar, R.; Thakar, M.; Ghate, M.; Patil, S.; Vyakarnam, A.; Gangakhedkar, R.; Bai, G.; et al. Elevated Levels of Galectin-9 but Not Osteopontin in HIV and Tuberculosis Infections Indicate Their Roles in Detecting MTB Infection in HIV Infected Individuals. Front. Microbiol. 2020, 11, 1685. [Google Scholar] [CrossRef] [PubMed]
- Inomata, S.; Shijubo, N.; Kon, S.; Maeda, M.; Yamada, G.; Sato, N.; Abe, S.; Uede, T. Circulating interleukin-18 and osteopontin are useful to evaluate disease activity in patients with tuberculosis. Cytokine 2005, 30, 203–211. [Google Scholar] [CrossRef]
- Nau, G.J.; Liaw, L.; Chupp, G.L.; Berman, J.S.; Hogan, B.L.; Young, R.A. Attenuated host resistance against Mycobacterium bovis BCG infection in mice lacking osteopontin. Infect. Immun. 1999, 67, 4223–4230. [Google Scholar] [CrossRef]
- Van Der Windt, G.J.; Wieland, C.W.; Wiersinga, W.J.; Florquin, S.; Van Der Poll, T. Osteopontin is not crucial to protective immunity during murine tuberculosis. Immunology 2009, 128 (Suppl. S1), e766–e776. [Google Scholar] [CrossRef]
- Ashkar, S.; Weber, G.F.; Panoutsakopoulou, V.; Sanchirico, M.E.; Jansson, M.; Zawaideh, S.; Rittling, S.R.; Denhardt, D.T.; Glimcher, M.J.; Cantor, H. Eta-1 (osteopontin): An early component of type-1 (cell-mediated) immunity. Science 2000, 287, 860–864. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, Q.; Alam, A.; Cui, J.; Suen, K.C.; Soo, A.P.; Eguchi, S.; Gu, J.; Ma, D. The role of osteopontin in the progression of solid organ tumour. Cell Death Dis. 2018, 9, 356. [Google Scholar] [CrossRef]
- Yan, Z.; Hu, X.; Tang, B.; Deng, F. Role of osteopontin in cancer development and treatment. Heliyon 2023, 9, e21055. [Google Scholar] [CrossRef]
- Castello, L.M.; Raineri, D.; Salmi, L.; Clemente, N.; Vaschetto, R.; Quaglia, M.; Garzaro, M.; Gentilli, S.; Navalesi, P.; Cantaluppi, V.; et al. Osteopontin at the Crossroads of Inflammation and Tumor Progression. Mediat. Inflamm. 2017, 2017, 4049098. [Google Scholar] [CrossRef]
- Chang, Y.S.; Kim, H.J.; Chang, J.; Ahn, C.M.; Kim, S.K.; Kim, S.K. Elevated circulating level of osteopontin is associated with advanced disease state of non-small cell lung cancer. Lung Cancer 2007, 57, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Lin, D.; Yuan, J.; Xiao, T.; Zhang, H.; Sun, W.; Han, N.; Ma, Y.; Di, X.; Gao, M.; et al. Overexpression of osteopontin is associated with more aggressive phenotypes in human non-small cell lung cancer. Clin. Cancer Res. 2005, 11, 4646–4652. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Luo, L.; Zhu, Y.; Deng, H.; Liao, H.; Shen, Y.; Zheng, Y. SPP1 facilitates cell migration and invasion by targeting COL11A1 in lung adenocarcinoma. Cancer Cell Int. 2022, 22, 324. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, H.; Castellone, M.D.; Malek, R.L.; Letwin, N.; Frank, B.; Gutkind, J.S.; Lee, N.H. Autocrine activation of an osteopontin-CD44-Rac pathway enhances invasion and transformation by H-RasV12. Oncogene 2005, 24, 489–501. [Google Scholar] [CrossRef]
- Chakraborty, G.; Jain, S.; Kundu, G.C. Osteopontin promotes vascular endothelial growth factor-dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res. 2008, 68, 152–161. [Google Scholar] [CrossRef]
- Dai, J.; Peng, L.; Fan, K.; Wang, H.; Wei, R.; Ji, G.; Cai, J.; Lu, B.; Li, B.; Zhang, D.; et al. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene 2009, 28, 3412–3422. [Google Scholar] [CrossRef]
- Dong, B.; Wu, C.; Huang, L.; Qi, Y. Macrophage-Related SPP1 as a Potential Biomarker for Early Lymph Node Metastasis in Lung Adenocarcinoma. Front. Cell Dev. Biol. 2021, 9, 739358. [Google Scholar] [CrossRef]
- Frey, A.B.; Wali, A.; Pass, H.; Lonardo, F. Osteopontin is linked to p65 and MMP-9 expression in pulmonary adenocarcinoma but not in malignant pleural mesothelioma. Histopathology 2007, 50, 720–726. [Google Scholar] [CrossRef]
- Koshimune, S.; Kosaka, M.; Mizuno, N.; Yamamoto, H.; Miyamoto, T.; Ebisui, K.; Toyooka, S.; Ohtsuka, A. Prognostic value of OCT4A and SPP1C transcript variant co-expression in early-stage lung adenocarcinoma. BMC Cancer 2020, 20, 521. [Google Scholar] [CrossRef]
- Rangaswami, H.; Kundu, G.C. Osteopontin stimulates melanoma growth and lung metastasis through NIK/MEKK1-dependent MMP-9 activation pathways. Oncol. Rep. 2007, 18, 909–915. [Google Scholar] [CrossRef]
- Hiraki, A.; Aoe, K.; Ueoka, H. Asbestos exposure and serum osteopontin. N. Engl. J. Med. 2006, 354, 304–305. [Google Scholar] [PubMed]
- Grigoriu, B.D.; Scherpereel, A.; Devos, P.; Chahine, B.; Letourneux, M.; Lebailly, P.; Grégoire, M.; Porte, H.; Copin, M.; Lassalle, P. Utility of osteopontin and serum mesothelin in malignant pleural mesothelioma diagnosis and prognosis assessment. Clin. Cancer Res. 2007, 13, 2928–2935. [Google Scholar] [CrossRef] [PubMed]
- Creaney, J.; Yeoman, D.; Demelker, Y.; Segal, A.; Musk, A.W.; Skates, S.J.; Robinson, B.W.S. Comparison of osteopontin, megakaryocyte potentiating factor, and mesothelin proteins as markers in the serum of patients with malignant mesothelioma. J. Thorac. Oncol. 2008, 3, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Papaporfyriou, A.; Loukides, S.; Kostikas, K.; Simoes, D.C.M.; Papatheodorou, G.; Konstantellou, E.; Hillas, G.; Papiris, S.; Koulouris, N.; Bakakos, P. Increased levels of osteopontin in sputum supernatant in patients with COPD. Chest 2014, 146, 951–958. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, S.H.; Kim, W.; Lim, S.; Lee, S.H.; Kim, Y.E.; Cho, Y.J.; Jeong, Y.Y.; Kim, H.C.; Lee, J.D.; et al. Increased plasma osteopontin in frequent exacerbator and acute exacerbation of COPD. Clin. Respir. J. 2014, 8, 305–311. [Google Scholar] [CrossRef]
- Chen, H.; Song, Z.; Qian, M.; Bai, C.; Wang, X. Selection of disease-specific biomarkers by integrating inflammatory mediators with clinical informatics in AECOPD patients: A preliminary study. J. Cell. Mol. Med. 2012, 16, 1286–1297. [Google Scholar] [CrossRef]
- Ali, M.N.; Mori, M.; Mertens, T.C.J.; Siddhuraj, P.; Erjefält, J.S.; Önnerfjord, P.; Hiemstra, P.S.; Egesten, A. Osteopontin Expression in Small Airway Epithelium in Copd is Dependent on Differentiation and Confined to Subsets of Cells. Sci. Rep. 2019, 9, 15566. [Google Scholar] [CrossRef]
- Schneider, D.J.; Lindsay, J.C.; Zhou, Y.; Molina, J.G.; Blackburn, M.R. Adenosine and osteopontin contribute to the development of chronic obstructive pulmonary disease. FASEB J. 2010, 24, 70–80. [Google Scholar] [CrossRef]
- Shi, L.; Zhu, B.; Xu, M.; Wang, X. Selection of AECOPD-specific immunomodulatory biomarkers by integrating genomics and proteomics with clinical informatics. Cell Biol. Toxicol. 2018, 34, 109–123. [Google Scholar] [CrossRef]
- Kohan, M.; Bader, R.; Puxeddu, I.; Levi-Schaffer, F.; Breuer, R.; Berkman, N. Enhanced osteopontin expression in a murine model of allergen-induced airway remodelling. Clin. Exp. Allergy 2007, 37, 1444–1454. [Google Scholar] [CrossRef]
- Xu, H.; Lou, W.; Fu, F. Association between osteopontin expression and asthma: A meta-analysis. J. Int. Med. Res. 2019, 47, 3513–3521. [Google Scholar] [CrossRef] [PubMed]
- Samitas, K.; Zervas, E.; Vittorakis, S.; Semitekolou, M.; Alissafi, T.; Bossios, A.; Gogos, H.; Economidou, E.; Lötvall, J.; Xanthou, G.; et al. Osteopontin expression and relation to disease severity in human asthma. Eur. Respir. J. 2011, 37, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Delimpoura, V.; Bakakos, P.; Tseliou, E.; Bessa, V.; Hillas, G.; Simoes, D.C.M.; Papiris, S.; Loukides, S. Increased levels of osteopontin in sputum supernatant in severe refractory asthma. Thorax 2010, 65, 782–786. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trinh, H.K.T.; Nguyen, T.V.T.; Kim, S.H.; Cao, T.B.T.; Luu, Q.Q.; Kim, S.; Park, H. Osteopontin contributes to late-onset asthma phenotypes in adult asthma patients. Exp. Mol. Med. 2020, 52, 253–265. [Google Scholar] [CrossRef]
- Simoes, D.C.; Xanthou, G.; Petrochilou, K.; Panoutsakopoulou, V.; Roussos, C.; Gratziou, C. Osteopontin deficiency protects against airway remodeling and hyperresponsiveness in chronic asthma. Am. J. Respir. Crit. Care Med. 2009, 179, 894–902. [Google Scholar] [CrossRef]
- Xanthou, G.; Alissafi, T.; Semitekolou, M.; Simoes, D.C.M.; Economidou, E.; Gaga, M.; Lambrecht, B.N.; Lloyd, C.M.; Panoutsakopoulou, V. Osteopontin has a crucial role in allergic airway disease through regulation of dendritic cell subsets. Nat. Med. 2007, 13, 570–578. [Google Scholar] [CrossRef]
- King, E.M.; Zhao, Y.; Moore, C.M.; Steinhart, B.; Anderson, K.C.; Vestal, B.; Moore, P.K.; McManus, S.A.; Evans, C.M.; Mould, K.J. Gpnmb and Spp1 mark a conserved macrophage injury response masking fibrosis-specific programming in the lung. JCI Insight 2024, 9, e182700. [Google Scholar] [CrossRef]
- Takahashi, F.; Takahashi, K.; Shimizu, K.; Cui, R.; Tada, N.; Takahashi, H.; Soma, S.; Yoshioka, M.; Fukuchi, Y. Osteopontin is strongly expressed by alveolar macrophages in the lungs of acute respiratory distress syndrome. Lung 2004, 182, 173–185. [Google Scholar] [CrossRef]
- Nyirenda, J.; Hardy, O.M.; Silva Filho, J.D.; Herder, V.; Attipa, C.; Ndovi, C.; Siwombo, M.; Namalima, T.R.; Suwedi, L.; Ilia, G.; et al. Spatially resolved single-cell atlas unveils a distinct cellular signature of fatal lung COVID-19 in a Malawian population. Nat. Med. 2024, 30, 3765–3777. [Google Scholar] [CrossRef]
- Wendisch, D.; Dietrich, O.; Mari, T.; von Stillfried, S.; Ibarra, I.L.; Mittermaier, M.; Mache, C.; Chua, R.L.; Knoll, R.; Timm, S.; et al. SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell 2021, 184, 6243–6261 e27. [Google Scholar] [CrossRef]
- Dinnon, K.H., 3rd; Leist, S.R.; Okuda, K.; Dang, H.; Fritch, E.J.; Gully, K.L.; De la Cruz, G.; Evangelista, M.D.; Asakura, T.; Gilmore, R.C.; et al. SARS-CoV-2 infection produces chronic pulmonary epithelial and immune cell dysfunction with fibrosis in mice. Sci. Transl. Med. 2022, 14, eabo5070. [Google Scholar] [CrossRef] [PubMed]
- Kanth, S.M.; Huapaya, J.A.; Gairhe, S.; Wang, H.; Tian, X.; Demirkale, C.Y.; Hou, C.; Ma, J.; Kuhns, D.B.; Fink, D.L.; et al. Longitudinal analysis of the lung proteome reveals persistent repair months after mild to moderate COVID-19. Cell Rep. Med. 2024, 5, 101642. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.I.; Puritz, C.H.; Senkow, K.J.; Markov, N.S.; Diaz, E.; Jonasson, E.; Yu, Z.; Swaminathan, S.; Lu, Z.; Fenske, S.; et al. Profibrotic monocyte-derived alveolar macrophages are expanded in patients with persistent respiratory symptoms and radiographic abnormalities after COVID-19. Nat. Immunol. 2024, 25, 2097–2109. [Google Scholar] [CrossRef] [PubMed]
| Lung Disease | SPP1 Function/Role | ||
|---|---|---|---|
| Interstitial Lung Disease | Idiopathic Pulmonary Fibrosis |
| |
| CTD-associated ILD |
| ||
| Pneumoconiosis |
| ||
| Granulomatous Lung Diseases (noninfectious) |
| ||
| Infectious Granulomatous Diseases (e.g., TB) |
| ||
| Lung and Pleural Malignancies |
| ||
| Obstructive Lung Disease and Reactive Airway Disease | COPD |
| |
| Asthma |
| ||
| COVID-19-associated Lung Disease |
| ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Coarfa, C.; Charania, A.N.; Larson-Casey, J.L.; Rosas, I.O.; He, C. Secreted Phosphoprotein 1 in Lung Diseases. Metabolites 2025, 15, 365. https://doi.org/10.3390/metabo15060365
Liu H, Coarfa C, Charania AN, Larson-Casey JL, Rosas IO, He C. Secreted Phosphoprotein 1 in Lung Diseases. Metabolites. 2025; 15(6):365. https://doi.org/10.3390/metabo15060365
Chicago/Turabian StyleLiu, Hongli, Cristian Coarfa, Arzoo N. Charania, Jennifer L. Larson-Casey, Ivan O. Rosas, and Chao He. 2025. "Secreted Phosphoprotein 1 in Lung Diseases" Metabolites 15, no. 6: 365. https://doi.org/10.3390/metabo15060365
APA StyleLiu, H., Coarfa, C., Charania, A. N., Larson-Casey, J. L., Rosas, I. O., & He, C. (2025). Secreted Phosphoprotein 1 in Lung Diseases. Metabolites, 15(6), 365. https://doi.org/10.3390/metabo15060365






