Urinary Hippuric Acid as a Sex-Dependent Biomarker for Fruit and Nut Intake Raised from the EAT-Lancet Index and Nuclear Magnetic Resonance Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. 1H-NMR Urine Metabolomics
2.3. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Soltani, S.; Abdolshahi, A.; Shab-Bidar, S. Healthy and Unhealthy Dietary Patterns and the Risk of Chronic Disease: An Umbrella Review of Meta-Analyses of Prospective Cohort Studies. Br. J. Nutr. 2020, 124, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Naska, A.; Lagiou, A.; Lagiou, P. Dietary Assessment Methods in Epidemiological Research: Current State of the Art and Future Prospects. F1000Research 2017, 6, 926. [Google Scholar] [CrossRef]
- Bailey, R.L. Overview of Dietary Assessment Methods for Measuring Intakes of Foods, Beverages, and Dietary Supplements in Research Studies. Curr. Opin. Biotechnol. 2021, 70, 91–96. [Google Scholar] [CrossRef]
- Gavrieli, A.; Trichopoulou, A.; Valsta, L.M.; Ioannidou, S.; Berry, R.; Roe, M.; Harvey, L.; Finglas, P.; Glibetic, M.; Gurinovic, M.; et al. Identifying Sources of Measurement Error in Assessing Dietary Intakes—Results of a Multi-Country Ring-Trial. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 127–134. [Google Scholar] [CrossRef]
- Morze, J.; Danielewicz, A.; Hoffmann, G.; Schwingshackl, L. Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: A Second Update of a Systematic Review and Meta-Analysis of Cohort Studies. J. Acad. Nutr. Diet. 2020, 120, 1998–2031. [Google Scholar] [CrossRef]
- García-Conesa, M.T.; Philippou, E.; Pafilas, C.; Massaro, M.; Quarta, S.; Andrade, V.; Jorge, R.; Chervenkov, M.; Ivanova, T.; Dimitrova, D.; et al. Exploring the Validity of the 14-Item Mediterranean Diet Adherence Screener (Medas): A Cross-National Study in Seven European Countries around the Mediterranean Region. Nutrients 2020, 12, 2960. [Google Scholar] [CrossRef]
- Knuppel, A.; Papier, K.; Key, T.J.; Travis, R.C. EAT-Lancet Score and Major Health Outcomes: The EPIC-Oxford Study. Lancet 2019, 394, 213–214. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Kuhnle, G.G.C. Nutritional Biomarkers for Objective Dietary Assessment. J. Sci. Food Agric. 2012, 92, 1145–1149. [Google Scholar] [CrossRef]
- Pande, S.; Kratasyuk, V.A.; Medvedeva, N.N.; Kolenchukova, O.A.; Salmina, A.B. Nutritional Biomarkers: Current View and Future Perspectives. Crit. Rev. Food Sci. Nutr. 2018, 58, 3055–3069. [Google Scholar] [CrossRef] [PubMed]
- de la O, V.; Fernández-Cruz, E.; Valdés, A.; Cifuentes, A.; Walton, J.; Martínez, J.A. Exhaustive Search of Dietary Intake Biomarkers as Objective Tools for Personalized Nutrimetabolomics and Precision Nutrition Implementation. Nutr. Rev. 2025, 83, 925–942. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, T.; Azab, S.M.; Teo, K.K.; Thabane, L.; Anand, S.S.; Morrison, K.M.; De Souza, R.J.; Britz-Mckibbin, P. Nutritional Metabolomics and the Classification of Dietary Biomarker Candidates: A Critical Review. Adv. Nutr. 2021, 12, 2333–2357. [Google Scholar] [CrossRef]
- Lang, R.; Lang, T.; Bader, M.; Beusch, A.; Schlagbauer, V.; Hofmann, T. High-Throughput Quantitation of Proline Betaine in Foods and Suitability as a Valid Biomarker for Citrus Consumption. J. Agric. Food Chem. 2017, 65, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Gibbons, H.; Rundle, M.; Frost, G.; McNulty, B.A.; Nugent, A.P.; Walton, J.; Flynn, A.; Brennan, L. The Relationship between Fish Intake and Urinary Trimethylamine-N-Oxide. Mol. Nutr. Food Res. 2020, 64, e1900799. [Google Scholar] [CrossRef]
- Kochlik, B.; Gerbracht, C.; Grune, T.; Weber, D. The Influence of Dietary Habits and Meat Consumption on Plasma 3-Methylhistidine—A Potential Marker for Muscle Protein Turnover. Mol. Nutr. Food Res. 2018, 62, e1701062. [Google Scholar] [CrossRef]
- Zhu, Y.; Shurlknight, K.L.; Chen, X.; Sang, S. Identification and Pharmacokinetics of Novel Alkylresorcinol Metabolites in Human Urine, New Candidate Biomarkers for Whole-Grain Wheat and Rye Intake. J. Nutr. 2014, 144, 114–122. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Le Sayec, M.; Yan, X.; Spector, T.D.; Steves, C.J.; Bell, J.T.; Small, K.S.; Menni, C.; Gibson, R.; et al. Development of a (Poly)phenol Metabolic Signature for Assessing (Poly)phenol-Rich Dietary Patterns. J. Agric. Food Chem. 2024, 72, 13439–13450. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef]
- Ticinesi, A.; Guerra, A.; Nouvenne, A.; Meschi, T.; Maggi, S. Disentangling the Complexity of Nutrition, Frailty and Gut Microbial Pathways during Aging: A Focus on Hippuric Acid. Nutrients 2023, 15, 1138. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Muli, S.; Huybrechts, I.; Nöthlings, U.; Ahrens, W.; Scalbert, A.; Floegel, A. Assessment of Fruit and Vegetables Intake with Biomarkers in Children and Adolescents and Their Level of Validation: A Systematic Review. Metabolites 2022, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Rabassa, M.; Zamora-Ros, R.; Palau-Rodriguez, M.; Tulipani, S.; Miñarro, A.; Bandinelli, S.; Ferrucci, L.; Cherubini, A.; Andres-Lacueva, C. Habitual Nut Exposure, Assessed by Dietary and Multiple Urinary Metabolomic Markers, and Cognitive Decline in Older Adults: The InCHIANTI Study. Mol. Nutr. Food Res. 2020, 64, e1900532. [Google Scholar] [CrossRef]
- Mora-Cubillos, X.; Tulipani, S.; Garcia-Aloy, M.; Bulló, M.; Tinahones, F.J.; Andres-Lacueva, C. Plasma metabolomic biomarkers of mixed nuts exposure inversely correlate with severity of metabolic syndrome. Mol. Nutr. Food Res. 2015, 59, 2480–2490. [Google Scholar] [CrossRef] [PubMed]
- Roy Chowdhury, C.; Kavitake, D.; Jaiswal, K.K.; Jaiswal, K.S.; Reddy, G.B.; Agarwal, V.; Shetty, P.H. NMR-Based Metabolomics as a Significant Tool for Human Nutritional Research and Health Applications. Food Biosci. 2023, 53, 102538. [Google Scholar] [CrossRef]
- Marino, M.; Masella, R.; Bulzomi, P.; Campesi, I.; Malorni, W.; Franconi, F. Nutrition and Human Health from a Sex-Gender Perspective. Mol. Aspects. Med. 2011, 32, 1–70. [Google Scholar] [CrossRef]
- Chen, Y.; Kim, M.; Paye, S.; Benayoun, B.A. Sex as a Biological Variable in Nutrition Research: From Human Studies to Animal Models. Annu. Rev. Nutr. 2022, 42, 227–250. [Google Scholar] [CrossRef]
- Iizuka, K.; Yanagi, K.; Deguchi, K.; Ushiroda, C.; Yamamoto-Wada, R.; Kobae, K.; Yamada, Y.; Naruse, H. Sex and Age Differences in the Effects of Food Frequency on Metabolic Parameters in Japanese Adults. Nutrients 2024, 16, 2931. [Google Scholar] [CrossRef]
- Rist, M.J.; Roth, A.; Frommherz, L.; Weinert, C.H.; Krüger, R.; Merz, B.; Bunzel, D.; Mack, C.; Egert, B.; Bub, A.; et al. Metabolite patterns predicting sex and age in participants of the Karlsruhe Metabolomics and Nutrition (KarMeN) study. PLoS ONE 2017, 12, e0183228. [Google Scholar] [CrossRef]
- Weisell, R.C. Body mass index as an indicator of obesity. Asia Pac. J. Clin. Nutr. 2002, 11, S681–S684. [Google Scholar] [CrossRef]
- Fernández-Ballart, J.D.; Piñol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Perez-Bauer, M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Martn-Moreno, J.M. Relative Validity of a Semi-Quantitative Food-Frequency Questionnaire in an Elderly Mediterranean Population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A Systematic Review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Dona, A.C.; Jiménez, B.; Schafer, H.; Humpfer, E.; Spraul, M.; Lewis, M.R.; Pearce, J.T.M.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Precision High-Throughput Proton NMR Spectroscopy of Human Urine, Serum, and Plasma for Large-Scale Metabolic Phenotyping. Anal. Chem. 2014, 86, 9887–9894. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Millán, E.; Ramos, S.; Álvarez-Cilleros, D.; Samino, S.; Amigó, N.; Correig, X.; Chagoyen, M.; Álvarez, C.; Martín, M.Á. Urinary Metabolomics Study on the Protective Role of Cocoa in Zucker Diabetic Rats via 1H-NMR-Based Approach. Nutrients 2022, 14, 4127. [Google Scholar] [CrossRef]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic Quotient Normalization as Robust Method to Account for Dilution of Complex Biological Mixtures. Application In 1H NMR Metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef]
- Fay, M.P.; Proschan, M.A. Wilcoxon–Mann–Whitney or t-test? On assumptions for hypothesis tests and multiple interpretations of decision rules. Stat. Surv. 2010, 4, 1–39. [Google Scholar] [CrossRef]
- Peter, C. Analysis of Covariance. In The International Encyclopedia of Communication Research Methods; Matthes, J., Davis, C.S., Potter, R.F., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Tassi, P. Sample Size. In International Encyclopedia of Statistical Science; Lovric, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 466–470. [Google Scholar] [CrossRef]
- de la O, V.; Fernández-Cruz, E.; Matía Matin, P.; Larrad-Sainz, A.; Espadas Gil, J.L.; Barabash, A.; Fernández-Díaz, C.M.; Calle-Pascual, A.L.; Rubio-Herrera, M.A.; Martínez, J.A. Translational Algorithms for Technological Dietary Quality Assessment Integrating Nutrimetabolic Data with Machine Learning Methods. Nutrients 2024, 16, 3817. [Google Scholar] [CrossRef]
- Menyhart, O.; Weltz, B.; Győrffy, B. MultipleTesting.com: A Tool for Life Science Researchers for Multiple Hypothesis Testing Correction. PLoS ONE 2021, 16, e0245824. [Google Scholar] [CrossRef]
- Toromanović, J.; Kovač-Bešović, E.; Šapčanin, A.; Tahirović, I.; Rimpapa, Z.; Kroyer, G.; Sofić, E. Urinary Hippuric Acid after Ingestion of Edible Fruits. Bosn. J. Basic Med. Sci. 2008, 8, 38–43. [Google Scholar] [CrossRef]
- Penczynski, K.J.; Krupp, D.; Bring, A.; Bolzenius, K.; Remer, T.; Buyken, A.E. Relative Validation of 24-h Urinary Hippuric Acid Excretion as a Biomarker for Dietary Flavonoid Intake from Fruit and Vegetables in Healthy Adolescents. Eur. J. Nutr. 2017, 56, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; Larrosa, M.; García-Conesa, M.T.; Tomás-Barberán, F.A. Biological Significance of Urolithins, the Gut Microbial Ellagic Acid-Derived Metabolites: The Evidence So Far. Evid.-Based Complement. Altern. Med. 2013, 2013, 270418. [Google Scholar] [CrossRef] [PubMed]
- González-Barrio, R.; Edwards, C.A.; Crozier, A. Colonic Catabolism of Ellagitannins, Ellagic Acid, and Raspberry Anthocyanins: In Vivo and In Vitro Studies. Drug Metab. Dispos. 2011, 39, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhong, H.; Li, Y.; Shi, Z.; Ren, H.; Zhang, Z.; Zhou, X.; Tang, S.; Han, X.; Lin, Y.; et al. Sex- and Age-Related Trajectories of the Adult Human Gut Microbiota Shared across Populations of Different Ethnicities. Nat. Aging 2021, 1, 87–100. [Google Scholar] [CrossRef]
- Jardon, K.M.; Goossens, G.H.; Most, J.; Galazzo, G.; Venema, K.; Penders, J.; Blaak, E.E. Examination of Sex-Specific Interactions between Gut Microbiota and Host Metabolism after 12-Week Combined Polyphenol Supplementation in Individuals with Overweight or Obesity. Gut Microbes 2024, 16, 2392875. [Google Scholar] [CrossRef]
- Caterino, M.; Ruoppolo, M.; Villani, G.R.D.; Marchese, E.; Costanzo, M.; Sotgiu, G.; Dore, S.; Franconi, F.; Campesi, I. Influence of Sex on Urinary Organic Acids: A Cross-Sectional Study in Children. Int. J. Mol. Sci. 2020, 21, 582. [Google Scholar] [CrossRef]
- Santos-Marcos, J.A.; Rangel-Zuñiga, O.A.; Jimenez-Lucena, R.; Quintana-Navarro, G.M.; Garcia-Carpintero, S.; Malagon, M.M.; Landa, B.B.; Tena-Sempere, M.; Perez-Martinez, P.; Lopez-Miranda, J.; et al. Influence of Gender and Menopausal Status on Gut Microbiota. Maturitas 2018, 116, 43–53. [Google Scholar] [CrossRef]
- Guerra, A.; Ticinesi, A.; Allegri, F.; Nouvenne, A.; Prati, B.; Pinelli, S.; Merli, R.; Tana, C.; Lauretani, F.; Aloe, R.; et al. Insights about Urinary Hippuric and Citric Acid as Biomarkers of Fruit and Vegetable Intake in Patients with Kidney Stones: The Role of Age and Sex. Nutrition 2019, 59, 83–89. [Google Scholar] [CrossRef]
- Liang, S.; Nasir, R.F.; Bell-Anderson, K.S.; Toniutti, C.A.; O’Leary, F.M.; Skilton, M.R. Biomarkers of Dietary Patterns: A Systematic Review of Randomized Controlled Trials. Nutr. Rev. 2022, 80, 1856–1895. [Google Scholar] [CrossRef]
- Ferrari, L.; Panaite, S.A.; Bertazzo, A.; Visioli, F. Animal- and Plant-Based Protein Sources: A Scoping Review of Human Health Outcomes and Environmental Impact. Nutrients 2022, 14, 5115. [Google Scholar] [CrossRef]
- Chadid, S.; Kreger, B.E.; Singer, M.R.; Loring Bradlee, M.; Moore, L.L. Anthropometric Measures of Body Fat and Obesity-Related Cancer Risk: Sex-Specific Differences in Framingham Offspring Study Adults. Int. J. Obes. 2020, 44, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, K.J.; Arieta, L.R.; Brewer, G.J.; Hoselton, A.L.; Gould, L.M.; Smith-Ryan, A.E. Sex Differences and Considerations for Female Specific Nutritional Strategies: A Narrative Review. J. Int. Soc. Sports Nutr. 2021, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Ravanbakhsh, S.; Liu, P.; Bjordahl, T.C.; Mandal, R.; Grant, J.R.; Wilson, M.; Eisner, R.; Sinelnikov, I.; Hu, X.; Luchinat, C.; et al. Accurate, Fully-Automated NMR Spectral Profiling for Metabolomics. PLoS ONE 2015, 10, e0124219. [Google Scholar] [CrossRef] [PubMed]
- Hertel, J.; Fässler, D.; Heinken, A.; Weiß, F.U.; Rühlemann, M.; Bang, C.; Franke, A.; Budde, K.; Henning, A.K.; Petersmann, A.; et al. NMR Metabolomics Reveal Urine Markers of Microbiome Diversity and Identify Benzoate Metabolism as a Mediator between High Microbial Alpha Diversity and Metabolic Health. Metabolites 2022, 12, 308. [Google Scholar] [CrossRef]
- Vernocchi, P.; Del Chierico, F.; Putignani, L. Gut Microbiota Profiling: Metabolomics Based Approach to Unravel Compounds Affecting Human Health. Front. Microbiol. 2016, 7, 1144. [Google Scholar] [CrossRef]
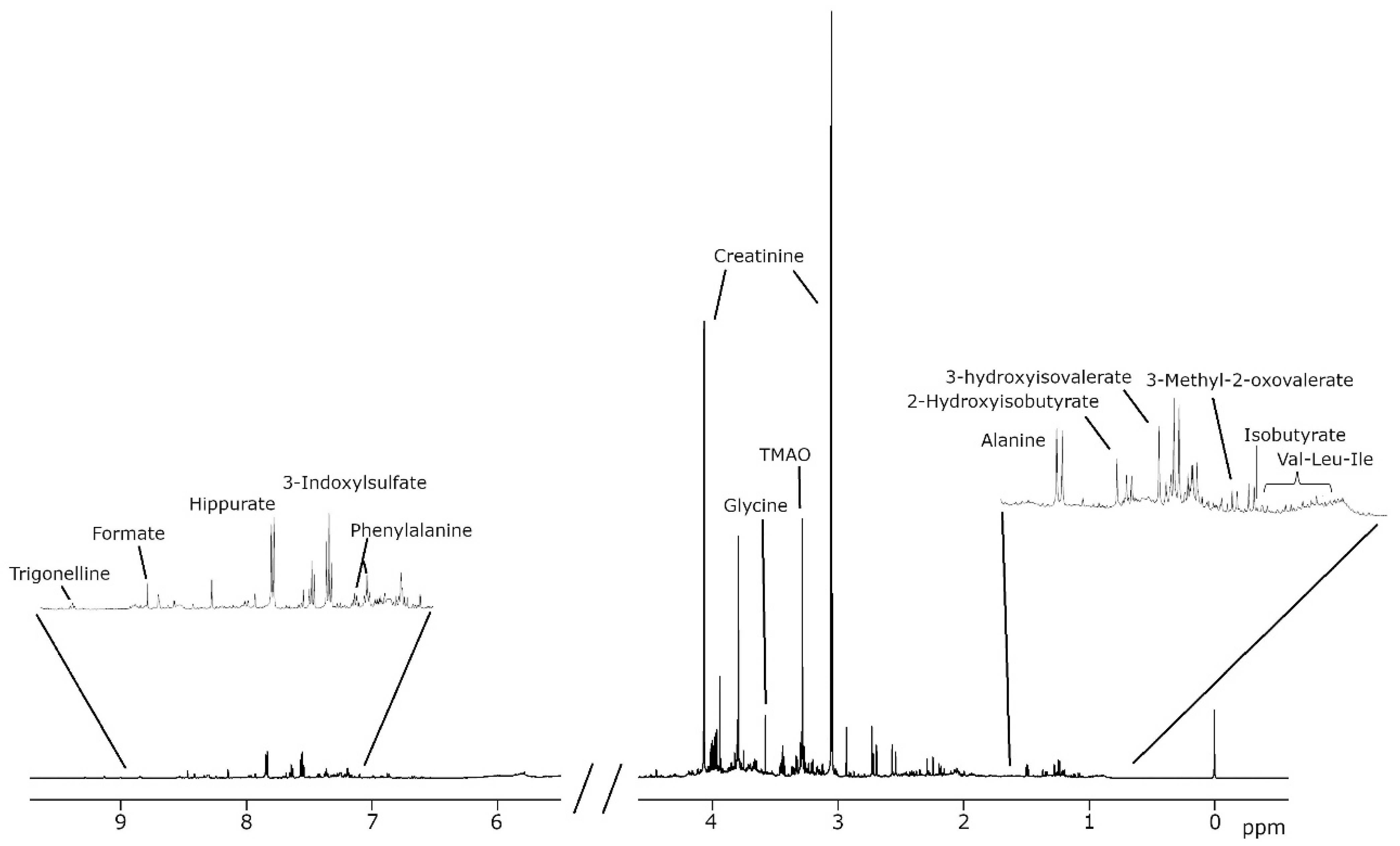
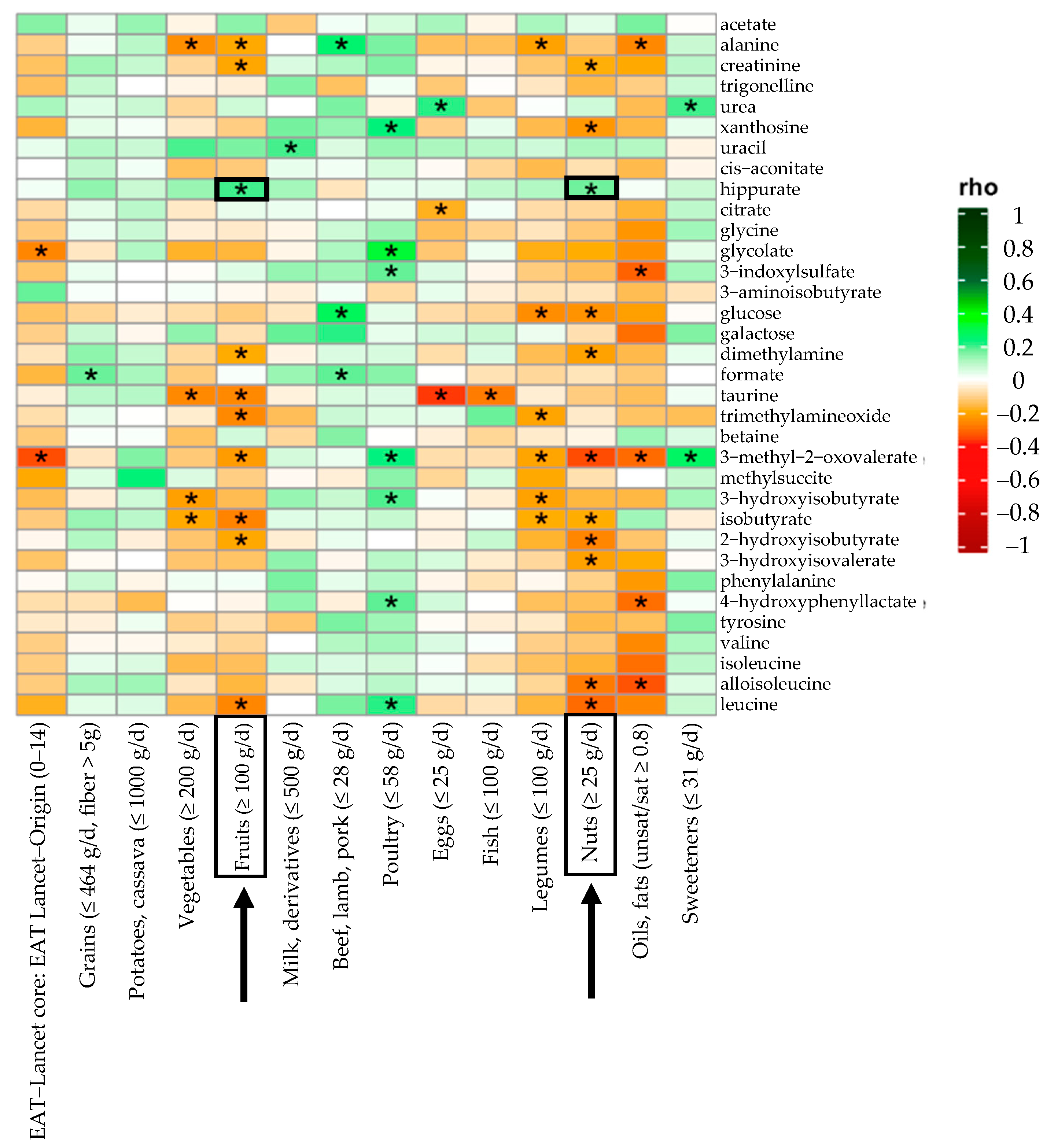
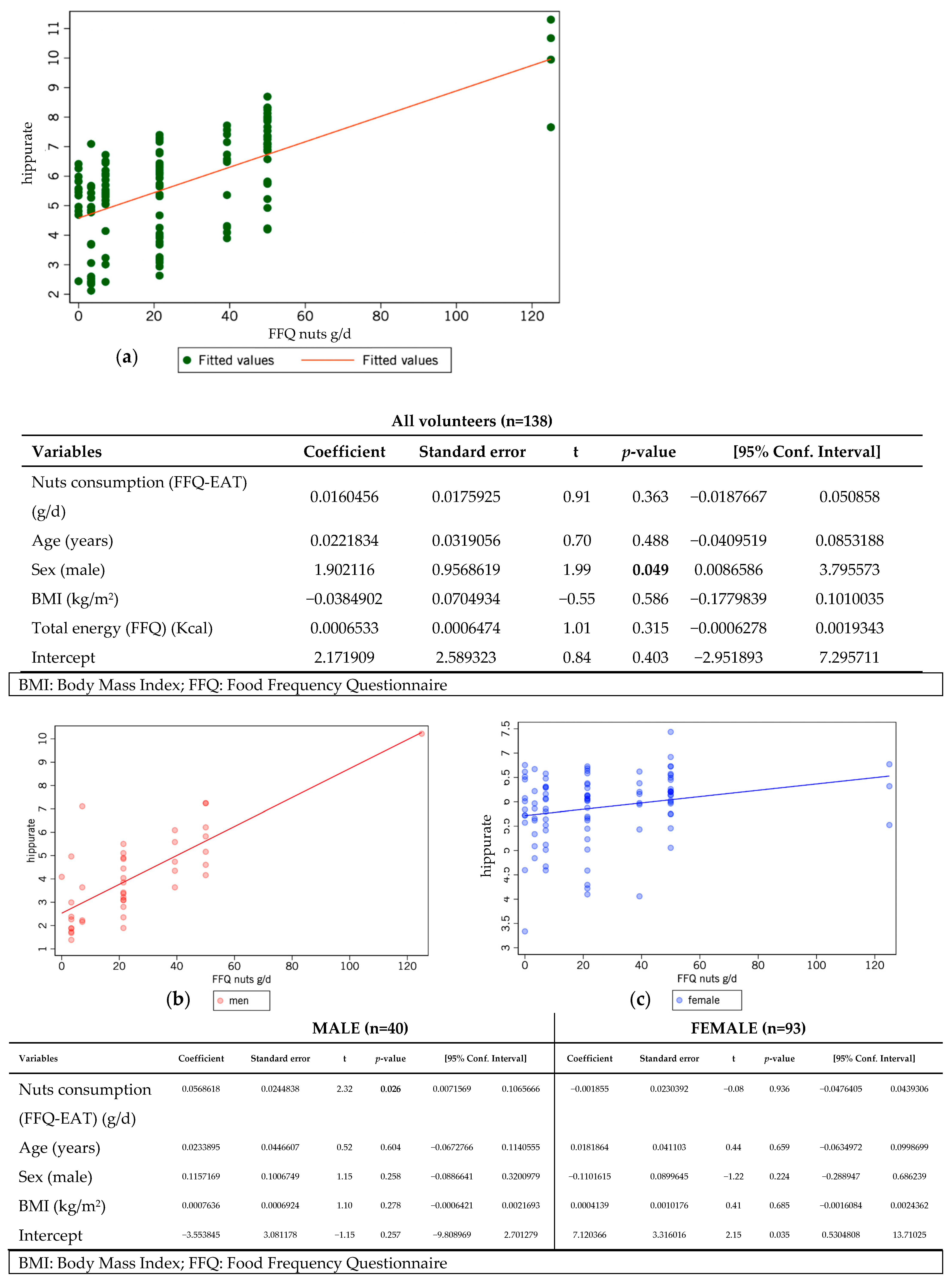

| Metabolism | Putative Associated Group | Compound Analyzed | Chemical Shift (ppm) | Signal |
|---|---|---|---|---|
| Amino acids or derived compounds | Aliphatic amino acids and derived compounds | Alanine | 1.5 | D |
| Alloisoleucine | 0.9 | D | ||
| Glycine | 3.6 | S | ||
| Isoleucine | 1.0 | D | ||
| Leucine | 1.0 | T | ||
| Valine | 1.0 | D | ||
| Aromatic amino acids and derived compounds | 4-Hydroxyphenyllactate | 6.9 | M | |
| Phenylalanine | 7.4 | M | ||
| Tyrosine | 6.9 | M | ||
| Sulfonic acid | Taurine | 3.3 | T | |
| Energy and carbohydrate metabolism | Krebs cycle | cis-Aconitate | 5.7 | M |
| Citrate | 2.7 | M | ||
| Methylsuccinate | 1.1 | D | ||
| Carbohydrate metabolism | Galactose | 5.3 | D | |
| Glucose | 5.2 | D | ||
| Glycolate | 4.0 | S | ||
| Gut microbiota metabolism | Carboxylic acids | Acetate | 1.9 | S |
| Formate | 8.5 | S | ||
| Metabolites derived from nitrogenous compounds | Dimethylamine | 2.7 | S | |
| Trimethylamine N-oxide (TMAO) | 3.3 | S | ||
| Phenolic compounds related to dietary polyphenols | Hippurate | 7.8 | M | |
| Sulfur-containing compounds derived from amino acids | 3-Indoxylsulfate | 7.7 | M | |
| Nitrogenous compounds | Nitrogen-related metabolites | Creatinine | 4.1 | S |
| Urea | 5.8 | S | ||
| Methylation-related metabolites | Betaine | 3.3 | S | |
| Trigonelline | 9.1 | S | ||
| Nucleotide metabolism | Precursors or intermediates of nucleotides | Uracil | 5.8 | D |
| Xanthosine | 5.9 | D | ||
| Degradation products of nucleotides | 3-Aminoisobutyrate | 1.2 | D | |
| Fatty acid metabolism | Intermediates in branched-chain fatty acid oxidation | 3-Methyl-2-oxovalerate | 1.1 | D |
| 3-Hydroxyisovalerate | 1.3 | S | ||
| Metabolites from beta-oxidation and alternative pathways | 3-Hydroxyisobutyrate | 1.1 | D | |
| Short-chain fatty acids (SCFAs) | Isobutyrate | 1.1 | D | |
| 2-Hydroxyisobutyrate | 1.4 | S |
| EAT Lancet Score (n = 138) | EAT-Lancet Score < P50 (n = 75) | EAT-Lancet Score ≥ P50 (n = 63) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 138) | Male (n = 29) | Female (n = 46) | Male (n = 13) | Female (n = 50) | |||||
| EAT Lancet food group (g/d) | P50 (IQR) | P50 (IQR) | P50 (IQR) | p | P50 (IQR) | P50 (IQR) | p | p-EAT | p-Sex |
| EAT-Lancet Score (0–14p) | 8 (7–9) | 8 (7–8) | 8 (7–8) | 0.732 | 9 (9–10) | 9 (9–10) | 0.826 | <0.001 | 0.316 |
| Grains | 120.6 (79.3–169.1) | 162 (103.2–191.4) | 100.8 (60–173.3) | 0.027 | 126.2 (103.3–171.3) | 107.6 (77–161.4) | 0.344 | 0.504 | 0.010 |
| Potatoes | 31.4 (20–74.3) | 42.8 (21.4–85.7) | 31.4 (20–64.3) | 0.151 | 53.6 (10–74.3) | 31.4 (20–64.3) | 0.737 | 0.385 | 0.148 |
| Vegetables | 473.1 (364.2–808.2) | 416.6 (307.6–594.5) | 504.6 (349.9–754.7) | 0.262 | 593.3 (399.9–776) | 469 (378.5–828.5) | 0.985 | 0.168 | 0.282 |
| Fruits | 345.4 (196.8–499.2) | 210.7 (124.6–286.8) | 358.5 (222.7–474.7) | 0.003 | 353.4 (267.8–542.3) | 372.8 (207.1–613.5) | 0.591 | 0.012 | 0.005 |
| Milk and derivatives | 347.1 (226–558.6) | 342.2 (240.1–607.1) | 503.3 (276.6–564.3) | 0.960 | 285.5 (202.8–365.1) | 299 (142.6–517.6) | 0.725 | 0.010 | 0.790 |
| Beef, lamb, pork | 115.2 (77.3–160) | 161.9 (113.5–189.3) | 104.2 (71.4–149.9) | 0.001 | 125 (84.7–178.8) | 98.8 (51.9–142.8) | 0.335 | 0.096 | 0.001 |
| Poultry | 64.3 (31.4–74.3) | 64.3 (64.3–74.3) | 64.3 (64.3–74.3) | 0.498 | 64.3 (31.4–74.3) | 31.4 (21.4–64.3) | 0.144 | <0.001 | 0.045 |
| Eggs | 25.7 (25.7–47.1) | 25.7 (25.7–47.1) | 25.7 (25.7–47.1) | 0.621 | 25.7 (25.7–47.1) | 25.7 (25.7–47.1) | 0.476 | 0.676 | 0.375 |
| Fish | 81.6 (56.2–117) | 78.7 (45.1–109.8) | 94.9 (62–137.8) | 0.037 | 89.5 (64.3–103) | 77.3 (56.8–98.9) | 0.239 | 0.139 | 0.349 |
| Legumes | 17.1 (12.6–29.7) | 16.8 (16–27.4) | 16.6 (12–25.7) | 0.600 | 25.4 (16–46.8) | 21.1 (12.6–29.7) | 0.200 | 0.110 | 0.364 |
| Nuts | 21.4 (7.1–39.3) | 21.4 (3.3–21.4) | 21.4 (7.1–21.4) | 0.828 | 44.6 (21.4–50) | 39.3 (7.1–50) | 0.484 | <0.001 | 0.934 |
| Oils, fats (unsat/sat ratio) | 18 (5.8–37.5) | 11.7 (4.7–18.5) | 22.6 (5.8–37.5) | 0.331 | 14.5 (14–35) | 24 (5–40.8) | 0.919 | 0.941 | 0.456 |
| Sweeteners | 1.4 (0–10) | 8.6 (0.3–25) | 1.3 (0–10.7) | 0.129 | 0 (0–7.9) | 1.4 (0–10) | 0.101 | 0.064 | 0.657 |
| EAT-Lancet < Median (n = 75) | EAT-Lancet ≥ Median (n = 63) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 138) | Male (n = 29) | Female (n = 46) | Male (n = 13) | Female (n = 50) | ||||||
| Metabolite (mmol/L) | Missing/Total | P50 (IR) | P50 (IQR) | P50 (IQR) | p | P50 (IQR) | P50 (IQR) | p | p-EAT | p-Sex |
| 3-indoxylsulfate | 14/138 | 0.347 (0.2–0.55) | 0.349 (0.178–0.609) | 0.38 (0.26–0.621) | 0.659 | 0.342 (0.133–0.46) | 0.292 (0.199–0.454) | 0.876 | 0.074 | 0.783 |
| Acetate | 28/138 | 0.041 (0.023–0.066) | 0.039 (0.015–0.058) | 0.035 (0.024–0.068) | 0.210 | 0.058 (0.032–0.127) | 0.042 (0.024–0.1) | 0.383 | 0.292 | 0.688 |
| Dimethylamine | 1/138 | 0.542 (0.371–0.776) | 0.617 (0.443–0.896) | 0.552 (0.374–0.778) | 0.461 | 0.522 (0.354–0.736) | 0.493 (0.361–0.732) | 0.926 | 0.249 | 0.475 |
| Formate | 8/138 | 0.249 (0.169–0.353) | 0.214 (0.174–0.401) | 0.289 (0.202–0.419) | 0.417 | 0.224 (0.182–0.297) | 0.196 (0.147–0.295) | 0.474 | 0.020 | 0.904 |
| Hippurate | 5/138 | 3.589 (2.252–6.8) | 3.367 (1.611–5.121) | 3.934 (2.784–7.294) | 0.045 | 2.871 (2.372–4.236) | 3.846 (2.105–7.668) | 0.218 | 0.909 | 0.020 |
| Trimethylamine-N-oxide | 35/138 | 0.681 (0.417–1.776) | 1.032 (0.438–1.436) | 0.621 (0.417–1.828) | 0.653 | 0.539 (0.279–0.873) | 0.612 (0.393–1.486) | 0.344 | 0.337 | 0.838 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Cruz, E.; de la O, V.; Fernández-Diaz, C.M.; Matía-Martín, P.; Rubio-Herrera, M.Á.; Amigó, N.; Calle-Pascual, A.L.; Martínez, J.A. Urinary Hippuric Acid as a Sex-Dependent Biomarker for Fruit and Nut Intake Raised from the EAT-Lancet Index and Nuclear Magnetic Resonance Analysis. Metabolites 2025, 15, 348. https://doi.org/10.3390/metabo15060348
Fernández-Cruz E, de la O V, Fernández-Diaz CM, Matía-Martín P, Rubio-Herrera MÁ, Amigó N, Calle-Pascual AL, Martínez JA. Urinary Hippuric Acid as a Sex-Dependent Biomarker for Fruit and Nut Intake Raised from the EAT-Lancet Index and Nuclear Magnetic Resonance Analysis. Metabolites. 2025; 15(6):348. https://doi.org/10.3390/metabo15060348
Chicago/Turabian StyleFernández-Cruz, Edwin, Víctor de la O, Cristina M. Fernández-Diaz, Pilar Matía-Martín, M. Ángel Rubio-Herrera, Nuria Amigó, Alfonso L. Calle-Pascual, and J. Alfredo Martínez. 2025. "Urinary Hippuric Acid as a Sex-Dependent Biomarker for Fruit and Nut Intake Raised from the EAT-Lancet Index and Nuclear Magnetic Resonance Analysis" Metabolites 15, no. 6: 348. https://doi.org/10.3390/metabo15060348
APA StyleFernández-Cruz, E., de la O, V., Fernández-Diaz, C. M., Matía-Martín, P., Rubio-Herrera, M. Á., Amigó, N., Calle-Pascual, A. L., & Martínez, J. A. (2025). Urinary Hippuric Acid as a Sex-Dependent Biomarker for Fruit and Nut Intake Raised from the EAT-Lancet Index and Nuclear Magnetic Resonance Analysis. Metabolites, 15(6), 348. https://doi.org/10.3390/metabo15060348








