Reducing Systemic Inflammation in IUGR-Born Neonatal Lambs via Daily Oral ω-3 PUFA Supplement Improved Skeletal Muscle Glucose Metabolism, Glucose-Stimulated Insulin Secretion, and Blood Pressure
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Surgical Preparation
2.3. In Vivo Metabolic Studies
2.3.1. Square-Wave Hyperglycemic Clamps
2.3.2. Hyperinsulinemic–Euglycemic Clamps
2.4. Blood Analyses
2.5. Ex Vivo Skeletal Muscle Metabolism
2.5.1. Glucose Uptake and Oxidation
2.5.2. Oxygen Consumption Rates
2.6. Skeletal Muscle Protein Expression
2.7. Muscle Glycogen Content
2.8. Statistical Analysis
3. Results
3.1. Weekly Blood Parameters
3.1.1. Plasma ω-3 PUFA and TNFα
3.1.2. Weekly Blood Gases and Metabolites
3.1.3. Weekly Blood Electrolytes
3.1.4. Leukocytes and Hematology
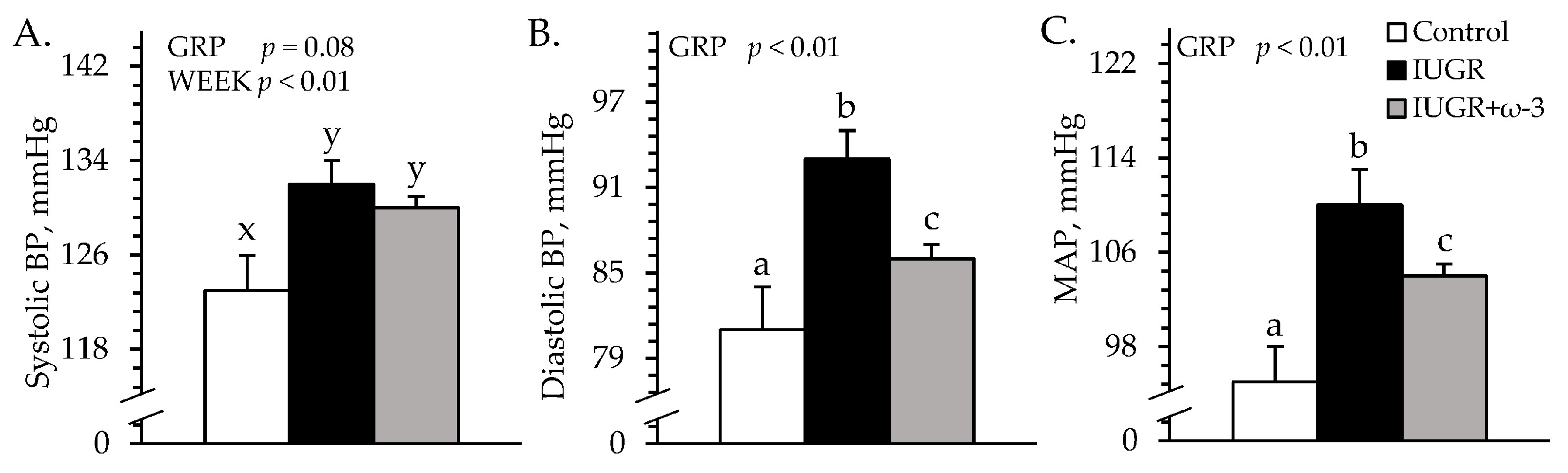
3.2. Cardiovascular Parameters
3.3. Square-Wave Hyperglycemic Clamps
3.3.1. Blood Gases and Metabolites
3.3.2. Blood Electrolytes
3.4. Hyperinsulinemic–Euglycemic Clamps
3.4.1. Hindlimb Glucose Metabolism
3.4.2. Blood Gases and Metabolites
3.4.3. Blood Electrolytes
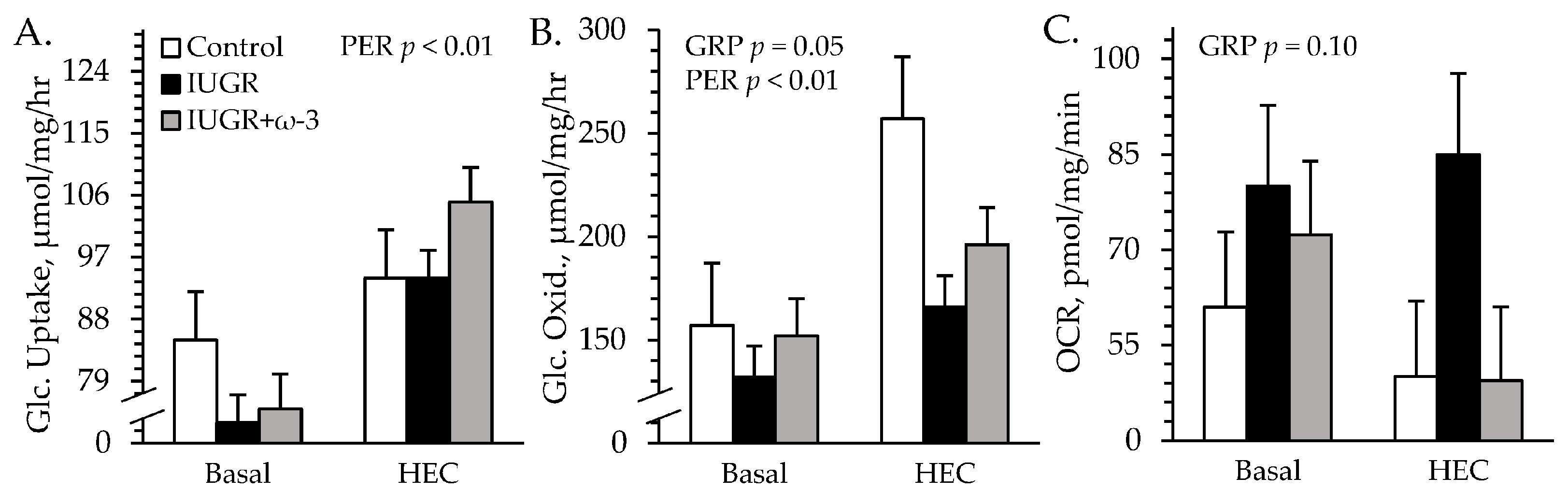
3.5. Ex Vivo Glucose Metabolism
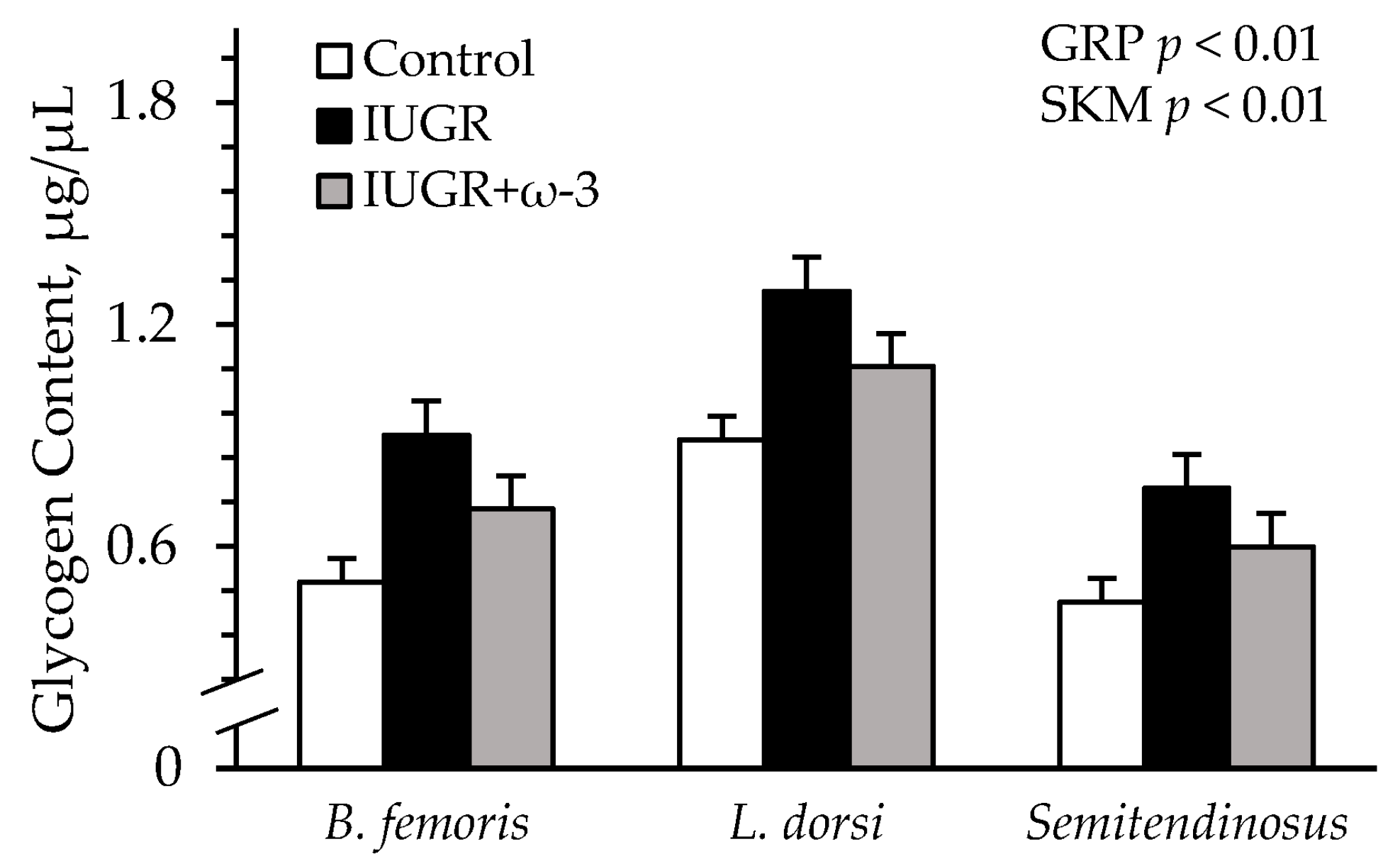
3.6. Muscle Protein and Glycogen Content
3.7. Effects of Sex and Birth Number
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barker, D.J.; Eriksson, J.G.; Forsen, T.; Osmond, C. Fetal origins of adult disease: Strength of effects and biological basis. Int. J. Epidemiol. 2002, 31, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Hales, C.N.; Fall, C.H.; Osmond, C.; Phipps, K.; Clark, P.M. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): Relation to reduced fetal growth. Diabetologia 1993, 36, 62–67. [Google Scholar] [CrossRef] [PubMed]
- White, M.R.; Yates, D.T. Dousing the flame: Reviewing the mechanisms of inflammatory programming during stress-induced intrauterine growth restriction and the potential for ω-3 polyunsaturated fatty acid intervention. Front. Physiol. 2023, 14, 1250134. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.L.; Yates, D.T. The Price of Surviving on Adrenaline: Developmental Programming Responses to Chronic Fetal Hypercatecholaminemia Contribute to Poor Muscle Growth Capacity and Metabolic Dysfunction in IUGR-Born Offspring. Front. Anim. Sci. 2021, 2, 769334. [Google Scholar] [CrossRef]
- Limesand, S.W.; Rozance, P.J.; Macko, A.R.; Anderson, M.J.; Kelly, A.C.; Hay, W.W., Jr. Reductions in insulin concentrations and beta-cell mass precede growth restriction in sheep fetuses with placental insufficiency. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E516–E523. [Google Scholar] [CrossRef]
- Macko, A.R.; Yates, D.T.; Chen, X.; Green, A.S.; Kelly, A.C.; Brown, L.D.; Limesand, S.W. Elevated plasma norepinephrine inhibits insulin secretion, but adrenergic blockade reveals enhanced beta-cell responsiveness in an ovine model of placental insufficiency at 0.7 of gestation. J. Dev. Orig. Health Dis. 2013, 4, 402–410. [Google Scholar] [CrossRef]
- Pendleton, A.L.; Antolic, A.T.; Kelly, A.C.; Davis, M.A.; Camacho, L.E.; Doubleday, K.; Anderson, M.J.; Langlais, P.R.; Lynch, R.M.; Limesand, S.W. Lower oxygen consumption and Complex I activity in mitochondria isolated from skeletal muscle of fetal sheep with intrauterine growth restriction. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E67–E80. [Google Scholar] [CrossRef]
- Zhao, W.; Kelly, A.C.; Luna-Ramirez, R.I.; Bidwell, C.A.; Anderson, M.J.; Limesand, S.W. Decreased Pyruvate but not Fatty Acid Driven Mitochondrial Respiration in Skeletal Muscle of Growth Restricted Fetal Sheep. Int. J. Mol. Sci. 2023, 24, 15760. [Google Scholar] [CrossRef]
- Jones, C.T.; Ritchie, J.W. The metabolic and endocrine effects of circulating catecholamines in fetal sheep. J. Physiol. 1978, 285, 395–408. [Google Scholar] [CrossRef]
- Jones, C.T.; Robinson, J.S. Studies on experimental growth retardation in sheep. Plasma catecholamines in fetuses with small placenta. J. Dev. Physiol. 1983, 5, 77–87. [Google Scholar]
- Jones, C.T.; Ritchie, J.W.; Walker, D. The effects of hypoxia on glucose turnover in the fetal sheep. J. Dev. Physiol. 1983, 5, 223–235. [Google Scholar] [PubMed]
- Chang, E.I.; Zárate, M.A.; Rabaglino, M.B.; Richards, E.M.; Keller-Wood, M.; Wood, C.E. Ketamine suppresses hypoxia-induced inflammatory responses in the late-gestation ovine fetal kidney cortex. J. Physiol. 2016, 594, 1295–1310. [Google Scholar] [CrossRef] [PubMed]
- Cadaret, C.N.; Merrick, E.M.; Barnes, T.L.; Beede, K.A.; Posont, R.J.; Petersen, J.L.; Yates, D.T. Sustained maternal inflammation during the early third-trimester yields intrauterine growth restriction, impaired skeletal muscle glucose metabolism, and diminished beta-cell function in fetal sheep. J. Anim. Sci. 2019, 97, 4822–4833. [Google Scholar] [CrossRef] [PubMed]
- Zarate, M.A.; Chang, E.I.; Wood, C.E. Effects of ketamine on the fetal transcriptomic response to umbilical cord occlusion: Comparison with hypoxic hypoxia in the cerebral cortex. J. Physiol. 2018, 596, 6063–6077. [Google Scholar] [CrossRef]
- Beer, H.N.; Lacey, T.A.; Gibbs, R.L.; Most, M.S.; Hicks, Z.M.; Grijalva, P.C.; Marks-Nelson, E.S.; Schmidt, T.B.; Petersen, J.L.; Yates, D.T. Daily Eicosapentaenoic Acid Infusion in IUGR Fetal Lambs Reduced Systemic Inflammation, Increased Muscle ADRβ2 Content, and Improved Myoblast Function and Muscle Growth. Metabolites 2024, 14, 340. [Google Scholar] [CrossRef]
- Posont, R.J.; Most, M.S.; Cadaret, C.N.; Marks-Nelson, E.S.; Beede, K.A.; Limesand, S.W.; Schmidt, T.B.; Petersen, J.L.; Yates, D.T. Primary myoblasts from intrauterine growth-restricted fetal sheep exhibit intrinsic dysfunction of proliferation and differentiation that coincides with enrichment of inflammatory cytokine signaling pathways. J. Anim. Sci. 2022, 100, skac145. [Google Scholar] [CrossRef]
- Gibbs, R.L.; Swanson, R.M.; Beard, J.K.; Hicks, Z.M.; Most, M.S.; Beer, H.N.; Grijalva, P.C.; Clement, S.M.; Marks-Nelson, E.S.; Schmidt, T.B.; et al. Daily injection of the β2 adrenergic agonist clenbuterol improved poor muscle growth and body composition in lambs following heat stress-induced intrauterine growth restriction. Front. Physiol. 2023, 14, 1252508. [Google Scholar] [CrossRef]
- Gibbs, R.L.; Wilson, J.A.; Swanson, R.M.; Beard, J.K.; Hicks, Z.M.; Beer, H.N.; Marks-Nelson, E.S.; Schmidt, T.B.; Petersen, J.L.; Yates, D.T. Daily Injection of the β2 Adrenergic Agonist Clenbuterol Improved Muscle Glucose Metabolism, Glucose-Stimulated Insulin Secretion, and Hyperlipidemia in Juvenile Lambs Following Heat-Stress-Induced Intrauterine Growth Restriction. Metabolites 2024, 14, 156. [Google Scholar] [CrossRef]
- Posont, R.J.; Cadaret, C.N.; Beard, J.K.; Swanson, R.M.; Gibbs, R.L.; Marks-Nelson, E.S.; Petersen, J.L.; Yates, D.T. Maternofetal inflammation induced for two weeks in late gestation reduced birthweight and impaired neonatal growth and skeletal muscle glucose metabolism in lambs. J. Anim. Sci. 2021, 99, skab102. [Google Scholar] [CrossRef]
- Bai, G.; Chen, J.; Liu, Y.; Chen, J.; Yan, H.; You, J.; Zou, T. Neonatal resveratrol administration promotes skeletal muscle growth and insulin sensitivity in intrauterine growth-retarded suckling piglets associated with activation of FGF21-AMPKα pathway. J. Sci. Food Agric. 2024, 104, 3719–3728. [Google Scholar] [CrossRef]
- Laskowska, M.; Laskowska, K.; Leszczyńska-Gorzelak, B.; Oleszczuk, J. Maternal and umbilical sTNF-R1 in preeclamptic pregnancies with intrauterine normal and growth retarded fetus. Hypertens. Pregnancy 2007, 26, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Rotter, V.; Nagaev, I.; Smith, U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J. Biol. Chem. 2003, 278, 45777–45784. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalili, L.; Bouzakri, K.; Glund, S.; Lonnqvist, F.; Koistinen, H.A.; Krook, A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol. Endocrinol. 2006, 20, 3364–3375. [Google Scholar] [CrossRef] [PubMed]
- Cadaret, C.N.; Beede, K.A.; Riley, H.E.; Yates, D.T. Acute exposure of primary rat soleus muscle to zilpaterol HCl (β2 adrenergic agonist), TNFa, or IL-6 in culture increases glucose oxidation rates independent of the impact on insulin signaling or glucose uptake. Cytokine 2017, 96, 107–113. [Google Scholar] [CrossRef]
- Hicks, Z.M. Mid-Gestation Maternofetal Inflammation Impacts Growth, Skeletal Muscle Glucose Metabolism, and Inflammatory Tone in the Ovine Fetus During Late Gestation. Ph.D. Thesis, The University of Nebraska-Lincoln, Lincoln, NE, USA, 2023. [Google Scholar]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef]
- Weldon, S.M.; Mullen, A.C.; Loscher, C.E.; Hurley, L.A.; Roche, H.M. Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. J. Nutr. Biochem. 2007, 18, 250–258. [Google Scholar] [CrossRef]
- Block, R.C.; Dier, U.; Calderonartero, P.; Shearer, G.C.; Kakinami, L.; Larson, M.K.; Harris, W.S.; Georas, S.; Mousa, S.A. The Effects of EPA+DHA and Aspirin on Inflammatory Cytokines and Angiogenesis Factors. World J. Cardiovasc. Dis. 2012, 2, 14–19. [Google Scholar] [CrossRef]
- Grytten, E.; Laupsa-Borge, J.; Cetin, K.; Bohov, P.; Nordrehaug, J.E.; Skorve, J.; Berge, R.K.; Strand, E.; Bjørndal, B.; Nygård, O.; et al. Inflammatory markers after supplementation with marine n-3 or plant n-6 PUFAs: A randomized double-blind crossover study. J. Lipid Res. 2025, 66, 100770. [Google Scholar] [CrossRef]
- Lacey, T.L.; Gibbs, R.L.; Most, M.S.; Beer, H.N.; Hicks, Z.M.; Grijalva, P.C.; Petersen, J.L.; Yates, D.T. Decreased fetal biometrics and impaired β-cell function in IUGR fetal sheep are improved by daily ω-3 PUFA infusion. Transl. Anim. Sci. 2021, 5, S41–S45. [Google Scholar] [CrossRef]
- Cadaret, C.N.; Posont, R.J.; Swanson, R.M.; Beard, J.K.; Gibbs, R.L.; Barnes, T.L.; Marks-Nelson, E.S.; Petersen, J.L.; Yates, D.T. Intermittent maternofetal oxygenation during late gestation improved birthweight, neonatal growth, body symmetry, and muscle metabolism in intrauterine growth-restricted lambs. J. Anim. Sci. 2022, 100, skab358. [Google Scholar] [CrossRef]
- Swanson, R.M.; Tait, R.G.; Galles, B.M.; Duffy, E.M.; Schmidt, T.B.; Petersen, J.L.; Yates, D.T. Heat stress-induced deficits in growth, metabolic efficiency, and cardiovascular function coincided with chronic systemic inflammation and hypercatecholaminemia in ractopamine-supplemented feedlot lambs. J. Anim. Sci. 2020, 98, skaa168. [Google Scholar] [CrossRef] [PubMed]
- Yates, D.T.; Camacho, L.E.; Kelly, A.C.; Steyn, L.V.; Davis, M.A.; Antolic, A.T.; Anderson, M.J.; Goyal, R.; Allen, R.E.; Papas, K.K.; et al. Postnatal beta2 adrenergic treatment improves insulin sensitivity in lambs with IUGR but not persistent defects in pancreatic islets or skeletal muscle. J. Physiol. 2019, 597, 5835–5858. [Google Scholar] [CrossRef] [PubMed]
- Camacho, L.E.; Chen, X.; Hay, W.W., Jr.; Limesand, S.W. Enhanced insulin secretion and insulin sensitivity in young lambs with placental insufficiency-induced intrauterine growth restriction. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2017, 313, R101–R109. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.L.; Burrack, R.M.; Schmidt, T.B.; Petersen, J.L.; Yates, D.T. Sustained heat stress elevated corneal and body surface temperatures and altered circulating leukocytes and metabolic indicators in wether lambs supplemented with ractopamine or zilpaterol. J. Anim. Sci. 2021, 99, skab236. [Google Scholar] [CrossRef]
- Barnes, T.L.; Cadaret, C.N.; Beede, K.A.; Schmidt, T.B.; Petersen, J.L.; Yates, D.T. Hypertrophic muscle growth and metabolic efficiency were impaired by chronic heat stress, improved by zilpaterol supplementation, and not affected by ractopamine supplementation in feedlot lambs. J. Anim. Sci. 2019, 97, 4101–4113. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Ginesu, G.C.; Tanda, C.; Feo, C.F.; Fancellu, A.; Fois, A.G.; Mangoni, A.A.; Sotgia, S.; Carru, C.; Porcu, A.; et al. Inflammatory cell indexes as preoperative predictors of hospital stay in open elective thoracic surgery. ANZ J. Surg. 2018, 88, 616–620. [Google Scholar] [CrossRef]
- Hu, B.; Yang, X.R.; Xu, Y.; Sun, Y.F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.M.; Qiu, S.J.; Zhou, J.; et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef]
- Qi, Q.; Zhuang, L.; Shen, Y.; Geng, Y.; Yu, S.; Chen, H.; Liu, L.; Meng, Z.; Wang, P.; Chen, Z. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer 2016, 122, 2158–2167. [Google Scholar] [CrossRef]
- Leslie, E.; Lopez, V.; Anti, N.A.O.; Alvarez, R.; Kafeero, I.; Welsh, D.G.; Romero, M.; Kaushal, S.; Johnson, C.M.; Bosviel, R.; et al. Gestational long-term hypoxia induces metabolomic reprogramming and phenotypic transformations in fetal sheep pulmonary arteries. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L770–L784. [Google Scholar] [CrossRef]
- Dong, Y.; Hou, W.; Wei, J.; Weiner, C.P. Chronic hypoxemia absent bacterial infection is one cause of the fetal inflammatory response syndrome (FIRS). Reprod. Sci. 2009, 16, 650–656. [Google Scholar] [CrossRef]
- von Schacky, C. Use of red blood cell fatty-acid profiles as biomarkers in cardiac disease. Biomark. Med. 2009, 3, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Ma, J.; Campos, H.; Hankinson, S.E.; Hu, F.B. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am. J. Clin. Nutr. 2007, 86, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Gerling, C.J.; Mukai, K.; Chabowski, A.; Heigenhauser, G.J.F.; Holloway, G.P.; Spriet, L.L.; Jannas-Vela, S. Incorporation of Omega-3 Fatty Acids into Human Skeletal Muscle Sarcolemmal and Mitochondrial Membranes Following 12 Weeks of Fish Oil Supplementation. Front. Physiol. 2019, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Hicks, Z.H.; Beer, H.N.; Herrera, N.J.; Gibbs, R.L.; Lacey, T.A.; Grijalva, P.C.; Most, M.S.; Yates, D.T. Hindlimb tissue composition shifts between the fetal and juvenile stages in the lamb. Transl. Anim. Sci. 2021, 5, S38–S40. [Google Scholar] [CrossRef]
- Stremming, J.; Chang, E.I.; Knaub, L.A.; Armstrong, M.L.; Baker, P.R., 2nd; Wesolowski, S.R.; Reisdorph, N.; Reusch, J.E.B.; Brown, L.D. Lower citrate synthase activity, mitochondrial complex expression, and fewer oxidative myofibers characterize skeletal muscle from growth-restricted fetal sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 322, R228–R240. [Google Scholar] [CrossRef]
- Selak, M.A.; Storey, B.T.; Peterside, I.; Simmons, R.A. Impaired oxidative phosphorylation in skeletal muscle of intrauterine growth-retarded rats. Am. J. Physiol.-Endocrinol. Metab. 2003, 285, E130–E137. [Google Scholar] [CrossRef]
- Chen, X.; Fahy, A.L.; Green, A.S.; Anderson, M.J.; Rhoads, R.P.; Limesand, S.W. β2-Adrenergic receptor desensitization in perirenal adipose tissue in fetuses and lambs with placental insufficiency-induced intrauterine growth restriction. J. Physiol. 2010, 588, 3539–3549. [Google Scholar] [CrossRef]
- Hicks, Z.M.; Gibbs, R.L.; Beer, H.N.; Grijalva, P.C.; Most, M.S.; Yates, D.T. PSVIII-B-18 Sustained Maternofetal Inflammation at mid-Gestation Causes Intrauterine Growth Restriction of the Sheep Fetus That is Characterized by Poor Muscle Mass and Asymmetric Body Composition Near Term. J. Anim. Sci. 2022, 100, 312. [Google Scholar] [CrossRef]
- Cadaret, C.N.; Posont, R.J.; Beede, K.A.; Riley, H.E.; Loy, J.D.; Yates, D.T. Maternal inflammation at midgestation impairs subsequent fetal myoblast function and skeletal muscle growth in rats, resulting in intrauterine growth restriction at term. Transl. Anim. Sci. 2019, 3, 867–876. [Google Scholar] [CrossRef]
- Glund, S.; Deshmukh, A.; Long, Y.C.; Moller, T.; Koistinen, H.A.; Caidahl, K.; Zierath, J.R.; Krook, A. Interleukin-6 directly increases glucose metabolism in resting human skeletal muscle. Diabetes 2007, 56, 1630–1637. [Google Scholar] [CrossRef]
- Tredget, E.E.; Yu, Y.M.; Zhong, S.; Burini, R.; Okusawa, S.; Gelfand, J.A.; Dinarello, C.A.; Young, V.R.; Burke, J.F. Role of interleukin 1 and tumor necrosis factor on energy metabolism in rabbits. Am. J. Physiol. 1988, 255, E760–E768. [Google Scholar] [CrossRef] [PubMed]
- López-Soriano, J.; Argilés, J.M.; López-Soriano, F.J. Effects of tumour necrosis factor-alpha on the enzymatic activities related to glucose metabolism. Biochem. Mol. Biol. Int. 1993, 30, 21–27. [Google Scholar] [PubMed]
- Anil, T.M.; Dandu, A.; Harsha, K.; Singh, J.; Shree, N.; Kumar, V.S.; Lakshmi, M.N.; Sunil, V.; Harish, C.; Balamurali, G.V.; et al. A novel 11β-hydroxysteroid dehydrogenase type1 inhibitor CNX-010-49 improves hyperglycemia, lipid profile and reduces body weight in diet induced obese C57B6/J mice with a potential to provide cardio protective benefits. BMC Pharmacol. Toxicol. 2014, 15, 43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adamska, A.; Nikołajuk, A.; Karczewska-Kupczewska, M.; Kowalska, I.; Otziomek, E.; Górska, M.; Strączkowski, M. Relationships between serum adiponectin and soluble TNF-α receptors and glucose and lipid oxidation in lean and obese subjects. Acta Diabetol. 2012, 49, 17–24. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, J.; Ling, Y.; McCall, C.E.; Liu, T.F. Mitochondrial Sirtuin 4 Resolves Immune Tolerance in Monocytes by Rebalancing Glycolysis and Glucose Oxidation Homeostasis. Front. Immunol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Taylor, D.J. Interleukin-1 stimulation of fibroblast glycolysis is accompanied by reduced glucose oxidation in the tricarboxylic acid cycle. Biochem. Soc. Trans. 1990, 18, 982–983. [Google Scholar] [CrossRef]
- Taylor, D.J.; Faragher, E.B.; Evanson, J.M. Inflammatory cytokines stimulate glucose uptake and glycolysis but reduce glucose oxidation in human dermal fibroblasts in vitro. Circ. Shock 1992, 37, 105–110. [Google Scholar]
- Jani, S.; Da Eira, D.; Hadday, I.; Bikopoulos, G.; Mohasses, A.; de Pinho, R.A.; Ceddia, R.B. Distinct mechanisms involving diacylglycerol, ceramides, and inflammation underlie insulin resistance in oxidative and glycolytic muscles from high fat-fed rats. Sci. Rep. 2021, 11, 19160. [Google Scholar] [CrossRef]
- Ramsay, T.G.; Blomberg, L.; Caperna, T.J. Methyl-β-cyclodextrin alters adipokine gene expression and glucose metabolism in swine adipose tissue. Animal 2013, 7, 1690–1696. [Google Scholar] [CrossRef]
- Alonso-Chamorro, M.; Nieto-Vazquez, I.; Montori-Grau, M.; Gomez-Foix, A.M.; Fernandez-Veledo, S.; Lorenzo, M. New emerging role of protein-tyrosine phosphatase 1B in the regulation of glycogen metabolism in basal and TNF-α-induced insulin-resistant conditions in an immortalised muscle cell line isolated from mice. Diabetologia 2011, 54, 1157–1168. [Google Scholar] [CrossRef]
- Araújo, A.M.; Arruda, S.F. Ameliorating the impairment of glucose utilization in a high-fat diet-induced obesity model through the consumption of Tucum-do-Cerrado (Bactris Setosa Mart.). PLoS ONE 2024, 19, e0293627. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.Q.; Duque-Guimaraes, D.E.; Machado, U.F.; Zierath, J.R.; Krook, A. Altered response of skeletal muscle to IL-6 in type 2 diabetic patients. Diabetes 2013, 62, 355–361. [Google Scholar] [CrossRef]
- Gudiksen, A.; Schwartz, C.L.; Bertholdt, L.; Joensen, E.; Knudsen, J.G.; Pilegaard, H. Lack of Skeletal Muscle IL-6 Affects Pyruvate Dehydrogenase Activity at Rest and during Prolonged Exercise. PLoS ONE 2016, 11, e0156460. [Google Scholar] [CrossRef]
- Klymenko, O.; Brecklinghaus, T.; Dille, M.; Springer, C.; de Wendt, C.; Altenhofen, D.; Binsch, C.; Knebel, B.; Scheller, J.; Hardt, C.; et al. Histone deacetylase 5 regulates interleukin 6 secretion and insulin action in skeletal muscle. Mol. Metab. 2020, 42, 101062. [Google Scholar] [CrossRef]
- Michaeli, B.; Martinez, A.; Revelly, J.P.; Cayeux, M.C.; Chioléro, R.L.; Tappy, L.; Berger, M.M. Effects of endotoxin on lactate metabolism in humans. Crit. Care 2012, 16, R139. [Google Scholar] [CrossRef]
- Wai, S.G.; Rozance, P.J.; Wesolowski, S.R.; Hay, W.W., Jr.; Brown, L.D. Prolonged amino acid infusion into intrauterine growth restricted fetal sheep increases leucine oxidation rates. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E1143–E1153. [Google Scholar] [CrossRef]
- Ross, J.C.; Fennessey, P.V.; Wilkening, R.B.; Battaglia, F.C.; Meschia, G. Placental transport and fetal utilization of leucine in a model of fetal growth retardation. Am. J. Physiol. 1996, 270, E491–E503. [Google Scholar] [CrossRef]
- Rozance, P.J.; Zastoupil, L.; Wesolowski, S.R.; Goldstrohm, D.A.; Strahan, B.; Cree-Green, M.; Sheffield-Moore, M.; Meschia, G.; Hay, W.W., Jr.; Wilkening, R.B.; et al. Skeletal muscle protein accretion rates and hindlimb growth are reduced in late gestation intrauterine growth-restricted fetal sheep. J. Physiol. 2018, 596, 67–82. [Google Scholar] [CrossRef]
- Carver, T.D.; Quick, A.A.; Teng, C.C.; Pike, A.W.; Fennessey, P.V.; Hay, W.W., Jr. Leucine metabolism in chronically hypoglycemic hypoinsulinemic growth-restricted fetal sheep. Am. J. Physiol. 1997, 272, E107–E117. [Google Scholar] [CrossRef]
- Lane, R.H.; Kelley, D.E.; Ritov, V.H.; Tsirka, A.E.; Gruetzmacher, E.M. Altered expression and function of mitochondrial beta-oxidation enzymes in juvenile intrauterine-growth-retarded rat skeletal muscle. Pediatr. Res. 2001, 50, 83–90. [Google Scholar] [CrossRef][Green Version]
- Patel, D.; Kalhan, S. Glycerol metabolism and triglyceride-fatty acid cycling in the human newborn: Effect of maternal diabetes and intrauterine growth retardation. Pediatr. Res. 1992, 31, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Brons, C.; Lilleore, S.K.; Astrup, A.; Vaag, A. Disproportionately increased 24-h energy expenditure and fat oxidation in young men with low birth weight during a high-fat overfeeding challenge. Eur. J. Nutr. 2016, 55, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Brons, C.; Jacobsen, S.; Hiscock, N.; White, A.; Nilsson, E.; Dunger, D.; Astrup, A.; Quistorff, B.; Vaag, A. Effects of high-fat overfeeding on mitochondrial function, glucose and fat metabolism, and adipokine levels in low-birth-weight subjects. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E43–E51. [Google Scholar] [CrossRef] [PubMed]
- Bruce, C.R.; Dyck, D.J. Cytokine regulation of skeletal muscle fatty acid metabolism: Effect of interleukin-6 and tumor necrosis factor-alpha. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E616–E621. [Google Scholar] [CrossRef]
- Pedersen, L.; Olsen, C.H.; Pedersen, B.K.; Hojman, P. Muscle-derived expression of the chemokine CXCL1 attenuates diet-induced obesity and improves fatty acid oxidation in the muscle. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E831–E840. [Google Scholar] [CrossRef]
- Wolsk, E.; Mygind, H.; Grøndahl, T.S.; Pedersen, B.K.; van Hall, G. IL-6 selectively stimulates fat metabolism in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E832–E840. [Google Scholar] [CrossRef]
- Knudsen, N.H.; Stanya, K.J.; Hyde, A.L.; Chalom, M.M.; Alexander, R.K.; Liou, Y.H.; Starost, K.A.; Gangl, M.R.; Jacobi, D.; Liu, S.; et al. Interleukin-13 drives metabolic conditioning of muscle to endurance exercise. Science 2020, 368, eaat3987. [Google Scholar] [CrossRef]
- Frisard, M.I.; McMillan, R.P.; Marchand, J.; Wahlberg, K.A.; Wu, Y.; Voelker, K.A.; Heilbronn, L.; Haynie, K.; Muoio, B.; Li, L.; et al. Toll-like receptor 4 modulates skeletal muscle substrate metabolism. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E988–E998. [Google Scholar] [CrossRef]
- Frisard, M.I.; Wu, Y.; McMillan, R.P.; Voelker, K.A.; Wahlberg, K.A.; Anderson, A.S.; Boutagy, N.; Resendes, K.; Ravussin, E.; Hulver, M.W. Low levels of lipopolysaccharide modulate mitochondrial oxygen consumption in skeletal muscle. Metabolism 2015, 64, 416–427. [Google Scholar] [CrossRef]
- Kolmus, K.; Tavernier, J.; Gerlo, S. β2-Adrenergic receptors in immunity and inflammation: Stressing NF-κB. Brain Behav. Immun. 2015, 45, 297–310. [Google Scholar] [CrossRef]
- Lorton, D.; Bellinger, D.L. Molecular Mechanisms Underlying β-Adrenergic Receptor-Mediated Cross-Talk between Sympathetic Neurons and Immune Cells. Int. J. Mol. Sci. 2015, 16, 5635–5665. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Gulick, T.S.; Rotondo, R.E.; Schreiner, G.F.; Lange, L.G. Mechanism of cytokine inhibition of beta-adrenergic agonist stimulation of cyclic AMP in rat cardiac myocytes. Impairment of signal transduction. Circ. Res. 1990, 67, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Gulick, T.; Chung, M.K.; Pieper, S.J.; Lange, L.G.; Schreiner, G.F. Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta-adrenergic responsiveness. Proc. Natl. Acad. Sci. USA 1989, 86, 6753–6757. [Google Scholar] [CrossRef] [PubMed]
- Bick, R.J.; Liao, J.P.; King, T.W.; LeMaistre, A.; McMillin, J.B.; Buja, L.M. Temporal effects of cytokines on neonatal cardiac myocyte Ca2+ transients and adenylate cyclase activity. Am. J. Physiol. 1997, 272, H1937–H1944. [Google Scholar] [CrossRef]
- Leos, R.A.; Anderson, M.J.; Chen, X.; Pugmire, J.; Anderson, K.A.; Limesand, S.W. Chronic exposure to elevated norepinephrine suppresses insulin secretion in fetal sheep with placental insufficiency and intrauterine growth restriction. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E770–E778. [Google Scholar] [CrossRef]
- Limesand, S.W.; Rozance, P.J.; Zerbe, G.O.; Hutton, J.C.; Hay, W.W., Jr. Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology 2006, 147, 1488–1497. [Google Scholar] [CrossRef]
- Camacho, L.E.; Davis, M.A.; Kelly, A.C.; Steffens, N.R.; Anderson, M.J.; Limesand, S.W. Prenatal Oxygen and Glucose Therapy Normalizes Insulin Secretion and Action in Growth-Restricted Fetal Sheep. Endocrinology 2022, 163, bqac053. [Google Scholar] [CrossRef]
- Macko, A.R.; Yates, D.T.; Chen, X.; Shelton, L.A.; Kelly, A.C.; Davis, M.A.; Camacho, L.E.; Anderson, M.J.; Limesand, S.W. Adrenal Demedullation and Oxygen Supplementation Independently Increase Glucose-Stimulated Insulin Concentrations in Fetal Sheep With Intrauterine Growth Restriction. Endocrinology 2016, 157, 2104–2115. [Google Scholar] [CrossRef]
- Yates, D.T.; Macko, A.R.; Chen, X.; Green, A.S.; Kelly, A.C.; Anderson, M.J.; Fowden, A.L.; Limesand, S.W. Hypoxaemia-induced catecholamine secretion from adrenal chromaffin cells inhibits glucose-stimulated hyperinsulinaemia in fetal sheep. J. Physiol. 2012, 590, 5439–5447. [Google Scholar] [CrossRef]
- Benjamin, J.S.; Culpepper, C.B.; Brown, L.D.; Wesolowski, S.R.; Jonker, S.S.; Davis, M.A.; Limesand, S.W.; Wilkening, R.B.; Hay, W.W., Jr.; Rozance, P.J. Chronic anemic hypoxemia attenuates glucose-stimulated insulin secretion in fetal sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R492–R500. [Google Scholar] [CrossRef]
- Chen, X.; Green, A.S.; Macko, A.R.; Yates, D.T.; Kelly, A.C.; Limesand, S.W. Enhanced insulin secretion responsiveness and islet adrenergic desensitization after chronic norepinephrine suppression is discontinued in fetal sheep. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E58–E64. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kelly, A.C.; Yates, D.T.; Macko, A.R.; Lynch, R.M.; Limesand, S.W. Islet adaptations in fetal sheep persist following chronic exposure to high norepinephrine. J. Endocrinol. 2017, 232, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Huang, H.; Limesand, S.W.; Chen, X. Pancreatic Islets Exhibit Dysregulated Adaptation of Insulin Secretion after Chronic Epinephrine Exposure. Curr. Issues Mol. Biol. 2021, 43, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Hadjivassiliou, V.; Green, M.H.; James, R.F.; Swift, S.M.; Clayton, H.A.; Green, I.C. Insulin secretion, DNA damage, and apoptosis in human and rat islets of Langerhans following exposure to nitric oxide, peroxynitrite, and cytokines. Nitric Oxide 1998, 2, 429–441. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, W.; Zhang, D.; Lin, C.; He, H.; Xie, F.; Gan, L.; Fu, W.; Wu, L.; Wu, Y. TNF-α Antagonizes the Effect of Leptin on Insulin Secretion through FOXO1-Dependent Transcriptional Suppression of LepRb in INS-1 Cells. Oxid. Med. Cell. Longev. 2022, 2022, 9142798. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Mandrup-Poulsen, T. A choice of death--the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia 2001, 44, 2115–2133. [Google Scholar] [CrossRef]
- Amirshahrokhi, K.; Zohouri, A. Carvedilol prevents pancreatic β-cell damage and the development of type 1 diabetes in mice by the inhibition of proinflammatory cytokines, NF-κB, COX-2, iNOS and oxidative stress. Cytokine 2021, 138, 155394. [Google Scholar] [CrossRef]
- Franco, M.d.C.P.; Arruda, R.M.M.P.; Dantas, A.P.V.; Kawamoto, E.M.; Fortes, Z.B.; Scavone, C.; Carvalho, M.H.C.; Tostes, R.C.A.; Nigro, D. Intrauterine undernutrition: Expression and activity of the endothelial nitric oxide synthase in male and female adult offspring. Cardiovasc. Res. 2002, 56, 145–153. [Google Scholar] [CrossRef]
- Grandvuillemin, I.; Buffat, C.; Boubred, F.; Lamy, E.; Fromonot, J.; Charpiot, P.; Simoncini, S.; Sabatier, F.; Dignat-George, F.; Peyter, A.C.; et al. Arginase upregulation and eNOS uncoupling contribute to impaired endothelium-dependent vasodilation in a rat model of intrauterine growth restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R509–R520. [Google Scholar] [CrossRef]
- Dinh, Q.N.; Drummond, G.R.; Sobey, C.G.; Chrissobolis, S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed. Res. Int. 2014, 2014, 406960. [Google Scholar] [CrossRef]
- Kalinowski, L.; Dobrucki, L.W.; Szczepanska-Konkel, M.; Jankowski, M.; Martyniec, L.; Angielski, S.; Malinski, T. Third-generation beta-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: A novel mechanism for antihypertensive action. Circulation 2003, 107, 2747–2752. [Google Scholar] [CrossRef] [PubMed]
- Bercea, C.I.; Cottrell, G.S.; Tamagnini, F.; McNeish, A.J. Omega-3 polyunsaturated fatty acids and hypertension: A review of vasodilatory mechanisms of docosahexaenoic acid and eicosapentaenoic acid. Br. J. Pharmacol. 2021, 178, 860–877. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Targher, G.; Montagnana, M.; Salvagno, G.L.; Zoppini, G.; Guidi, G.C. Relation Between Red Blood Cell Distribution Width and Inflammatory Biomarkers in a Large Cohort of Unselected Outpatients. Arch. Pathol. Lab. Med. 2009, 133, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Salvagno, G.L.; Sanchis-Gomar, F.; Picanza, A.; Lippi, G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab. Sci. 2015, 52, 86–105. [Google Scholar] [CrossRef]
- Rondanelli, M.; Perna, S.; Alalwan, T.A.; Cazzola, R.; Gasparri, C.; Infantino, V.; Perdoni, F.; Iannello, G.; Pepe, D.; Guido, D. A structural equation model to assess the pathways of body adiposity and inflammation status on dysmetabolic biomarkers via red cell distribution width and mean corpuscular volume: A cross-sectional study in overweight and obese subjects. Lipids Health Dis. 2020, 19, 154. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, M.R.; Gibbs, R.L.; Grijalva, P.C.; Hicks, Z.M.; Beer, H.N.; Marks-Nelson, E.S.; Yates, D.T. Reducing Systemic Inflammation in IUGR-Born Neonatal Lambs via Daily Oral ω-3 PUFA Supplement Improved Skeletal Muscle Glucose Metabolism, Glucose-Stimulated Insulin Secretion, and Blood Pressure. Metabolites 2025, 15, 346. https://doi.org/10.3390/metabo15060346
White MR, Gibbs RL, Grijalva PC, Hicks ZM, Beer HN, Marks-Nelson ES, Yates DT. Reducing Systemic Inflammation in IUGR-Born Neonatal Lambs via Daily Oral ω-3 PUFA Supplement Improved Skeletal Muscle Glucose Metabolism, Glucose-Stimulated Insulin Secretion, and Blood Pressure. Metabolites. 2025; 15(6):346. https://doi.org/10.3390/metabo15060346
Chicago/Turabian StyleWhite, Melanie R., Rachel L. Gibbs, Pablo C. Grijalva, Zena M. Hicks, Haley N. Beer, Eileen S. Marks-Nelson, and Dustin T. Yates. 2025. "Reducing Systemic Inflammation in IUGR-Born Neonatal Lambs via Daily Oral ω-3 PUFA Supplement Improved Skeletal Muscle Glucose Metabolism, Glucose-Stimulated Insulin Secretion, and Blood Pressure" Metabolites 15, no. 6: 346. https://doi.org/10.3390/metabo15060346
APA StyleWhite, M. R., Gibbs, R. L., Grijalva, P. C., Hicks, Z. M., Beer, H. N., Marks-Nelson, E. S., & Yates, D. T. (2025). Reducing Systemic Inflammation in IUGR-Born Neonatal Lambs via Daily Oral ω-3 PUFA Supplement Improved Skeletal Muscle Glucose Metabolism, Glucose-Stimulated Insulin Secretion, and Blood Pressure. Metabolites, 15(6), 346. https://doi.org/10.3390/metabo15060346





