Childhood Obesity and Overweight Are Associated with Higher Risk of Perceived Stress and Poor Sleep Quality: A Cross-Sectional Study in Children Aged 6–9 Years
Abstract
1. Introduction
2. Methods
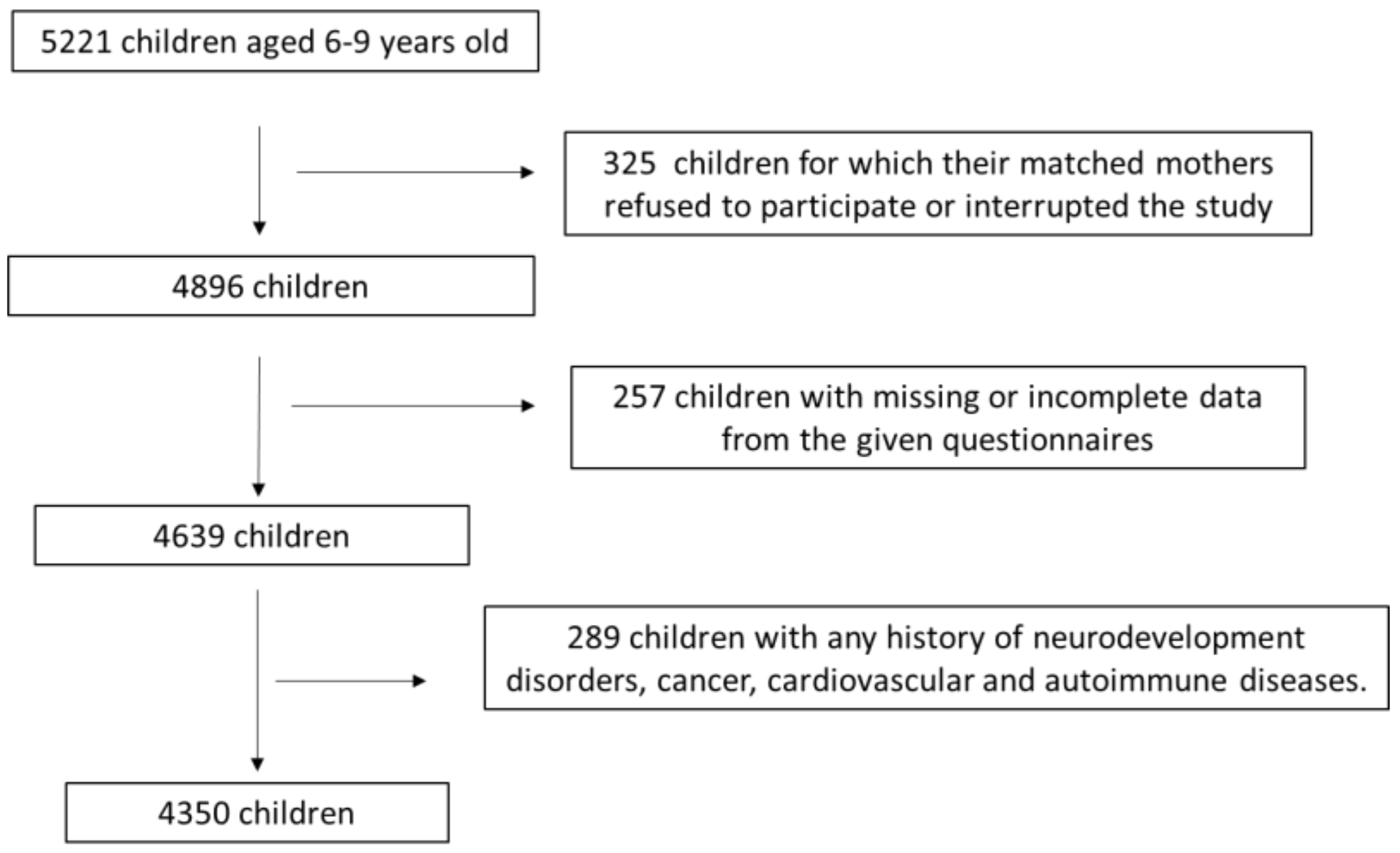
2.1. Study Population
2.2. Study Design
2.2.1. Sociodemographic Parameters
2.2.2. Perinatal Outcomes
2.2.3. Anthropometric Parameters
2.2.4. Breastfeeding Practices
2.2.5. Children’s Physical Activity
2.2.6. Children’s Perceived Stress and Sleep Quality
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zapata, J.K.; Gómez-Ambrosi, J.; Frühbeck, G. Childhood obesity: The threatening apprentice of the adiposity empire. Rev. Endocr. Metab. Disord. 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, Y.; Zhang, X.; Ye, J.; Zhang, J. Association of adverse childhood experiences with diabetes: A systematic review and meta-analysis. J. Diabetes Complicat. 2022, 36, 108289. [Google Scholar] [CrossRef]
- Hawton, K.; Shirodkar, D.; Siese, T.; Hamilton-Shield, J.P.; Giri, D. A recent update on childhood obesity: Aetiology, treatment and complications. J. Pediatr. Endocrinol. Metab. 2025; in press. [Google Scholar] [CrossRef]
- Nittari, G.; Scuri, S.; Petrelli, F.; Pirillo, I.; di Luca, N.M.; Grappasonni, I. Fighting obesity in children from European World Health Organization member states. Epidemiological data, medical-social aspects, and prevention programs. Clin. Ter. 2019, 170, e223–e230. [Google Scholar] [CrossRef]
- Spinelli, A.; Buoncristiano, M.; Nardone, P.; Starc, G.; Hejgaard, T.; Júlíusson, P.B.; Fismen, A.-S.; Weghuber, D.; Milanović, S.M.; García-Solano, M.; et al. Thinness, overweight, and obesity in 6-to 9-year-old children from 36 countries: The World Health Organization European Childhood Obesity Surveillance Initiative—COSI 2015–2017. Obes. Rev. 2021, 22, e13214. [Google Scholar] [CrossRef]
- Hassapidou, M.; Tzotzas, T.; Makri, E.; Pagkalos, I.; Kaklamanos, I.; Kapantais, E.; Abrahamian, A.; Polymeris, A.; Tziomalos, K. Prevalence and geographic variation of abdominal obesity in 7- and 9-year-old children in Greece; World Health Organization Childhood Obesity Surveillance Initiative 2010. BMC Public Health 2017, 17, 126. [Google Scholar] [CrossRef]
- Jacovides, C.; Pritsa, A.; Chrysafi, M.; Papadopoulou, S.K.; Kapetanou, M.G.; Lechouritis, E.; Mato, M.; Papadopoulou, V.G.; Tsourouflis, G.; Migdanis, A.; et al. Childhood Mediterranean Diet compliance Is associated with lower incidence of childhood obesity, specific sociodemographic, and lifestyle factors: A cross-sectional study in children Aged 6–9 Years. Pediatr. Rep. 2024, 16, 1207–1222. [Google Scholar] [CrossRef]
- Farajian, P.; Panagiotakos, D.B.; Risvas, G.; Karasouli, K.; Bountziouka, V.; Voutzourakis, N.; Zampelas, A. Socio-economic and demographic determinants of childhood obesity prevalence in Greece: The GRECO (Greek Childhood Obesity) study. Public Health Nutr. 2013, 16, 240–247. [Google Scholar] [CrossRef]
- Oudat, Q.; Messiah, S.E.; Ghoneum, A.D. A Multi-level approach to childhood pbesity prevention and management: Lessons from Japan and the United States. Nutrients 2025, 17, 838. [Google Scholar] [CrossRef]
- Rankin, J.; Matthews, L.; Cobley, S.; Han, A.; Sanders, R.; Wiltshire, H.D.; Baker, J.S. Psychological consequences of childhood obesity: Psychiatric comorbidity and prevention. Adolesc. Health Med. Ther. 2016, 7, 125–146. [Google Scholar] [CrossRef]
- Williams, J.; Buoncristiano, M.; Nardone, P.; Rito, A.I.; Spinelli, A.; Hejgaard, T.; Kierkegaard, L.; Nurk, E.; Kunešová, M.; Milanović, S.M.; et al. A snapshot of European children’s eating habits: Results from the fourth round of the WHO European Childhood Obesity Surveillance Initiative (COSI). Nutrients 2020, 12, 2481. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Kounatidis, D.; Tzivaki, I.; Zafeiri, G.C.M.; Rigatou, A.; Daskalopoulou, S.; Stratigou, T.; Karampela, I.; Dalamaga, M. Ultra-processed foods and childhood obesity: Current evidence and perspectives. Curr. Nutr. Rep. 2025, 14, 5. [Google Scholar] [CrossRef]
- Farrell, A.H.; Szatmari, P.; Vaillancourt, T. Epidemiology of mental health challenges in children and adolescents. Pediatr. Clin. N. Am. 2024, 71, 999–1011. [Google Scholar] [CrossRef]
- Singh, S.; Roy, D.; Sinha, K.; Parveen, S.; Sharma, G.; Joshi, G. Impact of COVID-19 and lockdown on mental health of children and adolescents: A narrative review with recommendations. Psychiatry Res. 2020, 293, 113429. [Google Scholar] [CrossRef]
- Deborah Omoleye, D.; Olubukola Abidakun, O.; Oluwadamilola Akinje, R.; Hannah Ademuyiwa, O.; Mofoluwaso Fasogbon, B. A review of the effects of the COVID-19 pandemic on children and adolescents’ mental health. Curr. Pediatr. Rev. 2024, 20, 129–136. [Google Scholar] [CrossRef]
- Meherali, S.; Punjani, N.; Louie-Poon, S.; Abdul Rahim, K.; Das, J.K.; Salam, R.A.; Lassi, Z.S. Mental health of children and adolescents amidst COVID-19 and past pandemics: A rapid systematic review. Int. J. Environ. Res. Public Health 2021, 18, 3432. [Google Scholar] [CrossRef]
- Zilidis, C.; Stuckler, D.; McKee, M. Use of amenable mortality indicators to evaluate the impact of financial crisis on health system performance in Greece. Eur. J. Public Health 2020, 30, 861–866. [Google Scholar] [CrossRef]
- Zilidis, C.; Angelopoulos, N.V. The impact of economic crisis on mortality due to mental health illnesses. J. Public Health 2022, 44, 92–99. [Google Scholar] [CrossRef]
- Marchionatti, L.E.; Schafer, J.L.; Karagiorga, V.E.; Balikou, P.; Mitropoulou, A.; Serdari, A.; Moschos, G.; Athanasopoulou, L.; Basta, M.; Simioni, A.; et al. The mental health care system for children and adolescents in Greece: A review and structure assessment. Front. Health Serv. 2024, 4, 1470053. [Google Scholar] [CrossRef]
- Davis, S.L.; Soistmann, H.C. Child’s perceived stress: A concept analysis. J. Pediatr. Nurs. 2022, 67, 15–26. [Google Scholar] [CrossRef]
- Achterberg, M.; Dobbelaar, S.; Boer, O.D.; Crone, E.A. Perceived stress as mediator for longitudinal effects of the COVID-19 lockdown on wellbeing of parents and children. Sci. Rep. 2021, 11, 2971. [Google Scholar] [CrossRef] [PubMed]
- Kornienko, D.S.; Rudnova, N.A.; Veraksa, A.N.; Gavrilova, M.N.; Plotnikova, V.A. Exploring the use of the perceived stress scale for children as an instrument for measuring stress among children and adolescents: A scoping review. Front. Psychol. 2024, 15, 1470448. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.C.; Parnarouskis, L.; Moore, M.D.; Minnick, A.M. Insomnia, short sleep, and their treatments: Review of their associations with weight. Curr. Obes. Rep. 2024, 13, 203–213. [Google Scholar] [CrossRef]
- El Halal, C.D.S.; Nunes, M.L. Sleep and weight-height development. J. Pediatr. 2019, 95, S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, H. Genetic sleep deprivation: Using sleep mutants to study sleep functions. EMBO Rep. 2019, 20, e46807. [Google Scholar] [CrossRef]
- Demichelis, O.P.; Grainger, S.A.; McKay, K.T.; Bourdaniotis, X.E.; Churchill, E.G.; Henry, J.D. Sleep, stress and aggression: Meta-analyses investigating associations and causality. Neurosci. Biobehav. Rev. 2022, 139, 104732. [Google Scholar] [CrossRef]
- Bruni, O.; Malorgio, E.; Doria, M.; Finotti, E.; Spruyt, K.; Melegari, M.G.; Villa, M.P.; Ferri, R. Changes in sleep patterns and disturbances in children and adolescents in Italy during the COVID-19 outbreak. Sleep Med. 2022, 91, 166–174. [Google Scholar] [CrossRef]
- Jahrami, H.; BaHammam, A.S.; Bragazzi, N.L.; Saif, Z.; Faris, M.; Vitiello, M.V. Sleep problems during the COVID-19 pandemic by population: A systematic review and meta-analysis. J. Clin. Sleep Med. 2021, 17, 299–313. [Google Scholar] [CrossRef]
- Jahrami, H.A.; Alhaj, O.A.; Humood, A.M.; Alenezi, A.F.; Fekih-Romdhane, F.; AlRasheed, M.M.; Saif, Z.Q.; Bragazzi, N.L.; Pandi-Perumal, S.R.; BaHammam, A.S.; et al. Sleep disturbances during the COVID-19 pandemic: A systematic review, meta-analysis, and meta-regression. Sleep Med. Rev. 2022, 62, 101591. [Google Scholar] [CrossRef]
- Sharma, M.; Aggarwal, S.; Madaan, P.; Saini, L.; Bhutani, M. Impact of COVID-19 pandemic on sleep in children and adolescents: A systematic review and meta-analysis. Sleep Med. 2021, 84, 259–267. [Google Scholar] [CrossRef]
- Kalmbach, D.A.; Anderson, J.R.; Drake, C.L. The impact of stress on sleep: Pathogenic sleep reactivity as a vulnerability to insomnia and circadian disorders. J. Sleep Res. 2018, 27, e12710. [Google Scholar] [CrossRef] [PubMed]
- Ordway, M.R.; Condon, E.M.; Basile Ibrahim, B.; Abel, E.A.; Funaro, M.C.; Batten, J.; Sadler, L.S.; Redeker, N.S. A systematic review of the association between sleep health and stress biomarkers in children. Sleep Med. Rev. 2021, 59, 101494. [Google Scholar] [CrossRef]
- Xu, Y.; Hua, J.; Wang, J.; Shen, Y. Sleep duration is associated with metabolic syndrome in adolescents and children: A systematic review and meta-analysis. J. Clin. Sleep Med. 2023, 19, 1835–1843. [Google Scholar] [CrossRef]
- Mentzelou, M.; Papadopoulou, S.K.; Papandreou, D.; Spanoudaki, M.; Dakanalis, A.; Vasios, G.K.; Voulgaridou, G.; Pavlidou, E.; Mantzorou, M.; Giaginis, C. Evaluating the Relationship between circadian rhythms and sleep, metabolic and cardiovascular disorders: Current clinical evidence in human studies. Metabolites 2023, 13, 370. [Google Scholar] [CrossRef]
- Barbouzas, A.E.; Malli, F.; Daniil, Z.; Gourgoulianis, K. Long-Term Impact of COVID-19 Pandemic in sleep quality and lifestyle in young adults. Int. J. Environ. Res. Public Health 2022, 19, 12333. [Google Scholar] [CrossRef]
- Piątkowska-Chmiel, I.; Krawiec, P.; Ziętara, K.J.; Pawłowski, P.; Samardakiewicz, M.; Pac-Kożuchowska, E.; Herbet, M. The impact of chronic stress related to COVID-19 on eating behaviors and the risk of obesity in children and adolescents. Nutrients 2023, 16, 54. [Google Scholar] [CrossRef]
- Hertiš Petek, T.; Marčun Varda, N. Childhood cardiovascular health, obesity, and some related disorders: Insights into chronic inflammation and oxidative stress. Int. J. Mol. Sci. 2024, 25, 9706. [Google Scholar] [CrossRef]
- Avogaro, A. Diabetes and obesity: The role of stress in the development of cancer. Endocrine 2024, 86, 48–57. [Google Scholar] [CrossRef]
- Kappes, C.; Stein, R.; Körner, A.; Merkenschlager, A.; Kiess, W. Stress, stress reduction and obesity in childhood and adolescence. Horm. Res. Paediatr. 2023, 96, 88–96. [Google Scholar] [CrossRef]
- Figorilli, M.; Velluzzi, F.; Redolfi, S. Obesity and sleep disorders: A bidirectional relationship. Nutr. Metab. Cardiovasc. Dis. 2025, 15, 104014. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, M.; Kohanmoo, A. Sleep deprivation in development of obesity, effects on appetite regulation, energy metabolism, and dietary choices. Nutr. Res. Rev. 2023, 31, 1–21. [Google Scholar] [CrossRef] [PubMed]
- McCoy, T.; Sochan, A.J.; Spaeth, A.M. The relationship between sleep and physical activity by age, race, and gender. Rev. Cardiovasc. Med. 2024, 25, 378. [Google Scholar] [CrossRef] [PubMed]
- Alenezi, M.A.; Alabdulathim, S.; Alhejaili, S.A.M.; Al Sheif, Z.A.A.; Aldossari, K.K.; Bakhsh, J.I.; Alharbi, F.M.; Ahmad, A.A.Y.; Aloufi, R.M.; Mushaeb, H. The association between obesity and the development and severity of obstructive sleep apnea: A systematic review. Cureus 2024, 16, e69962. [Google Scholar] [CrossRef]
- Chaput, J.P.; McHill, A.W.; Cox, R.C.; Broussard, J.L.; Dutil, C.; da Costa, B.G.G.; Sampasa-Kanyinga, H.; Wright, K.P., Jr. The role of insufficient sleep and circadian misalignment in obesity. Nat. Rev. Endocrinol. 2023, 19, 82–97. [Google Scholar] [CrossRef]
- Meneses-Echavez, J.F.; Iglesias-Gonzalez, L.E.; Loaiza-Betancur, A.F.; Guapo, N.C. Sedentary behavior and sleep for children and adolescents with obesity: A systematic review. Ann. N. Y. Acad. Sci. 2025, 1545, 66–75. [Google Scholar] [CrossRef]
- Mantzorou, M.; Papandreou, D.; Vasios, G.K.; Pavlidou, E.; Antasouras, G.; Psara, E.; Taha, Z.; Poulios, E.; Giaginis, C. Exclusive breastfeeding for at least four months is associated with a lower prevalence of overweight and obesity in mothers and their children after 2–5 years from delivery. Nutrients 2022, 14, 3599. [Google Scholar] [CrossRef]
- Papandreou, D.; Mantzorou, M.; Tyrovolas, S.; Pavlidou, E.; Antasouras, G.; Psara, E.; Poulios, E.; Vasios, G.K.; Giaginis, C. Pre-pregnancy excess weight association with maternal sociodemographic, anthropometric and lifestyle factors and maternal perinatal outcomes. Nutrients 2022, 14, 3810. [Google Scholar] [CrossRef]
- Gilmore, L.A.; Redman, L.M. Weight gain in pregnancy and application of the 2009 IOM guidelines: Toward a uniform approach. Obesity 2015, 23, 507–511. [Google Scholar] [CrossRef]
- Bacci, S.; Bartolucci, F.; Chiavarini, M.; Minelli, L.; Pieroni, L. Differences in birthweight outcomes: A longitudinal study based on siblings. Int. J. Environ. Res. Public Health 2014, 11, 6472–6484. [Google Scholar] [CrossRef]
- World Health Organization. The World Health Report: 2006: Working Together for Health. World Health Organization. 2006. Available online: https://apps.who.int/iris/handle/10665/43432 (accessed on 10 February 2025).
- Jamesm, W.P. WHO recognition of the global obesity epidemic. Int. J. Obes. 2008, 32, S120–S126. [Google Scholar] [CrossRef]
- Mantzorou, M.; Papandreou, D.; Pavlidou, E.; Papadopoulou, S.K.; Tolia, M.; Mentzelou, M.; Poutsidi, A.; Antasouras, G.; Vasios, G.K.; Giaginis, C. Maternal gestational diabetes is associated with high risk of childhood overweight and obesity: A cross-sectional study in pre-school children aged 2–5 years. Medicina 2023, 59, 455. [Google Scholar] [CrossRef] [PubMed]
- Antasouras, G.; Papadopoulou, S.K.; Alexatou, O.; Papandreou, D.; Mentzelou, M.; Migdanis, A.; Psara, E.; Migdanis, I.; Chrysafi, M.; Tyrovolas, S.; et al. Adherence to the Mediterranean diet during pregnancy: Associations with sociodemographic and anthropometric parameters, perinatal outcomes, and breastfeeding practices. Medicina 2023, 59, 1547. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1895. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Ribeiro Santiago, P.H.; Nielsen, T.; Smithers, L.G.; Roberts, R.; Jamieson, L. Measuring stress in Australia: Validation of the Perceived Stress Scale (PSS-14) in a national sample. Health Qual. Life Outcomes 2020, 18, 100. [Google Scholar] [CrossRef]
- Salahuddin, M.; Maru, T.T.; Kumalo, A.; Pandi-Perumal, S.R.; Bahammam, A.S.; Manzar, M.D. Validation of the Pittsburgh Sleep Quality Index in community dwelling Ethiopian adults. Health Qual. Life Outcomes 2017, 15, 58. [Google Scholar] [CrossRef]
- Park, S.E.; So, W.Y.; Kang, Y.S.; Yang, J.H. Relationship between perceived stress, obesity, and hypertension in Korean adults and older adults. Healthcare 2023, 11, 2271. [Google Scholar] [CrossRef]
- Roy, S.K.; Jahan, K.; Alam, N.; Rois, R.; Ferdaus, A.; Israt, S.; Karim, M.R. Perceived stress, eating behavior, and overweight and obesity among urban adolescents. J. Health Popul. Nutr. 2021, 40, 54. [Google Scholar] [CrossRef]
- Richardson, A.S.; Arsenault, J.E.; Cates, S.C.; Muth, M.K. Perceived stress, unhealthy eating behaviors, and severe obesity in low-income women. Nutr. J. 2015, 14, 122. [Google Scholar] [CrossRef]
- Barrington, W.E.; Ceballos, R.M.; Bishop, S.K.; McGregor, B.A.; Beresford, S.A. Perceived stress, behavior, and body mass index among adults participating in a worksite obesity prevention program, Seattle, 2005–2007. Prev. Chronic Dis. 2012, 9, E152. [Google Scholar] [CrossRef]
- Farag, N.H.; Moore, W.E.; Lovallo, W.R.; Mills, P.J.; Khandrika, S.; Eichner, J.E. Hypothalamic-pituitary-adrenal axis function: Relative contributions of perceived stress and obesity in women. J. Womens Health 2008, 17, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Baskind, M.J.; Taveras, E.M.; Gerber, M.W.; Fiechtner, L.; Horan, C.; Sharifi, M. Parent-perceived stress and its association with children’s weight and obesity-related behaviors. Prev. Chronic Dis. 2019, 16, E39. [Google Scholar] [CrossRef] [PubMed]
- Tenk, J.; Mátrai, P.; Hegyi, P.; Rostás, I.; Garami, A.; Szabó, I.; Hartmann, P.; Pétervári, E.; Czopf, L.; Hussain, A.; et al. Perceived stress correlates with visceral obesity and lipid parameters of the metabolic syndrome: A systematic review and meta-analysis. Psychoneuroendocrinology 2018, 95, 63–73. [Google Scholar] [CrossRef]
- Dakanalis, A.; Voulgaridou, G.; Alexatou, O.; Papadopoulou, S.K.; Jacovides, C.; Pritsa, A.; Chrysafi, M.; Papacosta, E.; Kapetanou, M.G.; Tsourouflis, G.; et al. Overweight and obesity Is associated with higher risk of perceived stress and poor sleep quality in young adults. Medicina 2024, 60, 983. [Google Scholar] [CrossRef]
- Elizabeth, B.; Wanda, D.; Apriyanti, E. The correlation between sleep quality and the prevalence of obesity in school-age children. J. Public Health Res. 2021, 10, jphr.2021.2331. [Google Scholar] [CrossRef]
- Turel, O.; Romashkin, A.; Morrison, K.M. A model linking video gaming, sleep quality, sweet drinks consumption and obesity among children and youth. Clin. Obes. 2017, 7, 191–198. [Google Scholar] [CrossRef]
- Yıldız, F.; Tuğrul Aksakal, M.Z.; Yıldız, R.; Baş, F. The relationship between sleep quality, sleep duration, social jet lag and obesity in adolescents. J. Clin. Res. Pediatr. Endocrinol. 2024, 16, 419–425. [Google Scholar] [CrossRef]
- Massoudi, M.; Pourghassem Gargari, B.; Asghari Jafarabadi, M.; Norouzi, S. Major dietary patterns and sleep quality in relation to overweight/obesity among school children: A case-control study. Health Promot. Perspect. 2023, 13, 330–338. [Google Scholar] [CrossRef]
- Anam, M.R.; Akter, S.; Hossain, F.; Bonny, S.Q.; Akter, J.; Zhang, C.; Rahman, M.M.; Mian, M.A.B. Association of sleep duration and sleep quality with overweight/obesity among adolescents of Bangladesh: A multilevel analysis. BMC Public Health 2022, 22, 374. [Google Scholar] [CrossRef]
- Hur, S.; Oh, B.; Kim, H.; Kwon, O. Associations of diet quality and sleep quality with obesity. Nutrients 2021, 13, 3181. [Google Scholar] [CrossRef]
- Doo, M.; Wang, C. Associations among Sleep Quality, Changes in eating habits, and overweight or obesity after studying abroad among international students in South Korea. Nutrients 2020, 12, 2020. [Google Scholar] [CrossRef]
- Hawes, N.J.; Wiggins, A.T.; Reed, D.B.; Hardin-Fanning, F. Poor sleep quality is associated with obesity and depression in farmers. Public Health Nurs. 2019, 36, 270–275. [Google Scholar] [CrossRef]
- Kanellopoulou, A.; Notara, V.; Magriplis, E.; Antonogeorgos, G.; Rojas-Gil, A.P.; Kornilaki, E.N.; Lagiou, A.; Yannakoulia, M.; Panagiotakos, D.B. Sleeping patterns and childhood obesity: An epidemiological study in 1,728 children in Greece. J. Clin. Sleep Med. 2021, 17, 1093–1101. [Google Scholar] [CrossRef]
- Flegal, K.M.; Kruszon-Moran, D.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016, 315, 2284–2291. [Google Scholar] [CrossRef]
- Salahuddin, M.; Pérez, A.; Ranjit, N.; Kelder, S.H.; Barlow, S.E.; Pont, S.J.; Butte, N.F.; Hoelscher, D.M. Predictors of severe obesity in low-income, predominantly Hispanic/Latino children: The Texas childhood obesity research demonstration study. Prev. Chronic Dis. 2017, 14, E141. [Google Scholar] [CrossRef][Green Version]
- Segal, A.B.; Huerta, M.C.; Aurino, E.; Sassi, F. The impact of childhood obesity on human capital in high-income countries: A systematic review. Obes. Rev. 2021, 22, e13104. [Google Scholar] [CrossRef]
- Pavlidou, E.; Papadopoulou, S.K.; Alexatou, O.; Tsourouflis, G.; Antasouras, G.; Louka, A.; Chatziprodromidou, I.P.; Mentzelou, M.; Sampani, A.; Chrysafi, M.; et al. Association of gestational hypertension with sociodemographic and anthropometric factors, perinatal outcomes, breastfeeding practices, and Mediterranean diet adherence: A cross-sectional study. Medicina 2023, 59, 2103. [Google Scholar] [CrossRef]
- Hernández-Barrera, L.; Trejo-Valdivia, B.; Téllez-Rojo, M.M.; Baccarelli, A.; Wright, R.; Cantoral, A.; Barquera, S. Pre-gestational obesity and gestational weight gain as predictors of childhood obesity. Arch. Med. Res. 2024, 55, 103006. [Google Scholar] [CrossRef]
- Papadopoulou, S.K.; Mentzelou, M.; Pavlidou, E.; Vasios, G.K.; Spanoudaki, M.; Antasouras, G.; Sampani, A.; Psara, E.; Voulgaridou, G.; Tsourouflis, G.; et al. Caesarean section delivery is associated with childhood overweight and obesity, low childbirth weight and postnatal complications: A cross-sectional Study. Medicina 2023, 59, 664. [Google Scholar] [CrossRef]
- Gorman, T.; Maher, G.M.; Al Khalaf, S.; Khashan, A.S. The association between caesarean section delivery and obesity at age 17 years. Evidence from a longitudinal cohort study in the United Kingdom. PLoS ONE 2024, 19, e0301684. [Google Scholar] [CrossRef]
- Sulley, I.; Saaka, M. Relationship between caesarean section delivery and risk of overweight/obesity among children aged 6–23 months in the Tamale Metropolis of Ghana. J. Nutr. Sci. 2022, 11, e43. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yuan, J.; Wang, L.; Qiao, Y.; Liu, E.; Wang, S.; Leng, J. The association between breastfeeding and childhood obesity/underweight: A population-based birth cohort study with repeated measured data. Int. Breastfeed. J. 2022, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Pojednic, R.; D’Arpino, E.; Halliday, I.; Bantham, A. The benefits of physical activity for people with obesity, independent of weight loss: A systematic review. Int. J. Environ. Res. Public Health 2022, 19, 4981. [Google Scholar] [CrossRef] [PubMed]
- Almonacid-Fierro, A.; González-Almonacid, J. The pandemic of childhood obesity: Challenges and possibilities from physical activity. Health Promot. Perspect. 2022, 12, 229–230. [Google Scholar] [CrossRef]
- Hidding, L.M.; Chinapaw, M.J.M.; van Poppel, M.N.M.; Mokkink, L.B.; Altenburg, T.M. An updated systematic review of childhood physical activity questionnaires. Sports Med. 2018, 48, 2797–2842. [Google Scholar] [CrossRef]
| Characteristics (n = 4350) | Childhood BMI | |||
|---|---|---|---|---|
| Normal Weight 3281 (75.4%) | Overweight 728 (16.7%) | Obesity 341 (7.9%) | p-Value | |
| Childhood age (mean ± SD; years) | 7.52 ± 1.03 | 7.50 ± 1.07 | 7.53 ± 1.08 | p = 0.3781 |
| Gender (n, %) | p ˂ 0.0001 | |||
| Male | 1738 (53.0%) | 275 (37.8%) | 153 (44.9%) | |
| Female | 1543 (47.0%) | 453 (62.2%) | 188 (55.1%) | |
| Nationality (n, %) | p = 0.1399 | |||
| Greek | 3152 (96.1%) | 690 (94.8%) | 322 (94.4%) | |
| Other | 129 (3.9%) | 38 (5.2%) | 19 (5.6%) | |
| Type of residence (n, %) | p = 0.6768 | |||
| Urban | 2381 (72.6%) | 540 (74.2%) | 239 (70.1%) | |
| Rural | 900 (27.4%) | 188 (25.8%) | 102 (29.9%) | |
| Maternal educational level (n, %) | p = 0.0045 | |||
| Low | 958 (29.2%) | 231 (31.7%) | 127 (37.2%) | |
| Moderate | 1402 (42.7%) | 306 (42.0%) | 146 (42.8%) | |
| High | 921 (21.2%) | 191 (6.3%) | 68 (20.0%) | |
| Family economic status (n, %) | p = 0.0031 | |||
| Low | 1354 (41.3%) | 313 (43.0%) | 160 (46.9%) | |
| Moderate | 1227 (37.4%) | 297 (40.8%) | 107 (31.4%) | |
| High | 700 (21.3%) | 118 (16.2%) | 74 (21.7%) | |
| Maternal age (mean ± SD; years) | 35.8 ± 6.4 | 35.2 ± 6.2 | 35.6 ± 6.7 | p = 0.7339 |
| Maternal smoking habits (n, %) | p ˂ 0.0001 | |||
| Non-smokers | 2485 (75.7%) | 533 (73.2%) | 209 (61.3%) | |
| Regular smokers | 796 (24.3%) | 195 (26.8%) | 132 (38.7%) | |
| Employment status (n, %) | p = 0.2596 | |||
| Employed | 2268 (69.1%) | 487 (66.9%) | 224 (65.7%) | |
| Unemployed | 1013 (23.3%) | 241 (33.1%) | 117 (34.3%) | |
| Marital status (n, %) | p = 0.7669 | |||
| Married | 2230 (68.0%) | 505 (69.4%) | 233 (68.3%) | |
| Divorced | 1051 (32.0%) | 223 (30.6%) | 108 (31.7%) | |
| Parity (n, %) | p ˂ 0.0001 | |||
| Nulliparity | 2185 (66.6%) | 414 (56.9%) | 216 (63.3%) | |
| Multiparity | 1096 (33.4%) | 314 (43.1%) | 125 (36.7%) | |
| Maternal pre-pregnancy BMI status (n, %) | p ˂ 0.0001 | |||
| Underweight | 115 (3.5%) | 11 (1.5%) | 0 (0.0%) | |
| Normal weight | 2496 (76.1%) | 570 (78.3%) | 219 (64.2%) | |
| Overweight | 560 (17.1%) | 90 (12.4%) | 91 (26.7%) | |
| Obese | 110 (3.3%) | 57 (7.8%) | 31 (9.1%) | |
| Maternal gestational weight gain (n, %) | p = 0.0001 | |||
| Low | 466 (14.2%) | 144 (19.8%) | 31 (9.1%) | |
| Normal | 1558 (47.5%) | 308 (42.3%) | 156 (45.7%) | |
| Excessive | 1257 (38.3%) | 276 (37.9%) | 154 (45.2%) | |
| Childbirth weight (n, %) | p ˂ 0.0001 | |||
| Low birth weight (<2500 gr) | 288 | 50 | 6 | |
| Normal birth weight (2500–4000 gr) | 2708 | 569 | 276 | |
| High birth weight (>4000 gr) | 285 | 109 | 59 | |
| Kind of delivery (n, %) | p ˂ 0.0001 | |||
| Vaginal | 1568 (47.8%) | 264 (36.3%) | 68 (19.9%) | |
| Cesarean section | 1713 (52.2%) | 464 (63.7%) | 273 (80.1%) | |
| Exclusive breastfeeding (n, %) | p ˂ 0.0001 | |||
| No | 1486 (45.3%) | 424 (58.2%) | 257 (75.4%) | |
| Yes | 1795 (54.7%) | 304 (41.8%) | 84 (24.6%) | |
| Children’s physical activity (n, %) | p ˂ 0.0001 | |||
| Low | 1459 (44.5%) | 374 (51.4%) | 215 (63.1%) | |
| Moderate | 1384 (42.2%) | 251 (34.5%) | 84 (24.6%) | |
| High | 438 (13.3%) | 103 (14.1%) | 42 (12.3%) | |
| Children’s perceived stress (n, %) | p ˂ 0.0001 | |||
| No | 2408 (73.4%) | 490 (67.3%) | 206 (60.4%) | |
| Yes | 873 (26.6%) | 238 (32.7%) | 135 (39.6%) | |
| Children’s sleep quality (n, %) | p ˂ 0.0001 | |||
| Adequate | 2372 (72.3%) | 582 (79.9%) | 276 (80.9%) | |
| Inadequate | 909 (27.7%) | 146 (20.1%) | 65 (19.1%) | |
| Characteristics | Children’s BMI (Overweight/Obesity vs. Normal Weight) | |
|---|---|---|
| OR * (95% CI **) | p-Value | |
| Childhood age (over/below mean value) | 0.95 (0.20–1.91) | p = 0.7032 |
| Gender (male/female) | 1.38 (0.97–1.75) | p = 0.0341 |
| Nationality (Greek/other) | 1.12 (0.32–1.99) | p = 0.8019 |
| Type of residence (rural/urban) | 1.09 (0.36–1.79) | p = 0.3011 |
| Maternal educational level (low/moderate and high) | 1.18 (0.64–1.72) | p = 0.2139 |
| Family economic status (low/moderate and high) | 1.39 (0.92–1.79) | p = 0.0293 |
| Maternal age (over/below mean value) | 1.03 (0.22–1.97) | p = 0.8282 |
| Maternal smoking status (regular smokers/non-smokers) | 1.31 (0.67–1.88) | p = 0.1254 |
| Employment status (unemployed/employed) | 1.29 (0.69–1.92) | p = 0.3004 |
| Marital status (married/divorced) | 0.95 (0.23–1.98) | p = 0.7081 |
| Parity (multiparity/nulliparity) | 1.41 (0.75–1.96) | p = 0.1202 |
| Maternal pre-pregnancy BMI status (overweight and obesity/underweight and normal weight) | 1.72 (1.03–2.45) | p = 0.0809 |
| Maternal gestational weight gain (low and excessive/normal) | 1.88 (1.41–2.33) | p = 0.0201 |
| Childbirth weight (low and high/normal) | 1.68 (1.45–2.06) | p = 0.0278 |
| Kind of delivery (cesarean section/vaginal) | 2.03 (1.78–2.39) | p = 0.0248 |
| Exclusive breastfeeding (no/yes) | 2.57 (2.24–2.78) | p = 0.0045 |
| Children’s physical activity (low/moderate and high) | 2.88 (2.58–3.31) | p = 0.0087 |
| Children’s perceived stress (yes/no) | 3.12 (2.93–3.58) | p = 0.0013 |
| Children’s sleep quality (inadequate/adequate) | 2.48 (2.25–2.92) | p = 0.0052 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mentzelou, M.; Louka, A.; Vorvolakos, T.; Kapetanou, M.G.; Seradri, A.; Antasouras, G.; Kontogiorgis, C.; Deligiannidou, G.-E.; Chrysafi, M.; Giaginis, C. Childhood Obesity and Overweight Are Associated with Higher Risk of Perceived Stress and Poor Sleep Quality: A Cross-Sectional Study in Children Aged 6–9 Years. Metabolites 2025, 15, 345. https://doi.org/10.3390/metabo15060345
Mentzelou M, Louka A, Vorvolakos T, Kapetanou MG, Seradri A, Antasouras G, Kontogiorgis C, Deligiannidou G-E, Chrysafi M, Giaginis C. Childhood Obesity and Overweight Are Associated with Higher Risk of Perceived Stress and Poor Sleep Quality: A Cross-Sectional Study in Children Aged 6–9 Years. Metabolites. 2025; 15(6):345. https://doi.org/10.3390/metabo15060345
Chicago/Turabian StyleMentzelou, Maria, Aikaterini Louka, Theophanis Vorvolakos, Maria G. Kapetanou, Aspasia Seradri, George Antasouras, Christos Kontogiorgis, Georgia-Eirini Deligiannidou, Maria Chrysafi, and Constantinos Giaginis. 2025. "Childhood Obesity and Overweight Are Associated with Higher Risk of Perceived Stress and Poor Sleep Quality: A Cross-Sectional Study in Children Aged 6–9 Years" Metabolites 15, no. 6: 345. https://doi.org/10.3390/metabo15060345
APA StyleMentzelou, M., Louka, A., Vorvolakos, T., Kapetanou, M. G., Seradri, A., Antasouras, G., Kontogiorgis, C., Deligiannidou, G.-E., Chrysafi, M., & Giaginis, C. (2025). Childhood Obesity and Overweight Are Associated with Higher Risk of Perceived Stress and Poor Sleep Quality: A Cross-Sectional Study in Children Aged 6–9 Years. Metabolites, 15(6), 345. https://doi.org/10.3390/metabo15060345








