Utilizing Untargeted Lipidomics Technology to Elucidate Differences in Lipid Compositions Among Sensitive Dry, Sensitive Oily and Healthy Skin Types
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Sample Collection and Preparation
2.2.1. Test Environmental Conditions
2.2.2. Chemical Reagents and Instruments
2.2.3. Physiological Indicator Measurements
2.2.4. Skin Lipid Sampling
2.3. Sample Processing
2.4. Statistical Analysis
3. Results
3.1. Differences in Skin Physiological Indicators of SDS, SOS, and HS Groups
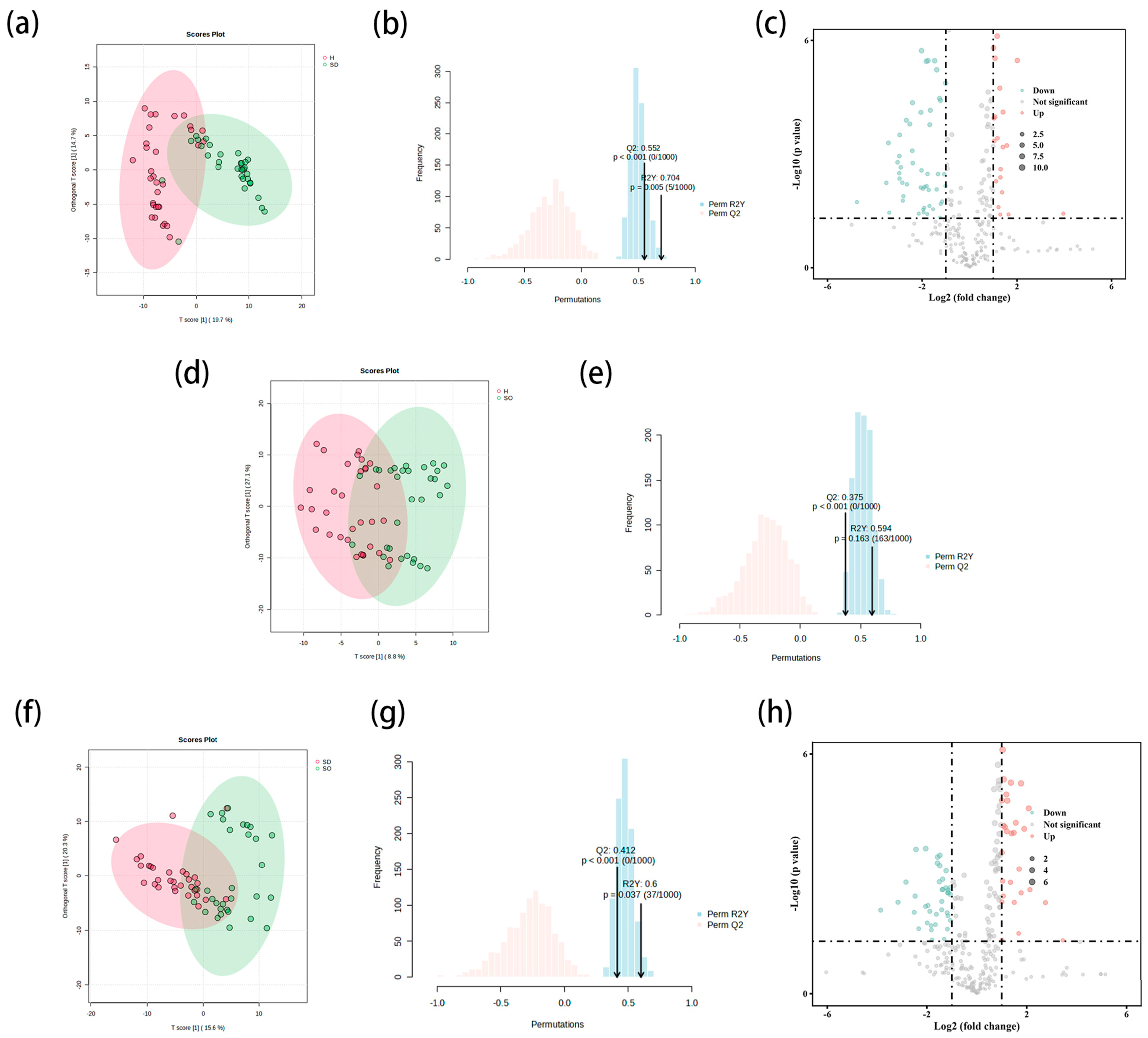
3.2. Comprehensive Pairwise Comparative Lipidomic Analysis of Sebaceous Glands Across Three Distinct Skin Types
3.2.1. Comparison of SDS and HS Groups in Sebaceous Glands
3.2.2. Comparison of SOS and HS Groups in Sebaceous Glands
3.2.3. Comparison of SDS and SOS Groups in Sebaceous Glands
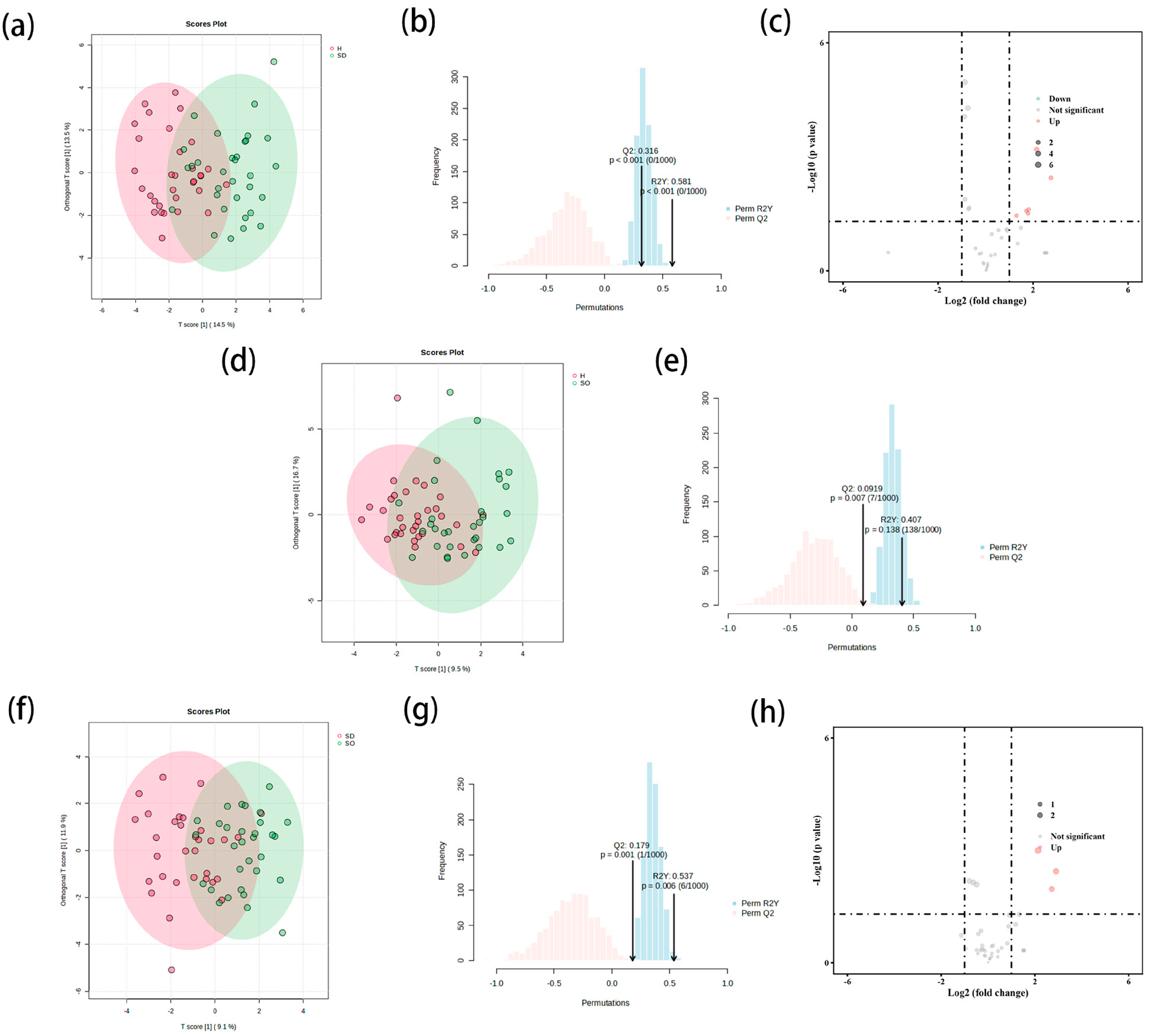
3.3. Comprehensive Pairwise Comparative Lipidomic Analysis of Stratum Corneum Intercellular Across Three Distinct Skin Types
3.3.1. Comparison of SDS and HS Groups in Stratum Corneum
3.3.2. Comparison of SOS and HS Groups in Stratum Corneum
3.3.3. Comparison of SDS and SOS Groups in Stratum Corneum
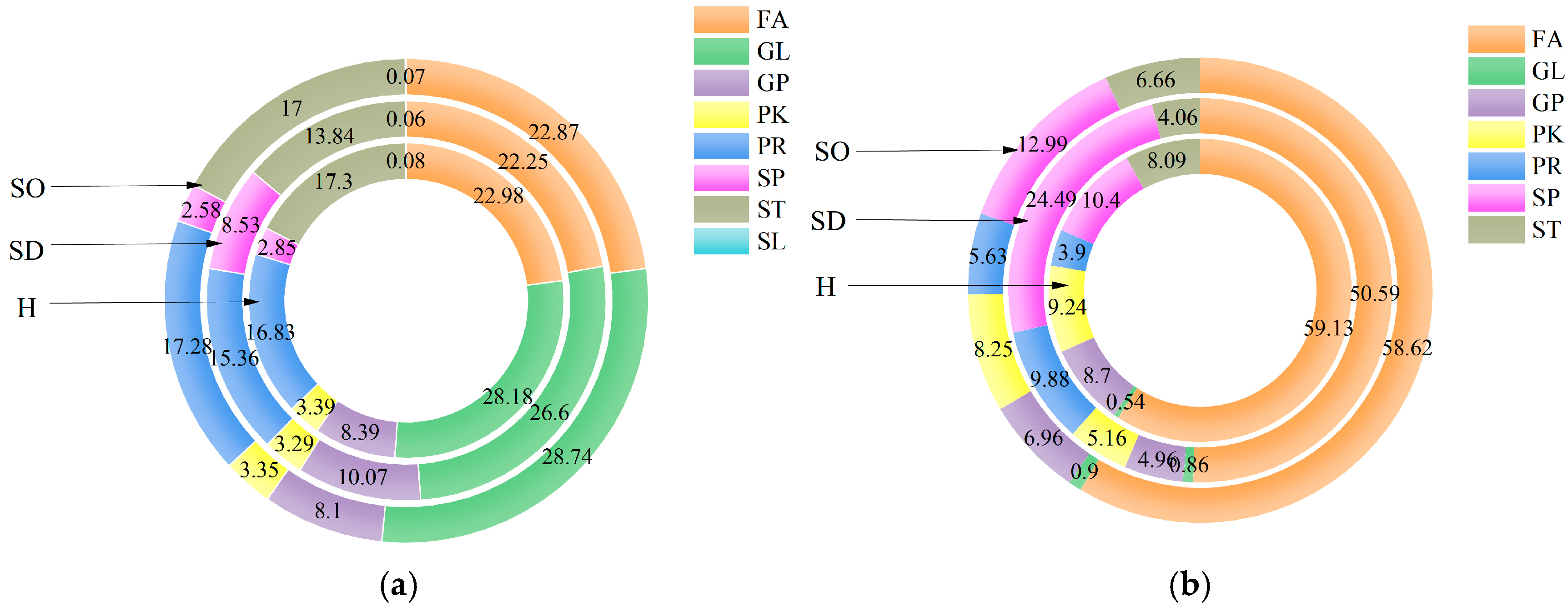
3.4. Analysis of the Lipids in the Sebaceous Glands of SDS, SOS and HS
3.5. Analysis of the Lipids in the Stratum Corneum of SDS, SOS, and HS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, L.; Jia, Y.; Cui, L.; Li, X.; He, C. Analysis of sensitive skin barrier function: Basic indicators and sebum composition. Int. J. Cosmet. Sci. 2018, 40, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Misery, L.; Loser, K.; Staender, S. Sensitive skin. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2–8. [Google Scholar] [CrossRef]
- Staender, S.; Schneider, S.W.; Weishaupt, C.; Luger, T.A.; Misery, L. Putative neuronal mechanisms of sensitive skin. Exp. Dermatol. 2009, 18, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cui, L.; Tian, Y.; He, C. Lipidomics analysis of facial lipid biomarkers in females with self-perceived skin sensitivity. Health Sci. Rep. 2022, 5, e632. [Google Scholar] [CrossRef]
- Fan, L.; He, C.; Jiang, L.; Bi, Y.; Dong, Y.; Jia, Y. Brief analysis of causes of sensitive skin and advances in evaluation of anti-allergic activity of cosmetic products. Int. J. Cosmet. Sci. 2016, 38, 120–127. [Google Scholar] [CrossRef]
- Proksch, E.; Brandner, J.M.; Jensen, J.-M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Bosko, C.A. Skin Barrier Insights: From Bricks and Mortar to Molecules and Microbes. J. Drugs Dermatol. 2019, 18, S63–S67. [Google Scholar] [PubMed]
- Madison, K.C. Barrier function of the skin: “La Raison d'Etre” of the epidermis. J. Investig. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef]
- Rajkumar, J.; Chandan, N.; Lio, P.; Shi, V. The Skin Barrier and Moisturization: Function, Disruption, and Mechanisms of Repair. Ski. Pharmacol. Physiol. 2023, 36, 174–185. [Google Scholar] [CrossRef]
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Moore, D.J.; Lane, M.E.; Lachmann, N.; Rawlings, A.V. Epidermal barrier function in dry, flaky and sensitive skin: A narrative review. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, F.; Wang, J.; Qin, Y.; Pan, Y. Unveiling the Metabolomic Profile of Oily Sensitive Skin: A Non-Invasive Approach. Int. J. Mol. Sci. 2024, 25, 11033. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.; Levi, H.; Menon, G.; Maria, R.; Upcher, A.; Kotlovski, Y.; Oss-Ronen, L.; Cohen, I. ZNF750 Regulates Skin Barrier Function by Driving Cornified Envelope and Lipid Processing. J. Investig. Dermatol. 2024, 144, 296–306. [Google Scholar] [CrossRef]
- Cui, L.; Jia, Y.; Cheng, Z.; Gao, Y.; Zhang, G.; Li, J.; He, C. Advancement in Maintaining Skin Barrier-Secretion and Composition of Lipid. Chin. J. Dermatovenereol. 2016, 30, 640–643. [Google Scholar]
- Voegeli, R.; Gierschendorf, J.; Summers, B.; Rawlings, A.V. Facial skin mapping: From single point bio-instrumental evaluation to continuous visualization of skin hydration, barrier function, skin surface pH, and sebum in different ethnic skin types. Int. J. Cosmet. Sci. 2019, 41, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lu, L.; Zhao, Z.; Cai, M.; Gao, N.; Han, G. Lipidomics profiling of skin surface lipids in senile pruritus. Lipids Health Dis. 2020, 19, 171. [Google Scholar] [CrossRef]
- Cha, H.J.; He, C.; Zhao, H.; Dong, Y.; An, I.-S.; An, S. Intercellular and intracellular functions of ceramides and their metabolites in skin (Review). Int. J. Mol. Med. 2016, 38, 16–22. [Google Scholar] [CrossRef]
- Danby, S.G.; Andrew, P.V.; Kay, L.J.; Pinnock, A.; Chittock, J.; Brown, K.; Williams, S.F.; Cork, M.J. Enhancement of stratum corneum lipid structure improves skin barrier function and protects against irritation in adults with dry, eczema-prone skin. Br. J. Dermatol. 2022, 186, 875–886. [Google Scholar] [CrossRef]
- Xu, X.; Qu, R.; Wu, W.; Jiang, C.; Shao, D.; Shi, J. Applications of microbial co-cultures in polyketides production. J. Appl. Microbiol. 2021, 130, 1023–1034. [Google Scholar] [CrossRef]
- Yang, D.; Eun, H.; Prabowo, C.P.S. Metabolic Engineering and Synthetic Biology Approaches for the Heterologous Production of Aromatic Polyketides. Int. J. Mol. Sci. 2023, 24, 8923. [Google Scholar] [CrossRef]
- King, R.J.; Singh, P.K.; Mehla, K. The cholesterol pathway: Impact on immunity and cancer. Trends Immunol. 2022, 43, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Frommer, K.W.; Hasseli, R.; Schäffler, A.; Lange, U.; Rehart, S.; Steinmeyer, J.; Rickert, M.; Sarter, K.; Zaiss, M.M.; Culmsee, C.; et al. Free Fatty Acids in Bone Pathophysiology of Rheumatic Diseases. Front. Immunol. 2019, 10, 2757. [Google Scholar] [CrossRef] [PubMed]
- Han, X. Lipidomics for studying metabolism. Nat. Rev. Endocrinol. 2016, 12, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Kind, T.; Cajka, T.; Hazen, S.L.; Tang, W.H.W.; Kaddurah-Daouk, R.; Irvin, M.R.; Arnett, D.K.; Barupal, D.K.; Fiehn, O. Systematic Error Removal Using Random Forest for Normalizing Large-Scale Untargeted Lipidomics Data. Anal. Chem. 2019, 91, 3590–3596. [Google Scholar] [CrossRef] [PubMed]
- Navas-Iglesias, N.; Carrasco-Pancorbo, A.; Cuadros-Rodriguez, L. From lipids analysis towards lipidomics, a new challenge for the analytical chemistry of the 21st century. Part II: Analytical lipidomics. Trac-Trends Anal. Chem. 2009, 28, 393–403. [Google Scholar] [CrossRef]
- Xu, T.; Hu, C.; Xuan, Q.; Xu, G. Recent advances in analytical strategies for mass spectrometry-based lipidomics. Anal. Chim. Acta 2020, 1137, 156–169. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367. [Google Scholar] [CrossRef]
- Heinrich, U.; Koop, U.; Leneveu-Duchemin, M.C.; Osterrieder, K.; Bielfeldt, S.; Chkarnat, C.; Degwert, J.; Hantschel, D.; Jaspers, S.; Nissen, H.P.; et al. Multicentre comparison of skin hydration in terms of physical-, physiological- and product-dependent parameters by the capacitive method (Corneometer CM 825). Int. J. Cosmet. Sci. 2003, 25, 45–53. [Google Scholar] [CrossRef]
- Keurentjes, A.J.; Jakasa, I.; Kezic, S. Research Techniques Made Simple: Stratum Corneum Tape Stripping. J. Investig. Dermatol. 2021, 141, 1129–1133. [Google Scholar] [CrossRef]
- Cajka, T.; Fiehn, O. Comprehensive analysis of lipids in biological systems by liquid chromatography-mass spectrometry. Trac-Trends Anal. Chem. 2014, 61, 192–206. [Google Scholar] [CrossRef]
- Su, Q.; Hu, X.; Yang, M.; He, H.; Jia, Y. Lipidomic analysis of facial skin surface lipids in acne in young women. Int. J. Cosmet. Sci. 2024, 46, 424–436. [Google Scholar] [CrossRef]
- Ayaki, S.; Mii, T.; Matsuno, K.; Tokuyama, T.; Tokuyama, T.; Tokuyama, T.; Uyama, T.; Ueda, N. β-1,4-Galactan suppresses lipid synthesis in sebaceous gland cells via TLR4. J. Biochem. 2023, 173, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Kirsten, N.; Körber, A.; Wilsmann-Theis, D.; Itschert, G.; Staubach-Renz, P.; Maul, J.-T.; Zander, N. Prevalence, predictors and comorbidity of dry skin in the general population. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 147–150. [Google Scholar] [CrossRef]
- Mekic, S.; Jacobs, L.C.; Gunn, D.A.; Mayes, A.E.; Ikram, M.A.; Pardo, L.M.; Nijsten, T. Prevalence and determinants for xerosis cutis in the middle-aged and elderly population: A cross-sectional study. J. Am. Acad. Dermatol. 2019, 81, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Roussaki-Schulze, A.V.; Zafiriou, E.; Nikoulis, D.; Klimi, E.; Rallis, E.; Zintzaras, E. Objective biophysical findings in patients with sensitive skin. Drugs Under Exp. Clin. Res. 2005, 31, 17–24. [Google Scholar]
- Sun, S.-J.; Tu, Y.; Chen, S.-H.; Yang, Z. Physiometry measurement of sensitive skin due to different causes and its clinical significance. J. Clin. Dermatol. 2010, 39, 695–697. [Google Scholar]
- Cui, L.; He, C.; Fan, L.; Jia, Y. Application of lipidomics to reveal differences in facial skin surface lipids between males and females. J. Cosmet. Dermatol. 2018, 17, 1254–1261. [Google Scholar] [CrossRef]
- Uchida, Y.; Park, K. Ceramides in Skin Health and Disease: An Update. Am. J. Clin. Dermatol. 2021, 22, 853–866. [Google Scholar] [CrossRef]
- Luczaj, W.; Wronski, A.; Domingues, P.; Domingues, M.R.; Skrzydlewska, E. Lipidomic Analysis Reveals Specific Differences between Fibroblast and Keratinocyte Ceramide Profile of Patients with Psoriasis Vulgaris. Molecules 2020, 25, 630. [Google Scholar] [CrossRef]
- Kendall, A.C.; Koszyczarek, M.M.; Jones, E.A.; Hart, P.J.; Towers, M.; Griffiths, C.E.M.; Morris, M.; Nicolaou, A. Lipidomics for translational skin research: A primer for the uninitiated. Exp. Dermatol. 2018, 27, 721–728. [Google Scholar] [CrossRef]
- Meckfessel, M.H.; Brandt, S. The structure, function, and importance of ceramides in skin and their use as therapeutic agents in skin-care products. J. Am. Acad. Dermatol. 2014, 71, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Zhi, L.; You, B.; Wang, G.; Wu, N.; Jia, Y. Skin homeostasis: Mechanism and influencing factors. J. Cosmet. Dermatol. 2024, 23, 1518–1526. [Google Scholar] [CrossRef]
- Baroni, A.; Perfetto, B.; Paoletti, I.; Ruocco, E.; Canozo, N.; Orlando, M.; Buommino, E. Malassezia furfur invasiveness in a keratinocyte cell line (HaCat): Effects on cytoskeleton and on adhesion molecule and cytokine expression. Arch. Dermatol. Res. 2001, 293, 414–419. [Google Scholar] [CrossRef]
- Lin, T.-K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2018, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Liu, J.; Li, H.; Sun, J.; Ding, W.; He, C.; Jia, Y. Lipidomics-based analysis of lipid differences between dry skin of women aged 22–28 years and 29–35 years. J. Cosmet. Dermatol. 2024, 23, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, J.; Mao, Z. Lipid Differences and Related Metabolism Present on the Hand Skin Surface of Different-Aged Asiatic Females-An Untargeted Metabolomics Study. Metabolites 2023, 13, 553. [Google Scholar] [CrossRef]
- Coderch, L.; López, O.; de la Maza, A.; Parra, J.L. Ceramides and skin function. Am. J. Clin. Dermatol. 2003, 4, 107–129. [Google Scholar] [CrossRef]
- Murphy, B.; Grimshaw, S.; Hoptroff, M.; Paterson, S.; Arnold, D.; Cawley, A.; Adams, S.E.; Falciani, F.; Dadd, T.; Eccles, R.; et al. Alteration of barrier properties, stratum corneum ceramides and microbiome composition in response to lotion application on cosmetic dry skin. Sci. Rep. 2022, 12, 5223. [Google Scholar] [CrossRef]
| Physiological Indicators | SDS | SOS | HS | P | ||
|---|---|---|---|---|---|---|
| SDS HS | SOS HS | SDS SOS | ||||
| Oil content (μg/cm2) | 38.22 ± 26.18 | 105.23 ± 52.93 | 45.26 ± 24.38 | 0.468 | <0.05 | <0.05 |
| Stratum corneum water content (a.u.) | 40.10 ± 9.32 | 52.69 ± 12.65 | 57.16 ± 7.96 | 0.141 | <0.05 | <0.05 |
| TEWL (g/m2 h) | 10.88 ± 2.79 | 10.13 ± 2.28 | 9.26 ± 2.48 | 0.618 | 0.343 | 0.173 |
| Lipid Type | Description | Formula | M/Z | p Value | FC | Highest Mean |
|---|---|---|---|---|---|---|
| GP | PS (P-20:0/21:0) | C47H92NO9P | 863.6881942 | 0.000 | 4.041 | SDS |
| PI (P-20:0/22:4(7Z,10Z,13Z,16Z)) | C51H91O12P | 944.6623105 | 0.000 | 3.374 | ||
| OHOOA-PS | C32H56NO12P | 678.3626075 | 0.000 | 3.362 | ||
| GL | TG (20:5(5Z,8Z,11Z,14Z,17Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)/20:5(5Z,8Z,11Z,14Z,17Z)) (d5) | C65H89D5O6 | 998.729861 | 0.000 | 3.386 | |
| PK | Protorifamycin I | C35H45NO10 | 622.2994686 | 0.000 | 3.839 | |
| PR | Glucopyranosyl-1-O-(4,4′-diapo-7′,8′,11′,12′-tetrahydrolycopen-4-oate)-6-O-(2-methylbutanoate) | C41H60O8 | 698.4636448 | 0.038 | 15.493 | |
| ST | 1alpha,25-dihydroxy-2beta-(6-hydroxyhexyl) vitamin D3 | C33H56O4 | 534.4499808 | 0.000 | 3.869 | |
| Estradiol-17beta 3-sulfate | C18H24O5S | 353.1432017 | 0.000 | 3.311 | ||
| GP | PA (O-16:0/13:0) | C32H65O7P | 610.4798065 | 0.006 | 11.392 | HS |
| PE (P-20:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | C47H82NO7P | 786.5796597 | 0.002 | 8.194 | ||
| PI (P-16:0/20:4(5Z,8Z,11Z,14Z)) | C45H79O12P | 825.5259817 | 0.003 | 7.818 | ||
| PC (O-16:0/0:0) | C24H52NO6P | 499.3872606 | 0.002 | 7.449 | ||
| PG (O-20:0/14:1(9Z)) | C40H79O9P | 752.5815589 | 0.006 | 7.131 | ||
| PG (O-16:0/12:0) | C34H69O9P | 653.4732924 | 0.006 | 6.24 | ||
| PC (18:4(6Z,9Z,12Z,15Z)/20:1(11Z)) | C46H82NO8P | 830.5638364 | 0.007 | 3.72 | ||
| PA (P-16:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | C41H69O7P | 687.4776526 | 0.000 | 3.629 | ||
| GL | 1-O-(2R-hydroxy-tetradecyl)-sn-glycerol | C17H36O4 | 327.2505918 | 0.036 | 10.465 | |
| 1-O-(2R-hydroxy-heneicosanyl)-sn-glycerol | C24H50O4 | 425.3594323 | 0.001 | 7.783 | ||
| MGMG (16:3(7Z,10Z,13Z)/0:0) | C25H42O9 | 485.2773161 | 0.017 | 7.035 | ||
| MGDG (18:3(9Z,12Z,15Z)/18:4(6Z,9Z,12Z,15Z)) | C45H72O9 | 774.5488086 | 0.000 | 4.139 | ||
| DG (12:0/16:1(9Z)/0:0) [iso2] | C31H58O5 | 493.4229286 | 0.000 | 4.068 | ||
| 1-O-(2R-hydroxy-docosanyl)-sn-glycerol | C25H52O4 | 439.3755923 | 0.035 | 3.824 | ||
| 1-O-(2R-hydroxy-eicosanyl)-sn-glycerol | C23H48O4 | 411.3449934 | 0.026 | 3.757 | ||
| 2-(8-[5]-ladderane-octanyl)-sn-glycerol | C23H38O3 | 385.2705996 | 0.003 | 3.419 | ||
| DG (12:0/16:1(9Z)/0:0) [iso2] | C31H58O5 | 493.4229286 | 0.000 | 4.068 | ||
| 1-O-(2R-hydroxy-docosanyl)-sn-glycerol | C25H52O4 | 439.3755923 | 0.035 | 3.824 | ||
| 1-O-(2R-hydroxy-eicosanyl)-sn-glycerol | C23H48O4 | 411.3449934 | 0.026 | 3.757 | ||
| 2-(8-[5]-ladderane-octanyl)-sn-glycerol | C23H38O3 | 385.2705996 | 0.003 | 3.419 | ||
| DG (14:0/16:1(9Z)/0:0) [iso2] | C33H62O5 | 521.4540388 | 0.000 | 3.369 | ||
| PK | Poinsettifolin B | C30H34O5 | 497.2314437 | 0.018 | 27.124 | |
| Uvaricin | C39H68O7 | 671.4831433 | 0.007 | 3.507 | ||
| SP | Cer (d18:1/27:0) | C45H89NO3 | 674.6836807 | 0.001 | 10.722 | |
| N,N,N-trimethyl-sphingosine | C21H44NO2+ | 360.3720076 | 0.008 | 3.897 | ||
| PR | Zeaxanthin sulfate | C40H56O5S | 666.4216044 | 0.038 | 4.422 | |
| Ustusolate G | C21H28O7 | 415.1726074 | 0.000 | 4.294 | ||
| Rhodoquinone-9 | C53H81NO3 | 797.6572202 | 0.000 | 3.539 | ||
| ST | Limnantheoside C | C38H62O16 | 775.4124783 | 0.013 | 6.923 | |
| 1alpha,2alpha:4alpha,5alpha-diepoxy-3alpha,6beta-dihydroxy-ergosta-7,24(28)-dien-6-one-21-oic acid | C28H40O6 | 490.3169262 | 0.000 | 6.343 | ||
| 19-oxodesacetylcinobufagin | C24H30O6 | 437.1944656 | 0.000 | 5.737 | ||
| 1beta,3beta,5alpha,6beta-tetrahydroxyandrostan-17-one | C19H30O5 | 337.2034079 | 0.001 | 5.335 | ||
| Suberoretisteroid E | C29H44O7 | 527.2968084 | 0.000 | 4.221 | ||
| Lamellosterol A | C27H48O7S | 499.3110574 | 0.003 | 4.076 | ||
| Fasciculic acid C | C38H63NO11 | 710.4474808 | 0.029 | 3.865 | ||
| Vitamin D3 glucosiduronate | C33H52O7 | 583.3593119 | 0.000 | 3.452 | ||
| Bufogarlide A | C24H26O4 | 379.192273 | 0.009 | 3.302 | ||
| FA | Oleoyl-EA (d2) | C20H37D2NO2 | 310.3084792 | 0.004 | 7.741 | |
| karalicin | C14H20O6 | 267.121575 | 0.000 | 7.708 | ||
| (Z)-7-(5-((1E,3E,6Z,9Z)-dodeca-1,3,6,9-tetraen-1-yl)-1,2-dioxolan-3-yl)-7-hydroperoxyhept-4-enoic acid | C22H32O6 | 415.2071569 | 0.000 | 5.301 | ||
| 5-hydroperoxy-7-[3,5-epidioxy-2-(2-octenyl)-cyclopentyl]-6-heptenoic acid | C19H30O6 | 372.2395883 | 0.002 | 5.256 | ||
| 2E,4E-undecadien-8,10-diynoic acid phenethylamide | C19H19NO | 553.2839167 | 0.008 | 4.162 | ||
| Lanoceric acid | C30H60O4 | 507.4382958 | 0.000 | 4.03 | ||
| Bromovulone I | C21H29BrO4 | 425.1328207 | 0.000 | 3.754 | ||
| Erucamide | C22H43NO | 360.3234737 | 0.000 | 3.644 | ||
| O-(17-carboxyheptadecanoyl) carnitine | C25H47NO6 | 458.3453896 | 0.039 | 3.441 |
| Lipid Type | Description | Formula | M/Z | p Value | FC | Highest Mean |
|---|---|---|---|---|---|---|
| GP | PS (P-20:0/21:0) | C47H92NO9P | 863.6882 | 0.000 | 2.954 | SDS |
| PI (O-20:0/20:0) | C49H97O12P | 926.7039 | 0.000 | 2.336 | ||
| GL | TG (20:5(5Z,8Z,11Z,14Z,17Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)/20:5(5Z,8Z,11Z,14Z,17Z)) (d5) | C65H89D5O6 | 998.7299 | 0.000 | 2.948 | |
| DG (19:0/0:0/19:0) (d5) | C41H75D5O5 | 658.6372 | 0.000 | 2.269 | ||
| SP | Cer (d18:1/28:0) | C46H91NO3 | 688.6937 | 0.005 | 6.736 | |
| Cer (m18:1(4E)/26:1(17Z)) | C44H85NO2 | 660.6625 | 0.003 | 4.378 | ||
| Cer (t18:0/27:0(2OH)) | C45H91NO5 | 708.6876 | 0.000 | 3.732 | ||
| Cer (t20:0/26:0) | C46H93NO4 | 706.704 | 0.003 | 3.424 | ||
| Hypoculoside | C30H61NO8 | 586.4293 | 0.000 | 3.417 | ||
| 1-O-carboceroyl-Cer (d18:1/18:0) | C64H125NO4 | 994.9454 | 0.001 | 3.241 | ||
| Cer(d18:1/29:0) | C47H93NO3 | 702.7119 | 0.031 | 3.196 | ||
| Cer(d18:2/25:0) | C43H83NO3 | 662.6417 | 0.000 | 2.798 | ||
| Cer(d18:2/15:0) | C33H63NO3 | 522.4865 | 0.000 | 2.617 | ||
| Cer(d18:0/16:0) | C34H69NO3 | 540.5373 | 0.002 | 2.549 | ||
| Cer(t18:0/21:0) | C39H79NO4 | 626.6078 | 0.000 | 2.297 | ||
| PR | Glucopyranosyl-1-O-(4,4′-diapo-7′,8′,11′,12′-tetrahydrolycopen-4-oate)-6-O-(2-methylbutanoate) | C41H60O8 | 698.4636 | 0.047 | 10.917 | |
| Menaquinone-9 | C56H80O2 | 767.6089 | 0.000 | 2.578 | ||
| FA | N-(20-hydroxyarachidoyl)-5-hydroxytryptamine | C30H50N2O3 | 509.3724 | 0.000 | 4.233 | |
| ascr#11 | C10H18O6 | 235.1183 | 0.005 | 2.831 | ||
| GP | PI(P-16:0/20:4(5Z,8Z,11Z,14Z)) | C45H79O12P | 825.526 | 0.005 | 8.912 | SOS |
| PG(O-16:0/12:0) | C34H69O9P | 653.4733 | 0.009 | 5.681 | ||
| PA(O-16:0/13:0) | C32H65O7P | 610.4798 | 0.024 | 5.02 | ||
| PE(P-20:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | C47H82NO7P | 786.5797 | 0.026 | 3.527 | ||
| PC(18:4(6Z,9Z,12Z,15Z)/20:1(11Z)) | C46H82NO8P | 830.5638 | 0.045 | 3.463 | ||
| PA(P-16:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | C41H69O7P | 687.4777 | 0.002 | 2.36 | ||
| GL | MGTS(18:0/0:0) | C28H55NO6 | 519.4373 | 0.000 | 5.414 | |
| DG(13:0/16:1(9Z)/0:0)[iso2] | C32H60O5 | 507.4397 | 0.000 | 4.129 | ||
| MGDG(18:3(9Z,12Z,15Z)/18:4(6Z,9Z,12Z,15Z)) | C45H72O9 | 774.5488 | 0.010 | 3.072 | ||
| DG(12:0/16:1(9Z)/0:0)[iso2] | C31H58O5 | 493.4229 | 0.000 | 2.968 | ||
| DG(O-16:0/18:1(9Z)) | C37H72O4 | 603.5314 | 0.005 | 2.642 | ||
| TG(22:5(7Z,10Z,13Z,16Z,19Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z))[iso3] | C69H100O6 | 513.3835 | 0.043 | 2.48 | ||
| PK | Poinsettifolin B | C30H34O5 | 497.2314 | 0.008 | 14.435 | |
| catechin 3-dodecanoate | C27H36O7 | 495.235 | 0.002 | 2.833 | ||
| 5-((5Z,8Z,11Z,14Z)-heptadeca-5,8,11,14-tetraen-1-yl)resorcinol | C23H32O2 | 339.2318 | 0.001 | 2.799 | ||
| SP | Cer(d18:1/27:0) | C45H89NO3 | 674.6837 | 0.004 | 5.548 | |
| Rhizochalinin D | C35H70N2O8 | 647.5215 | 0.007 | 4.151 | ||
| N,N,N-trimethyl-sphingosine | C21H44NO2+ | 360.372 | 0.006 | 3.882 | ||
| PR | Nonaflavuxanthin | C45H62O | 601.4773 | 0.002 | 7.145 | |
| Rhodoquinone-9 | C53H81NO3 | 797.6572 | 0.001 | 2.981 | ||
| Ustusolate G | C21H28O7 | 415.1726 | 0.003 | 2.616 | ||
| Glisoprenin B | C45H82O6 | 741.5997 | 0.001 | 2.582 | ||
| 2,2′-Diapocarotene-dial | C34H40O2 | 463.3014 | 0.001 | 2.344 | ||
| ST | 19-oxodesacetylcinobufagin | C24H30O6 | 437.1945 | 0.020 | 3.551 | |
| 1alpha,2alpha:4alpha,5alpha-diepoxy-3alpha,6beta-dihydroxy-ergosta-7,24(28)-dien-6-one-21-oic acid | C28H40O6 | 490.3169 | 0.019 | 3.155 | ||
| Lamellosterol A | C27H48O7S | 499.3111 | 0.008 | 2.648 | ||
| Suberoretisteroid E | C29H44O7 | 527.2968 | 0.003 | 2.565 | ||
| FA | O-(17-carboxyheptadecanoyl)carnitine | C25H47NO6 | 458.3454 | 0.017 | 3.963 | |
| (Z)-7-(5-((1E,3E,6Z,9Z)-dodeca-1,3,6,9-tetraen-1-yl)-1,2-dioxolan-3-yl)-7-hydroperoxyhept-4-enoic acid | C22H32O6 | 415.2072 | 0.009 | 3.684 | ||
| Lanoceric acid | C30H60O4 | 507.4383 | 0.000 | 2.794 | ||
| Bromovulone I | C21H29BrO4 | 425.1328 | 0.010 | 2.274 |
| Lipid Type | Description | Formula | M/Z | p Value | FC | Highest Mean |
|---|---|---|---|---|---|---|
| PK | Lasalocid A | C34H54O8 | 608.41604 | 0.000 | 2.171 | HS |
| GL | TG(16:0/18:1(9Z)/20:2(11Z,14Z))[iso6] | C57H104O6 | 902.8153729 | 0.027 | 3.293 | SDS |
| SP | Cer(d18:1/23:0) | C41H81NO3 | 618.6210692 | 0.004 | 6.737 | |
| Cer(d18:1/25:0) | C43H85NO3 | 646.6491518 | 0.001 | 4.458 | ||
| Cer(t18:1(6OH)/23:0(2OH)) | C41H81NO5 | 650.6071059 | 0.031 | 3.468 | ||
| Cer(t18:0/23:0) | C41H83NO4 | 654.6418605 | 0.035 | 2.468 | ||
| FA | (21-Methyl-8Z-pentatriacontene | C36H72 | 522.5977113 | 0.024 | 3.519 |
| Lipid Type | Description | Formula | M/Z | p Value | FC | Highest Mean |
|---|---|---|---|---|---|---|
| SP | Cer (d18:1/23:0) | C41H81NO3 | 618.6210692 | 0.004 | 7.471 | SDS |
| Cer (t18:1(6OH)/23:0(2OH)) | C41H81NO5 | 650.6071059 | 0.011 | 6.569 | ||
| Cer (d18:1/25:0) | C43H85NO3 | 646.6491518 | 0.001 | 4.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, A.; Zhang, X.; Huang, Q.; Wu, J. Utilizing Untargeted Lipidomics Technology to Elucidate Differences in Lipid Compositions Among Sensitive Dry, Sensitive Oily and Healthy Skin Types. Metabolites 2025, 15, 292. https://doi.org/10.3390/metabo15050292
Xie A, Zhang X, Huang Q, Wu J. Utilizing Untargeted Lipidomics Technology to Elucidate Differences in Lipid Compositions Among Sensitive Dry, Sensitive Oily and Healthy Skin Types. Metabolites. 2025; 15(5):292. https://doi.org/10.3390/metabo15050292
Chicago/Turabian StyleXie, Agui, Xingjiang Zhang, Qing Huang, and Jianxin Wu. 2025. "Utilizing Untargeted Lipidomics Technology to Elucidate Differences in Lipid Compositions Among Sensitive Dry, Sensitive Oily and Healthy Skin Types" Metabolites 15, no. 5: 292. https://doi.org/10.3390/metabo15050292
APA StyleXie, A., Zhang, X., Huang, Q., & Wu, J. (2025). Utilizing Untargeted Lipidomics Technology to Elucidate Differences in Lipid Compositions Among Sensitive Dry, Sensitive Oily and Healthy Skin Types. Metabolites, 15(5), 292. https://doi.org/10.3390/metabo15050292





