Development and Application of an In-Capillary CE-DAD Method for the Inhibitory Screening of Natural Extracts Towards Acetylcholinesterase Enzyme
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Sample Preparation
2.2.1. Stock and Working Solutions
2.2.2. Plant Extracts Preparation
2.3. Electrophoretic System and Methods
2.3.1. Instrumentation and Operating Conditions
2.3.2. Injection Method
2.4. Kinetic Study
2.5. Inhibition Study
2.6. Screening of Plant Extracts
3. Results
3.1. Kinetic Studies
3.2. Inhibition Studies
3.3. Evaluation of Inhibitory Activity of Natural Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AChE | Acetylcholinesterase |
| CZE-UV/Vis | Electrophoresis method with direct ultraviolet detection |
| DTNB | [5,5′-dithiobis-(2-nitrobenzoic acid)] |
| ACh | Acetylcholine |
| AChEI | Inhibitors of the enzyme acetylcholinesterase |
| CE | Capillary electrophoresis |
| CZE-UV | UV-detection capillary zone electrophoresis |
| EMMA | Electrophoretic mediated microanalysis |
| BGE | Background electrolyte |
| IMER | Immobilised enzymatic micro reactor |
| LbL | Layer-by-layer |
| AThCh | Acetylthiocholine |
| DMC | Dichloromethane |
| ACN | Acetonitrile |
| TNB | 2-nitro-5-thiobenzoic acid |
References
- Kim, A.Y.; Al Jerdi, S.; MacDonald, R.; Triggle, C.R. Alzheimer’s Disease and Its Treatment–Yesterday, Today, and Tomorrow. Front. Pharmacol. 2024, 15, 1399121. [Google Scholar] [CrossRef] [PubMed]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of Acetylcholinesterase Inhibitors in Alzheimer’s Disease. Neuropharmacology 2021, 190, 177–193. [Google Scholar] [CrossRef]
- Murray, A.P.; Biscussi, B.; Cavallaro, V.; Donozo, M.; Rodriguez, S.A. Naturally Occurring Cholinesterase Inhibitors from Plants, Fungi, Algae, and Animals: A Review of the Most Effective Inhibitors Reported in 2012–2022. Curr. Neuropharmacol. 2023, 22, 1621–1649. [Google Scholar] [CrossRef]
- Anand, P.; Singh, B. A Review on Cholinesterase Inhibitors for Alzheimer’s Disease. Arch. Pharm. Res. 2013, 36, 375–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, K.; Adams, E.; Schepdael, A. Van Recent Advances in CE-Mediated Microanalysis for Enzyme Study. Electrophoresis 2014, 35, 119–127. [Google Scholar] [CrossRef]
- Bartolini, M.; Bertucci, C.; Gotti, R.; Tumiatti, V.; Cavalli, A.; Recanatini, M.; Andrisanò, V.A. D Etermination of the Dissociation Constants (PK) of Basic a Acetylcholinesterase Inhibitors by Reversed-Phase Liquid Chromatography. J. Chromatogr. A 2002, 958, 59–67. [Google Scholar] [CrossRef]
- Cheng, M.; Chen, Z. Recent Advances in Screening of Enzymes Inhibitors Based on Capillary Electrophoresis. J. Pharm. Anal. 2018, 8, 226–233. [Google Scholar] [CrossRef]
- Scriba, G.K.E.; Belal, F. Advances in Capillary Electrophoresis-Based Enzyme Assays. Chromatographia 2015, 78, 947–970. [Google Scholar] [CrossRef]
- Gattu, S.; Crihfield, C.L.; Lu, G.; Bwanali, L.; Veltri, L.M.; Holland, L.A. Advances in Enzyme Substrate Analysis with Capillary Electrophoresis. Methods 2018, 146, 93–106. [Google Scholar] [CrossRef]
- Zhao, H.H.; Liu, Y.Q.; Chen, J. Screening Acetylcholinesterase Inhibitors from Traditional Chinese Medicines by Paper-Immobilized Enzyme Combined with Capillary Electrophoresis Analysis. J. Pharm. Biomed. Anal. 2020, 190, 113547. [Google Scholar] [CrossRef]
- Rozenski, J.; Asfaw, A.A.; Van Schepdael, A. Overview of In-Capillary Enzymatic Reactions Using Capillary Electrophoresis. Electrophoresis 2022, 43, 57–73. [Google Scholar] [CrossRef]
- Nagori, K.; Nakhate, K.T.; Yadav, K.; Ajazuddin; Pradhan, M. Unlocking the Therapeutic Potential of Medicinal Plants for Alzheimer’s Disease: Preclinical to Clinical Trial Insights. Future Pharmacol. 2023, 3, 877–907. [Google Scholar] [CrossRef]
- Siebert, D.A.; Caon, N.B.; Alberton, M.D.; Vitali, L.; Parize, A.L.; Micke, G.A. Immobilized Acetylcholinesterase in Magnetic Nanoparticles for In-Line Inhibition Studies Using a Capillary Electrophoresis System. Anal. Chim. Acta. 2023, 1275, 341566. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, J.; Yang, L. Capillary Electrophoresis-Integrated Immobilized Enzyme Reactors. Rev. Anal. Chem. 2016, 35, 115–131. [Google Scholar] [CrossRef]
- Min, W.; Wang, W.; Chen, J.; Wang, A.; Hu, Z. On-Line Immobilized Acetylcholinesterase Microreactor for Screening of Inhibitors from Natural Extracts by Capillary Electrophoresis. Anal. Bioanal. Chem. 2012, 404, 2397–2405. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, T.; Kang, J. Immobilized Capillary Enzyme Reactor Based on Layer-by-Layer Assembling Acetylcholinesterase for Inhibitor Screening by CE. Electrophoresis 2007, 28, 2981–2987. [Google Scholar] [CrossRef] [PubMed]
- Van Nguyen, H.; Trong Le, N.; Nguyen Le, N.T.; Duong, T.D.; Thuc Le, T.; Thi Nguyen, H.T.; Phung, H.T.; Thi Nguyen, H. Extraction, Purification, and Evaluation of Bioactivities of Total Triterpenoids from Persimmon (Diospyros kaki L.f.) Leaves. Process Biochem. 2024, 139, 70–80. [Google Scholar] [CrossRef]
- Campos, G.P.; Lima, N.M.; Campos, J.S.; Micke, G.A.; de Oliveira, M.A.L. Alternative Method for Microenzymatic Inhibition Activities Monitoring from Baccharis Trimera Extracts by CZE-UV. Phytochem. Anal. 2022, 33, 1190–1197. [Google Scholar] [CrossRef]
- Calleri, E.; Temporini, C.; Perani, E.; Stella, C.; Rudaz, S.; Lubda, D.; Mellerio, G.; Veuthey, J.L.; Caccialanza, G.; Massolini, G. Development of a Bioreactor Based on Trypsin Immobilized on Monolithic Support for the On-Line Digestion and Identification of Proteins. J. Chromatogr. A 2004, 1045, 99–109. [Google Scholar] [CrossRef]
- Andrisano, V.; Bartolini, M.; Gotti, R.; Cavrini, V.; Felix, G. Determination of Inhibitors’ Potency (IC) by a Direct High-50 Performance Liquid Chromatographic Method on an Immobilised Acetylcholinesterase Column. J. Chromatogr. B Biomed. Sci. Appl. 2001, 753, 375–383. [Google Scholar] [CrossRef]
- Siebert, D.A.; Campos, J.S.; Alberton, M.D.; Vitali, L.; Micke, G.A. Dual Electrophoretically-Mediated Microanalysis in Multiple Injection Mode for the Simultaneous Determination of Acetylcholinesterase and α-Glucosidase Activity Applied to Selected Polyphenols. Talanta 2021, 224, 121773. [Google Scholar] [CrossRef] [PubMed]
- Ressaissi, A.; Mannai, A.; Ben-Attia, M.; Serralheiro, M.L.; El-Bok, S. Diospyros Kaki Leaves Decoction Phytochemical Characterization and Bioactivities Evaluation: LC-MS-Qtof Identification, Antioxidant Activity, Enzyme Inhibition and Cytotoxicity Toward HepG2 and Mcf-7 Cell Lines. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Jeong, D.W.; Cho, C.H.; Lee, J.S.; Lee, S.H.; Kim, T.; Kim, D.O. Deastringent Peel Extracts of Persimmon (Diospyros kaki Thunb. Cv. Cheongdo-Bansi) Protect Neuronal PC-12 and SH-SY5Y Cells against Oxidative Stress. J. Microbiol. Biotechnol. 2018, 28, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Rostamiasrabadi, P.; Shahpiri, Z.; Marques, A.M.; Rahimi, R.; Farzaei, M.H. Aloysia Citrodora Paláu (Lemon verbena): A Review of Phytochemistry and Pharmacology. J. Ethnopharmacol. 2018, 222, 34–51. [Google Scholar] [CrossRef]
- Haryalchi, K.; Kazemi Aski, S.; Mansour Ghanaie, M.; Fotouhi, M.; Mansoori, R.; Sadraei, S.M.; Yaghobi, Y.; Olangian-Tehrani, S. Effects of the Aroma of Lemone Verbena (Aloysia citriodora Paláu) Essential Oil on Anxiety and the Hemodynamic Profile before Cesarean Section: A Randomized Clinical Trial. Health Sci. Rep. 2023, 6, e1282. [Google Scholar] [CrossRef]
- Afrasiabian, F.; Mirabzadeh Ardakani, M.; Rahmani, K.; Azadi, N.A.; Alemohammad, Z.B.; Bidaki, R.; Karimi, M.; Emtiazy, M.; Hashempur, M.H. Aloysia Citriodora Palau (Lemon verbena) for Insomnia Patients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Efficacy and Safety. Phytother. Res. 2019, 33, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Politeo, O.; Cajic, I.; Simic, A.; Ruscic, M.; Bektasevic, M. Comparative Study of Chemical Composition and Cholinesterase Inhibition Potential of Essential Oils Isolated from Artemisia Plants from Croatia. Separations 2023, 10, 546. [Google Scholar] [CrossRef]
- Chougouo, R.D.K.; Nguekeu, Y.M.M.; Dzoyem, J.P.; Awouafack, M.D.; Kouamouo, J.; Tane, P.; McGaw, L.J.; Eloff, J.N. Anti-Inflammatory and Acetylcholinesterase Activity of Extract, Fractions and Five Compounds Isolated from the Leaves and Twigs of Artemisia Annua Growing in Cameroon. Springerplus 2016, 5, 1–7. [Google Scholar] [CrossRef]
- Dong, X.; Zhou, S.; Nao, J. Kaempferol as a Therapeutic Agent in Alzheimer’s Disease: Evidence from Preclinical Studies. Ageing Res. Rev. 2023, 87, 101910. [Google Scholar] [CrossRef]
- Liao, Y.; Mai, X.; Wu, X.; Hu, X.; Luo, X.; Zhang, G. Exploring the Inhibition of Quercetin on Acetylcholinesterase by Multispectroscopic and In Silico Approaches and Evaluation of Its Neuroprotective Effects on PC12 Cells. Molecules 2022, 27, 7971. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 2020, 10, 59. [Google Scholar] [CrossRef] [PubMed]

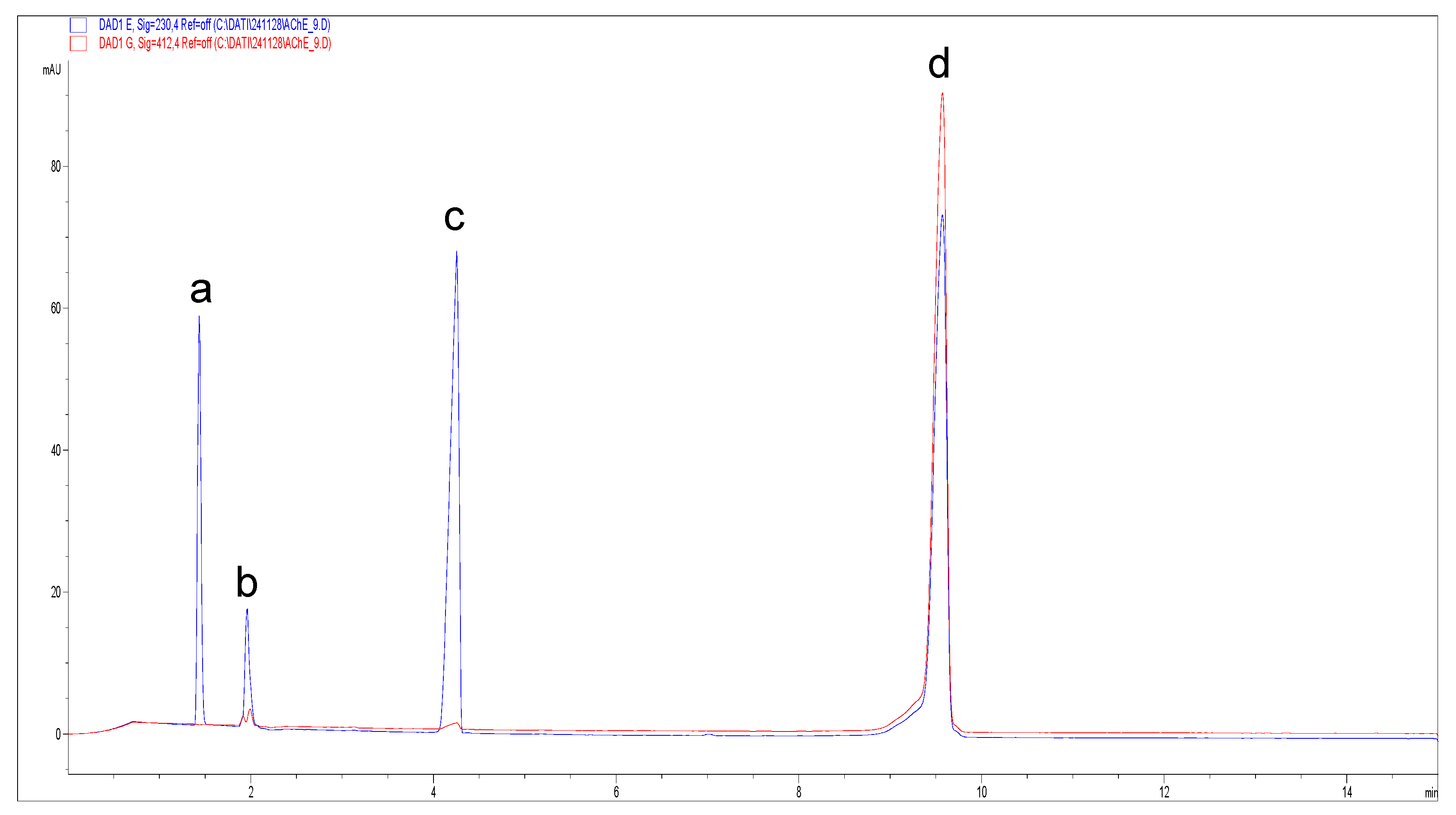
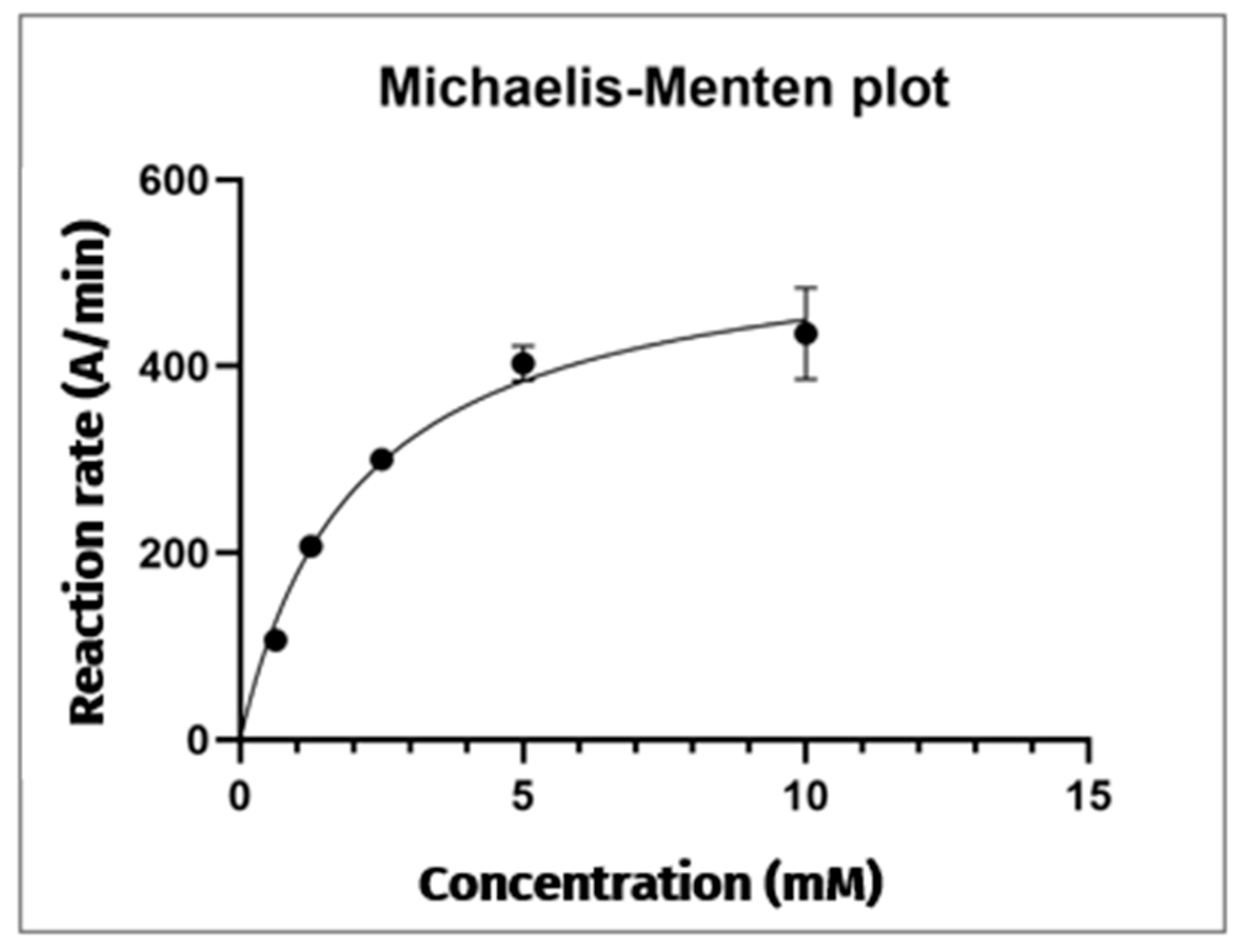
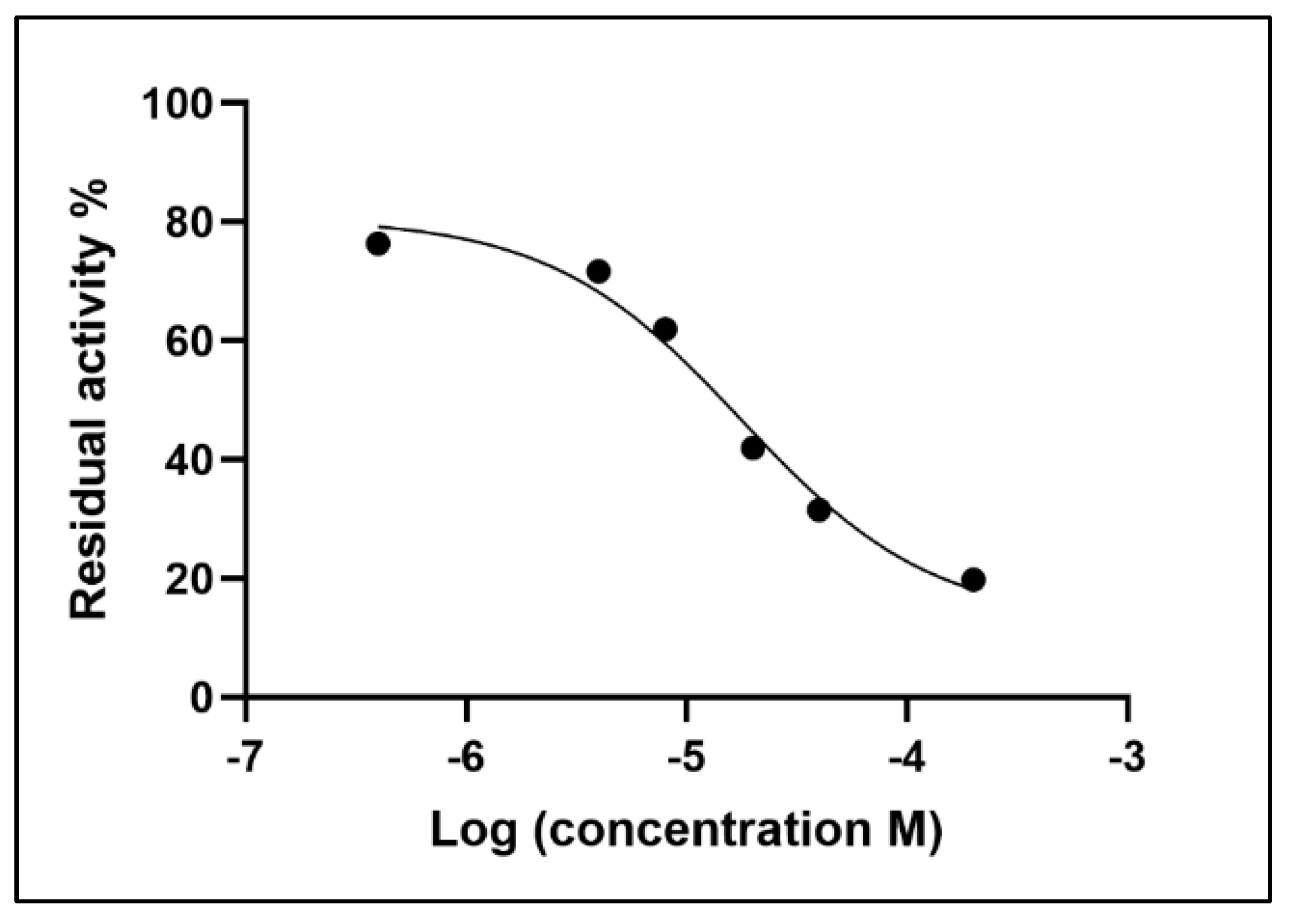

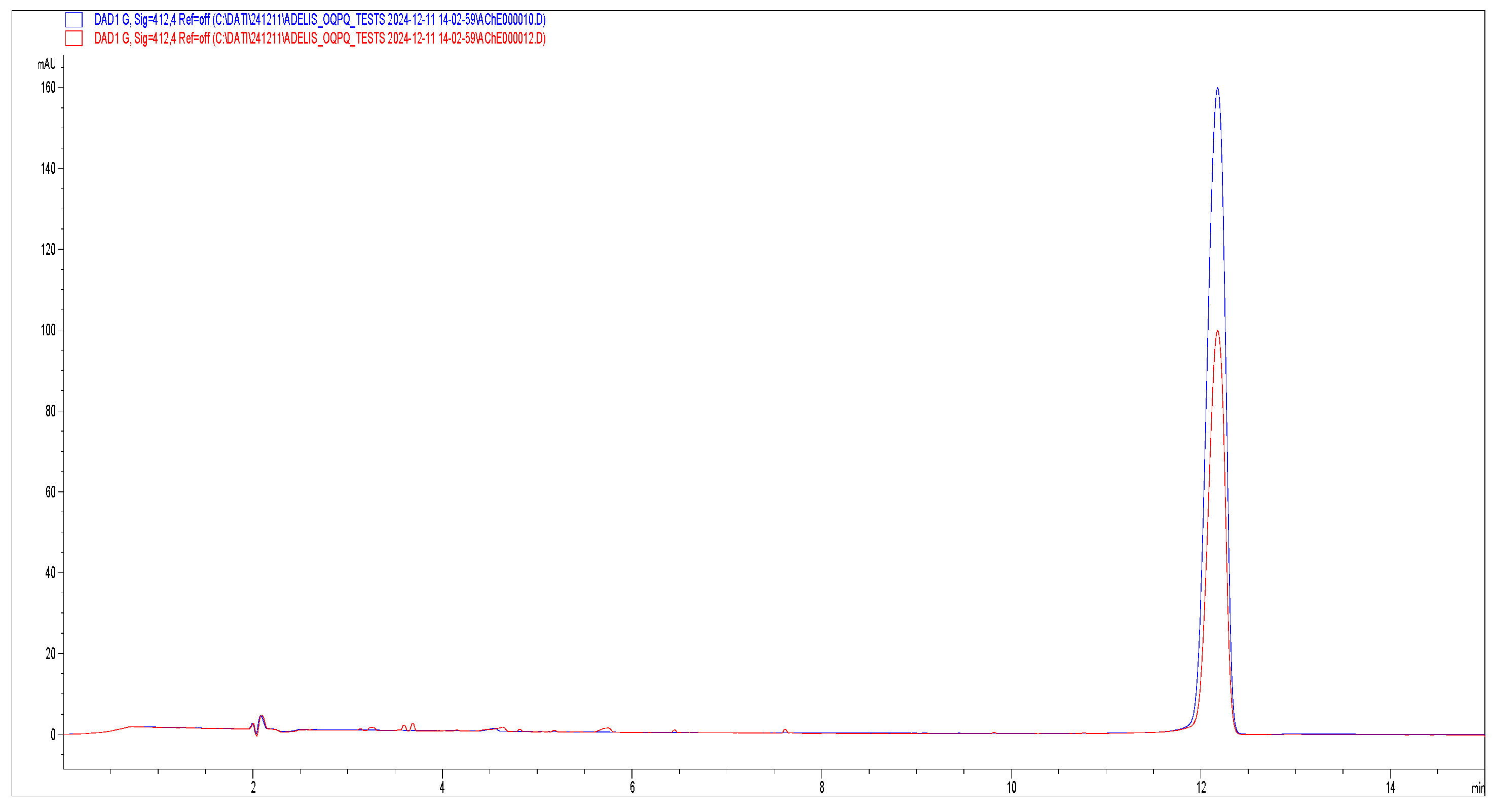
| [AThCh] (mM) | Area TNB (mAUxs) | SD Area TNB 412 nm (mAUxs) |
|---|---|---|
| 0.625 | 213.800 | 6.855 |
| 1.25 | 414.733 | 6.014 |
| 2.5 | 600.367 | 7.146 |
| 5 | 806.967 | 37.100 |
| 10 | 870.767 | 98.146 |
| [Neostigmine] (μM) | Area TNB (mAU*s) | Inhibition (%) | Residual Activity (%) |
|---|---|---|---|
| 0.4 | 609.3 | 23.592 | 76.408 |
| 4 | 571.5 | 28.333 | 71.667 |
| 8 | 493.9 | 38.064 | 61.936 |
| 20 | 334.4 | 58.065 | 41.935 |
| 40 | 251.8 | 68.424 | 31.576 |
| 200 | 157.5 | 80.249 | 19.751 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi, F.; Salerno, S.; Frigoli, E.; De Soricellis, G.; Brusotti, G.; Negri, S.; Radice, M.; Merlo, F.; Speltini, A.; Cena, H.; et al. Development and Application of an In-Capillary CE-DAD Method for the Inhibitory Screening of Natural Extracts Towards Acetylcholinesterase Enzyme. Metabolites 2025, 15, 283. https://doi.org/10.3390/metabo15040283
Rinaldi F, Salerno S, Frigoli E, De Soricellis G, Brusotti G, Negri S, Radice M, Merlo F, Speltini A, Cena H, et al. Development and Application of an In-Capillary CE-DAD Method for the Inhibitory Screening of Natural Extracts Towards Acetylcholinesterase Enzyme. Metabolites. 2025; 15(4):283. https://doi.org/10.3390/metabo15040283
Chicago/Turabian StyleRinaldi, Francesca, Sofia Salerno, Elena Frigoli, Giulia De Soricellis, Gloria Brusotti, Stefano Negri, Matteo Radice, Francesca Merlo, Andrea Speltini, Hellas Cena, and et al. 2025. "Development and Application of an In-Capillary CE-DAD Method for the Inhibitory Screening of Natural Extracts Towards Acetylcholinesterase Enzyme" Metabolites 15, no. 4: 283. https://doi.org/10.3390/metabo15040283
APA StyleRinaldi, F., Salerno, S., Frigoli, E., De Soricellis, G., Brusotti, G., Negri, S., Radice, M., Merlo, F., Speltini, A., Cena, H., & Calleri, E. (2025). Development and Application of an In-Capillary CE-DAD Method for the Inhibitory Screening of Natural Extracts Towards Acetylcholinesterase Enzyme. Metabolites, 15(4), 283. https://doi.org/10.3390/metabo15040283










