Studies of Piper auritum Kuntz’s Mutagenic and Antimutagenic Properties Using the Ames Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Collection and Extract Preparation
2.3. Chemical Identification
2.4. The Ames Test
2.5. Bacterial Membranes Containing Human CYP1A1
2.6. Kinetics of Human CYP1A1 Inhibition
2.7. Molecular Docking Assays
3. Results
3.1. Chemical Identification
3.2. The Ames Test
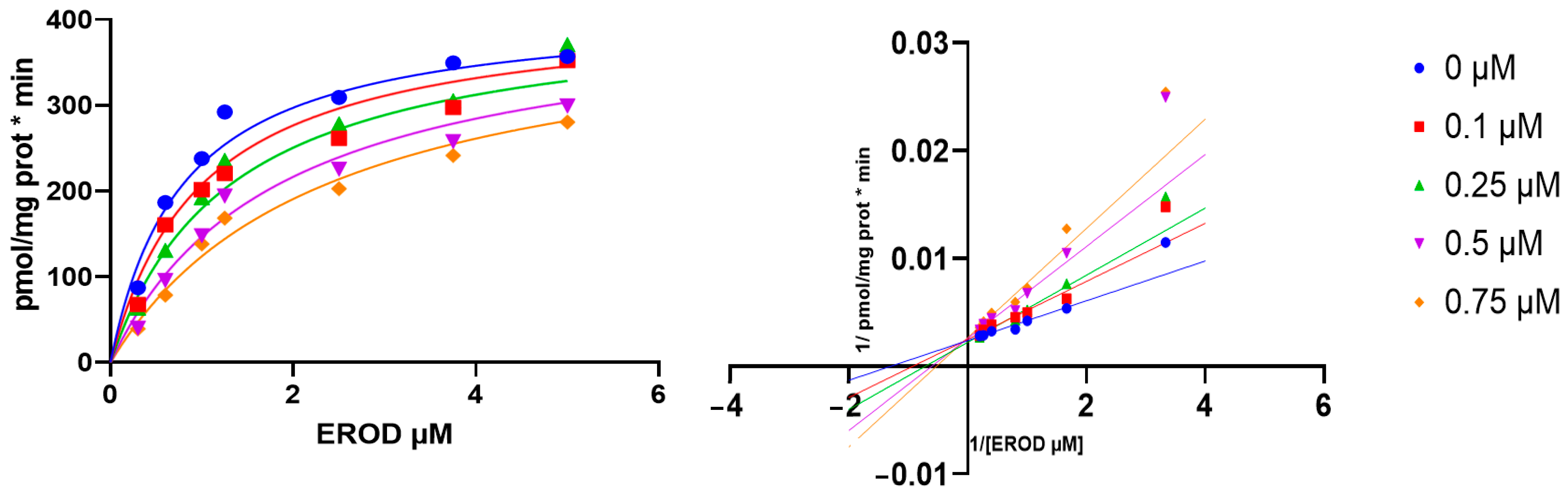
3.3. Kinetics of Human CYP1A1 Inhibition
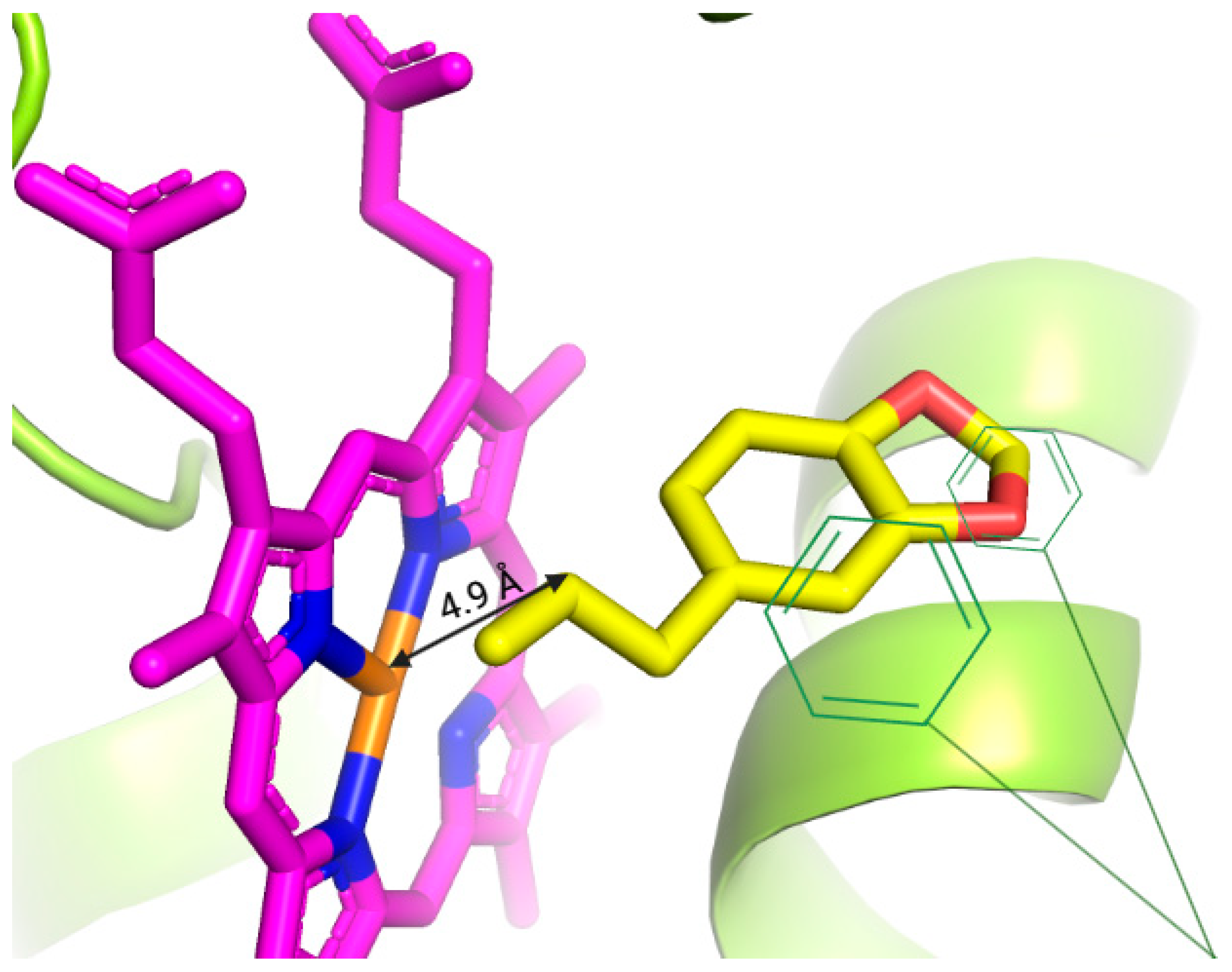
3.4. Molecular Docking Assays
4. Discussion
| Strain | Histidine Mutation | Reversion Event | LPS | DNA Excision Repair | Plasmid |
|---|---|---|---|---|---|
| TA98 | D3052 | Frameshift | rfa | Δ uvrB | pKM101 |
| TA100 | G46 | Base-pair substitution | rfa | Δ uvrB | pKM101 |
| TA1535 | G46 | Base-pair substitution | rfa | Δ uvrB | - |
| TA1537 | C3076 | Frameshift | rfa | Δ uvrB | - |
| TA102 | G428 | Transitions/transversions | rfa | Wild-type genes | pKM101, pAQ1 |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Mbadiko, C.; Bongo, G.; Ngbolua, K.; Ngombe, N.; Kapepula, P.; Yandju, M.C.; Mpiana, P.; Mbemba, T. Uses, phytochemistry and biological activity of Piper genus: A review. J. Med. Herbs 2023, 14, 1–17. [Google Scholar]
- Shah, S.K.; Gard, G.; Jhade, D.; Patel, N. Piper betle: Phytochemicals, pharmacological and nutritional value in health management. Int. J. Pharm. Sci. Rev. Res. 2016, 34, 181–189. [Google Scholar]
- Woon, C.K.; Ahmad, F.B.; Zamakshshari, N.H. Chemical Constituents and Biological Activities of Piper as anticancer agents: A review. Chem. Biodivers. 2023, 20, e202300166. [Google Scholar] [CrossRef] [PubMed]
- Salleh, W.M.N.H.W. A systematic review of botany, phytochemicals and pharmacological properties of “Hoja santa” (Piper auritum Kunth). Z. Für Naturforschung C 2020, 76, 93–102. [Google Scholar] [CrossRef]
- Conde-Hernández, L.A.; Guerrero-Beltrán, J.Á. Total phenolics and antioxidant activity of Piper auritum and Porophyllum ruderale. Food Chem. 2014, 142, 455–460. [Google Scholar] [CrossRef]
- Montalvo, L.R.V.; Parra, L.A.L. Evaluation of the anti-inflammatory effect of Piper auritum HBK extract and acute oral toxicity. Rev. Cuba. Plantas Med. 1999, 4, 11–14. [Google Scholar]
- Pérez-Gutierrez, R.M. Effect of the hexane extract of Piper auritum on insulin release from β-cell and oxidative stress in streptozotocin-induced diabetic rat. Pharmacogn. Mag. 2012, 8, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Khairan, K.; Ginting, B.; Sufriadi, E.; Amalia, A.; Sofyan, H.; Muhammad, S.; Diah, M.; Ernawati, E. Studies on the antioxidant activity of safrole, myristicin and terpeniol from myristica fragrans houtt: A review. IOP Conf. Ser. Earth Environ. Sci. 2023, 1183, 012062. [Google Scholar] [CrossRef]
- Lima, L.M. Safrole and the versatility of a natural biophore. Rev. Virtual Quim. 2015, 7, 495–538. [Google Scholar] [CrossRef]
- Gocke, E.; King, M.T.; Eckhardt, K.; Wild, D. Mutagenicity of cosmetics ingredients licensed by the European communities. Mutat. Res. Genetic Toxicol. 1981, 90, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res./Environ. Mutagen. Relat. Subj. 1982, 113, 173–215. [Google Scholar]
- Mortelmans, K.; Zeiger, E. The Ames Salmonella/microsome mutagenicity assay. Mutat. Res./Environ. Mutagen. Relat. Subj. 2000, 455, 29–60. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ojeda, S.L.; Espinosa-Aguirre, J.J.; Camacho-Carranza, R.; Amacosta-Castillo, J.; Cárdenas-Ávila, R. Piper auritum etanol extract is a potent antimutagen against food-borne aromatic amines: Mechanisms of action and chemical composition. Mutagenesis 2024, 39, 301–309. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 471: Bacterial Reverse Mutation Test, OECD Guidelines for the Testing of Chemicals, Seccion 4; OECD Publishing: Paris, France, 2020. [Google Scholar] [CrossRef]
- Ikken, Y.; Morales, P.; Martínez, A.; Haza, A.I.; Cambero, M.I. Antimutagenic effect of fruit and vegetable ethanolic extracts against N-nitrosamines evaluated by the Ames test. J. Agric. Food Chem. 1999, 4, 3257–3264. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Aguirre, J.J.; Camacho-Carranza, R.; Hernández-Ojeda, S.L.; Cárdenas-Ávila, R.I.; Santes-Palacios, R. Apiole, an important constituent of parsley, is a mixed-type inhibitor of the CYP1A subfamily. Mutat. Res. 2024, 829, 111881. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Duran, A.; Barrera-Cortés, J.; Lina-García, L.P.; Santillan, R.; Soto-Hernández, R.M.; Ramos-Valdivia, A.C.; Ponce-Noyola, T.; Ríos-Leal, E. Biological activity of phytochemicals from agricultural wastes and weeds on Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Sustainability 2021, 13, 13896. [Google Scholar] [CrossRef]
- De Flora, S.; Camoirano, A.; D’Agostini, F.; Balansky, R. Modulation of the mutagenic response in prokaryotes. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1992, 267, 183–192. [Google Scholar] [CrossRef]
- Parikh, A.; Gillam, E.M.J.; Guengerich, F.P. Drug metabolism by Escherichia coli expressing human cytochromes P450. Nat. Biotechnol. 1997, 15, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.Y.; Gillam, E.M.J.; Ohmori, S.; Tukey, R.H.; Guengerich, F.P. Expression of modified human cytochrome P450 1A1 in Escherichia coli: Effects of 5′ substitution, stabilization, purification, spectral characterization, and catalytic properties. Arch. Biochem. Biophys. 1994, 312, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.D.; Thompson, S.; Weaver, R.J.; Wolf, C.R.; Mayer, R.T. Cytochrome P450 specificities of alkoxyresorufin O-dealkylation in human and rat liver. Biochem. Pharmacol. 1994, 48, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.A.; Szklarz, G.D.; Scott, E.E. Human cytochrome P450 1A1 structure and utility in understanding drug and xenobiotic metabolism. J. Biol. Chem. 2013, 288, 12932–12943. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor lexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking methods, expanded force field, and python bindings. J. Chem. Inf. Model. 2021, 68, 3891–3898. [Google Scholar] [CrossRef]
- Mortelmans, K.A. A perspective on the development of the Ames Salmonella/mammalian-microsome mutagenicity assay. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2019, 841, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Dantas, F.G.D.S.; Castilho, P.F.; Almeida-Apolonio, A.A.; Araújo, R.P.; Oliveira, K.M.P. Mutagenic potential of medicinal plants evaluated by the Ames Salmonella/microsome assay: A systematic review. Mutat. Res. Rev. Mutat. Res. 2000, 786, 108338. [Google Scholar] [CrossRef] [PubMed]
- Vijay, U.; Gupta, S.; Mathur, P.; Suravajhala, P.; Bhatnagar, P. Microbial Mutagenicity Assay: Ames Test. Bio-Protocol 2018, 8, e2763. [Google Scholar] [CrossRef] [PubMed]
- Vizoso-Parra, A.; García-López, A.; Ramos-Ruiz, A. Ausencia de potencial genotóxico in vitro e in vivo de un extracto fluido de Piper auritum H.K.B. Rev. Cuba. Plantas Med. 1999, 3, 57–64. [Google Scholar]
- Álvarez-González, I.; Madrigal-Bujaidar, E.; Castro-García, S. Antigenotoxic capacity of beta-caryophyllene in mouse, and evaluation of its antioxidant and GST induction activities. J. Toxicol. Sci. 2014, 39, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Arruda, C.; Aldana Mejía, J.A.; Ribeiro, V.P.; Gambeta Borges, C.H.; Martins, C.H.G.; Sola Veneziani, R.C.; Ambrósio, S.R.; Bastos, J.K. Occurrence, chemical composition, biological activities and analytical methods on Copaifera genus—A review. Biomed Pharmacother. 2019, 109, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Batista, Â.G.; Ferrari, A.S.; da Cunha, D.C.; da Silva, J.K.; Cazarin, C.B.; Correa, L.C.; Prado, M.A.; Carvalho-Silva, L.B.; Esteves, E.A.; Maróstica Júnior, M.R. Polyphenols, antioxidants, and antimutagenic effects of Copaifera langsdorffii fruit. Food Chem. 2016, 197 Pt B, 1153–1159. [Google Scholar] [CrossRef]
- Ouedrhiri, W.; Mechchate, H.; Moja, S.; Mothana, R.A.; Noman, O.M.; Grafov, A.; Greche, H. Boosted antioxidant effect using a combinatory approach with essential oils from origanum compactum, Origanum majorana, Thymus serpyllum, Mentha spicata, Myrtus communis, and Artemisia herba-alba: Mixture design optimization. Plants 2021, 10, 2817. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; L D Jayaweera, S.; A Dias, D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α- and β-Pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Tekgüler, B.; Koca, I.; Karadeniz, B.; Zannou, O.; Pashazadeh, H. Antioxidant properties and monoterpene composition of 13 different pine resin samples from Turkey. Commagene J. Biol. 2021, 5, 208–213. [Google Scholar] [CrossRef]
- Toledo, A.; de-Lara, J.; Borges-da Silva, J.; Favreto, W.; Costa, W.; Pinto, F. Chemical composition, antimicrobial and antioxidant activity of the essential oil of leaves of Eugenia involucrata DC. J. Biosci. 2019, 36, 568–577. [Google Scholar] [CrossRef]
- Toscano-Garibay, J.D.; Arriaga-Alba, M.; Sánchez-Navarrete, J.; Mendoza-García, M.; Flores-Estrada, J.J.; Moreno-Eutimio, M.A.; Espinosa-Aguirre, J.J.; González-Ávila, M.; Ruiz-Pérez, N.J. Antimutagenic and antioxidant activity of the essential oils of Citrus sinensis and Citrus latifolia. Sci. Rep. 2017, 7, 11479. [Google Scholar] [CrossRef]
- Türkez, H.; Celik, K.; Toğar, B. Effects of copaene, a tricyclic sesquiterpene, on human lymphocytes cells in vitro. Cytotechnology 2014, 66, 597–603. [Google Scholar] [CrossRef]
- Walasek-Janusz, M.; Grzegorczyk, A.; Malm, A.; Nurzyńska-Wierdak, R.; Zalewski, D. Chemical composition, and antioxidant and antimicrobial activity of oregano essential oil. Molecules 2024, 16, 435. [Google Scholar] [CrossRef] [PubMed]
- Yeo, D.; Hwang, S.J.; Song, Y.S.; Lee, H.J. Humulene inhibits acute gastric mucosal injury by enhancing mucosal integrity. Antioxidants 2021, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Zahin, M.; Ahmad, I.; Gupta, R.C.; Aqil, F. Punicalagin and ellagic acid demonstrate antimutagenic activity and inhibition of benzo[a]pyrene induced DNA adducts. Biomed Res. Int. 2014, 2014, 467465. [Google Scholar] [CrossRef]
- Bukowska, B.; Mokra, K.; Michałowicz, J. Benzo[a]pyrene-Environmental occurrence, human exposure, and mechanisms of toxicity. Int. J. Mol. Sci. 2022, 23, 6348. [Google Scholar] [CrossRef] [PubMed]
- Kapelyukh, Y.; Henderson, C.J.; Scheer, N.; Rode, A.; Wolf, C.R. Defining the Contribution of CYP1A1 and CYP1A2 to drug metabolism using humanized CYP1A1/1A2 and Cyp1a1/Cyp1a2 knockout mice. Drug Metab. Dispos. 2019, 47, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.G. Phenylpropenes. Occurrence, distribution, and biosynthesis in fruits. J. Agric. Food Chem. 2018, 66, 2259–2272. [Google Scholar] [CrossRef] [PubMed]
- Jeurissen, S.M.; Punt, A.; Boersma, M.G.; Bogaards, J.J.; Fiamegos, Y.C.; Schilter, B.; van Bladeren, P.J.; Cnubben, N.H.; Rietjens, I.M. Human cytochrome p450 enzyme specificity for the bioactivation of estragole and related alkenylbenzenes. Chem. Res. Toxicol. 2007, 20, 798–806. [Google Scholar] [CrossRef]
- Daimon, H.; Sawada, S.; Asakura, S.; Sagami, F. In vivo genotoxicity and DNA adduct levels in the liver of rats treated with safrole. Carcinogenesis 1998, 19, 141–146. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jorge-Nebert, L.F.; Jiang, Z.; Chakraborty, R.; Watson, J.; Jin, L.; McGarvey, S.T.; Deka, R.; Nebert, D.W. Analysis of human CYP1A1 and CYP1A2 genes and their shared bidirectional promoter in eight world populations. Hum. Mutat. 2010, 31, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Pedroni, L.; Louisse, J.; Punt, A.; Dorne, J.L.C.M.; Dall’Asta, C.; Dellafiora, L. A computational inter-species study on Safrole phase I metabolism-dependent bioactivation: A mechanistic insight into the study of possible differences among species. Toxins 2023, 15, 94. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wu, Y.; Xiong, Y.; Wu, J.; Wang, M.; Sun, X.; Ding, X.; Yang, L.; Sun, X.; Ge, G. CYP1A inhibitors: Recent progress, current challenges, and future perspectives. Med. Res. Rev. 2024, 44, 169–234. [Google Scholar] [CrossRef] [PubMed]
- IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man; Vol 10: Some naturally occurring substances; IARC Press: Lyon, France, 1976; pp. 1–342. [Google Scholar]


| Compounds | Retention Time (s) | Abundance (%) | Concentration (ppm) |
|---|---|---|---|
| Alfa-Pinene | 5.473 | 1.25 | - |
| Beta-Pinene | 5.953 | 2.50 | - |
| Carene | 6.365 | 1.92 | - |
| Gama-Terpinene | 6.771 | 7.14 | 33.44 |
| 2-Carene | 7.033 | 4.90 | - |
| Safrole | 8.654 | 68.71 | 364.53 |
| Copaene | 9.246 | 4.11 | 14.87 |
| Gama-Muurolene | 9.336 | 0.57 | - |
| Cariophyllene | 9.554 | 4.41 | 19.93 |
| Humulene | 9.764 | 1.0 | - |
| Pentadecane | 9.914 | 0.01 | - |
| Germacrene | 9.944 | 1.65 | - |
| Muurolene isomere | 10.019 | 0.29 | - |
| Alfa-Copaene | 10.041 | 0.62 | - |
| B-Bisabolone | 10.364 | 0.50 | - |
| mg/Plate | S. typhimurium (His+ Revertants) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TA98 | TA100 | TA1535 | TA1537 | TA102 | ||||||
| PAEE | (−S9) | (+S9) | (−S9) | (+S9) | (−S9) | (+S9) | (−S9) | (+S9) | (−S9) | (+S9) |
| 0.002739 | 25 ± 2 | 36 ± 4 | 125 ± 17 | 124 ± 17 | 14 ± 2 | 15 ± 1 | 10 ± 3 | 11 ± 2 | 537 ± 14 | 567 ± 11 |
| 0.02739 | 28 ± 2 | 42 ± 6 | 122 ± 6 | 141 ± 11 | 17 ± 5 | 15 ± 2 | 10 ± 2 | 10 ± 2 | 562 ± 15 | 533 ± 27 |
| 0.2739 | 20 ± 1 | 41 ± 7 | 119 ± 7 | 133 ± 13 | 15 ± 6 | 17 ± 1 | 10 ± 3 | 10 ± 3 | 533 ± 15 | 540 ± 10 |
| 2.739 | 23 ± 4 | 40 ± 9 | 115 ± 13 | 131 ± 4 | 20 ± 9 | 16 ± 2 | 11 ± 3 | 12 ± 5 | 528 ± 5 | 548 ± 26 |
| 27.39 | 29 ± 5 | 33 ± 5 | 133 ± 11 | 122 ± 14 | 16 ± 5 | 14 ± 3 | 11 ± 4 | 10 ± 3 | 501 ± 24 | 557 ± 22 |
| Spontaneous reversion | 25 ± 3 | 41 ± 3 | 120 ± 13 | 119 ± 4 | 14 ± 3 | 11 ± 4 | 11 ± 3 | 9 ± 3 | 519 ± 6 | 572 ± 13 |
| Positive mutagens | 2419 ± 169 1 | 765 ± 109 2 | 1909 ± 428 1 | 2399 ± 293 2 | 481 ± 28 3 | 371 ± 20 4 | 63 ± 2 5 | 120 ± 12 4 | 2341 ± 149 6 | 2080 ± 27 7 |
| μg/Plate | S. typhimurium (His+ Revertants) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TA98 | TA100 | TA1535 | TA1537 | TA102 | ||||||
| SA | (−S9) | (+S9) | (−S9) | (+S9) | (−S9) | (+S9) | (−S9) | (+S9) | (−S9) | (+S9) |
| 10 | 19 ± 3 | 20 ± 2 | 110 ± 16 | 97 ± 18 | 16 ± 3 | 13 ± 1 | 10 ± 3 | 12 ± 3 | 502 ± 4 | 573 ± 6 |
| 50 | 22 ± 7 | 34 ± 8 | 120 ± 17 | 108 ± 18 | 16 ± 6 | 13 ± 4 | 14 ± 4 | 10 ± 3 | 489 ± 19 | 558 ± 14 |
| 100 | 27 ± 4 | 38 ± 9 | 107 ± 10 | 105 ± 9 | 20 ± 4 | 11 ± 1 | 10 ± 1 | 10 ± 1 | 484 ± 13 | 520 ± 10 |
| 500 | 18 ± 3 | 26 ± 9 | 100 ± 17 | 108 ± 9 | 19 ± 3 | 15 ± 5 | 12 ± 3 | 11 ± 4 | 451 ± 18 | 477 ± 17 |
| 1000 | 22 ± 9 | 36 ± 7 | 112 ± 6 | 102 ± 9 | 17 ± 2 | 18 ± 2 | 11 ± 4 | 9 ± 3 | 512 ± 40 | 504 ± 12 |
| Spontaneous reversion | 26 ± 5 | 29 ± 6 | 114 ± 9 | 103 ± 7 | 14 ± 3 | 11 ± 4 | 11 ± 3 | 9 ± 3 | 519 ± 6 | 572 ± 13 |
| Positive mutagens | 2070 ± 44 1 | 476 ± 24 2 | 1909 ± 428 1 | 2399 ± 293 2 | 481 ± 28 3 | 371 ± 20 4 | 63 ± 2 5 | 120 ± 12 4 | 2341 ± 149 6 | 2080 ± 27 7 |
| Compound | Structure | Enzyme (CYP1A1) | ||
|---|---|---|---|---|
| Binding Energy (kcal/mol) | Interactions | Distance to the Hem Group | ||
| Safrole | 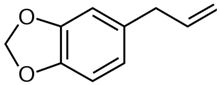 | −7.879 | Ser-122, Phe-123, Phe-224, Leu-312, Asp-313, Gly-316, Ala-317, Thr-321, Ile-386, Leu-496, Thr-497, Hem-601 | 4.9 Å |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Carrillo, L.S.; Madrigal-Bujaidar, E.; Hernández-Ojeda, S.L.; Morales-González, J.A.; Madrigal-Santillán, E.O.; Álvarez-González, I.; Espinosa-Aguirre, J.J. Studies of Piper auritum Kuntz’s Mutagenic and Antimutagenic Properties Using the Ames Test. Metabolites 2025, 15, 164. https://doi.org/10.3390/metabo15030164
Muñoz-Carrillo LS, Madrigal-Bujaidar E, Hernández-Ojeda SL, Morales-González JA, Madrigal-Santillán EO, Álvarez-González I, Espinosa-Aguirre JJ. Studies of Piper auritum Kuntz’s Mutagenic and Antimutagenic Properties Using the Ames Test. Metabolites. 2025; 15(3):164. https://doi.org/10.3390/metabo15030164
Chicago/Turabian StyleMuñoz-Carrillo, Luis S., Eduardo Madrigal-Bujaidar, Sandra L. Hernández-Ojeda, José A. Morales-González, Eduardo O. Madrigal-Santillán, Isela Álvarez-González, and J. Javier Espinosa-Aguirre. 2025. "Studies of Piper auritum Kuntz’s Mutagenic and Antimutagenic Properties Using the Ames Test" Metabolites 15, no. 3: 164. https://doi.org/10.3390/metabo15030164
APA StyleMuñoz-Carrillo, L. S., Madrigal-Bujaidar, E., Hernández-Ojeda, S. L., Morales-González, J. A., Madrigal-Santillán, E. O., Álvarez-González, I., & Espinosa-Aguirre, J. J. (2025). Studies of Piper auritum Kuntz’s Mutagenic and Antimutagenic Properties Using the Ames Test. Metabolites, 15(3), 164. https://doi.org/10.3390/metabo15030164






