Metabolic Syndrome Spectrum in Children with Classic Congenital Adrenal Hyperplasia—A Comprehensive Review
Abstract
1. Introduction
2. Methods
2.1. Study Identification and Search Strategy
2.2. Study Selection and Data Collection
2.3. Independent Variables
2.4. Outcome Variables
2.5. Risk of Bias
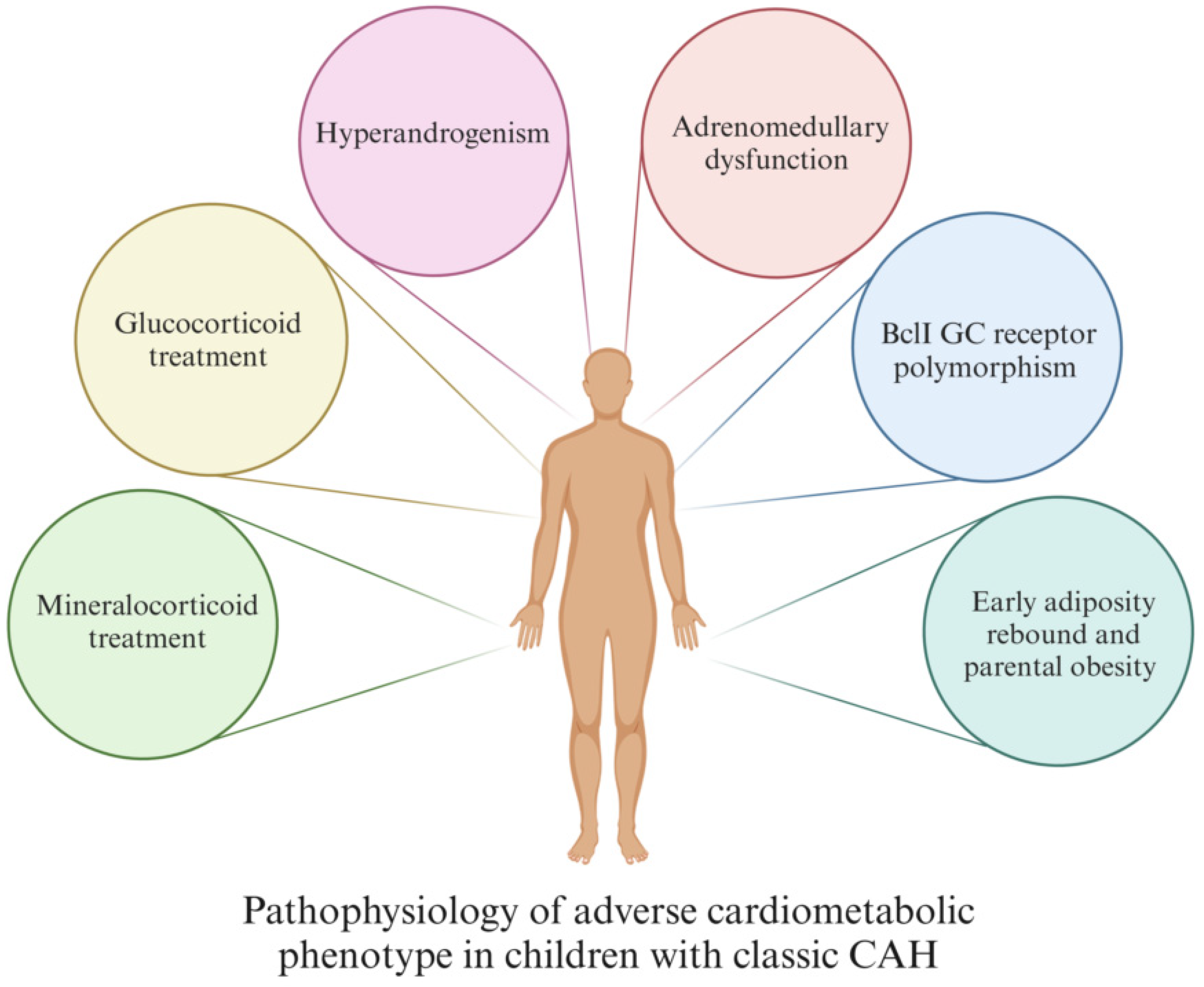
3. Pathophysiological Mechanisms
- GC treatment
- MC treatment
- Hyperandrogenism
- Adrenomedullary dysfunction
- BclI GC receptor polymorphism
- Early adiposity rebound and parental obesity
- GC treatment: The therapeutic range of GC is tight, and precise and individualized follow-ups are needed for patients to avoid both under- and over-treatment, as both can increase the CV risk in CCAH patients [35,40,41,42,43,44]. GC treatment has been shown to be strongly correlated with obesity [40,41,42,43], HTA [41,42], impaired insulin sensitivity [8,40,41], and increased CV mortality [40].
- MC treatment: Mineralocorticoid treatment increases blood pressure (BP) by acting on the MC receptor [8]; hence, management with excessively high doses of MC may result in high BP [35,36,40,41,42,44]. Therefore, it is important to individualize MC therapy based on BP, growth, and electrolyte values, as a precise dosing of MC treatment would minimize the risk of overtreatment [8,40,42].
- Hyperandrogenism: Many CCAH patients have a certain degree of hyperandrogenism, even when they are receiving regular treatment. Androgen excess is a well-known CV risk factor [45,46,47]. In addition, excess androgens are linked to reduced insulin sensitivity, which is also a significant CV risk factor [40,41,43]. Insulin further promotes adrenal and ovarian steroidogenesis and acts in a positive feedback loop as a major driving pathophysiologic mechanism behind hyperandrogenism [48].
- Adrenomedullary dysfunction: Patients with CCAH experience adrenomedullary dysfunction leading to reduced release of catecholamines, such as adrenaline, which normally facilitates lipolysis and suppresses insulin secretion via adrenergic receptors, thereby preventing a surge in fat mass [41,43,48,49]. This could be explained as the consequence of antenatal adrenomedullary maldevelopment due to decreased intra-adrenal GC [35,41,48]. Additionally, prolonged adrenomedullary dysfunction may also lead to increased insulin levels and IR [48].
- BclI GC receptor polymorphism: Classic CAH patients carrying BclI variants of the GC receptor gene, which enhances the receptor’s transactivation process, are at increased risk of systolic hypertension and higher BMI and WC compared to the wild-type CCAH controls [50].
- Early adiposity rebound and parental obesity: Early adiposity rebound (AR) is a well-known risk factor for childhood obesity and MetS [36,40], with potentially greater significance in children with CCAH, because in these children, AR has been found to occur at an earlier age than usual (at 1.7 years of age in the UK, 3 years of age in Japan, and 3.3–3.8 years of age in the USA) [16].
4. Obesity and Body Composition
5. Insulin Resistance and Glucose Metabolism
6. Lipid Metabolism
7. Hypertension
8. Other Cardiovascular Risk Factors
9. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sartorato, P.; Zulian, E.; Benedini, S.; Mariniello, B.; Schiavi, F.; Bilora, F.; Pozzan, G.; Greggsio, N.; Pagnan, A.; Mantero, F.; et al. Cardiovascular risk factors and ultrasound evaluation of intima-media thickness at common carotids, carotid bulbs, and femoral and abdominal aorta arteries in patients with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 2007, 92, 1015–1018. [Google Scholar] [CrossRef]
- Mooij, C.F.; Kroese, J.M.; Sweep, F.C.; Hermus, A.R.; Tack, C.J. Adult patients with congenital adrenal hyperplasia have elevated blood pressure but otherwise a normal cardiovascular risk profile. PLoS ONE 2011, 6, e24204. [Google Scholar] [CrossRef] [PubMed]
- Hashemi Dehkordi, E.; Khaheshi, S.; Mostofizadeh, N.; Hashemipour, M. Cardiovascular Risk Factors in Children and Adolescents with Congenital Adrenal Hyperplasia. Adv. Biomed. Res. 2021, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Metwalley, K.A.; Farghaly, H.S.; Abdelhamid, A. Epicardial Fat Thickness in Children with Classic Congenital Adrenal Hyperplasia. J. Clin. Res. Pediatr. Endocrinol. 2019, 11, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Falhammar, H.; Filipsson Nystrom, H.; Wedell, A.; Thoren, M. Cardiovascular risk, metabolic profile, and body composition in adult males with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur. J. Endocrinol. 2011, 164, 285–293. [Google Scholar] [CrossRef]
- Harrington, J.; Pena, A.S.; Gent, R.; Hirte, C.; Couper, J. Adolescents with congenital adrenal hyperplasia because of 21-hydroxylase deficiency have vascular dysfunction. Clin. Endocrinol. 2012, 76, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, R.; Korkmaz, H.A.; Kucuk, M.; Karadeniz, C.; Mese, T.; Ozkan, B. Assessment of early atherosclerosis and left ventricular dysfunction in children with 21-hydroxylase deficiency. Clin. Endocrinol. 2017, 86, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Barbot, M.; Mazzeo, P.; Lazzara, M.; Ceccato, F.; Scaroni, C. Metabolic syndrome and cardiovascular morbidity in patients with congenital adrenal hyperplasia. Front. Endocrinol. 2022, 13, 934675. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.G.; Mendonca, B.B.; Bachega, T. Long-term cardio-metabolic outcomes in patients with classical congenital adrenal hyperplasia: Is the risk real? Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 155–161. [Google Scholar] [CrossRef]
- Vijayan, R.; Bhavani, N.; Pavithran, P.V.; Nair, V.; Menon, U.V.; Menon, A.S.; Abraham, N.; Bhadran, K.; Narayanan, P.; Kumar, H. Metabolic profile, cardiovascular risk factors and health-related quality of life in children, adolescents and young adults with congenital adrenal hyperplasia. J. Pediatr. Endocrinol. Metab. 2019, 32, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Falhammar, H.; Frisen, L.; Hirschberg, A.L.; Norrby, C.; Almqvist, C.; Nordenskjold, A.; Nordenstrom, A. Increased Cardiovascular and Metabolic Morbidity in Patients with 21-Hydroxylase Deficiency: A Swedish Population-Based National Cohort Study. J. Clin. Endocrinol. Metab. 2015, 100, 3520–3528. [Google Scholar] [CrossRef]
- Espinosa Reyes, T.M.; Pesantez Velepucha, A.K.; Cabrera Rego, J.O.; Valdes Gomez, W.; Dominguez Alonso, E.; Falhammar, H. Cardiovascular risk in Cuban adolescents and young adults with congenital adrenal hyperplasia. BMC Endocr. Disord. 2023, 23, 241. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choi, S.; Lee, Y.A.; Lee, J.; Kim, S.G. Epidemiology and Long-Term Adverse Outcomes in Korean Patients with Congenital Adrenal Hyperplasia: A Nationwide Study. Endocrinol. Metab. 2022, 37, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Moreira, R.P.; Villares, S.M.; Madureira, G.; Mendonca, B.B.; Bachega, T.A. Obesity and familial predisposition are significant determining factors of an adverse metabolic profile in young patients with congenital adrenal hyperplasia. Horm. Res. Paediatr. 2013, 80, 111–118. [Google Scholar] [CrossRef]
- Bhullar, G.; Tanawattanacharoen, V.K.; Yeh, M.Y.; Kim, W.S.; Vidmar, A.P.; Geffner, M.E.; Hwang, D.H.; Kim, M.S. Early Adiposity Rebound Predicts Obesity and Adiposity in Youth with Congenital Adrenal Hyperplasia. Horm. Res. Paediatr. 2020, 93, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Akyurek, N.; Atabek, M.E.; Eklioglu, B.S.; Alp, H. Ambulatory blood pressure and subclinical cardiovascular disease in patients with congenital adrenal hyperplasia: A preliminary report. J. Clin. Res. Pediatr. Endocrinol. 2015, 7, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.M.; Improda, N.; Capalbo, D.; Salzano, A.; Arcopinto, M.; De Paulis, A.; Alessio, M.; Lenzi, A.; Isidori, A.M.; Cittadini, A.; et al. Cardiovascular abnormalities and impaired exercise performance in adolescents with congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 2015, 100, 644–652. [Google Scholar] [CrossRef]
- Amr, N.H.; Ahmed, A.Y.; Ibrahim, Y.A. Carotid intima media thickness and other cardiovascular risk factors in children with congenital adrenal hyperplasia. J. Endocrinol. Invest. 2014, 37, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Bacila, I.; Lawrence, N.R.; Mahdi, S.; Alvi, S.; Cheetham, T.D.; Crowne, E.; Das, U.; Dattani, M.T.; Davies, J.H.; Gevers, E.; et al. Health status of children and young persons with congenital adrenal hyperplasia in the UK (CAH-UK): A cross-sectional multi-centre study. Eur. J. Endocrinol. 2022, 187, 543–553. [Google Scholar] [CrossRef]
- Kurnaz, E.; Cetinkaya, S.; Ozalkak, S.; Bayramoglu, E.; Demirci, G.; Ozturk, H.S.; Erdeve, S.S.; Aycan, Z. Serum Fetuin-A and Insulin Levels in Classic Congenital Adrenal Hyperplasia. Horm. Metab. Res. 2020, 52, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.M.; Barra, C.B.; Santos, J.L.; Goulart, E.M.; Ferreira, A.V.; Silva, I.N. Cardiovascular risk factors and increased carotid intima-media thickness in young patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Arch. Endocrinol. Metab. 2015, 59, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Ryabets-Lienhard, A.; Dao-Tran, A.; Mittelman, S.D.; Gilsanz, V.; Schrager, S.M.; Geffner, M.E. Increased Abdominal Adiposity in Adolescents and Young Adults With Classical Congenital Adrenal Hyperplasia due to 21-Hydroxylase Deficiency. J. Clin. Endocrinol. Metab. 2015, 100, E1153–E1159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Yang, J.; Zhang, M.N.; Liu, C.Q.; Xu, M.; Li, X.J.; Yang, S.Y.; Li, X.Y. Metabolic disorders in newly diagnosed young adult female patients with simple virilizing 21-hydroxylase deficiency. Endocrine 2010, 38, 260–265. [Google Scholar] [CrossRef]
- Abdel Meguid, S.E.; Soliman, A.T.; De Sanctis, V.; Abougabal, A.M.S.; Ramadan, M.; Hassan, M.; Hamed, N.; Ahmed, S. Growth and Metabolic Syndrome (MetS) criteria in young children with classic Congenital Adrenal Hyperplasia (CAH) treated with corticosteroids (CS). Acta Biomed. 2022, 93, e2022304. [Google Scholar] [CrossRef]
- Subbarayan, A.; Dattani, M.T.; Peters, C.J.; Hindmarsh, P.C. Cardiovascular risk factors in children and adolescents with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clin. Endocrinol. 2014, 80, 471–477. [Google Scholar] [CrossRef]
- Roche, E.F.; Charmandari, E.; Dattani, M.T.; Hindmarsh, P.C. Blood pressure in children and adolescents with congenital adrenal hyperplasia (21-hydroxylase deficiency): A preliminary report. Clin. Endocrinol. 2003, 58, 589–596. [Google Scholar] [CrossRef]
- Volkl, T.M.; Simm, D.; Dotsch, J.; Rascher, W.; Dorr, H.G. Altered 24-hour blood pressure profiles in children and adolescents with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 2006, 91, 4888–4895. [Google Scholar] [CrossRef] [PubMed]
- Volkl, T.M.; Simm, D.; Beier, C.; Dorr, H.G. Obesity among children and adolescents with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics 2006, 117, e98–e105. [Google Scholar] [CrossRef]
- Bonfig, W.; Roehl, F.W.; Riedl, S.; Dorr, H.G.; Bettendorf, M.; Bramswig, J.; Schonau, E.; Riepe, F.; Hauffa, B.; Holl, R.W.; et al. Blood Pressure in a Large Cohort of Children and Adolescents With Classic Adrenal Hyperplasia (CAH) Due to 21-Hydroxylase Deficiency. Am. J. Hypertens. 2016, 29, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Sarafoglou, K.; Forlenza, G.P.; Yaw Addo, O.; Kyllo, J.; Lteif, A.; Hindmarsh, P.C.; Petryk, A.; Gonzalez-Bolanos, M.T.; Miller, B.S.; Thomas, W. Obesity in children with congenital adrenal hyperplasia in the Minnesota cohort: Importance of adjusting body mass index for height-age. Clin. Endocrinol. 2017, 86, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Doerr, H.G.; Pichl, C.; Penger, T.; Marx, M.; Rauh, M. Lipid profiles in Children and Adolescents with Classic Congenital Adrenal Hyperplasia due to 21-Hydroxylase Deficiency (CAH). J. Horm. Disord. Endocrinol. Res. 2020, 1, 105. [Google Scholar]
- Botero, D.; Arango, A.; Danon, M.; Lifshitz, F. Lipid profile in congenital adrenal hyperplasia. Metabolism 2000, 49, 790–793. [Google Scholar] [CrossRef]
- Maccabee-Ryaboy, N.; Thomas, W.; Kyllo, J.; Lteif, A.; Petryk, A.; Gonzalez-Bolanos, M.T.; Hindmarsh, P.C.; Sarafoglou, K. Hypertension in children with congenital adrenal hyperplasia. Clin. Endocrinol. 2016, 85, 528–534. [Google Scholar] [CrossRef]
- El-Maouche, D.; Arlt, W.; Merke, D.P. Congenital adrenal hyperplasia. Lancet 2017, 390, 2194–2210. [Google Scholar] [CrossRef] [PubMed]
- Pofi, R.; Ji, X.; Krone, N.P.; Tomlinson, J.W. Long-term health consequences of congenital adrenal hyperplasia. Clin. Endocrinol. 2023, 101, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Merke, D.P.; Mallappa, A.; Arlt, W.; Brac de la Perriere, A.; Linden Hirschberg, A.; Juul, A.; Newell-Price, J.; Perry, C.G.; Prete, A.; Rees, D.A.; et al. Modified-Release Hydrocortisone in Congenital Adrenal Hyperplasia. J. Clin. Endocrinol. Metab. 2021, 106, e2063–e2077. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Mallappa, A.; Reisch, N.; Nikolaou, N.; Krone, N.; Hughes, B.A.; O’Neil, D.M.; Whitaker, M.J.; Tomlinson, J.W.; Storbeck, K.H.; et al. Modified-Release and Conventional Glucocorticoids and Diurnal Androgen Excretion in Congenital Adrenal Hyperplasia. J. Clin. Endocrinol. Metab. 2017, 102, 1797–1806. [Google Scholar] [CrossRef]
- Sarafoglou, K.; Kim, M.S.; Lodish, M.; Felner, E.I.; Martinerie, L.; Nokoff, N.J.; Clemente, M.; Fechner, P.Y.; Vogiatzi, M.G.; Speiser, P.W.; et al. Phase 3 Trial of Crinecerfont in Pediatric Congenital Adrenal Hyperplasia. N. Engl. J. Med. 2024, 391, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Mooij, C.F.; Webb, E.A.; Claahsen van der Grinten, H.L.; Krone, N. Cardiovascular health, growth and gonadal function in children and adolescents with congenital adrenal hyperplasia. Arch. Dis. Child. 2017, 102, 578–584. [Google Scholar] [CrossRef]
- Mooij, C.F.; Kroese, J.M.; Claahsen-van der Grinten, H.L.; Tack, C.J.; Hermus, A.R. Unfavourable trends in cardiovascular and metabolic risk in paediatric and adult patients with congenital adrenal hyperplasia? Clin. Endocrinol. 2010, 73, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Balagamage, C.; Lawrence, N.R.; Krone, R.; Bacila, I.A.; Krone, N.P. Blood Pressure in Children with Congenital Adrenal Hyperplasia due to 21-Hydroxylase Deficiency. Horm. Res. Paediatr. 2024, 97, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Improda, N.; Barbieri, F.; Ciccarelli, G.P.; Capalbo, D.; Salerno, M. Cardiovascular Health in Children and Adolescents With Congenital Adrenal Hyperplasia Due to 21-Hydroxilase Deficiency. Front. Endocrinol. 2019, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Auer, M.K.; Nordenstrom, A.; Lajic, S.; Reisch, N. Congenital adrenal hyperplasia. Lancet 2023, 401, 227–244. [Google Scholar] [CrossRef]
- Mooij, C.F.; van Herwaarden, A.E.; Sweep, F.; Roeleveld, N.; de Korte, C.L.; Kapusta, L.; Claahsen-van der Grinten, H.L. Cardiovascular and metabolic risk in pediatric patients with congenital adrenal hyperplasia due to 21 hydroxylase deficiency. J. Pediatr. Endocrinol. Metab. 2017, 30, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Merke, D.P. Cardiovascular disease risk in adult women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Semin. Reprod. Med. 2009, 27, 316–321. [Google Scholar] [CrossRef]
- Macut, D.; Antic, I.B.; Bjekic-Macut, J. Cardiovascular risk factors and events in women with androgen excess. J. Endocrinol. Invest. 2015, 38, 295–301. [Google Scholar] [CrossRef]
- Charmandari, E.; Chrousos, G.P. Metabolic syndrome manifestations in classic congenital adrenal hyperplasia: Do they predispose to atherosclerotic cardiovascular disease and secondary polycystic ovary syndrome? Ann. N Y Acad. Sci. 2006, 1083, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Fraga, N.R.; Minaeian, N.; Geffner, M.E. Components of Metabolic Syndrome in Youth With Classical Congenital Adrenal Hyperplasia. Front. Endocrinol. 2022, 13, 848274. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.P.; Gomes, L.G.; Mendonca, B.B.; Bachega, T.A. Impact of glucocorticoid receptor gene polymorphisms on the metabolic profile of adult patients with the classical form of 21-hydroxylase deficiency. PLoS ONE 2012, 7, e44893. [Google Scholar] [CrossRef]
- Mnif, M.F.; Kamoun, M.; Mnif, F.; Charfi, N.; Naceur, B.B.; Kallel, N.; Rekik, N.; Mnif, Z.; Sfar, M.H.; Sfar, M.T.; et al. Metabolic profile and cardiovascular risk factors in adult patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Indian. J. Endocrinol. Metab. 2012, 16, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.R.; Silva, S.O.; Soares, S.C. The Use of High Sensitivity C-Reactive Protein in Cardiovascular Disease Detection. J. Pharm. Pharm. Sci. 2018, 21, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Vanreusel, I.; Taeymans, J.; Van Craenenbroeck, E.; Segers, V.F.M.; Van Berendoncks, A.; Briede, J.J.; Hens, W. Elevated oxidative stress in patients with congenital heart disease and the effect of cyanosis: A meta-analysis. Free Radic. Res. 2023, 57, 470–486. [Google Scholar] [CrossRef] [PubMed]
- Tamhane, S.; Rodriguez-Gutierrez, R.; Iqbal, A.M.; Prokop, L.J.; Bancos, I.; Speiser, P.W.; Murad, M.H. Cardiovascular and Metabolic Outcomes in Congenital Adrenal Hyperplasia: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2018, 103, 4097–4103. [Google Scholar] [CrossRef]
- Falhammar, H.; Frisen, L.; Norrby, C.; Hirschberg, A.L.; Almqvist, C.; Nordenskjold, A.; Nordenstrom, A. Increased mortality in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 2014, 99, E2715–E2721. [Google Scholar] [CrossRef]

| Metabolic Factors Analyzed | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | Autor | Year | Age Group | Study Population | Control Group | Obesity | Glucose Metabolism | Lipids | Blood Preassure | Other |
| 1 | Hashemi Dehkordi et al. [3] | 2021 | <18 y | 78 patients (51 SW, 27 SV) | No control group | BMI | HOMA IR | TG, TC, LDL, HDL | single measurments | |
| 2 | Metwalley et al. [4] | 2019 | <18 y | 36 patients (SW 30, SV 6) | 36 healthy controls matched for age, gender, pubertal, socioeconomic status | BMI ˚, WC ˚ | HOMA IR ˚ | TG ˚, TC ˚, LDL ˚, HDL ˚ | single measurments ˚ | hsCRP ˚, cIMT ˚ |
| 3 | Harrington et al. [6] | 2012 | <18 y | 14 patients (3 SV, 11 SW) | 28 obese controls and 53 healthy controls | BMI, WC | HOMA IR˚ | TG, TC, HDL | single measurments ˚ | hsCRP ˚, cIMT, endothelial function ˚ |
| 4 | Özdemir et al. [7] | 2016 | <18 y | 25 patients ˜ | 25 matched controls in terms of age, gender, and body size | BMI | HOMA IR | TG, TC, LDL, HDL | single measurments ˚ | cIMT ˚ |
| 5 | Vijayan et al. [10] | 2019 | <25 y | 52 patients ˜ | 28 healthy age- and sex-matched controls | BMI ˚ | HOMA IR ˚ | TG, TC, HDL, LDL | single measurments | |
| 6 | Moreira et al. [15] | 2013 | <18 y | 33 patients (11 SV, 22 SW) | 33 controls matched on BMI, age and sex | BMI, WC | HOMA IR ˚ | TG, TC, HDL ˚, LDL | ||
| 7 | Bhullar et al. [16] | 2020 | <18 y | 42 patients (38 SW, 4 SV) | No control group | BMI, WC, DXA, MRI | TG, HDL | single measurments | ||
| 8 | Akyürek et al. [17] | 2015 | <18 y | 25 SW patients | 25 age- and sex-matched healthy controls with normal weight and height percentiles | BMI ˚, WC | HOMA IR | TG, TC, LDL, HDL | 24 h ABPM ˚ | cIMT ˚ |
| 9 | Marra et al. [18] | 2015 | <18 y | 20 patients (15 SW, 5 SV) | 20 healthy adolescents, statistically not different for sex, pubertal status, and physical activity; 18 age- and BMI-matched patients affected by JIA | BMI ˚, WC ˚, DXA ˚ | HOMA IR ˚ | TG, TC, LDL, HDL | single measurments | |
| 10 | Amr et al. [19] | 2014 | <18 y | 32 patients, (24 SW, 8 SV) | 32 healthy controls | BMI ˚ | HOMA IR ˚, OGTT ˚ | TG, TC, LDL, HDL | cIMT ˚ | |
| 11 | Bacila et al. [20] | 2022 | <18 y | 107 patients ˜ | 83 healthy age- and sex matched controls differed by ethnicity | BMI ˚, WC ˚ | HOMA IR | TG, TC, HDL ˚, LDL | single measurments | |
| 12 | Kurnaz et al. [21] | 2020 | <18 y | 56 patients, (36 SW, 20 SV) | 70 age- and sex-matched healthy controls | BMI ˚ | HOMA IR ˚ | TG ˚, TC, HDL, LDL | hsCRP | |
| 13 | Rodrigues et al. [22] | 2015 | <25 y | 40 patients (29 SW, 11 SV) | 73 healthy, normal-weight children and adolescents | BMI ˚ | HOMA IR | TG, TC, HDL ˚, LDL | single measurments ˚ | cIMT ˚ |
| 14 | Kim et al. [23] | 2015 | <18 y | 28 patients (20 SW, 8 SV) | 28 healthy controls matched for age, sex, ethnicity, and BMI | BMI, WC measured from CT images ˚, VAT ˚, SAT ˚ | HOMA IR | TG, TC, HDL, LDL, VLDL | single measurments | hsCRP |
| 15 | Zhang et al. [24] | 2010 | <25 y | 30 patients (30 untreated SV women) | 30 controls | BMI | HOMA IR ˚, OGTT ˚ | TG ˚, TC, HDL ˚, LDL | single measurments | hsCRP |
| 16 | Abdel Meguid et al. [25] | 2022 | <18 y | 30 patients ˜ | 66 age-matched obese children | BMI | HOMA IR | TG, TC, HDL, LDL ˚ | single measurments | cIMT ˚ |
| 17 | Subbarayan et al. [26] | 2014 | <25 y | 107 patients (85 SW, 22 SV) | No control group | BMI ˚ | HOMA IR | TG, TC | single measurments ˚ | |
| 18 | Roche et al. [27] | 2003 | <18 y | 38 SW patients | No control group | BMI ˚, triceps and subscapular skinfold thickness ˚ | 24 h ABPM ˚ | |||
| 19 | Völkl et al. [28] | 2006 | <25 y | 55 patients SW 45, SV 10 | No control group | BMI ˚ | 24 h ABPM | |||
| 20 | Völkl et al. [29] | 2015 | <18 y | 89 patients ˜ | No control group | BMI ˚ | single measurments | |||
| 21 | Bonfig et al. [30] | 2015 | <18 y | 716 patients (571 SW, 145 SV) | No control group | BMI | single measurments ˚ | |||
| 22 | Sarafoglou et al. [31] | 2017 | <18 y | 194 patients (124 SW, 70 SV) | No control group | BMI | ||||
| 23 | Doerr et al. [32] | 2020 | <18 y | 43 patients (37 SW, 6 SV) | No control group | BMI | TG, TC, HDL, LDL, non-HDL | |||
| 24 | Botero et al. [33] | 2000 | <18 y | 14 patients ˜ | 14 prepubertal children | TG, TC, HDL, LDL | ||||
| 25 | Maccabee-Ryaboy et al. [34] | 2016 | <18 y | 180 patients (120 SW, 60 SV) | No control group | BMI | single measurments | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panic Zaric, S.; Milenkovic, T.; Todorovic, S.; Mitrovic, K.; Cvetkovic, D.; Cehic, M.; Vekic, J.; Dumic, K.; Vukovic, R. Metabolic Syndrome Spectrum in Children with Classic Congenital Adrenal Hyperplasia—A Comprehensive Review. Metabolites 2025, 15, 89. https://doi.org/10.3390/metabo15020089
Panic Zaric S, Milenkovic T, Todorovic S, Mitrovic K, Cvetkovic D, Cehic M, Vekic J, Dumic K, Vukovic R. Metabolic Syndrome Spectrum in Children with Classic Congenital Adrenal Hyperplasia—A Comprehensive Review. Metabolites. 2025; 15(2):89. https://doi.org/10.3390/metabo15020089
Chicago/Turabian StylePanic Zaric, Sanja, Tatjana Milenkovic, Sladjana Todorovic, Katarina Mitrovic, Dimitrije Cvetkovic, Maja Cehic, Jelena Vekic, Katja Dumic, and Rade Vukovic. 2025. "Metabolic Syndrome Spectrum in Children with Classic Congenital Adrenal Hyperplasia—A Comprehensive Review" Metabolites 15, no. 2: 89. https://doi.org/10.3390/metabo15020089
APA StylePanic Zaric, S., Milenkovic, T., Todorovic, S., Mitrovic, K., Cvetkovic, D., Cehic, M., Vekic, J., Dumic, K., & Vukovic, R. (2025). Metabolic Syndrome Spectrum in Children with Classic Congenital Adrenal Hyperplasia—A Comprehensive Review. Metabolites, 15(2), 89. https://doi.org/10.3390/metabo15020089







