Comparative Phytochemical Analysis of Five Species of the Genus Arthrophytum Schrenk (Amaranthaceae) from the Flora of Kazakhstan
Abstract
1. Introduction
2. Materials and Methods
2.1. Geobotany Methods
2.2. Morphological Methods
2.3. Chemical Methods
3. Results
3.1. Distribution Analyses
3.2. Morphological Analysis
3.3. Phytochemical Analysis
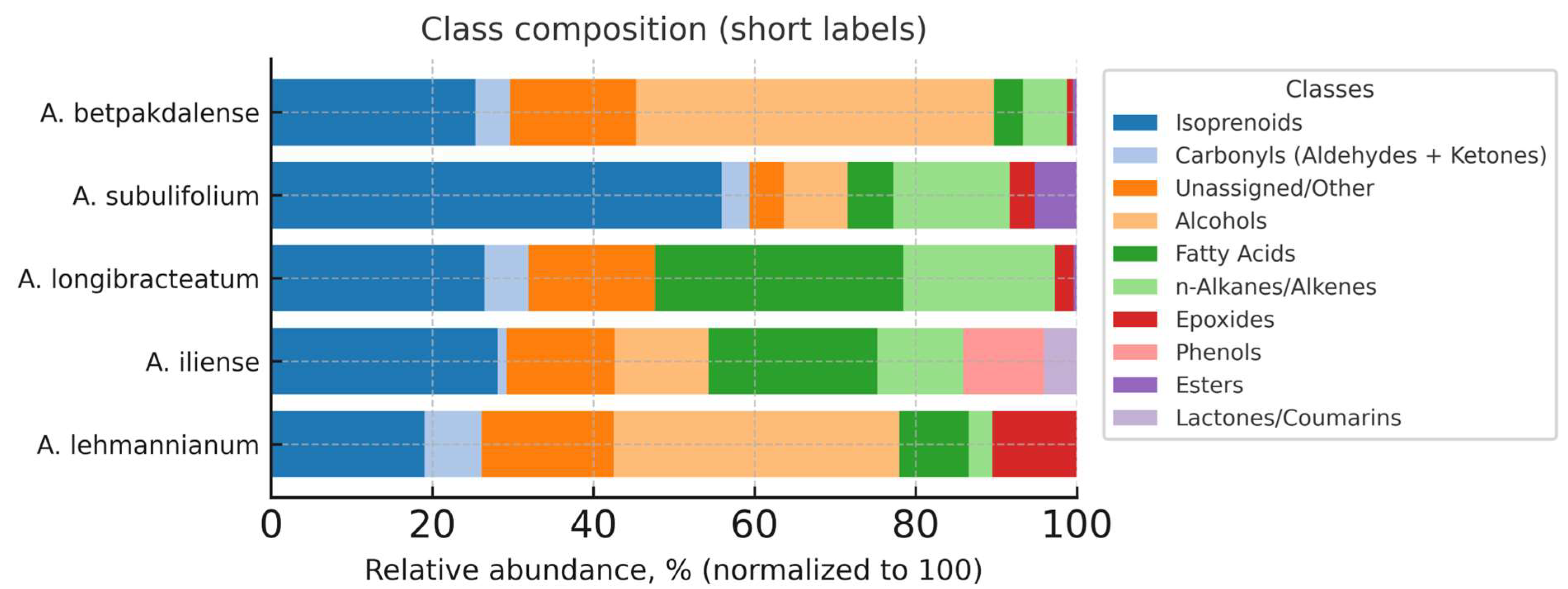
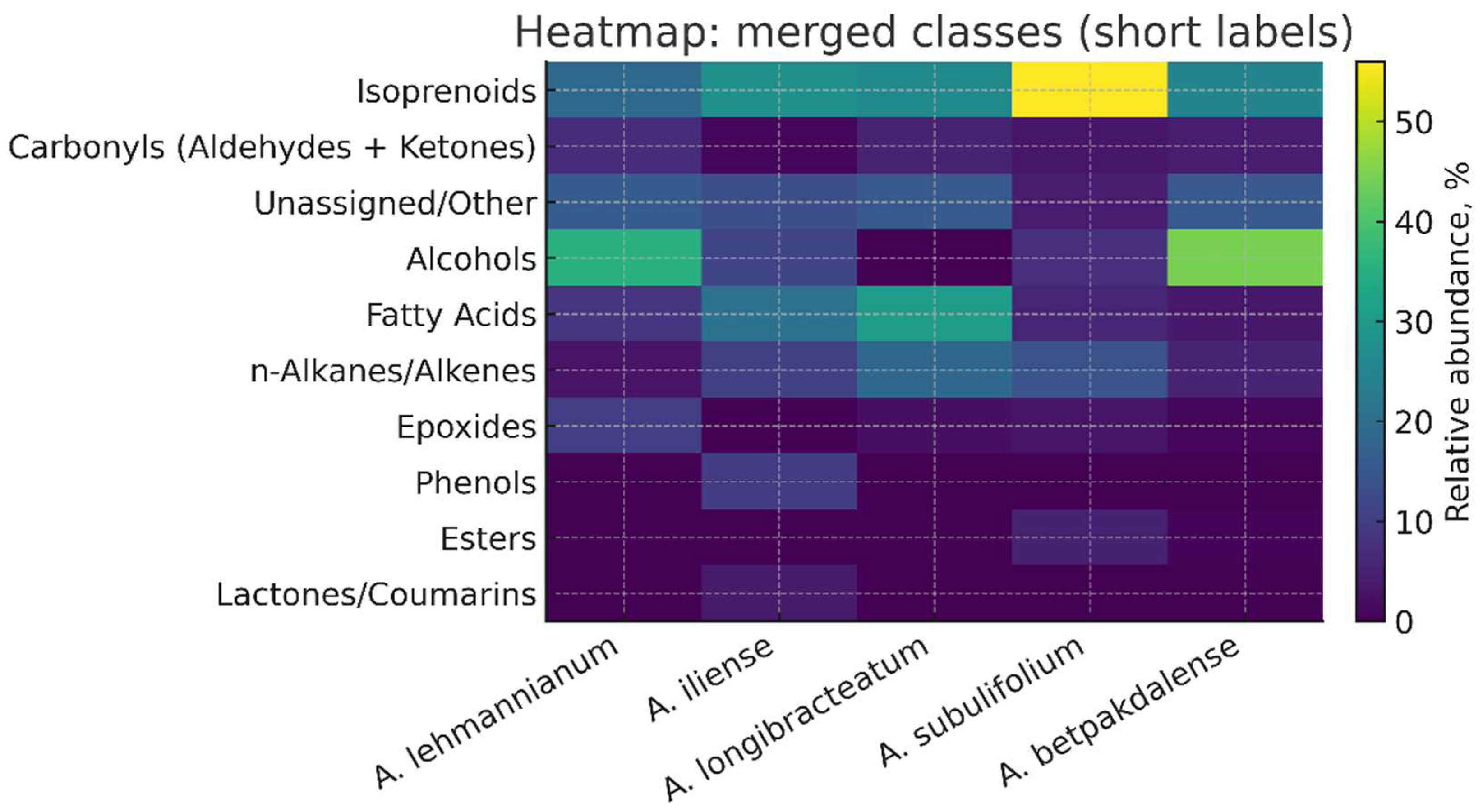
3.4. Class Composition of Volatile Components by Arthrophytum Species
4. Discussion
4.1. Correlation with Biological Effects from Reviews
4.2. Ecological and Methodological Factors of Variability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| ABTS | 2,2-azinobis 3-ethylbenzothiazoline 6-sulfonate |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| LC | Liquid Chromatography |
| IC50 | Half Maximal Inhibitory Concentration |
| RMS | Root Mean Square |
Appendix A
| № | Retention Time, min | Compounds | Content, % | RMS |
|---|---|---|---|---|
| 1 | 18.24 | Caryophyllene | 0.91 | 0.11 |
| 2 | 18.95 | 4-Methyl-1-(acetoxy)benzene | 3.23 | 0.20 |
| 3 | 23.98 | Caryophyllene oxide | 1.10 | 0.20 |
| 4 | 24.61 | Guanosine | 3.93 | 0.15 |
| 5 | 26.01 | D-Glucopyranose, 1,6-anhydro- | 0.96 | 0.29 |
| 6 | 29.20 | Mome inositol | 2.81 | 0.19 |
| 7 | 30.40 | n-Hexadecanoic acid | 6.70 | 0.20 |
| 8 | 32.69 | Phytol | 1.00 | 0.26 |
| 9 | 33.21 | Dimethyl 6-(trimethylsilyl)pyrazolo [1,5-a]pyridine-2,3-dicarboxylate | 0.37 | 0.11 |
| 10 | 34.10 | cis-Vaccenic acid | 1.19 | 0.28 |
| 11 | 34.42 | 9,12-Octadecadienoic acid | 0.76 | 0.17 |
| 12 | 34.80 | Oxirane, hexadecyl- | 0.52 | 0.17 |
| 13 | 35.80 | 1-Octadecanol | 1.53 | 0.16 |
| 14 | 36.49 | Tetradecanal | 0.37 | 0.05 |
| 15 | 37.26 | Octacosane | 0.95 | 0.22 |
| 16 | 38.11 | Hexadecanal | 0.78 | 0.07 |
| 17 | 39.04 | Behenic alcohol | 3.16 | 0.25 |
| 18 | 40.30 | Octacosane | 1.08 | 0.18 |
| 19 | 41.17 | Octadecanal | 2.12 | 0.17 |
| 20 | 42.06 | Lignoceric alcohol | 9.33 | 0.29 |
| 21 | 43.13 | Hexatriacontane | 1.33 | 0.32 |
| 22 | 44.03 | Octadecanal | 3.83 | 0.30 |
| 23 | 44.22 | Squalene | 4.62 | 0.16 |
| 24 | 44.86 | n-Tetracosanol-1 | 10.57 | 0.62 |
| 25 | 45.46 | 1,6,10,14-Hexadecatetraen-3-ol, 3,7,11,15-tetramethyl- | 1.02 | 0.30 |
| 26 | 45.77 | Tetratetracontane | 0.89 | 0.28 |
| 27 | 46.11 | 1-docosanol | 0.38 | 0.04 |
| 28 | 46.70 | Oxirane, heptadecyl- | 9.94 | 0.75 |
| 29 | 47.49 | Octacosanol | 10.51 | 0.77 |
| 30 | 49.19 | 1,30-Triacontanediol | 2.72 | 0.20 |
| 31 | 50.08 | Vitamin E | 2.97 | 0.16 |
| 32 | 52.59 | Stigmasterol | 2.03 | 0.24 |
| 33 | 53.57 | β-Sitosterol | 6.39 | 0.44 |
| № | Retention Time, min | Compounds | Content, % | RMS |
|---|---|---|---|---|
| 1 | 18.23 | Caryophyllene | 1.22 | 0.11 |
| 2 | 18.93 | 2-Methoxy-4-vinylphenol | 8.08 | 0.06 |
| 3 | 21.10 | Phenol, 2,6-dimethoxy- | 1.10 | 0.06 |
| 4 | 23.98 | Caryophyllene oxide | 1.57 | 0.09 |
| 5 | 24.14 | 2-methoxy-4-(n-propyl)phenol v | 0.82 | 0.10 |
| 6 | 24.58 | Coumarin | 4.10 | 0.06 |
| 7 | 24.81 | Ethanone, 1-(4-hydroxy-3-methoxyphenyl)- | 1.51 | 0.10 |
| 8 | 25.81 | 2-Propanone, 1-(4-hydroxy-3-methoxyphenyl)- | 0.81 | 0.10 |
| 9 | 25.89 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 0.60 | 0.06 |
| 10 | 26.00 | D-Allose | 1.40 | 0.10 |
| 11 | 26.19 | 2H-1-Benzopyran-3,4-diol, 2-(3,4-dimethoxyphenyl)-3,4-dihydro-6-methyl-, (2α,3α,4α)- | 0.42 | 0.05 |
| 12 | 26.41 | Tetradecanoic acid | 1.08 | 0.07 |
| 13 | 26.96 | 2(1H)-Pyridinone, 1-cyclohexyl-3,4,5,6-tetramethyl- | 0.94 | 0.11 |
| 14 | 27.40 | 2-Pentadecanone, 6,10,14-trimethyl- | 0.56 | 0.15 |
| 15 | 30.39 | n-Hexadecanoic acid | 9.54 | 0.41 |
| 16 | 34.10 | cis-Vaccenic acid | 6.26 | 0.30 |
| 17 | 34.42 | 9,12-Octadecadienoic acid | 4.02 | 0.20 |
| 18 | 35.79 | n-Heptadecanol-1 | 2.28 | 0.15 |
| 19 | 37.25 | Hexacosane | 2.49 | 0.06 |
| 20 | 38.11 | Octadecanal | 0.53 | 0.05 |
| 21 | 38.32 | 4,8,12,16-Tetramethylheptadecan-4-olide | 0.91 | 0.11 |
| 22 | 39.04 | Behenic alcohol | 3.42 | 0.25 |
| 23 | 40.29 | Hexacosane | 2.06 | 0.25 |
| 24 | 42.04 | n-Tetracosanol-1 | 8.23 | 0.13 |
| 25 | 43.12 | Octacosane | 3.19 | 0.10 |
| 26 | 44.20 | Squalene | 7.41 | 0.15 |
| 27 | 45.45 | 1,6,10,14-Hexadecatetraen-3-ol, 3,7,11,15-tetramethyl- | 2.58 | 0.20 |
| 28 | 45.77 | Hentriacontane | 2.95 | 0.10 |
| 29 | 46.18 | 9,19-Cycloergost-24(28)-en-3-ol, 4,14-dimethyl-, acetate, (3β,4α,5α)- | 1.29 | 0.20 |
| 30 | 46.50 | 1,6,10,14,18,22-Tetracosahexaen-3-ol, 2,6,10,15,19,23-hexamethyl- | 1.97 | 0.40 |
| 31 | 50.07 | Vitamin E | 2.73 | 0.15 |
| 32 | 52.57 | Stigmasterol | 4.05 | 0.03 |
| 33 | 53.55 | β-Sitosterol | 9.87 | 0.19 |
| № | Retention Time, min | Compounds | Content, % | RMS |
|---|---|---|---|---|
| 1 | 18.23 | Caryophyllene | 2.23 | 0.06 |
| 2 | 19.02 | Ethanone, 1-(2-hydroxy-5-methylphenyl)- | 1.34 | 0.16 |
| 3 | 23.98 | Caryophyllene oxide | 2.45 | 0.31 |
| 4 | 24.57 | Sucrose | 9.78 | 0.25 |
| 5 | 25.90 | E-6-Octadecen-1-ol acetate | 0.41 | 0.09 |
| 6 | 27.40 | 2-Pentadecanone, 6,10,14-trimethyl- | 1.21 | 0.09 |
| 7 | 28.83 | n-Heptadecanol-1 | 0.41 | 0.09 |
| 8 | 30.38 | n-Hexadecanoic acid | 16.77 | 0.72 |
| 9 | 32.52 | Trichloroacetic acid, pentadecyl ester | 0.42 | 0.07 |
| 10 | 32.68 | Phytol | 1.29 | 0.12 |
| 11 | 32.96 | 9-Octadecenoic acid, methyl ester | 0.48 | 0.04 |
| 12 | 33.51 | Phthalic acid, 6-ethyl-3-octyl butyl ester | 0.41 | 0.08 |
| 13 | 34.10 | Oleic Acid | 7.05 | 0.22 |
| 14 | 34.42 | 9,12-Octadecadienoic acid | 5.73 | 0.21 |
| 15 | 34.79 | Tetradecanal | 1.20 | 0.10 |
| 16 | 35.79 | n-Heptadecanol-1 | 2.91 | 0.20 |
| 17 | 37.25 | Heptadecane | 1.63 | 0.16 |
| 18 | 38.10 | Pentadecanal- | 1.41 | 0.10 |
| 19 | 38.32 | 4,8,12,16-Tetramethylheptadecan-4-olide | 1.24 | 0.15 |
| 20 | 39.04 | 1-Nonadecene | 5.29 | 0.10 |
| 21 | 40.29 | Heptadecane | 1.51 | 0.08 |
| 22 | 41.17 | Tetradecanal | 1.63 | 0.11 |
| 23 | 42.05 | 1-Docosene | 7.30 | 0.09 |
| 24 | 43.12 | Hentriacontane | 1.83 | 0.06 |
| 25 | 44.20 | Squalene | 5.04 | 0.19 |
| 26 | 45.76 | Tetratetracontane | 1.22 | 0.07 |
| 27 | 46.49 | Oxirane, 2,2-dimethyl-3-(3,7,12,16,20-pentamethyl-3,7,11,15,19-heneicosapentaenyl)- | 2.33 | 0.15 |
| 28 | 50.06 | Vitamin E | 1.64 | 0.15 |
| 29 | 52.58 | Stigmasterol | 3.09 | 0.21 |
| 30 | 53.55 | β-Sitosterol | 10.75 | 0.58 |
| № | Retention Time, min | Compounds | Content, % | RMS |
|---|---|---|---|---|
| 1 | 18.24 | Caryophyllene | 1.28 | 0.25 |
| 2 | 23.98 | Caryophyllene oxide | 1.39 | 0.09 |
| 3 | 27.42 | 2-Pentadecanone, 6,10,14-trimethyl- | 1.26 | 0.15 |
| 4 | 32.27 | 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione | 1.09 | 0.20 |
| 5 | 33.20 | Indazol-4-one, 3,6,6-trimethyl-1-phthalazin-1-yl-1,5,6,7-tetrahydro- | 1.96 | 0.12 |
| 6 | 33.53 | Phthalic acid, butyl isohexyl ester | 0.69 | 0.10 |
| 7 | 35.80 | 1-Eicosanol | 2.49 | 0.26 |
| 8 | 37.26 | Heptadecane | 3.25 | 0.15 |
| 9 | 38.11 | Tetradecanal | 1.14 | 0.11 |
| 10 | 38.33 | 4,8,12,16-Tetramethylheptadecan-4-olide | 1.26 | 0.15 |
| 11 | 38.37 | Hexanedioic acid, bis(2-ethylhexyl) ester | 1.03 | 0.16 |
| 12 | 39.05 | 1-Heneicosyl formate | 5.24 | 0.07 |
| 13 | 40.29 | Octacosane | 3.82 | 0.27 |
| 14 | 41.17 | Hexadecanal | 1.04 | 0.15 |
| 15 | 42.05 | 1-Eicosanol | 5.36 | 0.15 |
| 16 | 42.16 | 1,2-Benzenedicarboxylic acid, diisooctyl ester | 4.00 | 0.10 |
| 17 | 43.12 | Hentriacontane | 4.31 | 0.12 |
| 18 | 44.20 | Squalene | 8.00 | 0.20 |
| 19 | 45.76 | Heneicosane | 3.03 | 0.11 |
| 20 | 46.49 | Oxirane, 2,2-dimethyl-3-(3,7,12,16,20-pentamethyl-3,7,11,15,19-heneicosapentaenyl)- | 3.11 | 0.10 |
| 21 | 50.07 | Vitamin E | 5.31 | 0.08 |
| 22 | 52.58 | Stigmasterol | 8.52 | 0.07 |
| 23 | 53.55 | β-Sitosterol | 22.13 | 0.32 |
| 24 | 56.60 | Stigmasta-3,5-dien-7-one | 5.16 | 0.15 |
| 25 | 57.37 | Stigmast-4-en-3-one | 4.14 | 0.14 |
| № | Retention Time, min | Compounds | Content, % | RMS |
|---|---|---|---|---|
| 1 | 13.36 | 2,6-Octadienal, 3,7-dimethyl- | 0.82 | 0.07 |
| 2 | 14.53 | Benzenemethanol, α,α,4-trimethyl- | 2.01 | 0.09 |
| 3 | 15.41 | Bicyclo [3.1.1]hept-3-en-2-one, 4,6,6-trimethyl- | 1.53 | 0.06 |
| 4 | 16.51 | 2-Cyclohexen-1-one, 3-methyl-6-(1-methylethyl)- | 1.83 | 0.06 |
| 5 | 18.11 | Ylangene | 0.32 | 0.07 |
| 6 | 18.23 | Caryophyllene | 5.41 | 0.27 |
| 7 | 19.74 | 2H-Inden-2-one, 1,4,5,6,7,7a-hexahydro-7a-methyl-, (S)- | 2.80 | 0.20 |
| 8 | 20.20 | 1,6-Cyclodecadiene, 1-methyl-5-methylene-8-(1-methylethyl)-, [s-(E,E)]- | 0.69 | 0.02 |
| 9 | 20.42 | 3-Cyclopenten-1-one, 2-hydroxy-3-(3-methyl-2-butenyl)- | 1.42 | 0.07 |
| 10 | 23.84 | 1H-Cycloprop[e]azulen-7-ol, decahydro-1,1,7-trimethyl-4-methylene-, [1ar-(1aα,4aα,7β,7aβ,7bα)]- | 1.21 | 0.09 |
| 11 | 23.97 | Caryophyllene oxide | 3.42 | 0.07 |
| 12 | 24.54 | Sucrose | 1.85 | 0.13 |
| 13 | 25.90 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 1.21 | 0.08 |
| 14 | 27.40 | 2-Pentadecanone, 6,10,14-trimethyl- | 0.51 | 0.10 |
| 15 | 30.43 | n-Hexadecanoic acid | 3.35 | 0.15 |
| 16 | 32.26 | 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione | 0.52 | 0.11 |
| 17 | 32.52 | 1-Nonadecene | 0.21 | 0.02 |
| 18 | 32.68 | Phytol | 0.51 | 0.10 |
| 19 | 33.52 | Phthalic acid, butyl cycloheptyl ester | 0.27 | 0.03 |
| 20 | 34.80 | Hexadecanal | 0.32 | 0.03 |
| 21 | 35.78 | Behenic alcohol | 5.11 | 0.20 |
| 22 | 37.26 | Heneicosane | 1.17 | 0.15 |
| 23 | 38.32 | 4,8,12,16-Tetramethylheptadecan-4-olide | 0.89 | 0.04 |
| 24 | 38.80 | Tetradecane, 2,6,10-trimethyl- | 0.41 | 0.08 |
| 25 | 39.03 | Behenic alcohol | 4.19 | 0.09 |
| 26 | 40.29 | Octacosane | 1.65 | 0.15 |
| 27 | 41.17 | Tetradecanal | 0.44 | 0.06 |
| 28 | 42.04 | n-Tetracosanol-1 | 9.25 | 0.17 |
| 29 | 43.11 | Hentriacontane | 1.11 | 0.10 |
| 30 | 43.45 | 1-Docosanol, acetate | 0.64 | 0.07 |
| 31 | 44.02 | Oxirane, hexadecyl- | 0.74 | 0.05 |
| 32 | 44.20 | Squalene | 0.37 | 0.05 |
| 33 | 44.85 | 1-Octacosanol | 15.53 | 0.15 |
| 34 | 45.76 | Tetratetracontane | 1.30 | 0.10 |
| 35 | 46.11 | Triacontyl acetate | 0.50 | 0.01 |
| 36 | 46.67 | Hexadecanal | 1.50 | 0.10 |
| 37 | 47.47 | 1-Octacosanol | 9.69 | 0.19 |
| 38 | 50.06 | Vitamin E | 1.08 | 0.07 |
| 39 | 52.57 | Stigmasterol | 2.14 | 0.15 |
| 40 | 53.56 | β-Sitosterol | 8.50 | 0.10 |
| 41 | 55.11 | Stigmast-7-en-3-ol, (3β,5α,24S)- | 1.42 | 0.14 |
| 42 | 56.60 | Stigmasta-3,5-dien-7-one | 2.17 | 0.16 |
Appendix B

References
- Osmonali, B.B.; Vesselova, P.V.; Kudabayeva, G.M.; Ussen, S.; Abdildanov, D.S.; Friesen, N. Contributions to the flora of Kazakhstan, genera Arthrophytum and Haloxylon. Plant Syst. Evol. 2025, 311, 6. [Google Scholar] [CrossRef]
- Kubentayev, S.A.; Alibekov, D.T.; Perezhogin, Y.V.; Lazkov, G.A.; Kupriyanov, A.N.; Ebel, A.L.; Kubentayeva, B.B. Revised checklist of endemic vascular plants of Kazakhstan. PhytoKeys 2024, 238, 241. [Google Scholar] [CrossRef] [PubMed]
- Hosseyni, M.S.; Safaie, N.; Soltani, J.; Pasdaran, A. Endophytic association of bioactive and halotolerant Humicola fuscoatra with halophytic plants, and its capability of producing anthraquinone and anthranol derivatives. Antonie Leeuwenhoek 2020, 113, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.S.; Devi, S.; Ram, K.; Kumar, S.; Kumar, N.; Mann, A.; Chand, G. Halophytes: The plants of therapeutic medicine. In Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes; Springer: Singapore, 2019; pp. 271–287. [Google Scholar]
- Stanković, M.; Jakovljević, D. Phytochemical diversity of halophytes. In Handbook of Halophytes: From Molecules to Ecosystems Towards Biosaline Agriculture; Springer: Cham, Switzerland, 2021; pp. 2089–2114. [Google Scholar]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magne, C.; Isoda, H.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2011, 32, 289–326. [Google Scholar] [CrossRef]
- Todorović, M.; Zlatić, N.; Bojović, B.; Kanjevac, M. Biological properties of selected Amaranthaceae halophytic species: A review. Braz. J. Pharm. Sci. 2023, 58, e21229. [Google Scholar] [CrossRef]
- Dif, M.M.; Benchohra, H.A.; Dellal, A.; Akkal, N. Phytochemical study of phenolic compounds and biological activities of Arthrophytum schmittianum. J. Hortic. For. Biotechnol. 2022, 26, 49–55. [Google Scholar]
- Available online: https://powo.science.kew.org/taxon/165856-1 (accessed on 12 October 2025).
- Chao, H.C.; Najjaa, H.; Villareal, M.O.; Ksouri, R.; Han, J.; Neffati, M.; Isoda, H. Arthrophytum scoparium inhibits melanogenesis through the down-regulation of tyrosinase and melanogenic gene expressions in B16 melanoma cells. Exp. Dermatol. 2013, 22, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Osmonali, B.B.; Vesselova, P.V.; Kudabayeva, G.M.; Duisenbayev, S.; Taukebayev, O.; Zulpykharov, K.; Ussen, S.; Abdiildanov, D.S. Salt resistance of species of the Chenopodiaceae family (Amaranthaceae sl) in the desert part of the Syrdarya River Valley, Kazakhstan. Biodiversitas, J. Biol. Divers. 2024, 25, 4162–4170. [Google Scholar]
- Tleubayeva, M.I.; Abdullabekova, R.M.; Datkhayev, U.; Ishmuratova, M.Y.; Alimzhanova, M.B.; Kozhanova, K.K.; Seitaliyeva, A.M.; Zhakipbekov, K.S.; Iskakova, Z.B.; Serikbayeva, E.A.; et al. Investigation of CO2 Extract of Portulaca oleracea for Antioxidant Activity from Raw Material Cultivated in Kazakhstan. Int. J. Biomater. 2022, 2022, 6478977. [Google Scholar] [CrossRef]
- Ikhsanov, Y.S.; Nauryzbaev, M.; Musabekova, A.; Alimzhanova, M.; Burashev, E. Study of Nicotiana tabacum L. extraction, by methods of liquid and supercritical fluid extraction. J. Appl. Eng. Sci. 2019, 17, 338–353. [Google Scholar] [CrossRef]
- Toderich, K.N.; Terletskaya, N.V.; Zorbekova, A.N.; Saidova, L.T.; Ashimuly, K.; Mamirova, A.; Shuyskaya, E.V. Abiotic stresses utilisation for altering the natural antioxidant biosynthesis in Chenopodium quinoa, L. Russ. J. Plant Physiol. 2023, 70, 155. [Google Scholar] [CrossRef]
- Pavlov, N.V. Flora of Kazakhstan; Academy of Sciences of the Kazakh SSR: Alma-Ata, Kazakh Soviet Socialist Republic, 1960; Volume 3, p. 460. (In Russian) [Google Scholar]
- Polyakov, P.P.; Goloskokov, V.P. Family Chenopodiaceae. In The Flora of Kazakhstan, 3rd ed.; Pavlov, P.P., Ed.; Academy of Sciences of the Kazakh SSR: Alma-Ata, Kazakh Soviet Socialist Republic, 1960; pp. 179–320. (In Russian) [Google Scholar]
- Pratov, U.; Bondarenko, O.N.; Nabiev, M.M. Family Chenopodiaceae. In The Plant Identifier of Plants of Central Asia, III; FAN Publishing House: Tashkent, Union of Soviet Socialist Republics, 1972; pp. 29–137. (In Russian) [Google Scholar]
- Sukhorukov, A.P.; Kushunina, M.A.; Stepanova, N.Y.; Kalmykova, O.G.; Golovanov, Y.M.; Sennikov, A.N. Taxonomic inventory and distributions of Chenopodiaceae (Amaranthaceae sl) in Orenburg Region, Russia. Biodivers. Data J. 2024, 12, e121541. [Google Scholar] [CrossRef] [PubMed]
- Kaddour, S.M.; Zerargui, F.; Arrar, L.; Baghiani, A. Acute, sub-acute and antioxidant activities of Arthrophytum scoparium aerial parts. Int. J. Pharm. Sci. Res. 2019, 10, 4167–4175. [Google Scholar]
- Kaddour, S.M.; Arrar, L.; Baghiani, A. Anti-inflammatory potential evaluation (in-vitro and in-vivo) of Arthrophytum scoparium aerial part. J. Drug Deliv. Ther. 2020, 10, 213–218. [Google Scholar]
- Ouadja, B.; Katawa, G.; Toudji, G.A.; Layland, L.; Gbekley, E.H.; Ritter, M.; Karou, S.D. Anti-inflammatory antibacterial antioxidant activities of Chenopodium ambrosioides L. (Chenopodiaceae) extracts. J. Appl. Biosci. 2021, 162, 16764. [Google Scholar] [CrossRef]
- Zohra, M.; Fawzia, A. Hemolytic activity of different herbal extracts used in Algeria. Int. J. Pharm. Sci. Res. 2014, 5, 495–500. [Google Scholar]
- Boubrima, Y.; Gouzi, H.; Stocker, P.; Kameli, A.; Yousfi, M. Inhibitory effect of phenolic extracts of four Algerian Atlas Saharan plants on α-glucosidase activity. Curr. Enzym. Inhib. 2018, 14, 196–202. [Google Scholar] [CrossRef]
- Kalai, F.Z.; Oueslati, S.; Ksouri, R.; Isoda, H. Antioxidant and antiadipogenic potential of four Tunisian extremophiles (Mesembryanthemum edule, Atriplex inflata, Rantherium suaveolens, Arthrophytum scoparium). Adv. Nutr. Food Sci. 2022, ANAFS-229. [Google Scholar]
- Benslama, A.; Harrar, A. Free radicals scavenging activity and reducing power of two Algerian Sahara medicinal plants extracts. Int. J. Herb. Med. 2016, 4, 158–161. [Google Scholar] [CrossRef]
- Shegebayev, Z.; Turgumbayeva, A.; Datkhayev, U.; Zhakipbekov, K.; Kalykova, A.; Kartbayeva, E.; Beyatli, A.; Tastambek, K.; Altynbayeva, G.; Dilbarkhanov, B.; et al. Pharmacological properties of four plant species of the genus Anabasis (Amaranthaceae). Molecules 2023, 28, 4454. [Google Scholar] [CrossRef] [PubMed]
- Smach, M.A.; Hafsa, J.; Charfeddine, B.; Dridi, H.; Limem, K.; Jihene, B.A. Arthrophytum scoparium extract improves memory impairment and affects acetylcholinesterase activity in mice brain. Curr. Pharm. Biotechnol. 2020, 21, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Datkhayev, U.; Shegebayev, Z.; Turgumbayeva, A.; Beyatli, A.; Diyas, M.; Zhakipbekov, K.; Shepetov, A.; Datkayeva, G.; Kodasbaev, A.; Pazilov, S.; et al. GC–MS analysis, HPLC–UV analysis, antimicrobial and antioxidant activities of extracts of wild-growing Anabasis salsa native to Kazakhstan desert lands. Phytochem. Rev. 2024, 24, 2651–2675. [Google Scholar] [CrossRef]
- Ataev, E.A. On some plant communities in the Kugitangtau piedmont plain and their relationship with soil type. Izv. Akad. Nauk. Turkm. SSR Biol. Nauk. 1973, 37–41. [Google Scholar]
- Li, C.J.; Li, Y.; Ma, J.; Fan, L.; Wang, Q. Spatial heterogeneity of soil chemical properties between Haloxylon persicum and Haloxylon ammodendron populations. J. Arid. Land 2010, 2, 257–265. [Google Scholar]
- Nadaf, M.; Abad, M.H.K.; Gholami, A.; Yazdi, M.E.T.; Iriti, M.; Mottaghipisheh, J. Phenolic content antioxidant activity of different Iranian populations of Anabasis aphylla L. Nat. Prod. Res. 2024, 38, 1606–1610. [Google Scholar] [CrossRef]
- Snieckus, V. The distribution of indole alkaloids in plants. In The Alkaloids: Chemistry and Physiology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 11. [Google Scholar]
- Glasby, J.S. Encyclopedia of the Alkaloids; Plenum Press: New York, NY, USA, 1977. [Google Scholar]
- Belabdelli, F.; Zohra, E.K.F.; Benaoula, S.A.; Bekhti, N.; Piras, A.; Taleb, I.; Majda, S.R. Phytoconstituents effects of traditionally used herbs on dissolution and inhibition of kidney stones (CaOx). Farmacia 2023, 71, 1224–1231. [Google Scholar] [CrossRef]
- Hamad, M.N. Investigation of alkaloids of Anabasis aphylla (Chenopodiaceae). Ibn Al-Haitham J. Pure Appl. Sci. 2010, 23, 297–304. [Google Scholar]
- Shakeri, A.; Hazeri, N.; Vlizadeh, J.; Ghasemi, A.; Tavallaei, F.Z. Phytochemical screening antimicrobial antioxidant activities of Anabasis aphylla L. extracts. Kragujev. J. Sci. 2012, 34, 71–78. [Google Scholar]
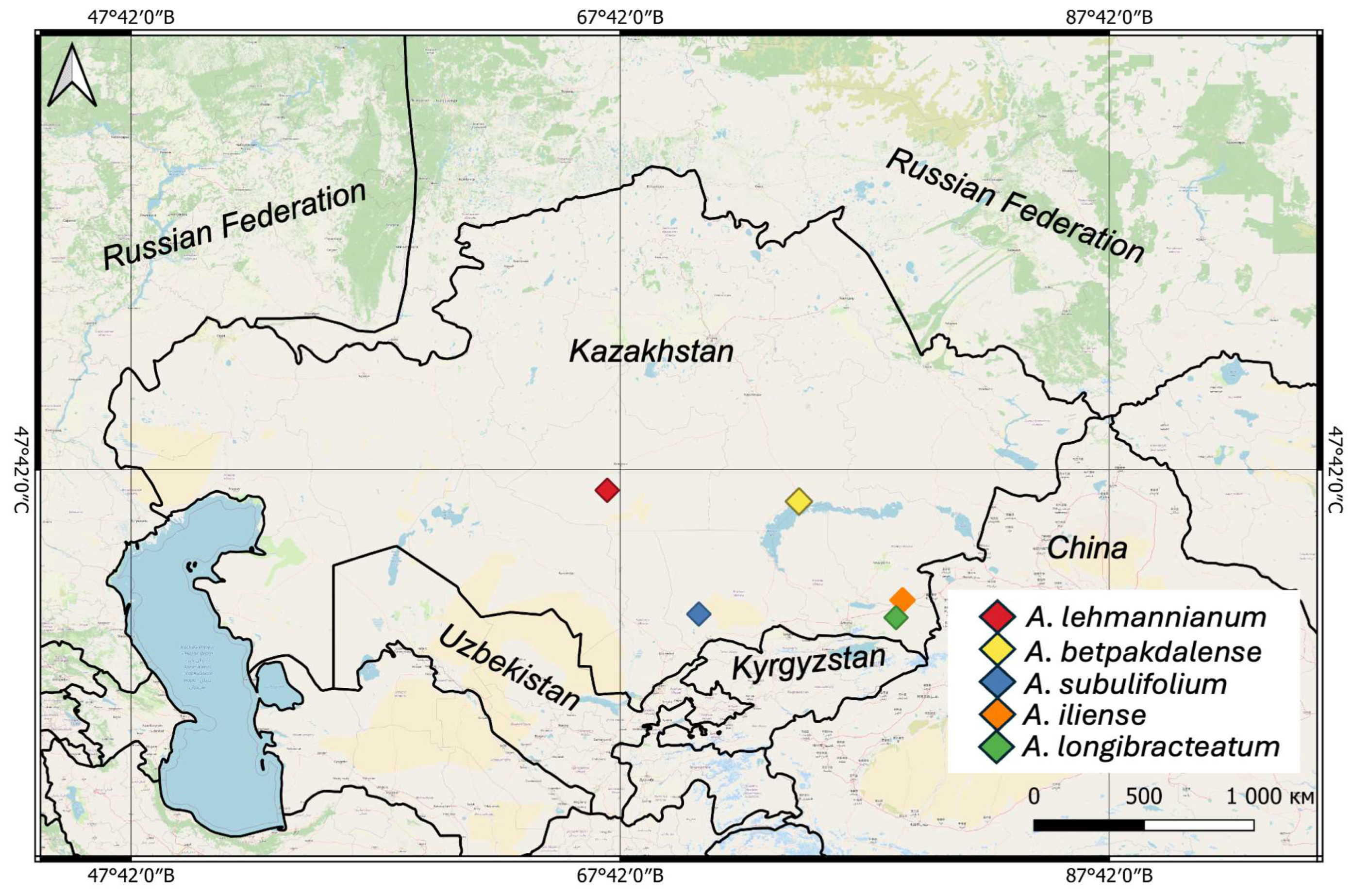
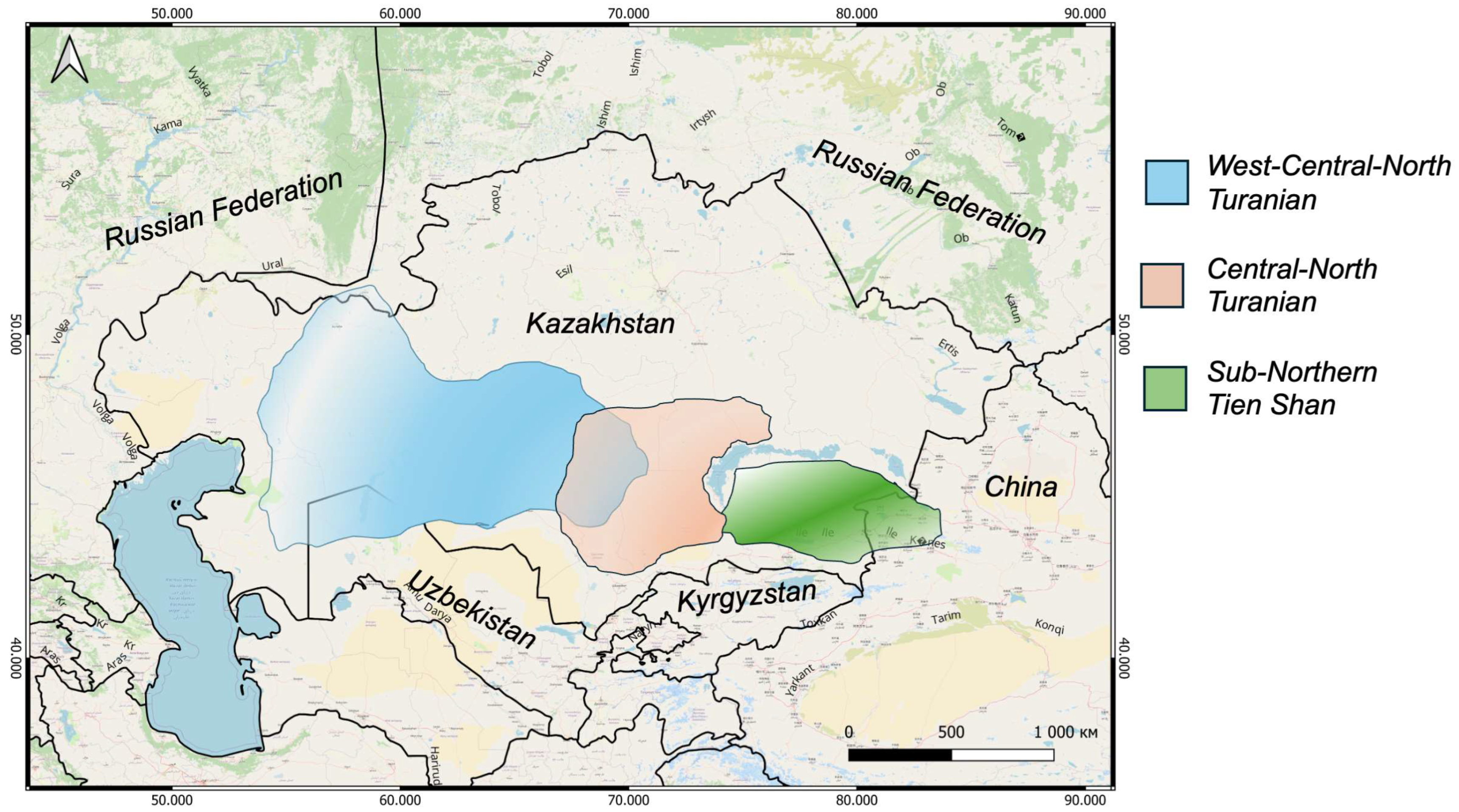
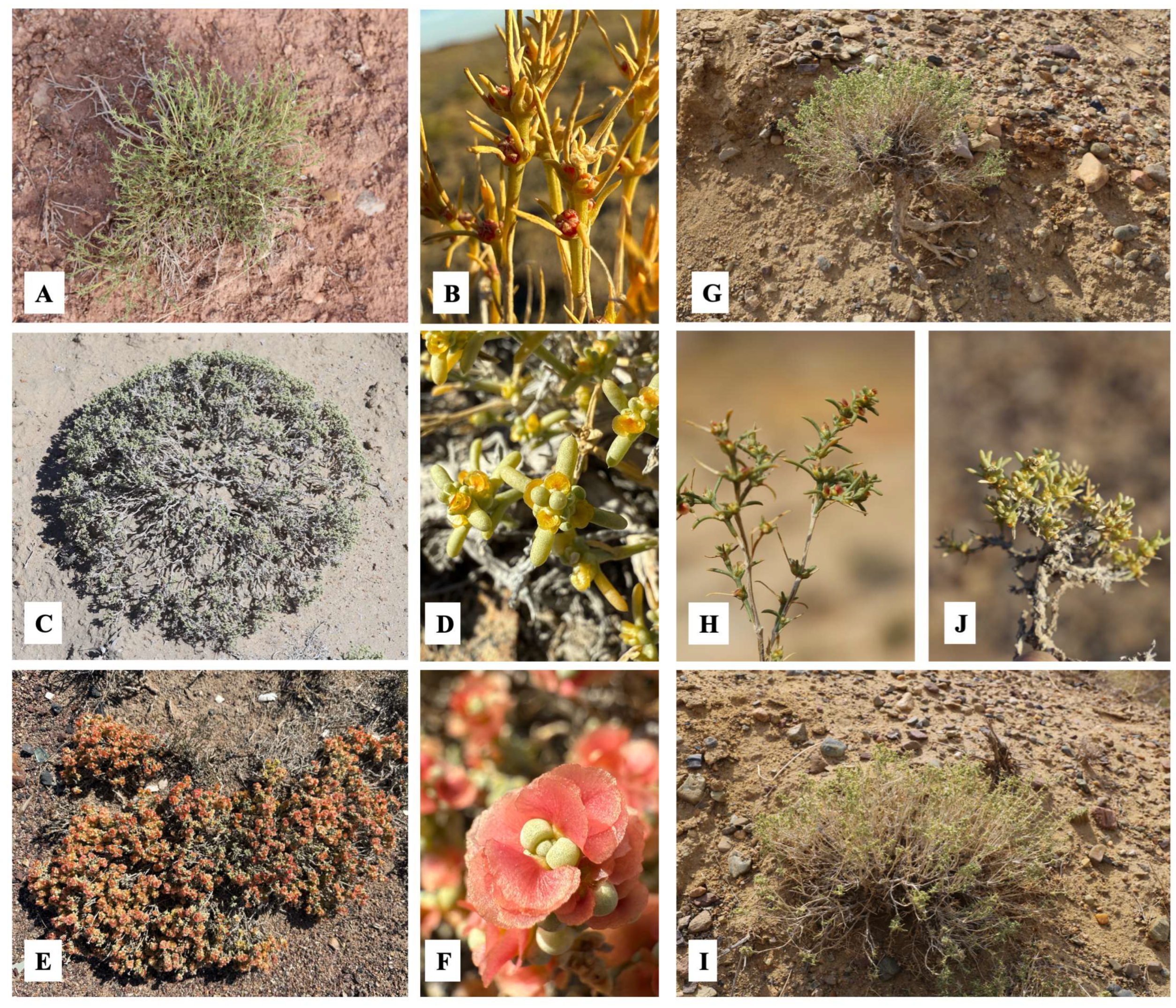

| № | Species | N | E | Administrative Districts | Voucher |
|---|---|---|---|---|---|
| 1 | A. lehmannianum | 47.133605 | 67.169902 | Ulytau region | 0003631 |
| 2 | A. iliense | 43.982948 | 79.242801 | Almaty Region, along the highway towards Chundzha | 0003629 |
| 3 | A. longibracteatum | 43.46725749 | 78.97789255 | Almaty Region, along the highway towards Chundzha | 0003635 |
| 4 | A. subulifolium | 43.57220281 | 70.91093101 | Zhambyl Region, Akkol | 0003622 |
| 5 | A. betpakdalense | 46.822778 | 75.008056 | Karaganda Region, 1 km from the city of Balkhash | 0003621 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ussen, S.; Vesselova, P.V.; Kudabayeva, G.M.; Sergazina, M.M.; Alimzhanova, M.B. Comparative Phytochemical Analysis of Five Species of the Genus Arthrophytum Schrenk (Amaranthaceae) from the Flora of Kazakhstan. Metabolites 2025, 15, 800. https://doi.org/10.3390/metabo15120800
Ussen S, Vesselova PV, Kudabayeva GM, Sergazina MM, Alimzhanova MB. Comparative Phytochemical Analysis of Five Species of the Genus Arthrophytum Schrenk (Amaranthaceae) from the Flora of Kazakhstan. Metabolites. 2025; 15(12):800. https://doi.org/10.3390/metabo15120800
Chicago/Turabian StyleUssen, Serikbay, Polina V. Vesselova, Gulmira M. Kudabayeva, Meruyert M. Sergazina, and Mereke B. Alimzhanova. 2025. "Comparative Phytochemical Analysis of Five Species of the Genus Arthrophytum Schrenk (Amaranthaceae) from the Flora of Kazakhstan" Metabolites 15, no. 12: 800. https://doi.org/10.3390/metabo15120800
APA StyleUssen, S., Vesselova, P. V., Kudabayeva, G. M., Sergazina, M. M., & Alimzhanova, M. B. (2025). Comparative Phytochemical Analysis of Five Species of the Genus Arthrophytum Schrenk (Amaranthaceae) from the Flora of Kazakhstan. Metabolites, 15(12), 800. https://doi.org/10.3390/metabo15120800






