Non-Targeted Plasma Lipidomic Profiling in Late Pregnancy and Early Postpartum Stages: An Observational Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
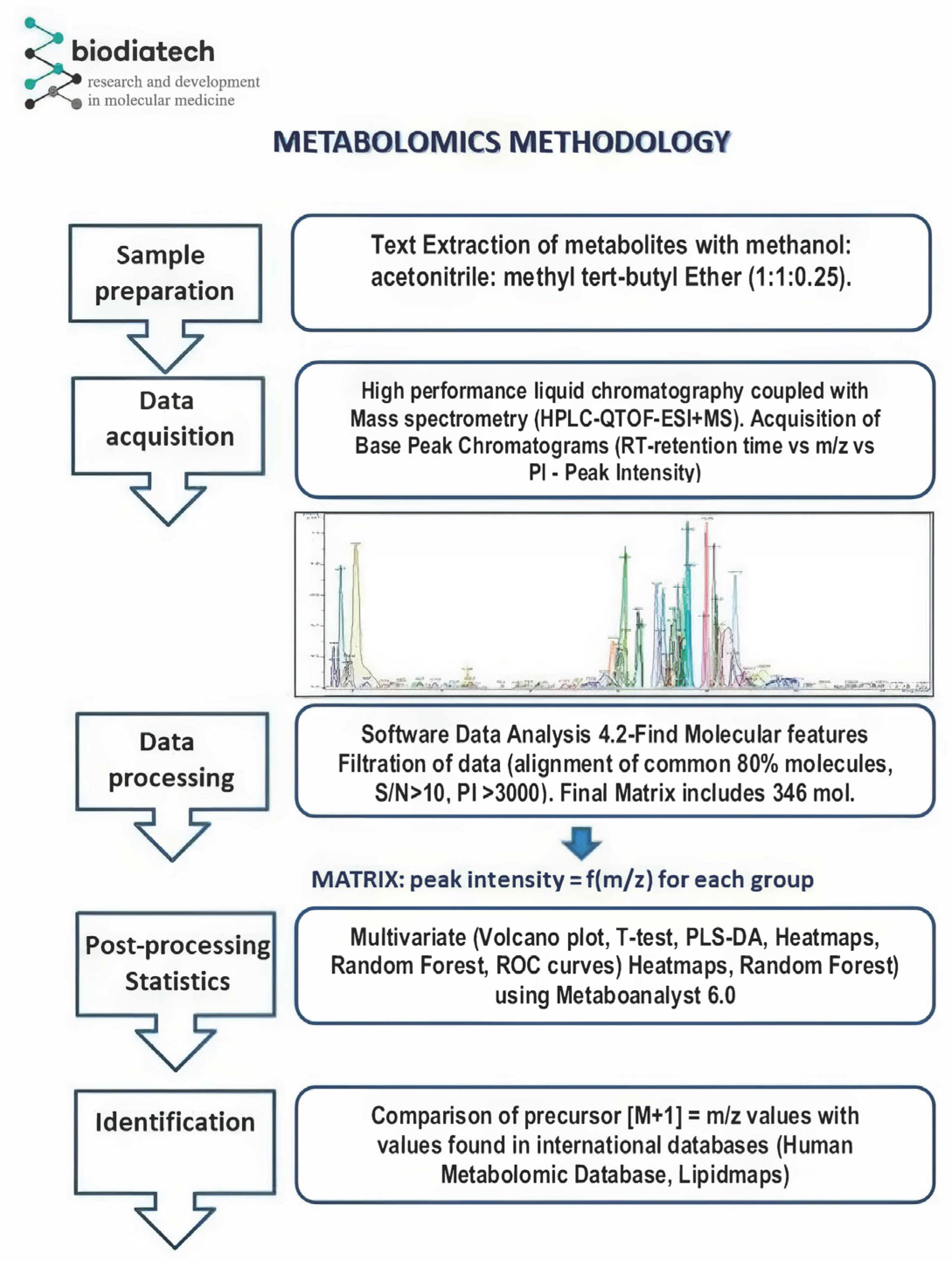
2.2. Plasma Sample Preparation
2.3. HPLC-QTOF-ESI+MS Instrumentation and Analysis
2.4. Data Processing and Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of Study Participants
3.2. PLS-DA (Partial Least Squares Discriminant Analysis)
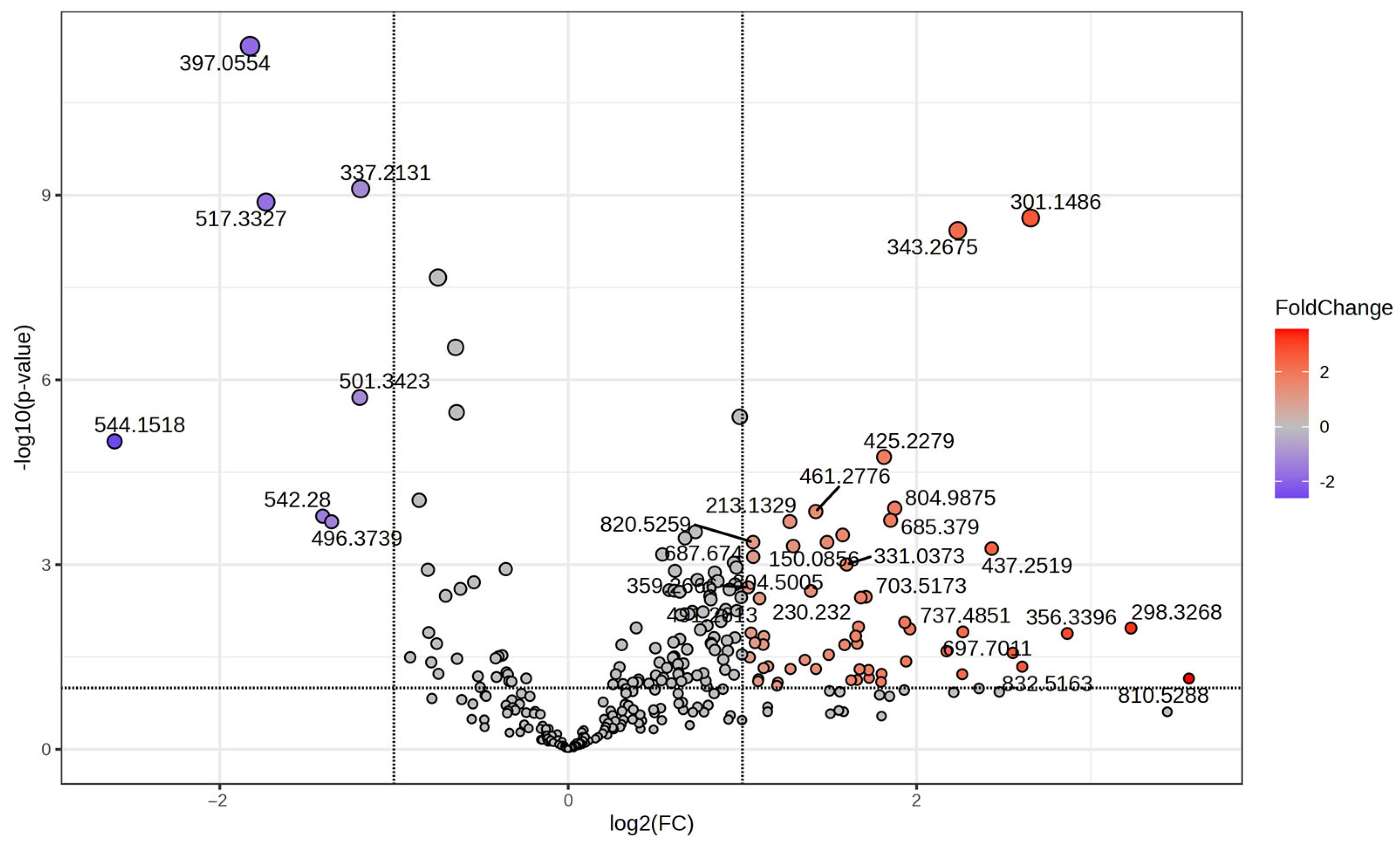
3.3. Volcano Plot and t-Test Analysis
3.4. Random Forest
3.5. Heatmap
3.6. Receiver Operating Characteristic (ROC) Analysis—Top Discriminating Lipids
3.7. Integrative Biomarker Panel and Lipid Class Categorization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kepley, J.M.; Bates, K.; Mohiuddin, S.S. Physiology, Maternal Changes. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539766/ (accessed on 5 September 2025).
- Wild, R.; Feingold, K.R. Effect of Pregnancy on Lipid Metabolism and Lipoprotein Levels. Available online: https://www.ncbi.nlm.nih.gov/books/NBK498654/ (accessed on 5 September 2025).
- Rich-Edwards, J.W.; Fraser, A.; Lawlor, D.A.; Catov, J.M. Pregnancy characteristics and women’s future cardiovascular health: An underused opportunity to improve women’s health? Epidemiol. Rev. 2014, 36, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Blaauwendraad, S.M.; Wahab, R.J.; van Rijn, B.B.; Koletzko, B.; Jaddoe, V.W.V.; Gaillard, R. Associations of Early Pregnancy Metabolite Profiles with Gestational Blood Pressure Development. Metabolites 2022, 12, 1169. [Google Scholar] [CrossRef] [PubMed]
- Kenny, L.C.; Broadhurst, D.; Brown, M.; Dunn, W.B.; Redman, C.W.G.; Kell, D.B.; Baker, P.N. Detection and Identification of Novel Metabolomic Biomarkers in Preeclampsia. Reprod. Sci. 2008, 15, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Skytte, H.N.; Christensen, J.J.; Gunnes, N.; Holven, K.B.; Lekva, T.; Henriksen, T.; Michelsen, T.M.; Roland, M.C.P. Metabolic profiling of pregnancies complicated by preeclampsia: A longitudinal study. Acta Obstet. Gynecol. Scand. 2023, 102, 334–343. [Google Scholar] [CrossRef]
- Bartho, L.A.; McKeating, D.R.; Walker, S.P.; Nijagal, B.; MacDonald, T.M.; Pritchard, N.; Hannan, N.J.; Perkins, A.V.; Tong, S.; Kaitu’u-Lino, T.J. Plasma metabolites are altered before and after diagnosis of preeclampsia or fetal growth restriction. Sci. Rep. 2024, 14, 15829. [Google Scholar] [CrossRef]
- Lee, S.M.; Kang, Y.; Lee, E.M.; Jung, Y.M.; Hong, S.; Park, S.J.; Park, C.-W.; Norwitz, E.R.; Lee, D.Y.; Park, J.S. Metabolomic biomarkers in midtrimester maternal plasma can accurately predict the development of preeclampsia. Sci. Rep. 2020, 10, 16142. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Lin, L. Maternal lipid profiles in women with and without gestational diabetes mellitus. Medicine 2019, 98, e15320. [Google Scholar] [CrossRef]
- Zhan, Y.; Wang, J.; He, X.; Huang, M.; Yang, X.; He, L.; Qiu, Y.; Lou, Y. Plasma metabolites, especially lipid metabolites, are altered in pregnant women with gestational diabetes mellitus. Clin. Chim. Acta 2021, 517, 139–148. [Google Scholar] [CrossRef]
- O’Neill, K.; Alexander, J.; Azuma, R.; Xiao, R.; Snyder, N.W.; Mesaros, C.A.; Blair, I.A.; Pinney, S.E. Gestational Diabetes Alters the Metabolomic Profile in 2nd Trimester Amniotic Fluid in a Sex-Specific Manner. Int. J. Mol. Sci. 2018, 19, 2696. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Wu, P.; Ye, Y.; Sun, F.; Yang, X.; Lu, Q.; Yuan, J.; Liu, Y.; Zeng, H.; et al. Plasma lipidomics in early pregnancy and risk of gestational diabetes mellitus: A prospective nested case-control study in Chinese women. Am. J. Clin. Nutr. 2021, 114, 1763–1773. [Google Scholar] [CrossRef]
- Lv, Y.; Xu, L.; He, Z.; Liu, X.; Guo, Y. The association between pregnancy levels of blood lipids and the risk of preterm birth. Sci. Rep. 2024, 14, 10800. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.T.; Ashrap, P.; Watkins, D.J.; Mukherjee, B.; Rosario, Z.; Vélez-Vega, C.M.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Maternal lipidomic signatures in relation to spontaneous preterm birth and large-for-gestational age neonates. Sci. Rep. 2021, 11, 8115. [Google Scholar] [CrossRef] [PubMed]
- Morillon, A.C.; Yakkundi, S.; Thomas, G.; Gethings, L.A.; Langridge, J.I.; Baker, P.N.; Kenny, L.C.; English, J.A.; McCarthy, F.P. Association between phospholipid metabolism in plasma and spontaneous preterm birth: A discovery lipidomic analysis in the Cork pregnancy cohort. Metabolomics 2020, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Lager, S.; Powell, T.L. Regulation of nutrient transport across the placenta. J. Pregnancy 2012, 2012, 179827. [Google Scholar] [CrossRef]
- Song, Y.; Lu, R.; Yu, G.; Rahman, M.L.; Chen, L.; Zhu, Y.; Tsai, M.Y.; Fiehn, O.; Chen, Z.; Zhang, C. Longitudinal lipidomic profiles during pregnancy and associations with neonatal anthropometry: Findings from a multiracial cohort. EBioMedicine 2023, 98, 104881. [Google Scholar] [CrossRef]
- Burugupalli, S.; Smith, A.A.T.; Oshlensky, G.; Huynh, K.; Giles, C.; Wang, T.; George, A.; Paul, S.; Nguyen, A.; Duong, T.; et al. Ontogeny of circulating lipid metabolism in pregnancy and early childhood–a longitudinal population study. eLife 2022, 11, e72779. [Google Scholar] [CrossRef]
- Chen, L.; Mir, S.A.; Bendt, A.K.; Chua, E.W.L.; Narasimhan, K.; Tan, K.M.-L.; Loy, S.L.; Tan, K.H.; Shek, L.P.; Chan, J.; et al. Plasma lipidomic profiling reveals metabolic adaptations to pregnancy and signatures of cardiometabolic risk: A preconception and longitudinal cohort study. BMC Med. 2023, 21, 53. [Google Scholar] [CrossRef]
- Handelman, S.K.; Romero, R.; Tarca, A.L.; Pacora, P.; Ingram, B.; Maymon, E.; Chaiworapongsa, T.; Hassan, S.S.; Erez, O. The plasma metabolome of women in early pregnancy differs from that of non-pregnant women. PLoS ONE 2019, 14, e0224682. [Google Scholar] [CrossRef]
- Morel, Y.; Roucher, F.; Plotton, I.; Goursaud, C.; Tardy, V.; Mallet, D. Evolution of steroids during pregnancy: Maternal, placental and fetal synthesis. Ann. Endocrinol. 2016, 77, 82–89. [Google Scholar] [CrossRef]
- Orczyk-Pawilowicz, M.; Jawien, E.; Deja, S.; Hirnle, L.; Zabek, A.; Mlynarz, P. Metabolomics of Human Amniotic Fluid and Maternal Plasma during Normal Pregnancy. PLoS ONE 2016, 11, e0152740. [Google Scholar] [CrossRef]
- Romero, R.; Erez, O.; Maymon, E.; Chaemsaithong, P.; Xu, Z.; Pacora, P.; Chaiworapongsa, T.; Done, B.; Hassan, S.S.; Tarca, A.L. The maternal plasma proteome changes as a function of gestational age in normal pregnancy: A longitudinal study. Am. J. Obstet. Gynecol. 2017, 217, 67.e1–67.e21. [Google Scholar] [CrossRef] [PubMed]
- Mitro, S.D.; Wu, J.; Rahman, M.L.; Cao, Y.; Zhu, Y.; Chen, Z.; Chen, L.; Li, M.; Hinkle, S.N.; Bremer, A.A.; et al. Longitudinal Plasma Metabolomics Profile in Pregnancy—A Study in an Ethnically Diverse U.S. Pregnancy Cohort. Nutrients 2021, 13, 3080. [Google Scholar] [CrossRef] [PubMed]
- Tinius, R.A.; Yoho, K.; Blankenship, M.M.; Maples, J.M. Postpartum Metabolism: How Does It Change from Pregnancy and What Are the Potential Implications? Int. J. Womens Health 2021, 13, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Matsukawa, N.; Saigusa, D.; Motoike, I.N.; Ono, C.; Okamura, Y.; Onuma, T.; Takahashi, Y.; Sakai, M.; Kudo, H.; et al. Plasma metabolic disturbances during pregnancy and postpartum in women with depression. iScience 2022, 25, 105666. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Chen, Y.; Li, E.M.; Xu, L.Y. Guide to Metabolomics Analysis: A Bioinformatics Workflow. Metabolites 2022, 12, 357. [Google Scholar] [CrossRef]
- Antonelli, J.; Claggett, B.L.; Henglin, M.; Kim, A.; Ovsak, G.; Kim, N.; Deng, K.; Rao, K.; Tyagi, O.; Watrous, J.D.; et al. Statistical Workflow for Feature Selection in Human Metabolomics Data. Metabolites 2019, 9, 143. [Google Scholar] [CrossRef]
- Chen, T.; Cao, Y.; Zhang, Y.; Liu, J.; Bao, Y.; Wang, C.; Jia, W.; Zhao, A. Random forest in clinical metabolomics for phenotypic discrimination and biomarker selection. Evid.-Based Complement. Altern. Med. 2013, 2013, 298183. [Google Scholar] [CrossRef]
- Kumar, N.; Hoque, M.A.; Sugimoto, M. Robust volcano plot: Identification of differential metabolites in the presence of outliers. BMC Bioinform. 2018, 19, 128. [Google Scholar] [CrossRef]
- Want, E.J. LC-MS untargeted analysis. Methods Mol. Biol. 2018, 1738, 99–116. [Google Scholar] [CrossRef]
- Noyola-Martínez, N.; Halhali, A.; Barrera, D. Steroid hormones and pregnancy. Gynecol. Endocrinol. 2019, 35, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Duttaroy, A.K.; Basak, S. Maternal Fatty Acid Metabolism in Pregnancy and Its Consequences in the Feto-Placental Development. Front. Physiol. 2021, 12, 787848. [Google Scholar] [CrossRef]
- Nagamatsu, T.; Iwasawa-Kawai, Y.; Ichikawa, M.; Kawana, K.; Yamashita, T.; Osuga, Y.; Fujii, T.; Schust, D.J. Emerging roles for lysophospholipid mediators in pregnancy. Am. J. Reprod. Immunol. 2014, 72, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, T.; Yasuda, K.; Neya, M.; Okada, H.; Tokumura, A. Lysophosphatidic acid production from lysophosphatidylcholine by lysophospholipase D activity of autotaxin in plasma of women with normal and adverse pregnancies. Prostaglandins Other Lipid Mediat. 2022, 163, 106670. [Google Scholar] [CrossRef] [PubMed]
- Fakhr, Y.; Brindley, D.N.; Hemmings, D.G. Physiological and pathological functions of sphingolipids in pregnancy. Cell. Signal. 2021, 85, 110041. [Google Scholar] [CrossRef]
- Lantzanaki, M.; Vavilis, T.; Harizopoulou, V.C.; Bili, H.; Goulis, D.G.; Vavilis, D. Ceramides during Pregnancy and Obstetrical Adverse Outcomes. Metabolites 2023, 13, 1136. [Google Scholar] [CrossRef]
- Saadat, N.; Aguate, F.; Nowak, A.L.; Hyer, S.; Lin, A.B.; Decot, H.; Koch, H.; Walker, D.S.; Lydic, T.; Padmanabhan, V.; et al. Changes in Lipid Profiles with the Progression of Pregnancy in Black Women. J. Clin. Med. 2024, 13, 2795. [Google Scholar] [CrossRef]
- Zhang, Z.; Lai, M.; Piro, A.L.; Alexeeff, S.E.; Allalou, A.; Röst, H.L.; Dai, F.F.; Wheeler, M.B.; Gunderson, E.P. Intensive lactation among women with recent gestational diabetes significantly alters the early postpartum circulating lipid profile: The SWIFT study. BMC Med. 2021, 19, 241. [Google Scholar] [CrossRef]
- Scommegna, A.; Burd, L.; Goodman, C.; Bieniarz, J. The Effect of Pregnenolone Sulfate on Uterine Contractility. Am. J. Obstet. Gynecol. 1970, 108, 1023–1029. [Google Scholar] [CrossRef]
- Tsutsui, K.; Haraguchi, S. Pregnenolone Sulfate. In Handbook of Hormones, 2nd ed.; Hinoroni, A., Kazuyoshi, U., Shinji, N., Eds.; Academic Press: Cambridge, MA, USA, 2021; Subchapter 124A; pp. 959–960. [Google Scholar]
- Pasqualini, J.R.; Kincl, F.A. Biosynthesis and Metabolism of Pregnenolone, 17-Hydroxypregnenolone and Its Sulfates. In Hormones in the Fetus; Elsevier Ltd.: Oxford, UK, 1985; Chapter 2.2; pp. 73–172. [Google Scholar] [CrossRef]
- Hashimoto, M.; Hossain, S. Fatty Acids: From Membrane Ingredients to Signaling Molecules. In Biochemistry and Health Benefits of Fatty Acids; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Klemetti, M.M.; Alahari, S.; Post, M.; Caniggia, I. Distinct Changes in Placental Ceramide Metabolism Characterize Type 1 and 2 Diabetic Pregnancies with Fetal Macrosomia or Preeclampsia. Biomedicines 2023, 11, 932. [Google Scholar] [CrossRef]
- Herrera, E.; Ortega-Senovilla, H. Lipid metabolism during pregnancy and its implications for fetal growth. Curr. Pharm. Biotechnol. 2014, 15, 24–31. [Google Scholar] [CrossRef]
- Delhaes, F.; Giza, S.A.; Koreman, T.; Eastabrook, G.; McKenzie, C.A.; Bedell, S.; Regnault, T.R.; de Vrijer, B. Altered maternal and placental lipid metabolism and fetal fat development in obesity: Current knowledge and advances in non-invasive assessment. Placenta 2018, 69, 118–124. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, R.; Zhang, Y.; Yuan, W. Early potential metabolic biomarkers of primary postpartum haemorrhage based on serum metabolomics. Ginekol. Pol. 2019, 90, 607–615. [Google Scholar] [CrossRef]
- Troisi, J.; Symes, S.J.K.; Lombardi, M.; Cavallo, P.; Colucci, A.; Scala, G.; Adair, D.C.; Guida, M.; Richards, S.M. Placental Metabolomics of Fetal Growth Restriction. Metabolites 2023, 13, 235. [Google Scholar] [CrossRef]
- Birchenall, K.A.; Welsh, G.I.; López Bernal, A. Metabolite Changes in Maternal and Fetal Plasma Following Spontaneous Labour at Term in Humans Using Untargeted Metabolomics Analysis: A Pilot Study. Int. J. Environ. Res. Public Health 2019, 16, 1527. [Google Scholar] [CrossRef]





| Variable | Pregnant Group (G) | Postpartum Group (L) | p Value |
|---|---|---|---|
| Participants | 65 | 42 | - |
| Maternal age (years) | 27.9 ± 5 | 28.9 ± 5.9 | 0.366 |
| Gestational age at sample (weeks) | 34 ± 3.6 | - | - |
| Height (cm) | 163.6 ± 2.7 | 162.9 ± 3.7 | 0.293 |
| Pre-pregnancy weight (kg) | 63.7 ± 5.4 | 62.5 ± 7.3 | 0.362 |
| Pre-pregnancy BMI (body mass index) (kg/m2) | 23.7 ± 1.6 | 23.5 ± 2.6 | 0.656 |
| BMI category-Normal (19–25) | 74.2% | 73.8% | 0.960 |
| BMI category-Overweight (25–30) | 25.8% | 26.2% | 0.960 |
| Education-Bachelor’s degree | 22.7% | 11.9% | 0.269 |
| Education-post-secondary studies | 18.2% | 30.9% | 0.269 |
| Education-High school graduate | 39.4% | 42.9% | 0.269 |
| Education-General education | 19.7% | 14.3% | 0.269 |
| Marital status-Single | 28.8% | 31% | 0.810 |
| Marital status-Married | 71.2% | 69% | 0.810 |
| Tobacco use-Yes | 33.3% | 21.4% | 0.182 |
| Tobacco use-No | 66.7% | 78.6% | 0.182 |
| Alcohol use-No | 62.1% | 73.8% | 0.209 |
| Alcohol use-Sometimes | 37.9% | 26.2% | 0.209 |
| Birth way-Natural birth | 45.5% | 40.5% | 0.611 |
| Birth way-Cesarean section | 54.5% | 59.5% | 0.611 |
| Infant sex-Female | 53% | 52.4% | 0.947 |
| Infant sex-Male | 47% | 47.6% | 0.947 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traila, A.; Abu-Awwad, S.-A.; Marta, C.-I.; Bacanoiu, M.V.; Maghiari, A.L.; Abu-Awwad, A.; Craina, M.L. Non-Targeted Plasma Lipidomic Profiling in Late Pregnancy and Early Postpartum Stages: An Observational Comparative Study. Metabolites 2025, 15, 798. https://doi.org/10.3390/metabo15120798
Traila A, Abu-Awwad S-A, Marta C-I, Bacanoiu MV, Maghiari AL, Abu-Awwad A, Craina ML. Non-Targeted Plasma Lipidomic Profiling in Late Pregnancy and Early Postpartum Stages: An Observational Comparative Study. Metabolites. 2025; 15(12):798. https://doi.org/10.3390/metabo15120798
Chicago/Turabian StyleTraila, Alexandra, Simona-Alina Abu-Awwad, Carmen-Ioana Marta, Manuela Violeta Bacanoiu, Anca Laura Maghiari, Ahmed Abu-Awwad, and Marius Lucian Craina. 2025. "Non-Targeted Plasma Lipidomic Profiling in Late Pregnancy and Early Postpartum Stages: An Observational Comparative Study" Metabolites 15, no. 12: 798. https://doi.org/10.3390/metabo15120798
APA StyleTraila, A., Abu-Awwad, S.-A., Marta, C.-I., Bacanoiu, M. V., Maghiari, A. L., Abu-Awwad, A., & Craina, M. L. (2025). Non-Targeted Plasma Lipidomic Profiling in Late Pregnancy and Early Postpartum Stages: An Observational Comparative Study. Metabolites, 15(12), 798. https://doi.org/10.3390/metabo15120798







